Abstract
Structural changes of MoO3 thin films deposited on thick copper substrates upon annealing at different temperatures were investigated via ex situ X-Ray Absorption Spectroscopy (XAS). From the analysis of the X-ray Absorption Near-Edge Structure (XANES) pre-edge and Extended X-ray Absorption Fine Structure (EXAFS), we show the dynamics of the structural order and of the valence state. As-deposited films were mainly disordered, and ordering phenomena did not occur for annealing temperatures up to 300 °C. At ~350 °C, a dominant α-MoO3 crystalline phase started to emerge, and XAS spectra ruled out the formation of a molybdenum dioxide phase. A further increase of the annealing temperature to ~500 °C resulted in a complex phase transformation with a concurrent reduction of Mo6+ ions to Mo4+. These original results suggest the possibility of using MoO3 as a hard, protective, transparent, and conductive material in different technologies, such as accelerating copper-based devices, to reduce damage at high gradients.
1. Introduction
Molybdenum-based oxides are amongst the most adaptable and functional oxides due to their unique characteristics and tunable properties [1,2,3]. Molybdenum trioxide (MoO3) is one of the thermodynamically stable molybdenum oxides, with the orthorhombic crystal structure α-MoO3 [3,4]. The latter phase consists of a set of layers, each one containing distorted MoO6 octahedra, characterized by three different oxygen sites: a single, a double, and a triple shared site. The dipole nature of the α-MoO3 layers is at the origin of its relatively high work function (WF) of about 6.5 eV [1,5]. Moreover, despite its insulator nature and high WF, previous studies pointed out that thin MoO3 films may exhibit a conductive behavior in the presence of defects and oxygen vacancies [6,7,8,9,10] or via interaction with a metallic substrate such as copper [7].
MoO3 is used in solar cells, batteries, and organic light-emitting diodes (OLEDs), and it is a promising material for protective coatings of accelerating radiofrequency (RF) cavities [5]. In fact, due to the low WF, the performance and the lifetime of a copper RF cavity are strongly affected by breakdown phenomena and thermal stress generated by electron emission from the surface [11]. A high WF conductive coating could be used to reduce these detrimental phenomena, extending the lifetime of the device and allowing it to operate at higher electric fields [1,5,11,12]. Hence, MoO3 coating can significantly enhance the electronic and mechanical properties of copper-based devices via its high WF and relatively higher hardness, compared with copper [5,13,14,15], without affecting the surface conductivity. Because the thermal evaporation deposition produces disordered and poorly adhesive MoO3 coatings [5], with the aim to obtain ordered MoO3 coatings, we tried to optimize the annealing procedure. This method minimized the formation of MoO2, which must be avoided, since the slightly off-axis position of Mo atoms in the MoO2 phase causes the lowering of the WF (~4.6 eV) [3,16]. To establish the optimal annealing temperature for an ordered MoO3 film on a copper substrate, we investigated the structural evolution of annealed MoO3 films by X-ray Absorption Spectroscopy (XAF) in X-ray Absorption Near-Edge Structure (XANES) and in the Extended X-ray Absorption Fine Structure (EXAFS) regions [17,18,19,20].
2. Materials and Methods
Molybdenum trioxide films were deposited in a dedicated vacuum sublimation set up on 5 mm-thick copper substrates [16]. Molybdenum trioxide powder (99.97% trace metal basis, Sigma-Aldrich®, St. Louis, MO, USA) was heated up to 600 °C in a tungsten crucible inside the evaporation chamber with a base pressure of 10−5 mbar. In order to anneal the coating without oxidizing the copper substrate, a heat treatment in a low vacuum environment was performed, with a base pressure of 5 × 10−1 mbar. We also considered a fast heating procedure to minimize the Mo reduction process and to further reduce copper oxidization. This setup allowed us to reach a temperature of up to 500 °C in less than 10 min, with a constant heating rate of ~1 °C/s. After a brief temperature decrease, the samples were exposed to air at 200 °C in order to increase the amount of oxygen in the film [16].
X-Ray Absorption Spectroscopy (XAS) measurements were performed at the B08 beamline at the European Synchrotron Radiation Facility (ESRF) in Grenoble, which works at the energy of 6 GeV and with a current of ~200 mA in the top-up mode. The B08 beamline is the Italian CRG (LISA), optimized for X-ray absorption measurements. Its optical layout covers a wide range of energies, from 5 to 40 keV, and, with the Si(111) crystals, delivers a flux to the sample of ~1011 ph/s within a spot of ~200 μm [21]. The acquisition at the Mo K-edge (19,999 eV) spectra was performed in the energy step-scan mode, and the fluorescence signal was collected by a 12-element Ge detector. The measurements were carried out with an energy resolution in the order of 2 eV, and the scan steps were set to 0.5 eV and 1 eV in the XANES and the EXAFS region, respectively.
