Dependency of the Charge–Discharge Rate on Lithium Reaction Distributions for a Commercial Lithium Coin Cell Visualized by Compton Scattering Imaging
Abstract
:1. Introduction
2. Experimental Study
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taminato, S.; Yonemura, M.; Shiotani, S.; Kamiyama, T.; Torii, S.; Nagao, M.; Ishikawa, Y.; Mori, K.; Fukunaga, T.; Onodera, Y.; et al. Real-time observations of lithium battery reactions–Operando neutron diffraction analysis during practical operation. Sci. Rep. 2016, 6, 28843. [Google Scholar] [CrossRef] [PubMed]
- Itou, M.; Orikasa, Y.; Gogyo, Y.; Suzuki, K.; Sakurai, H.; Uchimoto, Y.; Sakurai, Y. Compton scattering imaging of a working battery using synchrotron high-energy X-rays. J. Synchrotron Radiat. 2015, 22, 161–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Barbiellini, B.; Orikasa, Y.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Wang, Y.J.; Hafiz, H.; Uchimoto, Y.; Bansil, A.; et al. Non-destructive measurement of in-operando lithium concentration in batteries via X-ray Compton scattering. J. Appl. Phys. 2016, 119, 025103. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, A.; Ishikawa, T.; Itou, M.; Yamashige, H.; Orikasa, Y.; Uchimoto, Y.; Sakurai, Y.; Sakurai, H. In-operando quantitation of Li concentration for commercial Li-ion rechargeable battery using high-energy X-ray Compton scattering. J. Synchrotron Radiat. 