The Ecological Niches and Interspecific Associations of the Dominant Fishes in the Xiamen Seas, China
Abstract
1. Introduction
2. Materials and Methods
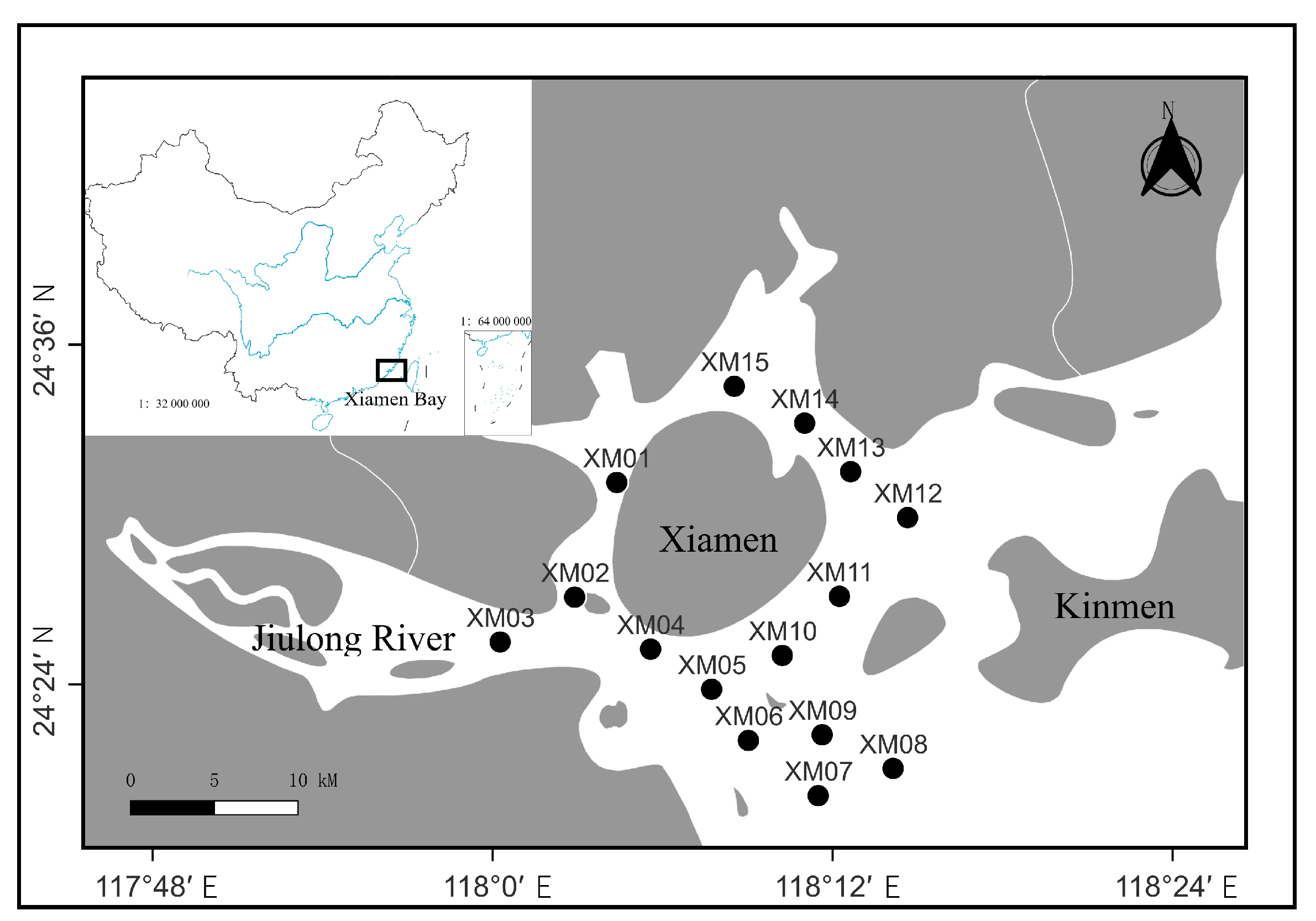
2.1. Study Area and Material Sources
2.2. Dominance Analysis
2.2.1. Index of Relative Importance (IRI)
2.2.2. Ecological Niche Width
2.2.3. Ecological Niche Overlap
2.3. Interspecific Association
2.4. Correlation Analysis
2.4.1. Association Coefficient
2.4.2. Pearson Correlation Analysis and Spearman’s Rank Correlation Analysis
2.5. Data Processing
3. Results
3.1. Relative Importance
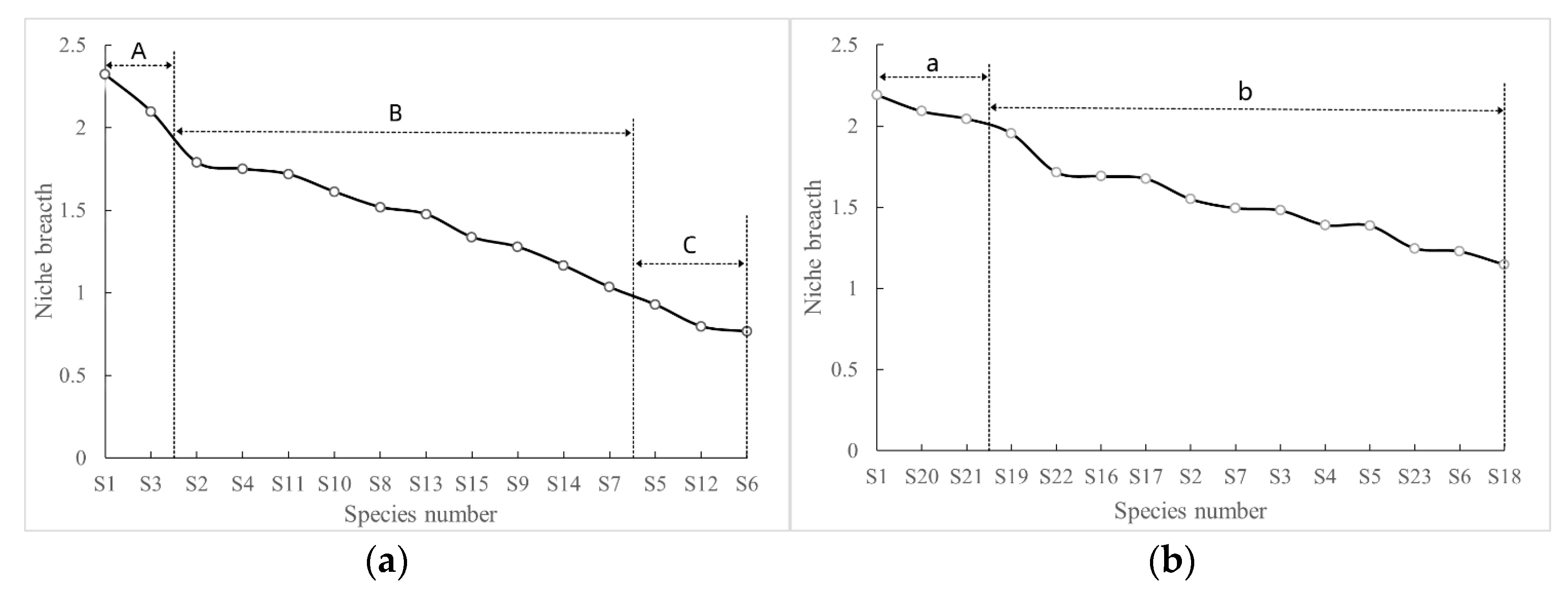
3.2. Main Species and Niche Width
3.3. Niche Overlap
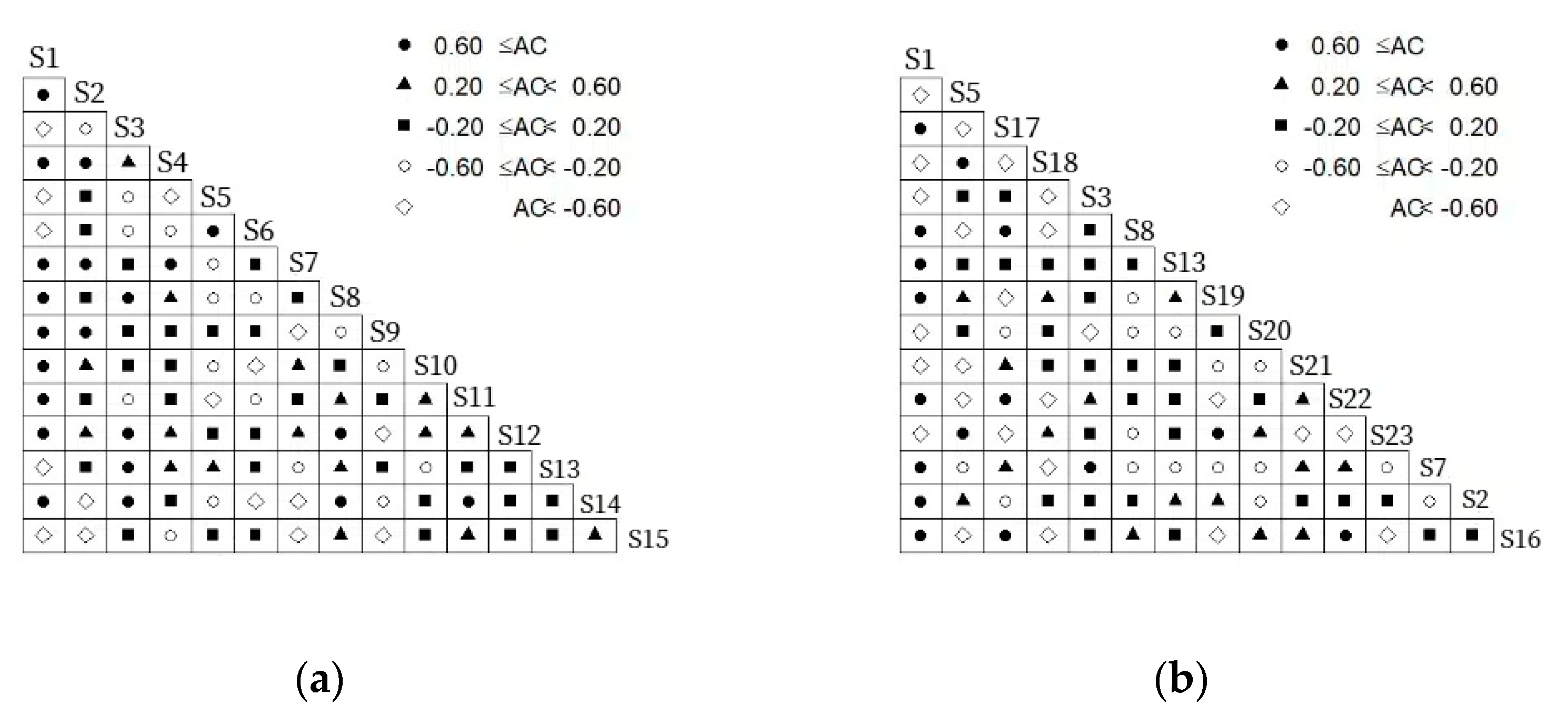
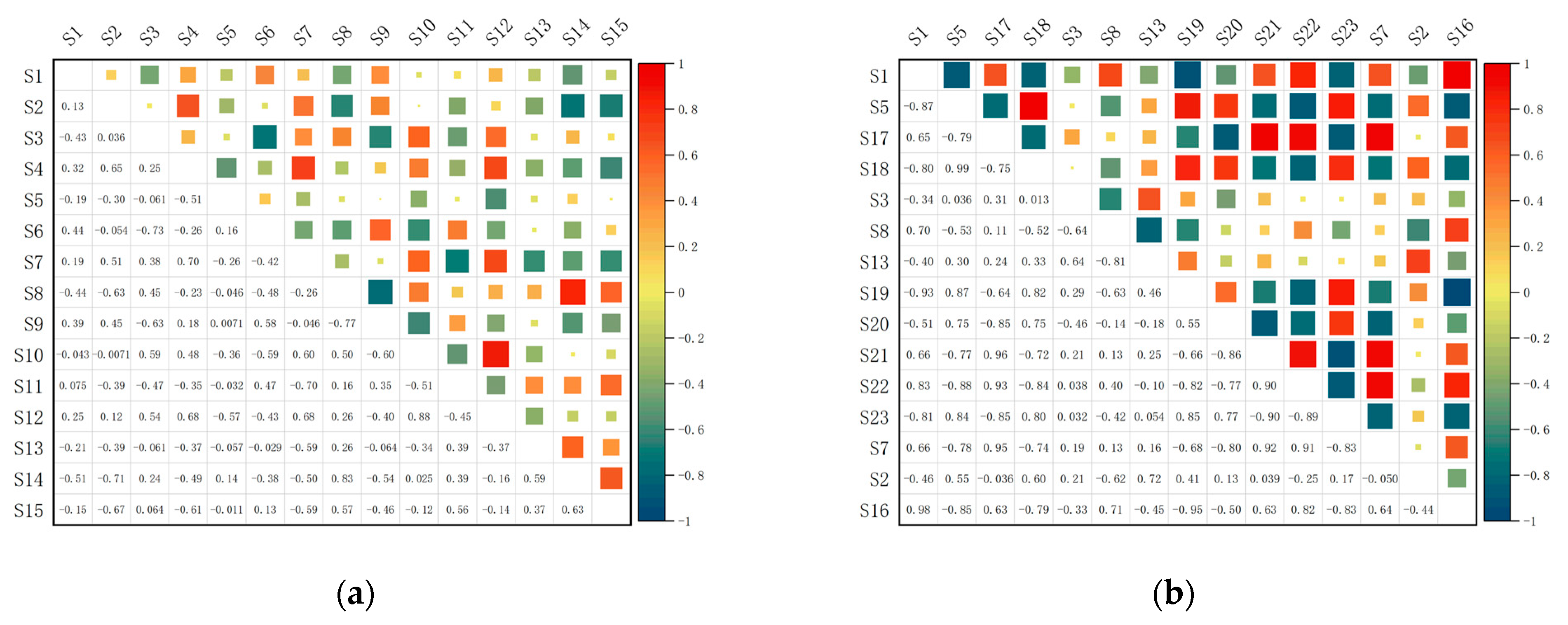
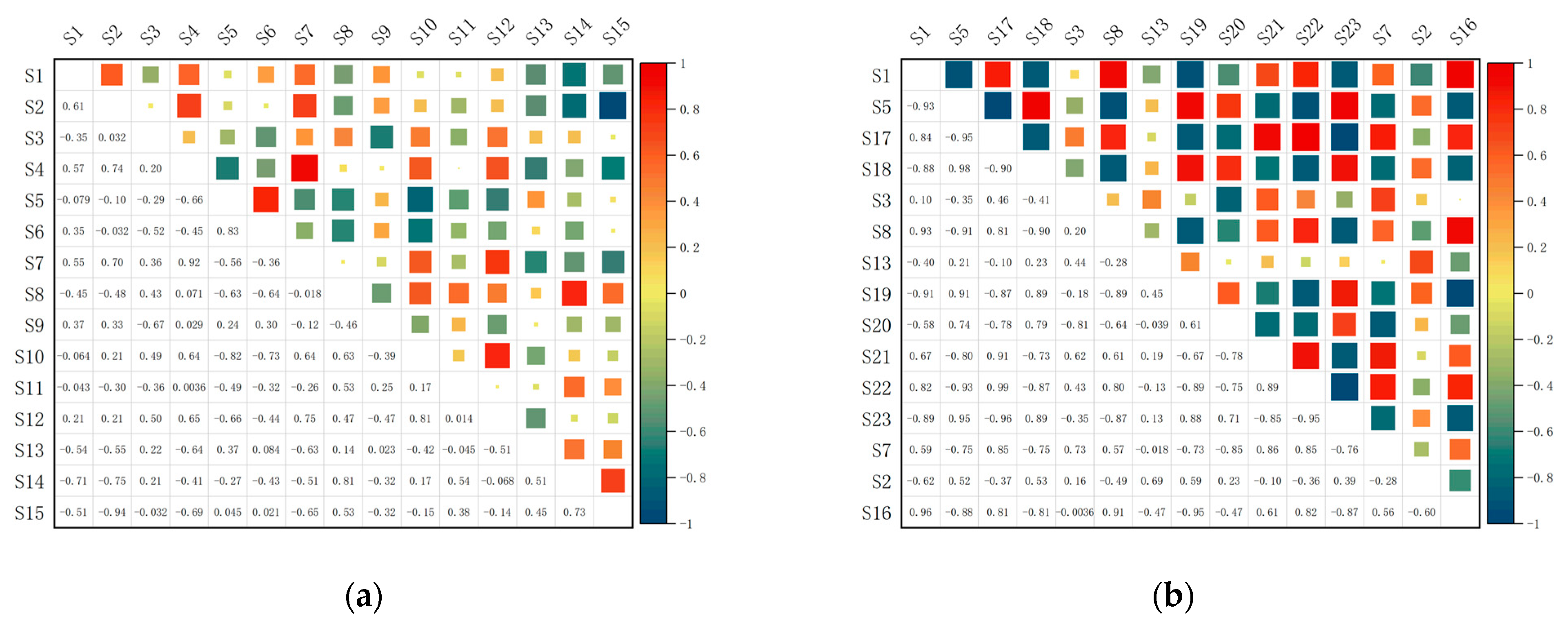
3.4. Overall Association Analysis
3.5. Interspecific Association Analysis
4. Discussion
4.1. Niche Width
4.2. Niche Overlap
4.3. Overall Association and Interspecific Association
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pocheville, A. The ecological niche: History and recent controversies. In Handbook of Evolutionary Thinking in the Sciences; Springer: New York, NY, USA, 2015; pp. 547–586. [Google Scholar]
- Parrish, J.; Bazzaz, F. Competitive interactions in plant communities of different successional ages. Ecology 1982, 63, 314–320. [Google Scholar] [CrossRef]
- Xue, W.X.; Li, C.H.; Ai, X.R.; Yao, L.; Guo, Q.J.; Zhu, J. Niche and interspecific association of dominant tree species in Liriodendron chinense natural forest. J. Agrofor. Environ. 2023, 43, 26–34. (In Chinese) [Google Scholar]
