Effect of Dietary Copper on Growth Performance, Antioxidant Capacity, and Immunity in Juvenile Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diet
2.2. Experimental Procedures
2.3. Sample Collection Analytical Methods
2.4. Chemical Analysis
2.5. Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
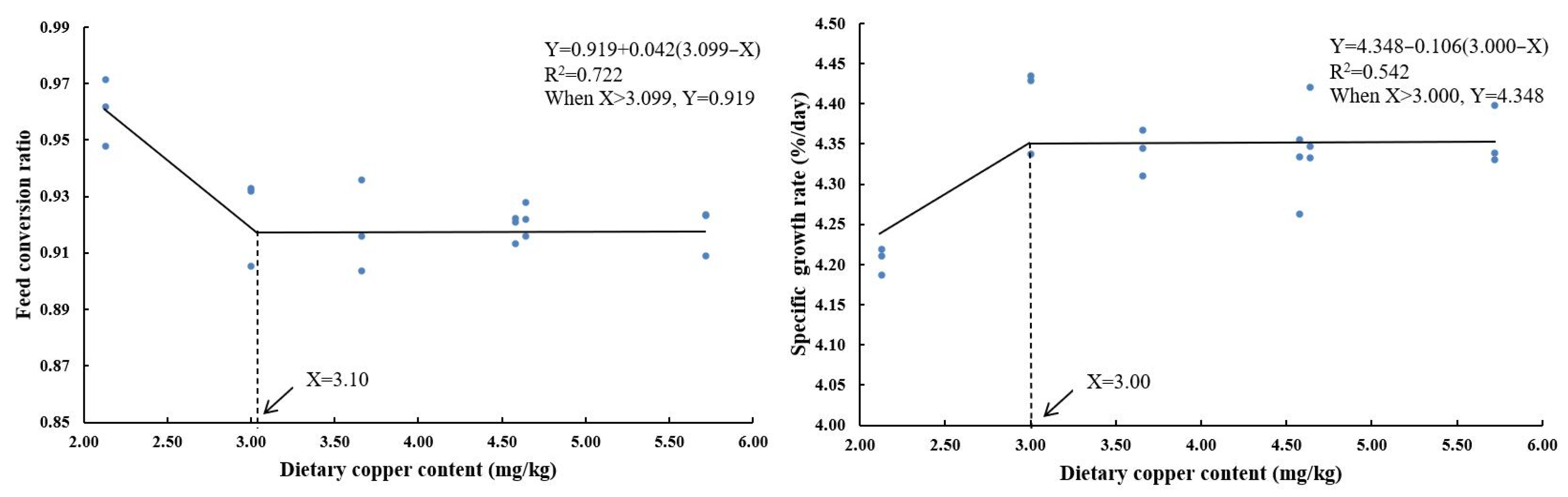
3.1. Growth Performance
3.2. Whole Body Composition
3.3. Plasma Biochemical Parameters
3.4. The Antioxidant Parameters of Liver
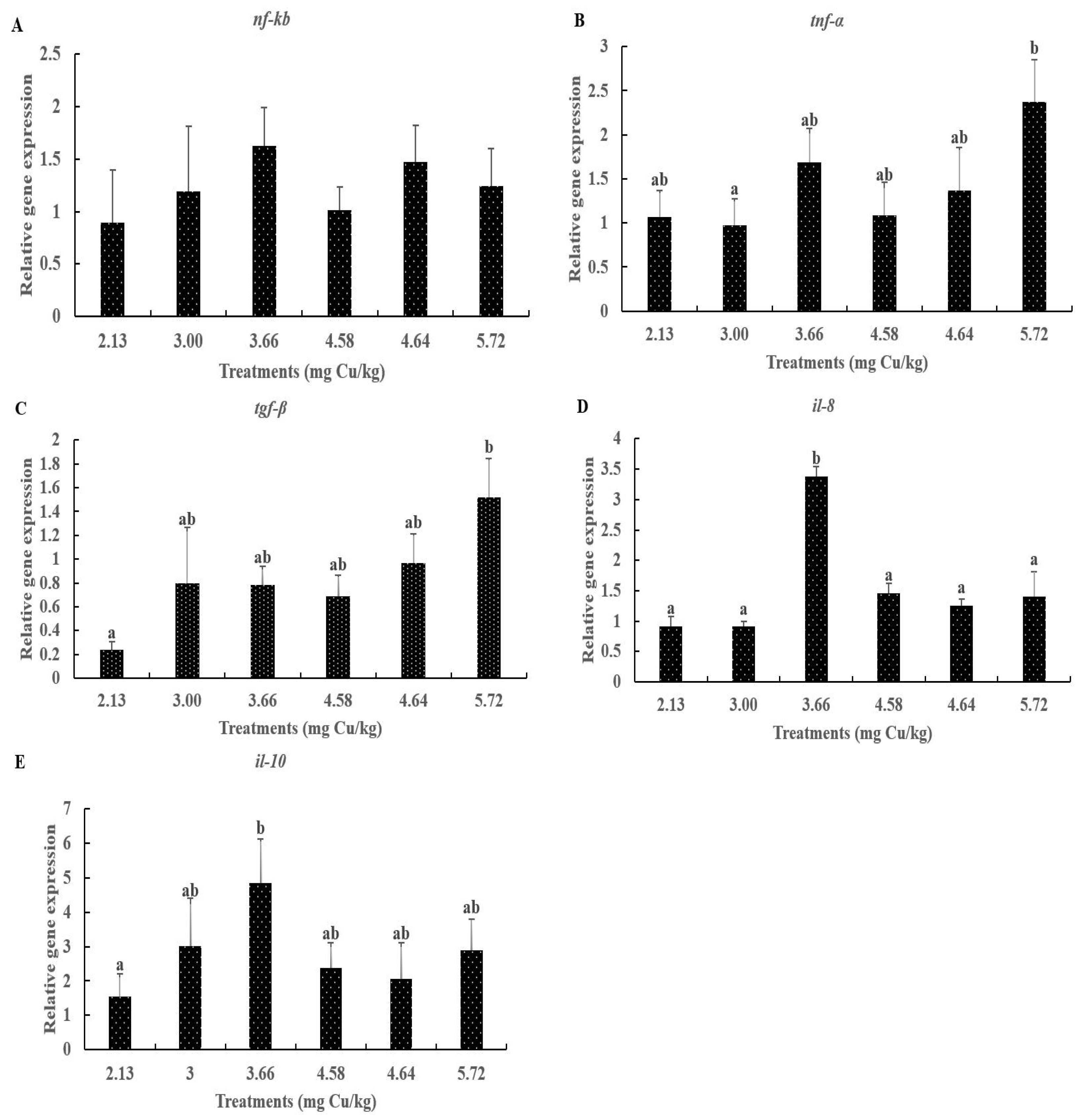
3.5. The Gene Expressions of the NF-κB Signaling Pathway in Liver
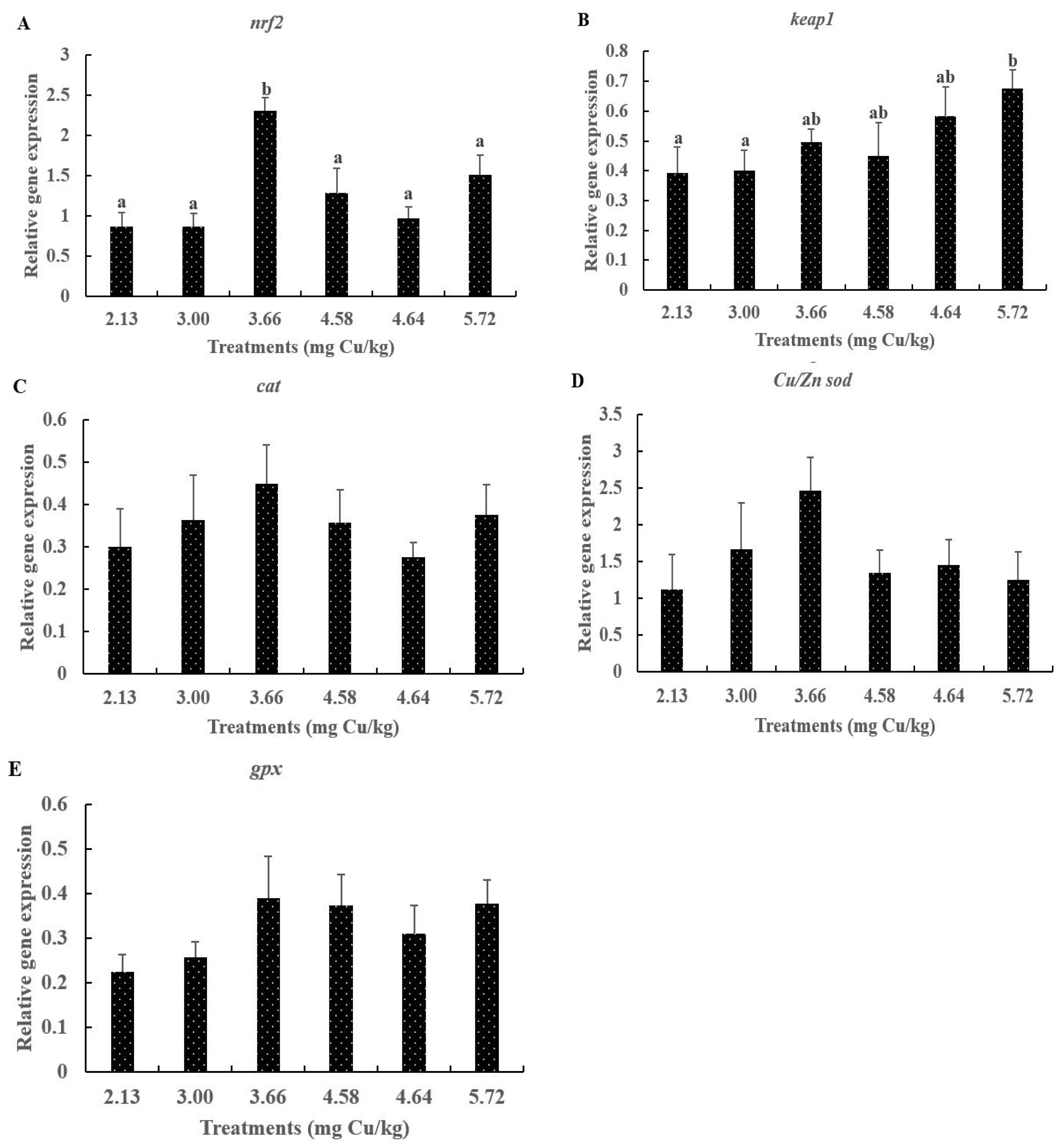
3.6. The Core Gene Expressions of Nrf2 Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.G.; Nebo, C.; Pádua, D.M.C.; Souto, C.N.; Guimarães, I.G. Apparent Digestibility of Minerals from Several Ingredients for Tambaqui, Colossoma macropomum, Juveniles. J. World Aquac. Soc. 2018, 49, 1026–1038. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67 (Suppl. S5), 952S–959S. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.C.; Ke, J.; Song, C.C.; Tan, X.Y.; Xu, Y.C.; Lv, W.H.; Song, Y.F.; Luo, Z. Effects of dietary copper (Cu) on growth performance, body composition, mineral content, hepatic histology and Cu transport of the GIFT strain of Nile tilapia (Oreochromis niloticus). Aquaculture 2023, 574, 739638. [Google Scholar] [CrossRef]
- Lin, Y.H.; Shie, Y.Y.; Shiau, S.Y. Dietary copper requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 2008, 274, 161–165. [Google Scholar] [CrossRef]
- Perez-Casanova, J.C.; Murray, H.M.; Gallant, J.W.; Ross, N.W.; Douglas, S.E.; Johnson, S.C. Development of the digestive capacity in larvae of haddock (Melanogrammus aeglefinus) and Atlantic cod (Gadus morhua). Aquaculture 2006, 251, 377–401. [Google Scholar] [CrossRef]
- Malhotra, N.; Ger, T.R.; Uapipatanakul, B.; Huang, J.C.; Chen, K.H.C.; Der Hsiao, C. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, T.; Wang, X.; Cheng, X.; Yuan, Y.; Jin, M.; Betancor, M.B.; Tocher, D.R.; Zhou, Q. Toxicological mechanism of excessive copper supplementation: Effects on coloration, copper bioaccumulation and oxidation resistance in mud crab Scylla paramamosain. J. Hazard. Mater. 2020, 395, 122600. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Huang, K.; Xie, J.; Yu, D.; Sun, L.; Huang, Q.; Bi, Y. Dietary copper affects antioxidant and immune activity in hybrid tilapia (Oreochromis niloticus × Oreochromis aureus). Aquac. Nutr. 2017, 23, 1003–1015. [Google Scholar] [CrossRef]