X-ray Absorption Spectroscopy (XAS) analysis were performed using the software package FEFF6 (Seattle, WA, USA) [22]. Background subtraction and normalization of the absorption spectra were performed by fitting the pre-edge region with a first-order polynomial, and the spectrum after the edge with a cubic polynomial. The Mo K edge absorption threshold was associated with the first maximum of the first derivative.
3. Results and Discussion
The comparison of XANES spectra at the Mo K edge of the 250 nm MoO3 films deposited on a copper substrate and annealed at 300 °C, 350 °C, and 500 °C is shown in Figure 1, along with the MoO3 reference spectrum. The latter has a well-defined pre-edge peak at 20,005.6 eV (peak A), with two other major components at 20,025.7 eV and 20,037.2 eV (peaks B and C). The broadened features of the as-deposited film, in comparison with the reference spectra of the α-MoO3 spectrum, confirmed that this film was mainly disordered [23,24,25]. Within the initial annealing stage at 300 °C, the ordering process (flagged by peak C) started to appear, although the film remained disordered. Increasing the annealing temperature to 350 °C triggered an ordering process within the film, leading to the observation of the typical MoO3 features (peaks B and C). At the annealing temperature of 500 °C, the spectrum indicated the occurrence of a phase transformation: a clear shift of 3.8 eV of the third component at the energy of 20,033.4 eV (peak C) was observed. This dynamic can be also associated with the partial reduction of Mo ions due to oxygen loss [23,26,27]. A closer look to the XANES spectra also revealed changes in the pre-edge peak as a function of the annealing temperature. Above 350 °C, we observed a broadening and a lowering of the pre-edge component that can be associated with the partial reduction of Mo6+ ions to Mo4+. Indeed, the reduction of the pre-edge intensity, that is a probe of the local and partial empty density of states around Mo atoms, was due to the direct 1s to 4d quadrupole-allowed transitions and to the dipole-allowed 1s hybridized (5p,4d) states.
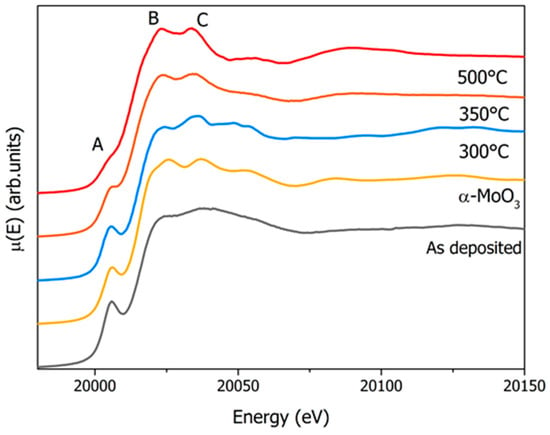
Figure 1.
Comparison of X-ray Absorption Near-Edge Structure (XANES) spectra at the molybdenum K edge for the standard α-MoO3 powder (yellow), the as-deposited MoO3 film 250 nm thick (grey), and those annealed at 300 °C (blue), 350 °C (orange), and 500 °C (red).
In order to probe the dependence of the structural changes of these oxide films on the annealing temperature, a more detailed XAS analysis was carried out. Figure 2 shows the comparison of the pseudo-radial distribution of the distances in these samples, obtained from the Fourier transform (FT) of the EXAFS Χ(k) from the as-deposited film, the annealed samples, and the α-MoO3 reference.
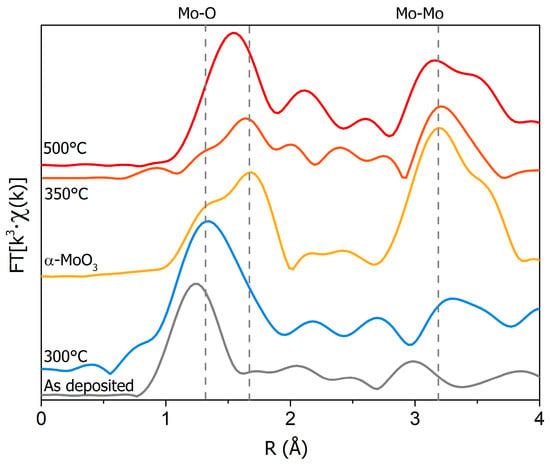
Figure 2.