- Albert, J.S.; Tagliacollo, V.A.; Dagosta, F. Diversification of Neotropical freshwater fishes. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 27–53. [Google Scholar] [CrossRef]
- Rota, E.; Caruso, T.; Bargagli, R. Community structure, diversity and spatial organization of enchytraeids in Mediterranean urban holm oak stands. Eur. J. Soil Biol. 2014, 62, 83–91. [Google Scholar] [CrossRef]
- Ma, Y.M.; Li, Q.H.; Pan, S.P.; Kuang, P.; Jin, S. Dynamic analysis of niche and interspecific association of dominant phytoplankton species in Huaxi Reservoir of Guizhou Plateau. J. Lake Sci. 2021, 33, 785–796. (In Chinese) [Google Scholar]
- Wang, Z.L.; Chen, Y.; Chen, X.M.; Wang, S.Y.; Zhao, J.J. Temporal-spatial niches of Chinese White Wax Scale insect (Ericerus pela) and its three dominant parasitoid wasps. Acta Ecol. Sin. 2011, 31, 914–922. (In Chinese) [Google Scholar]
- Grinnell, J. The niche-relationships of the California Thrasher. Auk 1917, 34, 427–433. [Google Scholar] [CrossRef]
- Elton, C.S. The Animal Community. In Animal Ecology; Macmillan Co.: New York, NY, USA, 1927; pp. 50–55. [Google Scholar]
- Hutchinson, G.E. Population studies: Animal ecology and demography. Bull. Math. Biol. 1991, 53, 193–213. [Google Scholar] [CrossRef]
- Pan, G. Study on the Biodiversity and Niche of Three Forest Communities Inhilly Red Soil Region of Southern China; Central South University of Forestry and Technology: Changsha, China, 2016. (In Chinese) [Google Scholar]