- Cao, J.; Miao, X.; Xu, W.; Li, J.; Zhang, W.; Mai, K. Dietary copper requirements of juvenile large yellow croaker Larimichthys croceus. Aquaculture 2014, 432, 346–350. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Rainbow, P.S. Regulation and Accumulation of Copper, Zinc and Cadmium by the Shrimp Palaemon elegans. Mar. Ecol.-Prog. Ser. 1982, 8, 95–101. [Google Scholar] [CrossRef]
- Craig, P.M.; Wood, C.M.; Mcclelland, G.B. Water Chemistry Alters Gene Expression and Physiological End Points of Chronic Waterborne Copper Exposure in Zebrafish, Danio rerio. Environ. Sci. Technol. 2010, 44, 2156. [Google Scholar] [CrossRef]
- Machado, A.A.; Hoff, M.L.M.; Klein, R.D.; Gordeiro, G.J.; Avila, J.M.L.; Costa, P.G.; Bianchini, A. Oxidative stress and DNA damage responses to phenanthrene exposure in the estuarine guppy Poecilia vivipar. Mar. Environ. Res. 2014, 98, 96–105. [Google Scholar] [CrossRef]
- Zimmer, A.M.; Fernanda, I.; Wood, C.M.; Bianchini, A. Waterborne copper exposure inhibits ammonia excretion and branchial carbonic anhydrase activity in euryhaline guppies acclimated to both fresh water and seawater. Aquat. Toxicol. 2012, 122–123, 172–180. [Google Scholar] [CrossRef]
- Yuan, Y.; Luo, J.; Zhu, T.; Jin, M.; Jiao, L.; Sun, P.; Ward, T.L.; Ji, F.; Xu, G.; Zhou, Q. Alteration of growth performance, meat quality, antioxidant and immune capacity of juvenile Litopenaeus vannamei in response to different dietary dosage forms of zinc: Comparative advantages of zinc amino acid complex. Aquaculture 2020, 522, 735120. [Google Scholar] [CrossRef]
- El Basuini, M.F.; El-Hais, A.M.; Dawood, M.A.O.; Abou-Zeid, A.E.S.; EL-Damrawy, S.Z.; Khalafalla, M.M.E.S.; Koshio, S.; Ishikawa, M.; Dossou, S. Effect of different levels of dietary copper nanoparticles and copper sulfate on growth performance, blood biochemical profiles, antioxidant status and immune response of red sea bream (Pagrus major). Aquaculture 2016, 455, 32–40. [Google Scholar] [CrossRef]
- Ogino, C.; Yang, G.Y. Requirements of Carp and Rainbow Trout for Dietary Manganese and Copper. Bull. Jpn. Soc. Sci. Fish 1980, 46, 455–458. [Google Scholar] [CrossRef]
- Liang, H.; Ji, K.; Ge, X.; Mi, H.; Xi, B.; Ren, M. Effects of dietary copper on growth, antioxidant capacity and immune responses of juvenile blunt snout bream (Megalobrama amblycephala) as evidenced by pathological examination. Aquac. Rep. 2020, 17, 100296. [Google Scholar] [CrossRef]
- Lorentzen, M.; Maage, A.; Julshamn, K. Supplementing copper to a fish meal based diet fed to Atlantic salmon parr affects liver copper and selenium concentrations. Aquac. Nutr. 1998, 4, 67–72. [Google Scholar] [CrossRef]
- Lall, S.P.; Hines, J.A. Iron and copper requirement of Atlantic salmon (Salmo salar) grown in sea water. In Proceedings of the International Symposium on Feeding and Nutrition of Fish, Bergen, Norway, 23–27 August 1987. [Google Scholar]