2017, 24, 1006. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.; Harding, E. Compton scatter imaging: A tool for historical exploration. Appl. Radiat. Isot. 2010, 68, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Schülke, W. The theory of Compton scattering. In X-ray Compton Scattering, 1st ed.; Cooper, M.J., Mijnarends, P.E., Shiotani, N., Sakai, N., Bansil, A., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 22–81. ISBN 978-0-19-850168-8. [Google Scholar]
- Barbiellini, B. A natural orbital method for the electron momentum distribution in mater. J. Phys. Chem. Solids 2000, 61, 341–344. [Google Scholar] [CrossRef]
- Barbiellini, B.; Bansil, A. Treatment of correlation effects in electron momentum density: Density functional theory and beyond. J. Phys. Chem. Solids 2001, 62, 2181–2189. [Google Scholar] [CrossRef]
- Suzuki, K.; Barbiellini, B.; Orikasa, Y.; Go, N.; Sakurai, H.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Uchimoto, Y.; Wang, Y.J.; et al. Extracting the redox orbitals in Li battery materials with high-resolution X-ray Compton scattering spectroscopy. Phys. Rev. Lett. 2015, 114, 087401. [Google Scholar] [CrossRef] [PubMed]
- Barbiellini, B.; Suzuki, K.; Orikasa, Y.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Wang, Y.J.; Hafiz, H.; Yamada, R.; Uchimoto, Y.; et al. Identifying a descriptor for d-orbital delocalization in cathodes of Li batteries based on X-ray Compton scattering. Appl. Phys. Lett. 2016, 109, 073102. [Google Scholar] [CrossRef]
- Hafiz, H.; Suzuki, K.; Barbiellini, B.; Orikasa, Y.; Callewaert, V.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Yamada, R.; Uchimoto, Y.; et al. Visualizing redox orbitals and their potentials in advanced lithium-ion battery materials using high-resolution X-ray Compton scattering. Sci. Adv. 2017, 3, e1700971. [Google Scholar] [CrossRef] [PubMed]
- Biggs, F.; Mendelsohn, L.B.; Mann, J.B. Hartree-Fock Compton profiles for the elements. At. Data Nucl. Data Tables 1975, 16, 201. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Zhu, Y.; Wu, Y.; Holze, R. An aqueous rechargeable lithium battery using coated Li metal as anode. Sci. Rep. 2013, 3, 1401. [Google Scholar] [CrossRef] [PubMed]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edström, K.; Vegge, T. Lithium salts for advanced lithium batteries: Li-metal, Li-O2, and Li-S. Energy Environ. Sci. 2015, 8, 1905. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Q.; Cheng, X.B.; Zhang, Q. Advances in interfaces between Li metal anode and electrolyte. Adv. Mater. Interfaces 2018, 5, 1701097. [Google Scholar] [CrossRef]