- Duan, Y.; Wang, A.Y.; Wang, X.L.; Liu, X.Z.; Chai, Y.; Lu, Y.; Wang, B.; Dong, J. Seasonal Variation and Community Structure of Zooplankton in Northern Yellow Sea in Liaoning in Spring and Summer. Fish. Sci. China 2017, 36, 456–461. [Google Scholar]
- Li, P.; Li, Y.L.; Fu, J.; Yu, X.G.; Zou, C.J. Niche characteristics of macrobenthic community in the intertidal zone on the west coast of Liaodong Bay. Adv. Mar. Sci. 2019, 43, 32–39. (In Chinese) [Google Scholar]
- Vannette, R.L.; Fukami, T. Historical contingency in species interactions: Towards niche-based predictions. Ecol. Lett. 2014, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, J.L.; Zhang, C.; Yang, T.; Pu, L.H.; Zhao, W.Q.; Chen, P.L. The niche of dominant plant species in the karst soil nutrient resource axis around FAST. China J. Ecol. 2023, 42, 1820–1828. (In Chinese) [Google Scholar]
- Liu, L.G.; Zhu, Z.Q.; Chen, X.; Liu, S.J. Ecological Niche and Interspecific Association of Dominant Species in the Initial Stage of Vegetation Restoration in Zhongba Village of Xide County. J. West China For. Sci. 2022, 51, 110–117. (In Chinese) [Google Scholar]
- Liu, R.H.; Chen, L.; Tu, H.R.; Liang, S.C.; Jiang, Y.; Li, Y.J.; Huang, D.L.; Nong, J.L. Niche and interspecific association of main species in shrub layer of Cyclobalanopsis glauca community in karst hills of Guilin, southwest China. Acta Ecol. Sin. 2020, 40, 2057–2071. (In Chinese) [Google Scholar]