- Wang, W.; Mai, K.; Zhang, W.; Ai, Q.; Yao, C.; Li, H.; Liufu, Z. Effects of dietary copper on survival, growth and immune response of juvenile abalone, Haliotis discus hannai Ino. Aquaculture 2009, 297, 122–127. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Wilson, R.P. Dietary copper requirement of fingerling channel catfish. Aquaculture 1986, 54, 277–285. [Google Scholar] [CrossRef]
- Brown, T.G.; Runciman, B.; Pollard, S.; Grant, A.D.A. (Micropterus salmoides). Lake 2002, 31, 1763–1769. [Google Scholar] [CrossRef]
- Wedemeyer, G.A. Physiology of Fish in Intensive Culture Systems; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Karin, M.; Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Mourente, G.; Diaz-Salvago, E.; Bell, J.; Tocher, D. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Wang, L.M.; Wang, J.; Bharadwaj, A.S.; Xue, M.; Qin, Y.C. Effects of dietary copper sources on growth, tissue copper accumulation and physiological responses of Japanese sea bass (Lateolabrax japonicus) (Cuvier, 1828) fed semipurified or practical diets. Aquac. Res. 2015, 46, 1619–1627. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2003; Volume 1. [Google Scholar]
- Gu, J.Z.; Liang, H.L.; Ge, X.P.; Xia, D.; Pan, L.K.; Mi, H.F.; Ren, M.C. A study of the potential effect of yellow mealworm (Tenebrio molitor) substitution for fish meal on growth, immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Fish & Shellfish Immunology 2022, 120, 214–221. [Google Scholar] [CrossRef]
- Yang, P.; Wang, W.Q.; Chi, S.Y.; Mai, K.S.; Song, F.; Wang, L. Effects of dietary lysine on regulating GH-IGF system, intermediate metabolism and immune response in largemouth bass (Micropterus salmoides). Aquaculture Reports 2020, 17, 100323. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Wang, J.L.; Meng, X.L.; Lu, R.H.; Wu, C.; Luo, Y.T.; Yan, X.; Li, X.J.; Kong, X.H.; Nie, G.X. Effects of Rehmannia glutinosa on growth performance, immunological parameters and disease resistance to Aeromonas hydrophila in common carp (Cyprinus carpio L.). Aquaculture 2015, 435, 293–300. [Google Scholar] [CrossRef]
- Mohseni, M.; Pourkazemi, M.; Bai, S.C. Effects of dietary inorganic copper on growth performance and immune responses of juvenile beluga, Huso huso. Aquac. Nutr. 2014, 20, 547–556. [Google Scholar] [CrossRef]
- Song, J.; Li, L.Y.; Chen, B.B.; Shan, L.L.; Yuan, S.Y.; Yu, H.R. Dietary copper requirements of postlarval coho salmon (Oncorhynchus kisutch). Aquac. Nutr. 2021, 27, 2084–2092. [Google Scholar] [CrossRef]
- Shahjahan, M.; Islam, M.J.; Hossain, M.T.; Mishu, M.A.; Hasan, J.; Brown, C. Blood biomarkers as diagnostic tools: An overview of climate-driven stress responses in fish. Sci. Total Environ. 2022, 843, 156910. [Google Scholar] [CrossRef]