Fourier transform (FT) of the k3·Χ(k) signal among the disordered MoO3 film (gray), the α-MoO3 (yellow), the 300 °C (blue), 350 °C (orange), and 500 °C (red) annealed MoO3 films deposited on Cu and the α-MoO3 powder (yellow). The vertical continuous lines refer to the position of MoO3 main peaks. It is clear that at 500 °C, the distribution is mainly related to MoO2, while at 350 °C, it corresponds to the α-MoO3 phase.
The as-deposited film and the one annealed at 300 °C exhibited only a large peak at around 1.7 Å, corresponding to the nearest oxygen neighbors (Mo–O) without additional shells. This is in agreement with previous XANES spectra of these same samples that pointed out the presence of disordered phases [23]. On the other hand, a structural ordering of the coating was observed at higher annealing temperatures (> 350 °C). As shown in Figure 2, at 350 °C, a split of the first peak appeared, similar to the spectrum of the reference, and a second major component associated with the nearest Mo neighbors (Mo–Mo second shell distance) also emerged at around 3.5 Å. A comparison with the α-MoO3 spectrum confirmed the formation of the dominant MoO3 phase, in agreement with the XANES spectra. At 500 °C, the FT was different from that of the film annealed at 350 °C, showing a single strong peak due to changes in the first Mo–O shells and in good agreement with the FT of the MoO2 phase [27]. The Mo–Mo peak at the distance of ~2.5 Å also appeared, in agreement with the edge-sharing octahedra characteristic of the distorted rutile structure of the MoO2 phase [27].
The EXAFS data of the α-MoO3 reference and annealed films were fitted using the FEFF package with MoO3 and MoO2 reference crystalline structures, respectively (see Figure 3). Structural refinements were performed by minimizing the difference of the raw absorption spectra with the simulation, including the structural oscillations, χ(κ), and a suitable background function. The fit was performed in two steps: first, the E0 and S02 parameters fitting only the first shell were calculated. In the second step, only the distances (R) and σ2 were left free. During this procedure, we used constant coordination numbers obtained from MoO3 and MoO2 crystal structures. The results (see Table 1) of the 350 °C annealed sample returned distances in good agreement with the α-MoO3 structure, with a reasonable mean-square relative displacement (σ2 = 0.0015 Å2).
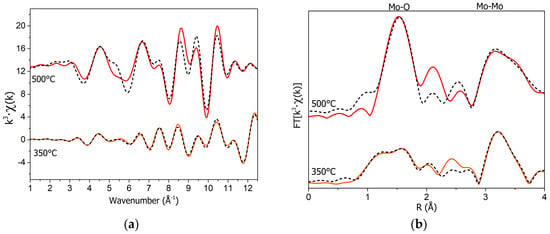
Figure 3.
(Left) Comparison of the k3·Χ(k) and the FT (right) of annealed MoO3 films deposited on Cu at 350 °C (orange) and 500 °C (red). Corresponding fits are the black dashed lines. The 350 °C signal was fitted with the α-MoO3 structure, while the 500 °C signal was fitted with the orthorhombic MoO2 structure.

Table 1.
Best-fit values of the samples from Extended X-ray Absorption Fine Structure (EXAFS) spectra. For the 350 °C sample, the fit was obtained from a refinement of the sum of theoretical EXAFS calculated for the orthorhombic MoO3 structure (k range = 3–12.5 Å−1, R = 0.5–4.2 Å, 8 single scattering paths, 16 free parameters, and two fixed, r-factor = 0.027, S02 = 0.6) and a monoclinic MoO2 structure for the 500 °C sample (k range = 3–12.5 Å−1, R = 0.5–4.2 Å, 6 single scattering paths, 12 free parameters, and two fixed, r-factor = 0.021, S02 = 0.8). The coordination numbers are those of the MoO3 and MoO2 structures.
On the contrary, the fit of the sample annealed at 500 °C showed a dominant MoO2 phase, with only the distances of the MoO2 structure ruling out the contribution of MoO3 phases. The small deviations of the fit from the experimental spectrum can be associated with the presence of minor amounts of other non-stoichiometric oxide phases.
4. Conclusions
The structural evolution of MoO3 films deposited on copper after annealing was investigated with XAS spectroscopy. At lower annealing temperatures, the experimental spectra did not change, showing that the as-deposited films were disordered up to ~300 °C. For the annealing procedure in a low vacuum regime (0.5 mbar) at around 350 °C, the spectra shows that the film underwent an ordering process, with the formation of the α-MoO3 phase, as confirmed by the EXAFS analysis. Moreover, XANES spectra at 500 °C showed that the molybdenum ions underwent a decrease of the pre-edge intensity, in agreement with a reduction from aMo6+ to a Mo4+ valence state. The fit of the EXAFS spectra showed that the phase of these films could be mainly associated with the presence of the MoO2 phase. These results represent a first important advancement for many foreseen applications of these coatings, in particular for compact RF devices made of copper.