- Xu, M.H.; Liu, M.; Zhai, D.T.; Liu, T. A review of contents and methods used to analyze various aspects of plant interspecific associations. Acta Ecol. Sin. 2016, 36, 8224–8233. (In Chinese) [Google Scholar]
- Liu, Y.; Zheng, Y.Y.; Ao, Y.Y. Niche and interspecific association of dominant bryophytes on different substrates. Acta Ecol. Sin. 2019, 39, 286–293. (In Chinese) [Google Scholar]
- Colwell, R.K.; Futuyma, D.J. On the measurement of niche breadth and overlap. Ecology 1971, 52, 567–576. [Google Scholar] [CrossRef]
- Mueller, L.D.; Altenberg, L. Statistical inference on measures of niche overlap. Ecology 1985, 66, 1204–1210. [Google Scholar] [CrossRef]
- Leibold, M.A. The niche concept revisited: Mechanistic models and community context. Ecology 1995, 76, 1371–1382. [Google Scholar] [CrossRef]
- Vandermeer, J. The niche construction paradigm in ecological time. Ecol. Modell. 2008, 214, 385–390. [Google Scholar] [CrossRef]
- Yu, S.X.; Ao, L.X. Meaning and measurement of foundational and realization ecological niches and their centroids. Acta Sci. Nat. Univ. Sunyatseni 1993, 34, 69–80. (In Chinese) [Google Scholar]
- Zhang, T.; Jia, R.L.; Gao, Y.H.; Sun, J.Y.; Zhao, Y.; Liu, L.C. Niche characteristics and interspecific associations of main biocrust- forming organisms during the succession of artificially sand-binding vegetation in Shapotou, Ningxia, China. J. Desert. Res. 2021, 41, 100–108. (In Chinese) [Google Scholar]