- Afshari, A.; Sourinejad, I.; Gharaei, A.; Johari, S.A.; Ghasemi, Z. The effects of diet supplementation with inorganic and nanoparticulate iron and copper on growth performance, blood biochemical parameters, antioxidant response and immune function of snow trout Schizothorax zarudnyi (Nikolskii, 1897). Aquaculture 2021, 539, 736638. [Google Scholar] [CrossRef]
- Minganti, V.; Drava, G.; De Pellegrini, R.; Siccardi, C. Trace elements in farmed and wild gilthead seabream, Sparus aurata. Mar. Pollut. Bull. 2010, 60, 2022–2025. [Google Scholar] [CrossRef]
- Yanpallewar, S.U.; Sen, S.; Tapas, S.; Kumar, M.; Raju, S.S.; Acharya, S.B. Effect of Azadirachta indica on paracetamol-induced hepatic damage in albino rats. Phytomedicine 2003, 10, 391–396. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Ganasen, S.; Batumalai, S.; Candasamy, M.; Krishnappa, P.; Dua, K.; Chellian, J.; Gupta, G. The Protective Action of the Aqueous Extract of Auricularia polytricha in Paracetamol Induced Hepatotoxicity in Rats. Recent Pat. Drug Deliv. Formul. 2016, 10, 72–76. [Google Scholar] [CrossRef]
- Eslamloo, K.; Falahatkar, B.; Yokoyama, S. Effects of dietary bovine lactoferrin on growth, physiological performance, iron metabolism and non-specific immune responses of Siberian sturgeon Acipenser baeri. Fish Shellfish Immunol. 2012, 32, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, D.; Huber, T.; Kurze, V.; Beyer, K.; Engelmann, B. Contribution of copper binding to the inhibition of lipid oxidation by plasmalogen phospholipids. Biochem. J. 1999, 340, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.; Khaiat, H. The Importance of Copper and the Effects of Its Deficiency and Toxicity in Animal Health. Int. J. Livest. Res. 2015, 5, 1–20. [Google Scholar] [CrossRef]
- Sevcikova, M.; Modra, H.; Blahova, J.; Dobsikova, R.; Plhalova, L.; Zitka, O.; Hynek, D.; Kizek, R.; Skoric, M.; Svobodova, Z. Biochemical, haematological and oxidative stress responses of common carp (Cyprinus carpio L.) after sub-chronic exposure to copper. Vet. Med. 2016, 61, 35–50. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac. Nutr. 2017, 23, 611–617. [Google Scholar] [CrossRef]
- Uyisenga, A.; Liang, H.; Ren, M.; Huang, D.; Xue, C.; Yin, H.; Mi, H. The Effects of Replacing Fish Meal with Enzymatic Soybean Meal on the Growth Performance, Whole-Body Composition, and Health of Juvenile Gibel Carp (Carassius auratus gibelio). Fishes 2023, 8, 423. [Google Scholar] [CrossRef]
- Musharraf, M.; Khan, M.A. Estimation of dietary copper requirement of fingerling Indian major carp, Labeo rohita (Hamilton). Aquaculture 2022, 549, 737742. [Google Scholar] [CrossRef]
- Wang, H.; Li, E.; Zhu, H.; Du, Z.; Qin, J.; Chen, L. Dietary copper requirement of juvenile Russian sturgeon Acipenser gueldenstaedtii. Aquaculture 2016, 454, 118–124. [Google Scholar] [CrossRef]
- Shi, B.; Lu, J.; Hu, X.; Betancor, M.B.; Zhao, M.; Tocher, D.R.; Zhou, Q.; Jiao, L.; Xu, F.; Jin, M. Dietary copper improves growth and regulates energy generation by mediating lipolysis and autophagy in hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2021, 537, 736505. [Google Scholar] [CrossRef]
- Parvez, S.; Sayeed, I.; Pandey, S.; Ahmad, A.; Bin-Hafeez, B.; Haque, R.; Ahmad, I.; Raisuddin, S. Modulatory effect of copper on nonenzymatic antioxidants in freshwater fish Channa punctatus (Bloch.). Biol. Trace Elem. Res. 2003, 93, 237–248. [Google Scholar] [CrossRef]
- Jiang, H.; Kong, X.; Wang, S.; Guo, H. Effect of Copper on Growth, Digestive and Antioxidant Enzyme Activities of Juvenile Qihe Crucian Carp, Carassius carassius, during Exposure and Recovery. Bull. Environ. Contam. Toxicol. 2016, 96, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, Y.; Li, S.H.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Effects of dietary copper on growth, digestive, and brush border enzyme activities and antioxidant defense of hepatopancreas and intestine for young grass carp (Ctenopharyngodon Idella). Biol. Trace Elem. Res. 2013, 155, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Kuete, V.; Eloff, J.N. Biochemical Parameters in Toxicological Studies in Africa: Significance, Principle of Methods, Data Interpretation, and Use in Plant Screenings. In Toxicological Survey of African Medicinal Plants; Elsevier Inc.: London, UK, 2014. [Google Scholar] [CrossRef]
- Fontagné-Dicharry, S.; Lataillade, E.; Surget, A.; Larroquet, L.; Cluzeaud, M.; Kaushik, S. Antioxidant defense system is altered by dietary oxidized lipid in first-feeding rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 424–425, 220–227. [Google Scholar] [CrossRef]
- Li, X.; Egervari, G.; Wang, Y.; Berger, S.L.; Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 2018, 19, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Mokrani, A.; Ji, K.; Ge, X.; Ren, M.; Pan, L.; Sun, A. Effects of dietary arginine on intestinal antioxidant status and immunity involved in Nrf2 and NF-κB signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Fish Shellfish Immunol. 2018, 82, 243–249. [Google Scholar] [CrossRef]
- Han, J.; Ulevitch, R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 2005, 6, 1198–1205. [Google Scholar] [CrossRef]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef]
- Ji, K.; Liang, H.; Ren, M.; Ge, X.; Liu, B.; Xi, B.; Pan, L.; Yu, H. Effects of dietary tryptophan levels on antioxidant status and immunity for juvenile blunt snout bream (Megalobrama amblycephala) involved in Nrf2 and TOR signaling pathway. Fish Shellfish Immunol. 2019, 93, 474–483. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, B.D.; Zhao, J.L.; Jiang, N.; Zhang, A.Z.; Wang, G.Q.; Li, M.Y. Amelioration of hexavalent chromium-induced bioaccumulation, oxidative stress, tight junction proteins and immune-related signaling factors by Allium mongolicum Regel flavonoids in Ctenopharyngodon idella. Fish Shellfish Immunol. 2020, 106, 993–1003. [Google Scholar] [CrossRef]
- Ali, Z.; Khan, I.; Iqbal, M.S.; Zhang, Q.; Ai, X.; Shi, H.; Ding, L.; Hong, M. Toxicological effects of copper on bioaccumulation and mRNA expression of antioxidant, immune, and apoptosis-related genes in Chinese striped-necked turtle (Mauremys sinensis). Front. Physiol. 2023, 14, 1296259. [Google Scholar] [CrossRef]
| Ingredients | Level (%) | Ingredients | Level (%) |
|---|---|---|---|
| Fish meal 1 | 20 | Choline chloride | 0.5 |
| Casein 1 | 28 | Vitamin premix 2 | 1 |
| Gelatin 1 | 7 | Mineral premix 3 (no copper) | 1 |
| Wheat Flour 1 | 16 | Calcium phosphate | 4 |
| Fish oil | 4 | Microcrystalline cellulose | 14.45 |
| Soybean oil | 4 | Vitamin C | 0.05 |
| Component Analysis | |||
| Crude protein (%) | 46.08 ± 0.21 | ||
| Crude lipid (%) | 9.97 ± 0.11 | ||
| Crude ash (%) | 4.05 ± 0.24 | ||
| Crude fiber | 13.69 ± 0.85 | ||
| Gross energy (KJ/g) | 15.35 ± 0.28 | ||
| Items | Methodologies |
|---|---|
| Moisture Crude protein Lipids Ash Gross energy Fiber | Dried sample in an oven at 105 °C Using the Kjeldahl procedure after acid digestion (multiplied by N × 6.25) Analysed through ether extraction using the Soxhlet system Examined by combusting at 550 °C for 5 h in an intelligent muffle furnace (model number XL-2A, Hangzhou, China: Zhang Chi Instruments Co., Ltd.) Examined by combusting in an oxygen bomb calorimeter: IKA C6000 (IKA Works, Guangzhou, China) Fibercarp method by Fiber analysis system (FiberCap™ 2021, FOSS, Hilleroed, Denmark) |
| Plasma total protein (TP, Mindray 105-000451-00) Albumin (ALB, Mindray 105-000450-00) Total cholesterol (TC, Mindray 105-000448-00) Glucose (GLU, Mindray 105-000460-00) Triglyceride (TG, Mindray 105-000449-00) Aspartate aminotransferase (AST, Mindray, 105-000443-00) Alanine aminotransferase (ALT, Mindray 105-000442-00) | Measured using a Mindray BS-400 Automatic Biochemical Analyser (Mindray Medical International Ltd., Shenzhen, China) |
| Malondialdehyde (MDA, A003-1-2) Glutathione (GSH, A006-2-1) Glutathione peroxidase (GPx, A005-1-2) Superoxide dismutase (SOD, A001-3-2) Total antioxidant capacity (T-AOC, A015-2-1) Catalase (CAT, A007-1-1) | Determined using biochemical kits from Nanjing Jiancheng Bioengineering Institute, Nanjing, China |
| Genes | Primer Sequence (5′-3′) | Reference | |
|---|---|---|---|
| gapdh | Forward | ACTGTCACTCCTCCATCTT | AZA04761.1 |
| Reverse | CACGGTTGCTGTATCCAA | ||
| tgf-β | Forward | GCTCAAAGAGAGCGAGGATG | [33] |
| Reverse | TCCTCTACCATTCGCAATCC | ||
| il-8 | Forward | CGTTGAACAGACTGGGAGAGATG | [34] |
| Reverse | AGTGGGATGGCTTCATTATCTTGT | ||
| il-10 | Forward | CGGCACAGAAATCCCAGAGC | [34] |
| Reverse | CAGCAGGCTCACAAAATAAACATCT | ||
| nrf2 | Forward | AGAGACATTCGCCGTAGA | NM_212855.2 |
| Reverse | TCGCAGTAGAGCAATCCT | ||
| keap1 | Forward | CGTACGTCCAGGCCTTACTC | XP_018520553.1 |
| Reverse | TGACGGAAATAACCCCCTGC | ||
| tnf-α | Forward | CTTCGTCTACAGCCAGGCATCG | [33] |
| Reverse | TTTGGCACACCGACCTCACC | ||
| Cu/Zn sod | Forward | TGGCAAGAACAAGAACCACA | [33] |
| Reverse | CCTCTGATTTCTCCTGTCACC | ||
| cat | Forward | CTATGGCTCTCACACCTTC | MK614708.1 |
| Reverse | TCCTCTACTGGCAGATTCT | ||
| gpx | Forward | GAAGGTGGATGTGAATGGA | MK614713.1 |
| Reverse | CCAACCAGGAACTTCTCAA | ||
| nf-κb | Forward | CCACTCAGGTGTTGGAGCTT | XP_027136364.1 |
| Reverse | TCCAGAGCACGACACACTTC |
| Dietary Cu Levels (mg/kg) | Growth Parameters | ||||
|---|---|---|---|---|---|
| IBW (g) | FBW (g) | FCR | WGR (%) | SGR (%/Day) | |
| 2.13 | 1.68 ± 0.01 | 17.71 ± 0.04 a | 0.96 ± 0.01 b | 953.92 ± 5.47 a | 4.21 ± 0.01 a |
| 3 | 1.68 ± 0.01 | 19.67 ± 0.28 c | 0.92 ± 0.01 a | 1075.60 ± 20.53 c | 4.40 ± 0.03 c |
| 4.58 | 1.67 ± 0.01 | 18.73 ± 0.36 b | 0.92 ± 0.00 a | 1022.39 ± 17.30 b | 4.31 ± 0.03 b |
| 4.64 | 1.67 ± 0.01 | 19.21 ± 0.27 bc | 0.92 ± 0.00 a | 1053.65 ± 17.60 bc | 4.37 ± 0.03 bc |
| 5.72 | 1.67 ± 0.01 | 19.15 ± 0.16 bc | 0.92 ± 0.00 a | 1046.59 ± 13.80 bc | 4.36 ± 0.02 bc |
| Dietary Cu levels (mg/kg) | Body Composition | |||
|---|---|---|---|---|
| Moisture (%) | Protein (%) | Lipid (%) | Ash (%) | |
| 2.13 | 71.99 ± 0.13 | 14.91 ± 0.76 | 6.41 ± 0.46 | 3.65 ± 0.07 |
| 3.00 | 71.63 ± 0.22 | 15.81 ± 0.14 | 7.09 ± 0.35 | 3.43 ± 0.14 |
| 3.66 | 71.45 ± 0.04 | 16.16 ± 0.09 | 6.39 ± 0.07 | 3.74 ± 0.11 |
| 4.58 | 71.92 ± 0.56 | 15.63 ± 0.35 | 7.78 ± 0.37 | 3.85 ± 0.13 |
| 4.64 | 71.37 ± 0.46 | 16.27 ± 0.47 | 6.72 ± 0.59 | 3.57 ± 0.06 |
| 5.72 | 71.78 ± 0.04 | 16.08 ± 0.13 | 6.27 ± 0.29 | 3.54 ± 0.19 |
| Parameters | Dietary Cu Levels (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 2.13 | 3.00 | 3.66 | 4.58 | 4.64 | 5.72 | |
| ALT (U/L) | 15.73 ± 3.39 | 12.03 ± 2.07 | 15.75 ± 2.98 | 20.88 ± 5.47 | 12.30 ± 2.78 | 24.50 ± 5.66 |
| AST (U/L) | 288.00 ± 59.11 | 251.80 ± 32.56 | 238.68 ± 34.52 | 297.45 ± 40.12 | 202.18 ± 33.62 | 285.55 ± 54.68 |
| TP (g/L) | 23.01 ± 2.16 a | 30.24 ± 1.71 b | 31.47 ± 2.04 b | 29.06 ± 1.26 b | 33.87 ± 1.71 b | 34.04 ± 1.39 b |
| ALB (g/L) | 2.30 ± 0.40 a | 4.03 ± 0.58 ab | 3.88 ± 0.74 ab | 4.23 ± 0.45 ab | 4.98 ± 0.68 b | 4.88 ± 0.96 b |
| TC (mmol/L) | 8.21 ± 0.90 a | 11.20 ± 0.74 b | 11.49 ± 0.53 b | 10.78 ± 0.76 b | 11.00 ± 1.35 b | 12.73 ± 0.74 b |
| TG (mmol/L) | 10.46 ± 1.24 a | 14.42 ± 1.20 ab | 15.02 ± 0.78 ab | 12.53 ± 2.04 ab | 17.08 ± 2.33 b | 17.32 ± 2.10 b |
| GLU (mmol/L) | 8.18 ± 0.68 b | 6.93 ± 0.53 ab | 5.85 ± 0.37 a | 7.97 ± 0.41 b | 6.23 ± 0.65 a | 5.40 ± 030 a |
| Parameters | Dietary Cu Levels (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 2.13 | 3 | 3.66 | 4.58 | 4.64 | 5.72 | |
| CAT (U/mgprot) | 4.25 ± 2.31 a | 13.84 ± 1.95 b | 13.85 ± 2.85 b | 5.99 ± 1.29 ab | 9.17 ± 3.91 ab | 6.18 ± 1.51 ab |
| SOD | 18.30 ± 2.81 ab | 11.30 ± 2.31 a | 12.07 ± 2.02 a | 13.23 ± 3.60 a | 16.16 ± 1.99 ab | 21.36 ± 1.72 b |
| (U/mgprot) | ||||||
| T-AOC (mmol/gprot) | 0.26 ± 0.04 | 0.41 ± 0.08 | 0.40 ± 0.04 | 0.39 ± 0.06 | 0.31 ± 0.02 | 0.35 ± 0.05 |
| GSH (μmol/gprot) | 19.38 ± 4.10 ab | 39.77 ± 6.23 c | 32.54 ± 5.24 bc | 14.90 ± 4.46 a | 27.21 ± 5.91 abc | 21.92 ± 3.05 ab |
| GPx | 65.36 ± 25.35 | 63.81 ± 23.46 | 39.45 ± 14.35 | 51.56 ± 15.72 | 40.54 ± 13.45 | 36.76 ± 10.27 |
| (U/mgprot) | ||||||
| MDA (nmol/mgprot) | 2.44 ± 0.48 | 2.40 ± 0.56 | 3.49 ± 0.54 | 2.50 ± 0.43 | 4.56 ± 0.97 | 4.48 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayiira, J.C.; Mi, H.; Liang, H.; Ren, M.; Huang, D.; Zhang, L.; Teng, T. Effect of Dietary Copper on Growth Performance, Antioxidant Capacity, and Immunity in Juvenile Largemouth Bass (Micropterus salmoides). Fishes 2024, 9, 369. https://doi.org/10.3390/fishes9090369
Kayiira JC, Mi H, Liang H, Ren M, Huang D, Zhang L, Teng T. Effect of Dietary Copper on Growth Performance, Antioxidant Capacity, and Immunity in Juvenile Largemouth Bass (Micropterus salmoides). Fishes. 2024; 9(9):369. https://doi.org/10.3390/fishes9090369
Chicago/Turabian StyleKayiira, John Cosmas, Haifeng Mi, Hualiang Liang, Mingchun Ren, Dongyu Huang, Lu Zhang, and Tao Teng. 2024. "Effect of Dietary Copper on Growth Performance, Antioxidant Capacity, and Immunity in Juvenile Largemouth Bass (Micropterus salmoides)" Fishes 9, no. 9: 369. https://doi.org/10.3390/fishes9090369
APA StyleKayiira, J. C., Mi, H., Liang, H., Ren, M., Huang, D., Zhang, L., & Teng, T. (2024). Effect of Dietary Copper on Growth Performance, Antioxidant Capacity, and Immunity in Juvenile Largemouth Bass (Micropterus salmoides). Fishes, 9(9), 369. https://doi.org/10.3390/fishes9090369