Author Contributions
Conceptualization, methodology, S.M., A.M., and I.D.; data analysis, S.M., J.R., and G.C.; Manuscript preparation: S.M., A.M., and J.R.; S.M., A.M., J.R., I.D., G.C., B.S., J.S., L.F. have reviewed the manuscript.
Funding
We acknowledge the INFN for the support within DEMETRA and NUCLEAAR, two projects funded by the INFN Vth Committee. We also acknowledge the financial support of the Bilateral Cooperation Agreement between Italy and Japan of the Italian Ministry of Foreign Affairs and of the International Cooperation (MAECI) in the framework of the project of major relevance N. PGR0072.
Acknowledgments
We acknowledge ESRF and CNR-IOM for providing beamtime (proposal n. 08-01 1056) at LISA, the Italian beamline at ESRF. We acknowledge A. Bianconi for many fruitful discussions and P. De Padova and M. Lucci for their contribution to the development of the annealing procedure and to the experimental setup.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, Y.; Robertson, J. Origin of the high work function and high conductivity of MoO3. Appl. Phys. Lett. 2014, 105, 222110. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition metal oxides for organic electronics: energetics, device physics and applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Watson, G.W.; Payne, D.J.; Atkinson, G.R.; Egdell, R.G.; Law, D.S.L. Theoretical and experimental study of the electronic structures of MoO3 and MoO2. J. Phys. Chem. C 2010, 114, 4636–4645. [Google Scholar] [CrossRef]
- Hanson, E.D.; Lajaunie, L.; Hao, S.; Myers, B.D.; Shi, F.; Murthy, A.A.; Dravid, V.P. Systematic study of oxygen vacancy tunable transport properties of few-layer MoO3−x enabled by vapor-based synthesis. Adv. Funct. Mater. 2017, 27, 1605380. [Google Scholar] [CrossRef]
- Macis, S.; Aramo, C.; Bonavolontà, C.; Cibin, G.; D’Elia, A.I.; Davoli, M.; De Lucia, M.; Lucci, S.; Lupi, M.; Miliucci, A.; et al. MoO3 films Grown on polycrystalline Cu: Morphological, structural, and electronic properties. J. Vac. Sci. Technol. A 2019, 37, 021513. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.S.; Sriram, T.D.; Kalantar-zadeh, K. Molybdenum oxides–from fundamentals to functionality. Adv. Mat. 2017, 29, 1701619. [Google Scholar] [CrossRef]
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.; Lu, Z.H. Metal/metal-oxide interfaces: How metal contacts affect the work function and band structure of MoO3. Adv. Funct. Mater. 2013, 23, 215–226. [Google Scholar] [CrossRef]
- Lambert, D.S.; Lennon, A.; Burr, P.A. Extrinsic defects in crystalline MoO3: Solubility and effect on the electronic structure. J. Phys. Chem. C 2018, 122, 27241–27249. [Google Scholar] [CrossRef]
- Lambert, D.S.; Murphy, S.T.; Lennon, A.; Burr, P.A. Formation of intrinsic and silicon defects in MoO3 under varied oxygen partial pressure and temperature conditions: An ab initio DFT investigation. RSC Adv. 2017, 7, 53810–53821. [Google Scholar] [CrossRef]
- Akande, S.O.; Chroneos, A.; Vasilopoulou, M.; Kennou, S.; Schwingenschlögl, U. Vacancy formation in MoO3: Hybrid density functional theory and photoemission experiments. J. Mater. Chem. C 2016, 4, 9526–9531. [Google Scholar] [CrossRef]
- Castorina, G.; Marcelli, A.; Monforte, F.; Sarti, S.; Spataro, B. An analytical model for evaluation of the properties of metallic coatings in RF structures. Condens. Matter 2016, 1, 12. [Google Scholar] [CrossRef]
- Marcelli, A.; Spataro, B.; Sarti, S.; Dolgashev, V.A.; Tantawi, S.; Yeremian, D.A.; Higashi, Y.; Parodi, R.; Notargiacomo, A.; Junqing, X.G.; et al. Characterization of thick conducting molybdenum films: Enhanced conductivity via thermal annealing. Surf. Coat. Tech. 2015, 261, 391–397. [Google Scholar] [CrossRef]