- Hao, J.F.; Li, Y.; Qi, J.Q.; Pei, Z.L.; Huang, Y.J.; Jiang, Q.; Chen, Y. Effects of anthropogenic disturbances on the species diversity and niche of the dominant populations in a Castanopsis fargesii secondary forest community in Bifengxia, Sichuan. Acta Ecol. Sin. 2016, 36, 7678–7688. [Google Scholar]
- Liang, Y.L.; Guo, Q.X. Determining niches of the major tree species based on a flow accumulation technique in a natural secondary forest in Northeastern China. Acta Ecol. Sin. 2010, 30, 2874–2882. (In Chinese) [Google Scholar]
- Allen, R.M.; Metaxas, A.; Snelgrove, P.V. Applying movement ecology to marine animals with complex life cycles. Annu. Rev. Mar. Sci. 2018, 10, 19–42. [Google Scholar] [CrossRef]
- Gelviz-Gelvez, S.M.; Pavón, N.P.; Illoldi-Rangel, P.; Ballesteros-Barrera, C. Ecological niche modeling under climate change to select shrubs for ecological restoration in Central Mexico. Ecol. Eng. 2015, 74, 302–309. [Google Scholar] [CrossRef]
- Nosil, P.; Sandoval, C.P. Ecological niche dimensionality and the evolutionary diversification of stick insects. PLoS ONE 2008, 3, 1907. [Google Scholar] [CrossRef]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Sánchez-Gómez, D. Ecophysiological traits associated with drought in Mediterranean tree seedlings: Individual responses versus interspecific trends in eleven species. Plant Biol. 2006, 8, 688–697. [Google Scholar] [CrossRef]
- Belshe, E.F.; Hoeijmakers, D.; Herran, N.; Mtolera, M.; Teichberg, M. Seagrass community-level controls over organic carbon storage are constrained by geophysical attributes within meadows of Zanzibar, Tanzania. Biogeosciences 2018, 15, 4609–4626. [Google Scholar] [CrossRef]
- Mouton, J.C.; Martin, T.E. Fitness consequences of interspecific nesting associations among cavity-nesting birds. Am. Nat. 2018, 192, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.C. Ecological Characteristics and Fishery Resources of Estuaries and Offshore Areas. In Life Below Water. Encyclopedia of the UN Sustainable Development Goals; Springer: Cham, Switzerland, 1992; pp. 24–30. (In Chinese) [Google Scholar]
- Huang, L.M.; Zhang, H.J.; Zhang, Y.Z.; Lin, T.; Li, J.; Wang, J.Q.; Chang, G.F. Current status and progress of research on the relationship between fish organisms and the aquatic environment in coastal estuaries. Chin. J. Oceanol. Limnol. 2013, 8, 61–68. (In Chinese) [Google Scholar]
- Lin, N.; Shen, C.C.; Zhong, J.S. Species composition and seasonal variation of fish larvae and juveniles in Jiulong River estuary. South China Fish. Sci. 2009, 5, 1–8. (In Chinese) [Google Scholar]
- Lin, N.; Shen, C.C.; Zhong, J.S. Study on the diversity and migration mechanism of fish larvae and juveniles in Jiulong River estuary. Mar. Fish. 2010, 32, 66–72. (In Chinese) [Google Scholar]
- Wang, J.Q.; Shih, Y.J.; Huang, L.M.; Li, J.; Li, W.W.; Shih, C.H.; Chu, T.J. Evaluating the effects related to restocking and stock replenishment of Penaeus penicillatus in the Xiamen Bay, China. J. Mar. Sci. Eng. 2021, 9, 1122. [Google Scholar] [CrossRef]
- Xu, C.Y.; Shen, C.C.; Liu, Y.; Ma, C. Species composition and quantitative distribution of spring fisheggs and juveniles in thesouthern sea of Xiamen, China. J. Fujian Fish. 2013, 6, 448–454. (In Chinese) [Google Scholar]
- Zhang, H.J.; Huang, L.M.; Li, J.; Wang, J.Q.; Zhang, Y.Z.; Lin, T.; Chang, G.F. Variations of fish community structure in the waters adjacent to Fugong in Jiulongjiang Estuary. J. Appl. Oceanogr. 2013, 32, 7. (In Chinese) [Google Scholar]
- Liu, Y.; Xu, C.Y.; Shen, C.C.; Ma, C. Characteristic of nekton community structure in spring and autumn in Jiulongjiang Estuary. J. Fish. Res. 2015, 37, 194–201. (In Chinese) [Google Scholar]
- Zhong, Z.H.; Lin, X.Z.; Yang, S.J.; Wang, Z.K.; Chen, S.B. Temporal and spatial distribution of nekton in the Xiamen Sea. J. Oceanogr. Taiwan Strait. 2010, 29, 241–249. (In Chinese) [Google Scholar]
- Du, J.G.; Liu, Z.H.; Yu, X.G.; Xu, Z.C.; Hu, W.J.; Chen, B.; Ma, Z.Y.; Lin, J.L. Fish species diversity and trophic level in the Jiulong Estuary. J. Trop. Oceanogr. 2012, 31, 76–82. (In Chinese) [Google Scholar]
- Ye, S.Z.; Ke, S.T.; Ma, C.; Luo, D.L.; Liu, Y.; Yang, F.; Guo, S.Y.; Shen, C.C.; Cai, J.D.; Xu, C.Y.; et al. Diversity and Distributional Characteristics of Fishery Biomes in the Jiulong River Estuary. Fish. Sci. 2020, 39, 359–368. (In Chinese) [Google Scholar]
- Huang, L.M.; Zhang, Y.Z.; Du, C.X.; Wei, Y.L. Analysis of the species composition and diversity of fish caught by drift gillnets in Xiamen East China Sea. J. Oceanogr. Taiwan Strait. 2007, 26, 261–269. (In Chinese) [Google Scholar]