- Brancewicz, M.; Itou, M.; Sakurai, Y.; Andrejczuk, A.; Chiba, S.; Kayahara, Y.; Inoue, T.; Nagamine, M. High transmission Ni compound refractive lens for high energy X-rays. Rev. Sci. Instrum. 2016, 87, 085106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
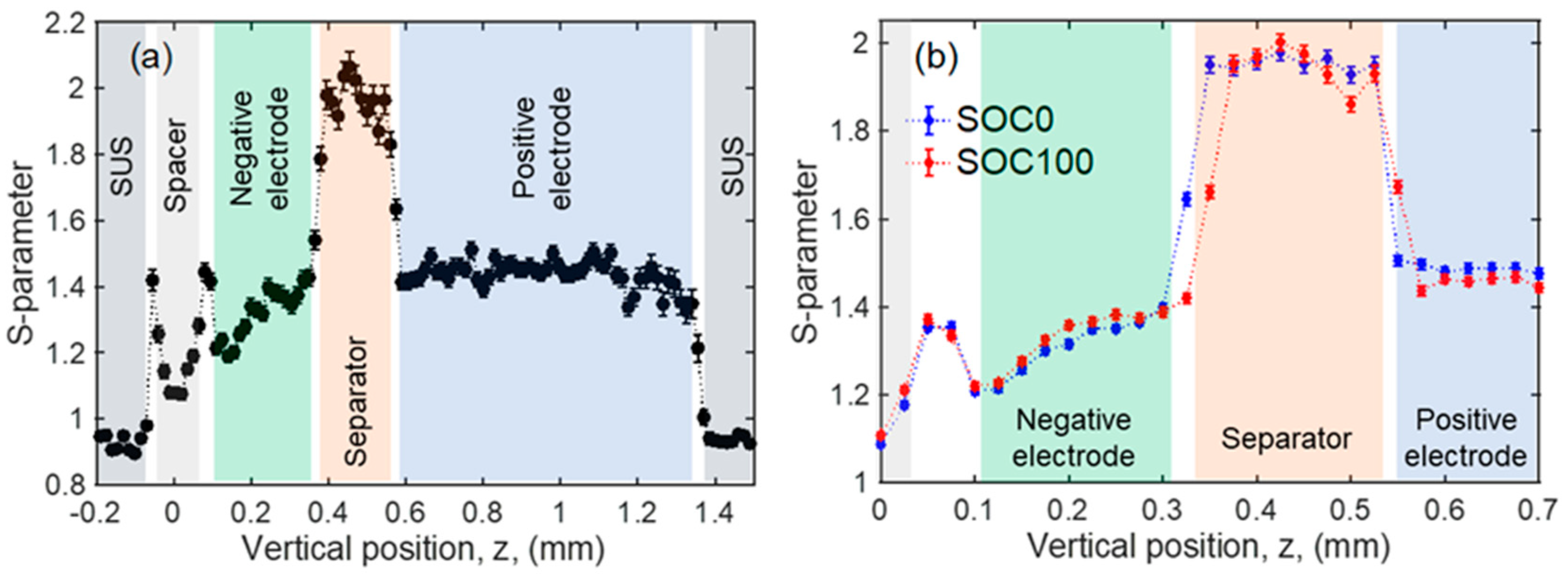
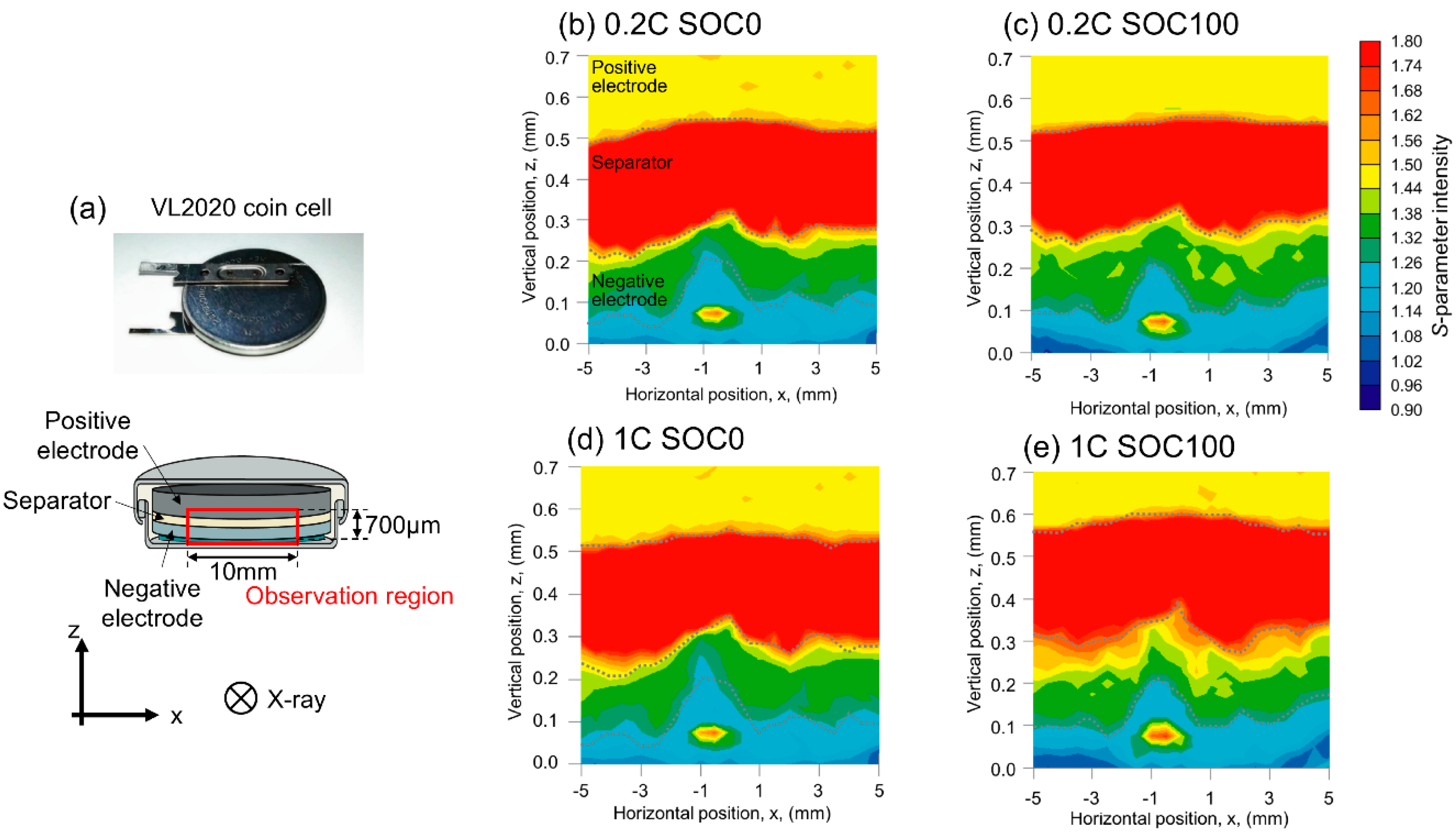
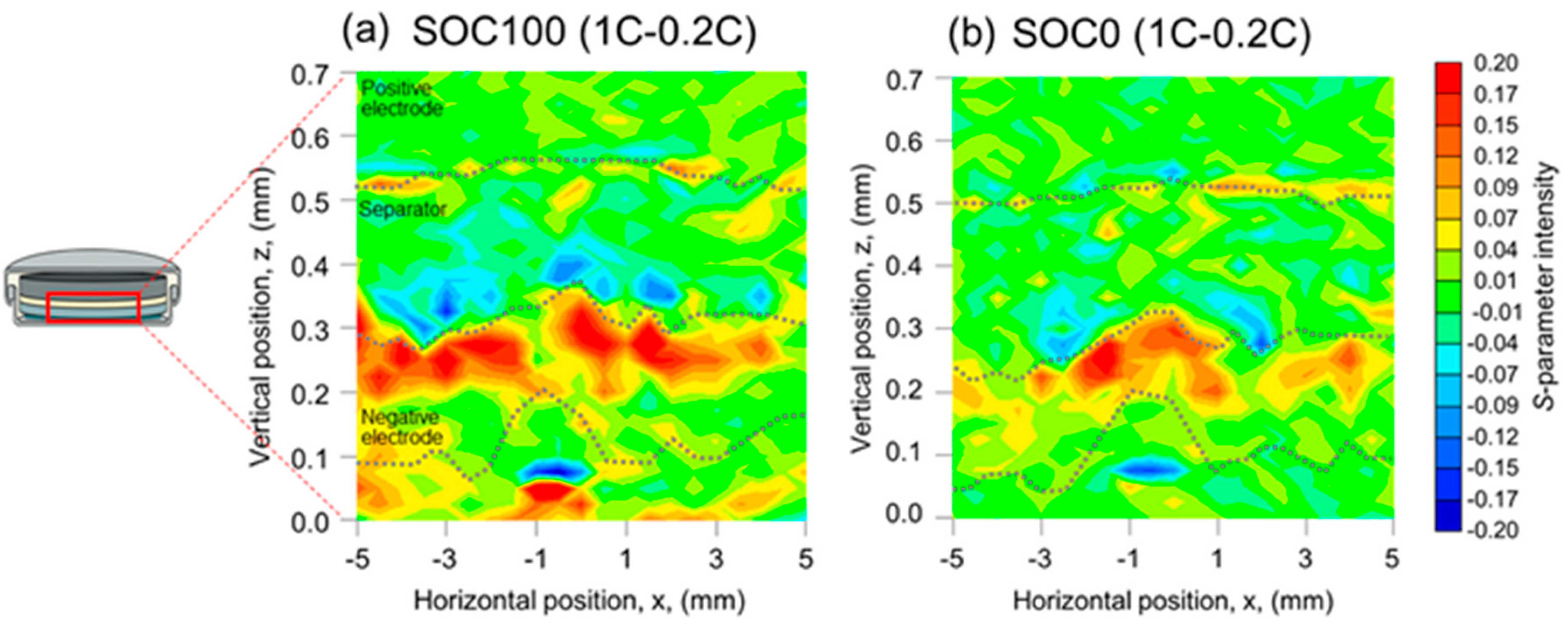
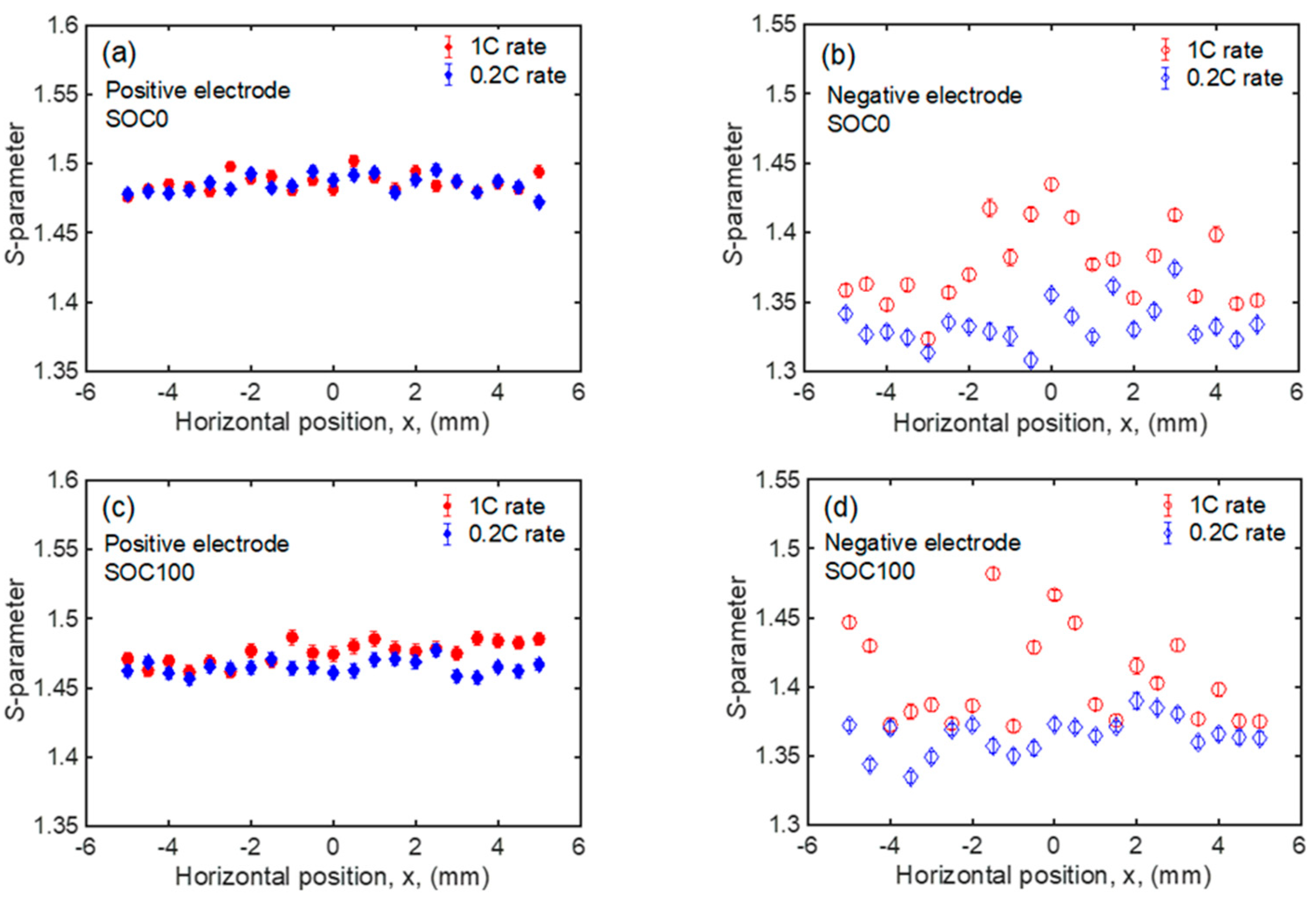

| Positive Electrode | Negative Electrode | ||||
|---|---|---|---|---|---|
| SOC0 | SOC100 | SOC0 | SOC100 | ||
| 1C rate | Average | 1.486 ± 0.001 | 1.476 ± 0.001 | 1.376 ± 0.001 | 1.405 ± 0.001 |
| Deviation | 0.006 | 0.008 | 0.028 | 0.033 | |
| 0.2C rate | Average | 1.485 ± 0.001 | 1.465 ± 0.001 | 1.334 ± 0.001 | 1.365 ± 0.001 |
| Deviation | 0.006 | 0.005 | 0.015 | 0.013 | |
| Positive Electrode | Negative Electrode | ||||
|---|---|---|---|---|---|
| SOC0 | SOC100 | SOC0 | SOC100 | ||
| 1C rate | Average | 1.088 ± 0.028 | 0.738 ± 0.035 | 0.412 ± 0.005 | 0.566 ± 0.005 |
| Deviation | 0.210 | 0.252 | 0.151 | 0.179 | |
| 0.2C rate | Average | 1.040 ± 0.028 | 0.387 ± 0.032 | 0.184 ± 0.005 | 0.349 ± 0.005 |
| Deviation | 0.198 | 0.158 | 0.080 | 0.070 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Kanai, R.; Tsuji, N.; Yamashige, H.; Orikasa, Y.; Uchimoto, Y.; Sakurai, Y.; Sakurai, H. Dependency of the Charge–Discharge Rate on Lithium Reaction Distributions for a Commercial Lithium Coin Cell Visualized by Compton Scattering Imaging. Condens. Matter 2018, 3, 27. https://doi.org/10.3390/condmat3030027
Suzuki K, Kanai R, Tsuji N, Yamashige H, Orikasa Y, Uchimoto Y, Sakurai Y, Sakurai H. Dependency of the Charge–Discharge Rate on Lithium Reaction Distributions for a Commercial Lithium Coin Cell Visualized by Compton Scattering Imaging. Condensed Matter. 2018; 3(3):27. https://doi.org/10.3390/condmat3030027
Chicago/Turabian StyleSuzuki, Kosuke, Ryo Kanai, Naruki Tsuji, Hisao Yamashige, Yuki Orikasa, Yoshiharu Uchimoto, Yoshiharu Sakurai, and Hiroshi Sakurai. 2018. "Dependency of the Charge–Discharge Rate on Lithium Reaction Distributions for a Commercial Lithium Coin Cell Visualized by Compton Scattering Imaging" Condensed Matter 3, no. 3: 27. https://doi.org/10.3390/condmat3030027
APA StyleSuzuki, K., Kanai, R., Tsuji, N., Yamashige, H., Orikasa, Y., Uchimoto, Y., Sakurai, Y., & Sakurai, H. (2018). Dependency of the Charge–Discharge Rate on Lithium Reaction Distributions for a Commercial Lithium Coin Cell Visualized by Compton Scattering Imaging. Condensed Matter, 3(3), 27. https://doi.org/10.3390/condmat3030027