- Xu, Y.; Spataro, B.; Sarti, S.; Dolgashev, V.A.; Tantawi, S.; Yeremian, A.D.; Higashi, Y.; Grimaldi, M.G.; Romano, L.; Ruffino, F. Structural and morphological characterization of Mo coatings for high gradient accelerating structures. J. Phys. Conf. Ser. 2013, 430, 012091. [Google Scholar] [CrossRef]
- Marcelli, A.; Spataro, B.; Castorina, G.; Xu, W.; Sarti, S.; Monforte, F.; Cibin, G. Materials and breakdown phenomena: Heterogeneous molybdenum metallic films. Condens. Matter 2017, 2, 18. [Google Scholar] [CrossRef]
- Dolgashev, V.A.; Tantawi, S.G.; Park, M.; Higashi, Y.; Spataro, B. Study of basic Rf breakdown phenomena in high gradient vacuum structures. In Proceedings of the 16th International Linear Accelerator Conference LINAC2010, Tsukuba, Japan, 12–17 September 2010; pp. 1043–1047. [Google Scholar]
- Macis, S. Deposition and Characterization of Thin MoO3 Films on Cu for Technological Applications. Ph.D. Thesis, Roma Tor Vergata University, Roma, Italy, 2019. [Google Scholar]
- Bianconi, A.; Marcelli, A. Surface X-Ray Absorption near-edge structure: XANES. In Synchrotron Radiation Research. Advances in Surface Science; Bachrach, R.Z., Ed.; Plenum Press: New York, NY, USA, 1992; Chapter 2; Volume 1. [Google Scholar]
- Marcelli, A. Phase separations in highly correlated materials. Acta Phys. Polonica A 2016, 129, 264–269. [Google Scholar] [CrossRef]
- Garcia, J.; Benfatto, M.; Natoli, C.R.; Bianconi, A.; Davoli, I.; Marcelli, A. Three particle correlation function of metal ions in tetrahedral coordination determined by XANES. Solid State Commun. 1986, 58, 595–599. [Google Scholar] [CrossRef]
- Giuli, G.; Paris, E.; Wu, Z.; Brigatti, M.F.; Cibin, G.; Mottana, A.; Marcelli, A. Experimental and theoretical XANES and EXAFS study of tetra-ferriphlogopit. Eur. J. Miner. 2001, 13, 1099–1108. [Google Scholar] [CrossRef]
- d’Acapito, F.; Lepore, G.O.; Puri, A.; Laloni, A.; la Manna, F.; Dettona, E.; de Luisa, A.; Martin, A. The LISA beamline at ESRF. J. Synchrotron Radiat. 2019, 26, 551–558. [Google Scholar]
- Zabinsky, S.I.; Rehr, J.J.; Ankudinov, A.; Albers, R.C.; Eller, M.J. Multiple scattering calculations of X-ray absorption spectra. Phys. Rev. B 1995, 52, 2995. [Google Scholar] [CrossRef]
- Kopachevska, N.S.; Melnyk, A.K.; Bacherikova, I.V.; Zazhigalov, V.A.; Wieczorek-Ciurowa, K. Determination of molybdenum oxidation state on the mechanochemically treated MoO3. Хімія Фізиката Технoлoгія Пoверхні 2015, 6, 474–480, ISSN 2079-1704. [Google Scholar]
- Di Cicco, A.; Bianconi, A.; Coluzza, C.; Rudolf, P.; Lagarde, P.; Flank, A.M.; Marcelli, A. XANES study of structural disorder in amorphous silicon. J. Non-Cryst. Solids 1990, 116, 27–32. [Google Scholar] [CrossRef]
- Bianconi, A.; Garcia, J.; Marcelli, A.; Benfatto, M.; Natoli, C.R.; Davoli, I. Probing higher order correlation functions in liquids by XANES (X-ray absorption Near Edge Structure). J. Phys. Colloq. 1985, 46, 101–106. [Google Scholar] [CrossRef]
- Ressler, T.; Jentoft, R.E.; Wienold, J.; Gu1nter, M.M.; Timpe, O. In situ XAS and XRD studies on the formation of Mo suboxides during reduction of MoO3. J. Phys. Chem. B 2000, 104, 6360–6370. [Google Scholar] [CrossRef]
- Ressler, T.; Wienold, J.; Jentoft, R.E. Formation of bronzes during temperature-programmed reduction of MoO3 with hydrogen—An in situ XRD and XAFS study. Solid State Ion. 2001, 141, 243–251. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).