- Huang, L.M.; Xie, Y.J.; Li, J.; Zhang, Y.Z.; Wang, J.Q. The taxonomic diversity of fish community in the coastal waters of Xiamen. Acta Oceanol. Sin. 2013, 35, 126–132. (In Chinese) [Google Scholar]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Food Habits of Albacore, Bluefin Tuna, and Bonito in California Waters; California Department of Fish and Game: Sacramento, CA, USA, 1971; Volume 152, pp. 1–105.
- Cheng, J.S. The Structure and Diversity of Demersal Fish Communities in Winter in the East China Sea and the Yellow Sea. Mar. Fish. Res. 2000, 28, 1–8. (In Chinese) [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pianka, R.E. The Structure of Lizard Communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Schluter, D. A variance test for detecting species associations, with some example applications. Ecology 1984, 65, 998–1005. [Google Scholar] [CrossRef]
- Zhang, J.T. A Quantitative Ecological Approach to Vegetation; China Science and Technology Press: Beijing, China, 1995; pp. 1–380. ISBN 7-5046-1313-4. [Google Scholar]
- Li, Y.D.; Xu, H.; Chen, D.X.; Luo, T.S.; Mo, J.H.; Mo, M.; Chen, H.Q.; Jiang, Z.L. Discussing on the Ecological Species Groups and Functional Groups Division Based on the Interspecific Association—A Case Study on the Arbor Layer Data in Tropical Lowland Rain Forest of Jianfenling, Hainan Island, China. Sci. Silvae Sin. 2007, 4, 9–16. (In Chinese) [Google Scholar]
- Xiao, Y.M.; Xie, J.Y.; Yao, Y.P.; Liang, S.C.; Gao, L.N.; Zhang, H.; Mo, S.Z. Niche of dominant species in arbor layer of evergreen deciduous broad-leaved mixed forest in karst hills of Guilin, southwestern China. