Fingerprint Oxygen Redox Reactions in Batteries through High-Efficiency Mapping of Resonant Inelastic X-ray Scattering
Abstract
1. Introduction
2. Results and Discussion
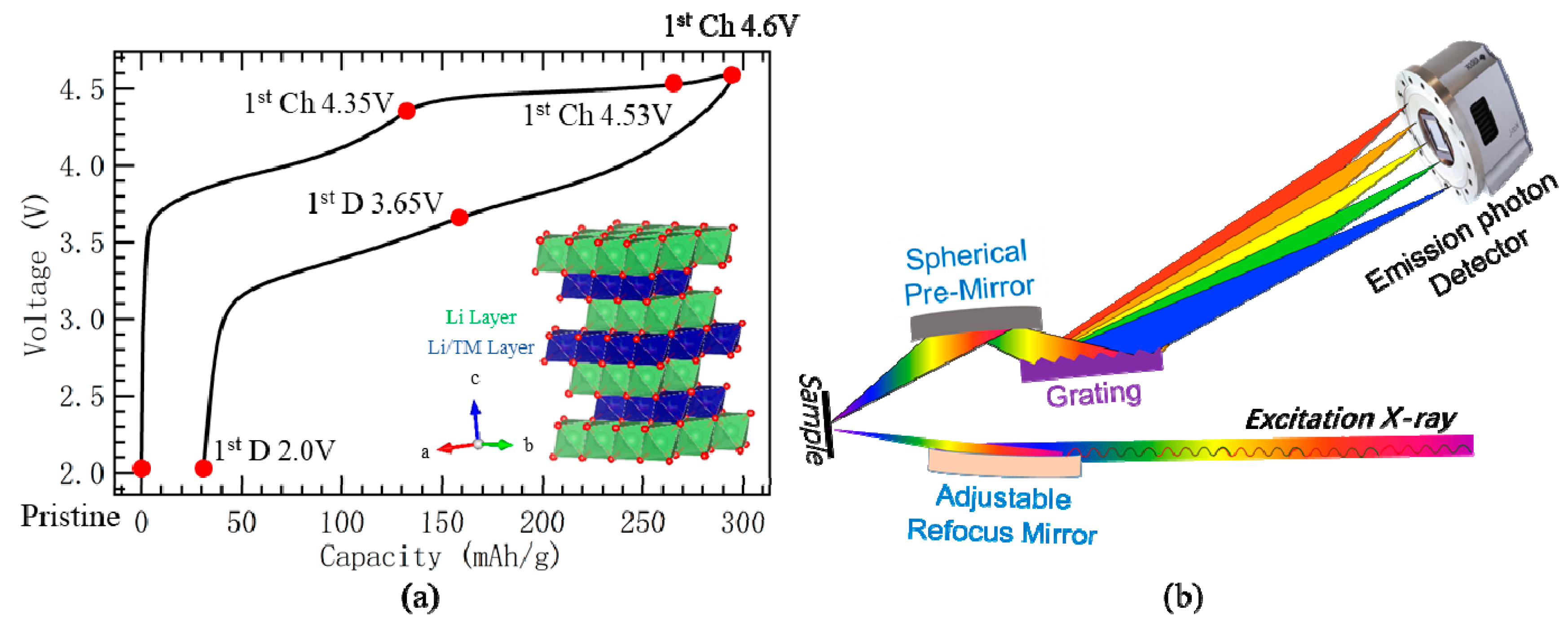
2.1. Materials and O-K mRIXS Technique
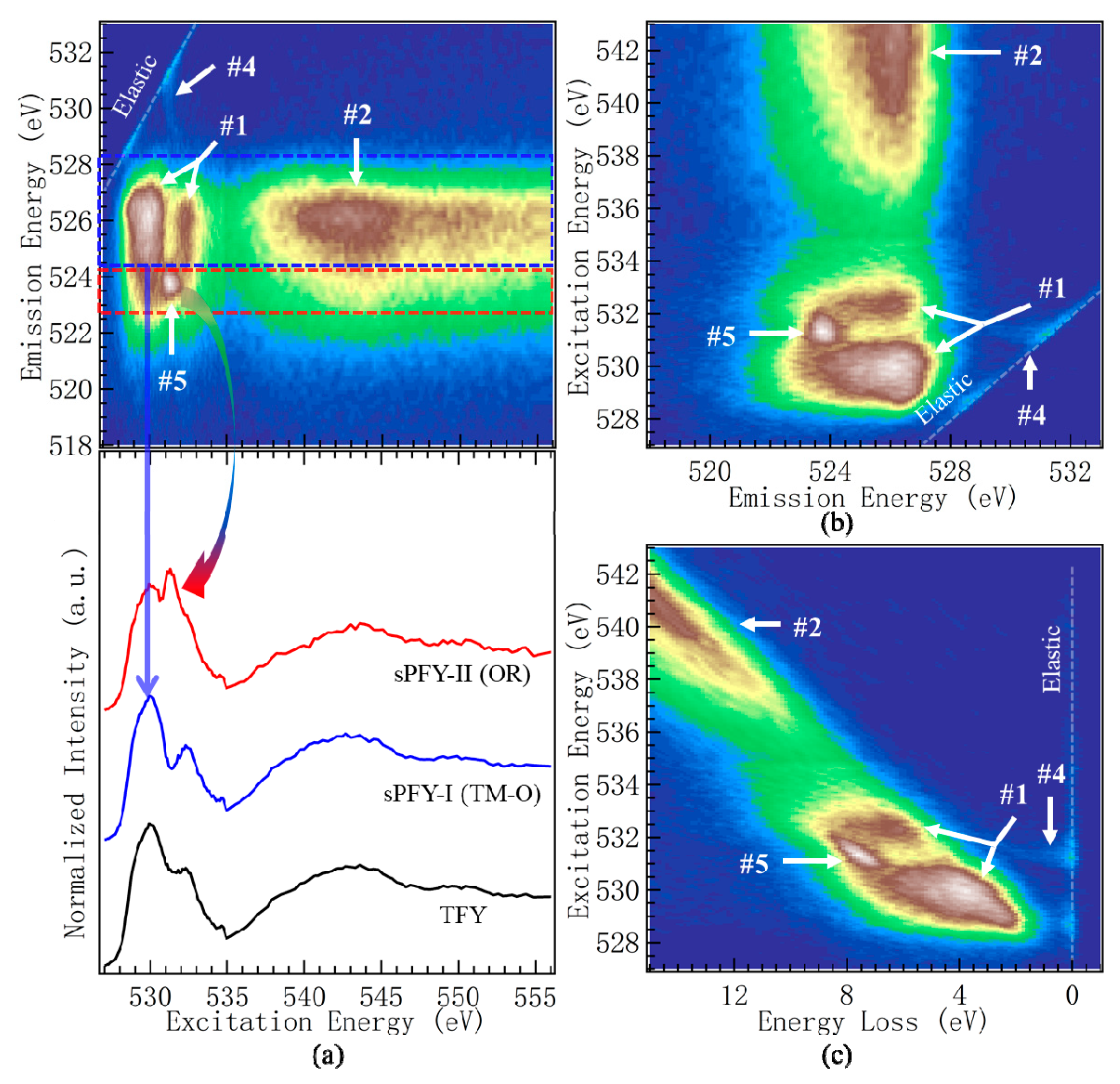
2.2. Presentations of O-K mRIXS
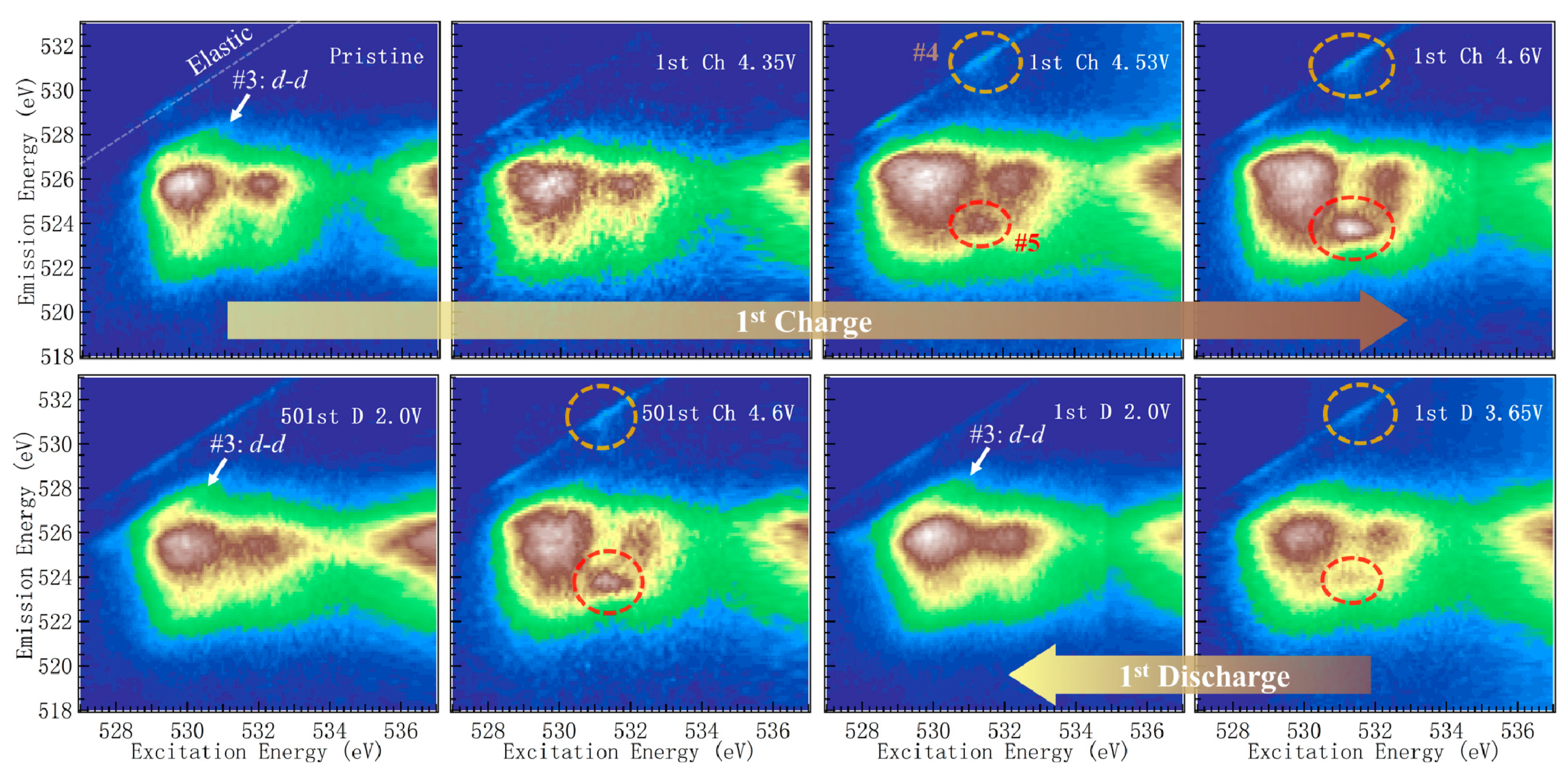
2.3. mRIXS Features of TM Characters Upon Electrochemical Cycling
2.4. Oxygen-Redox Features in mRIXS
2.4.1. Distinct Oxygen Redox Feature
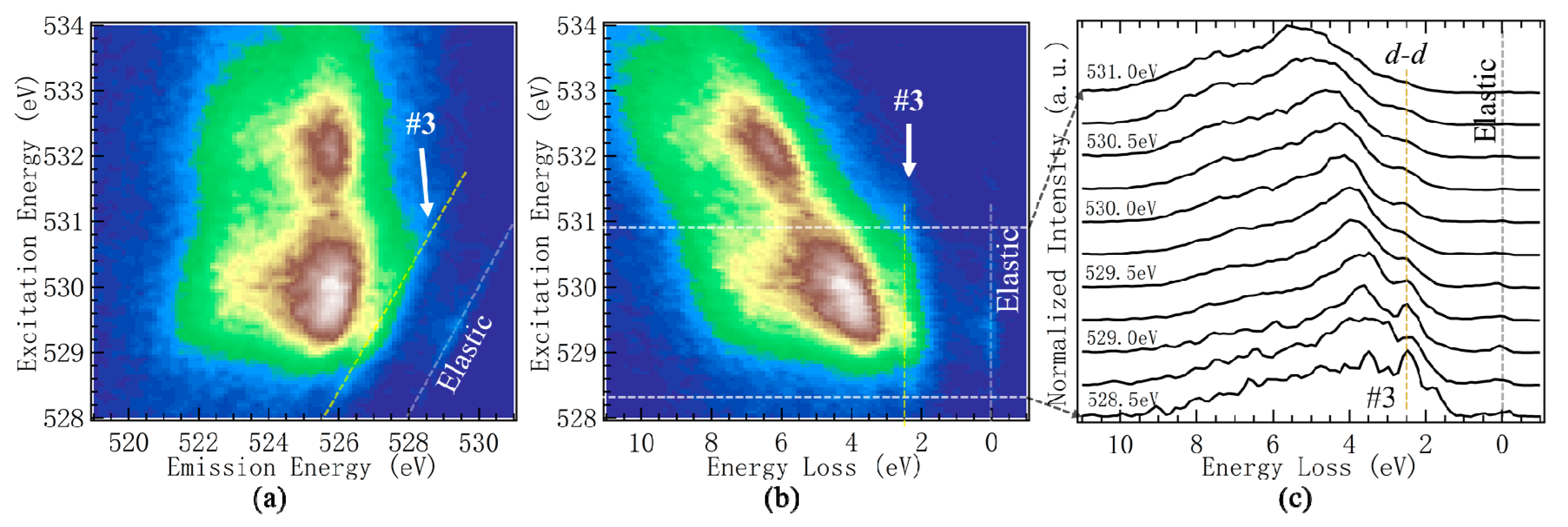
2.4.2. Low Energy-Loss Feature Associated with Oxygen Redox
3. Discussion
4. Materials and Methods
5. Conclusions
- The broad mRIXS features around the 525 eV emission energy are of (i) TM-3d character at the XAS pre-edge range of 527–534 eV excitation energy (Feature-1) and (ii) TM-4sp character at the 537–545 eV excitation energy (Feature-2), in addition to (iii) the XES signals with an O-2p character at higher excitation energies above 545 eV. These features are generally enhanced during the electrochemical charging. However, such enhancement is mostly from increased TM-O hybridization strength, which takes place in almost all battery electrodes and should not be counted as signatures of intrinsic oxygen redox reactions.
- A weak mRIXS feature with energy loss of about 2.4 eV (Feature-3) was observed in discharged electrodes. Its intensity also varied with electrochemical cycling, but was weakened in charged states. This stemmed from d-d excitations of the TM-3d states. The emergence of TM d-d features in O-K mRIXS is fundamentally interesting and deserves further theoretical studies. The reason behind the weakening during electrochemical charge remains unclear at this time, but was likely due to the overall broadening of spectroscopic features in a more covalent and amorphous phase at charged states.
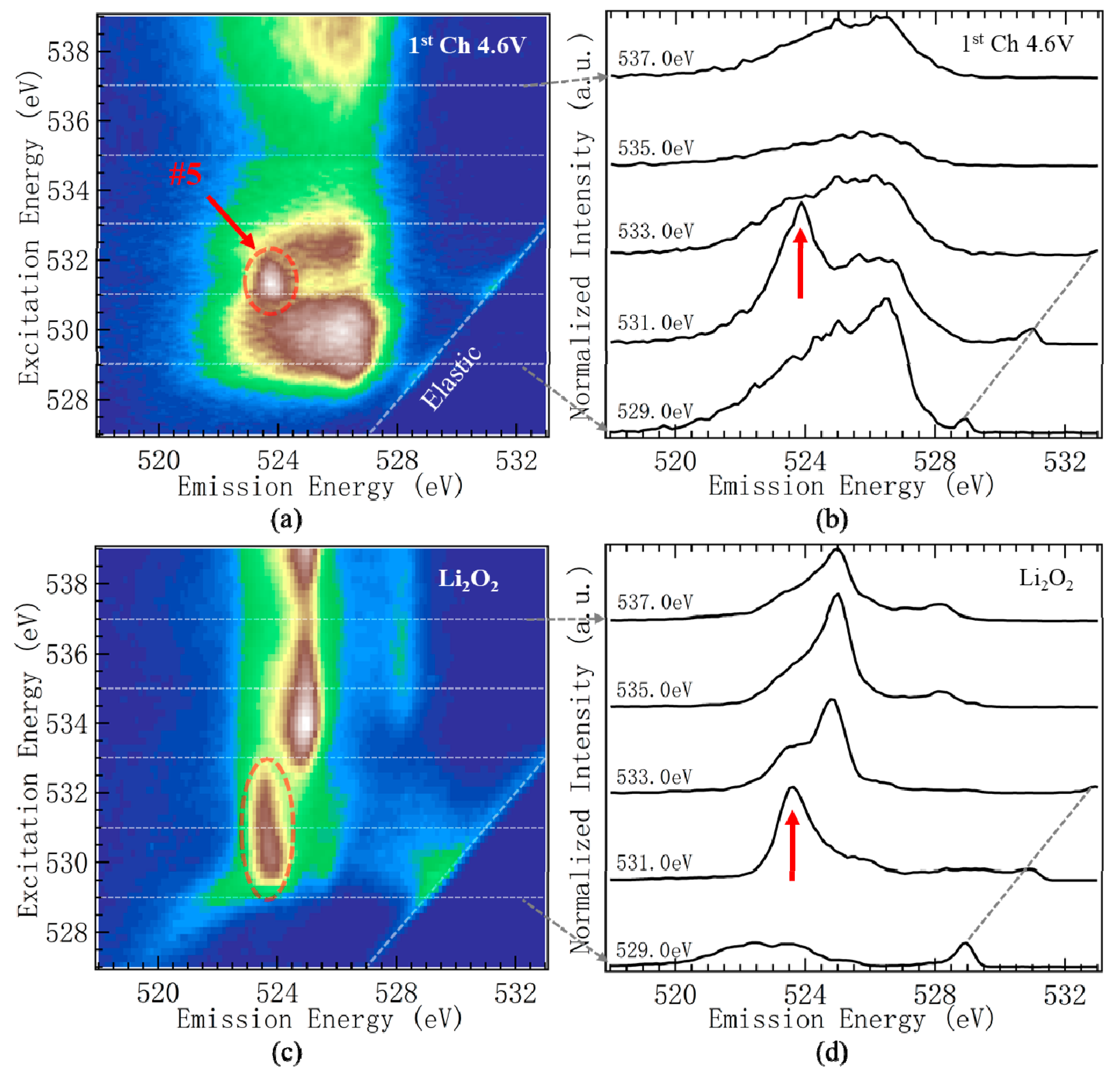
- A sharp mRIXS feature at the 531.0 excitation and 523.7 eV emission energy (Feature-5) emerged when the oxygen redox reaction took place above 4.35 V during the initial charge. The emission energy of this feature matched the spectroscopic behavior of oxidized oxygen in reference compounds, such as Li2O2. More importantly, the intensity of this feature followed closely the oxygen redox behavior during the electrochemical charge and discharge cycling, providing a reliable signature to fingerprint the oxygen redox reactions in batteries.
- Additionally, close to the elastic line in mRIXS results, a weak feature with less than a 1 eV energy loss (Feature-4) behaved in the same way as feature-5 upon electrochemical cycling. This provides another weak, but clean, indicator of oxygen redox reactions, and suggests that the oxidized oxygen states in charged electrodes spontaneously trigger low-energy excitations in its molecular configurations, likely through electron-phonon (vibronic mode) coupling. Feature-4 thus not only senses the oxygen redox reactions in the system, but also provides an important hint on the molecular model of the oxidized oxygen states.
- As previously reviewed [8], mRIXS results simultaneously included various fluorescence yield channels through different intensity integrations along emission energy axis. Although not a focused topic in this article, it is important to note that integrating the intensity around the characteristic 531 eV emission energy opens up the opportunity for quantifying the oxygen redox evolution upon electrochemical cycling [17]. With enough signal statistic, the same approach could be extended to the weak low-energy excitation feature-4, and could be employed to study almost all TM oxide based battery electrodes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-Gonzalez, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Assat, G.; Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 2018, 3, 373–386. [Google Scholar] [CrossRef]
- Yang, W.; Devereaux, T.P. Anionic and cationic redox and interfaces in batteries: Advances from soft X-ray absorption spectroscopy to resonant inelastic scattering. J. Power Sources 2018, 389, 188–197. [Google Scholar] [CrossRef]
- Qiao, R.; Chuang, Y.D.; Yan, S.; Yang, W. Soft x-ray irradiation effects of Li(2)O(2), Li(2)CO(3) and Li(2)O revealed by absorption spectroscopy. PLoS ONE 2012, 7, e49182. [Google Scholar] [CrossRef]
- Glans, P.; Gunnelin, K.; Skytt, P.; Guo, J.; Wassdahl, N.; Nordgren, J.; Agren, H.; Gel’mukhanov, F.K.; Warwick, T.; Rotenberg, E. Resonant x-ray emission spectroscopy of molecular oxygen. Phys. Rev. Lett. 1996, 76, 2448–2451. [Google Scholar] [CrossRef]
- De Groot, F.M.F.; Grioni, M.; Fuggle, J.C.; Ghijsen, J.; Sawatzky, G.A.; Petersen, H. Oxygen 1s x-ray-absorption edges of transition-metal oxides. Phys. Rev. B 1989, 40, 5715–5723. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.J.; Barbiellini, B.; Hafiz, H.; Basak, S.; Liu, J.; Richardson, T.; Shu, G.; Chou, F.; Weng, T.C.; et al. Why LiFePO4 is a safe battery electrode: Coulomb repulsion induced electron-state reshuffling upon lithiation. Phys. Chem. Chem. Phys. 2015, 17, 26369–26377. [Google Scholar] [CrossRef]
- Qiao, R.; Wray, L.A.; Kim, J.-H.; Pieczonka, N.P.W.; Harris, S.J.; Yang, W. Direct Experimental Probe of the Ni(II)/Ni(III)/Ni(IV) Redox Evolution in LiNi0.5Mn1.5O4Electrodes. J. Phys. Chem. C 2015, 119, 27228–27233. [Google Scholar] [CrossRef]
- Wu, J.; Sallis, S.; Qiao, R.; Li, Q.; Zhuo, Z.; Dai, K.; Guo, Z.; Yang, W. Elemental-sensitive Detection of the Chemistry in Batteries through Soft X-ray Absorption Spectroscopy and Resonant Inelastic X-ray Scattering. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Li, Q.; Zhuo, Z.; Sallis, S.; Fuchs, O.; Blum, M.; Weinhardt, L.; Heske, C.; Pepper, J.; Jones, M.; et al. High-efficiency in situ resonant inelastic X-Ray scattering (iRIXS) endstation at the Advanced Light Source. Rev. Sci. Instrum. 2017, 88, 033106. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Pemmaraju, C.D.; Vinson, J.; Jia, C.; Moritz, B.; Lee, I.; Sallies, S.; Li, Q.; Wu, J.; Dai, K.; et al. Spectroscopic Signature of Oxidized Oxygen States in Peroxides. J. Phys. Chem. Lett. 2018, 6378–6384. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Wu, J.; Zhuo, Z.; Li, Q.; Sallis, S.; Hussain, Z.; Liu, G.; Yang, W. High Reversibility of Lattice Oxygen Redox Quantified by Direct Bulk Probes of Both Anionic and Cationic Redox Reactions. Joule 2018, 3, 1–24. [Google Scholar] [CrossRef]
- Gent, W.E.; Lim, K.; Liang, Y.; Li, Q.; Barnes, T.; Ahn, S.J.; Stone, K.H.; McIntire, M.; Hong, J.; Song, J.H.; et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat. Commun. 2017, 8, 2091. [Google Scholar] [CrossRef]
- Xu, J.; Sun, M.; Qiao, R.; Renfrew, S.E.; Ma, L.; Wu, T.; Hwang, S.; Nordlund, D.; Su, D.; Amine, K.; et al. Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat. Commun. 2018, 9, 947. [Google Scholar] [CrossRef]
- Butorin, S.M.; Guo, J.; Wassdahl, N.; Nordgren, E.J. Tunable-excitation soft X-ray fluorescence spectroscopy of high-Tc superconductors: An inequivalent-site seeing story. J. Electron Spectrosc. Relat. Phenom. 2000, 110–111, 235–273. [Google Scholar] [CrossRef]
- Firouzi, A.; Qiao, R.; Motallebi, S.; Valencia, C.W.; Israel, H.S.; Fujimoto, M.; Wray, L.A.; Chuang, Y.D.; Yang, W.; Wessells, C.D. Monovalent manganese based anodes and co-solvent electrolyte for stable low-cost high-rate sodium-ion batteries. Nat. Commun. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Duda, L.C.; Schmitt, T.; Magnuson, M.; Forsberg, J.; Olsson, A.; Nordgren, J.; Okada, K.; Kotani, A. Resonant Inelastic X-Ray Scattering at the Oxygen K Resonance of NiO: Nonlocal Charge Transfer and Double-Singlet Excitations. Phys. Rev. Lett. 2006, 96, 067402. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lim, H.-D.; Lee, M.; Kim, S.-W.; Kim, H.; Oh, S.-T.; Chung, G.-C.; Kang, K. Critical Role of Oxygen Evolved from Layered Li–Excess Metal Oxides in Lithium Rechargeable Batteries. Chem. Mater. 2012, 24, 2692–2697. [Google Scholar] [CrossRef]
- Renfrew, S.E.; McCloskey, B.D. Residual Lithium Carbonate Predominantly Accounts for First Cycle CO2 and CO Outgassing of Li-Stoichiometric and Li-Rich Layered Transition-Metal Oxides. J. Am. Chem. Soc. 2017, 139, 17853–17860. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R.; Holzapfel, M.; Novák, P.; Johnson, C.S.; Kang, S.-H.; Thackeray, M.M.; Bruce, P.G. Demonstrating Oxygen Loss and Associated Structural Reorganization in the Lithium Battery Cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Ament, L.J.P.; van Veenendaal, M.; Devereaux, T.P.; Hill, J.P.; van den Brink, J. Resonant inelastic x-ray scattering studies of elementary excitations. Rev. Modern Phys. 2011, 83, 705–767. [Google Scholar] [CrossRef]
- Freelon, B.; Augustsson, A.; Guo, J.H.; Medaglia, P.G.; Tebano, A.; Balestrino, G. Electron correlation and charge transfer in superconducting superlattices. Phys. Rev. Lett. 2006, 96, 017003. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Li, Q.; Sallis, S.; Zhuo, Z.; Gent, W.E.; Chueh, W.C.; Yan, S.; Chuang, Y.-d.; Yang, W. Fingerprint Oxygen Redox Reactions in Batteries through High-Efficiency Mapping of Resonant Inelastic X-ray Scattering. Condens. Matter 2019, 4, 5. https://doi.org/10.3390/condmat4010005
Wu J, Li Q, Sallis S, Zhuo Z, Gent WE, Chueh WC, Yan S, Chuang Y-d, Yang W. Fingerprint Oxygen Redox Reactions in Batteries through High-Efficiency Mapping of Resonant Inelastic X-ray Scattering. Condensed Matter. 2019; 4(1):5. https://doi.org/10.3390/condmat4010005
Chicago/Turabian StyleWu, Jinpeng, Qinghao Li, Shawn Sallis, Zengqing Zhuo, William E. Gent, William C. Chueh, Shishen Yan, Yi-de Chuang, and Wanli Yang. 2019. "Fingerprint Oxygen Redox Reactions in Batteries through High-Efficiency Mapping of Resonant Inelastic X-ray Scattering" Condensed Matter 4, no. 1: 5. https://doi.org/10.3390/condmat4010005
APA StyleWu, J., Li, Q., Sallis, S., Zhuo, Z., Gent, W. E., Chueh, W. C., Yan, S., Chuang, Y.-d., & Yang, W. (2019). Fingerprint Oxygen Redox Reactions in Batteries through High-Efficiency Mapping of Resonant Inelastic X-ray Scattering. Condensed Matter, 4(1), 5. https://doi.org/10.3390/condmat4010005




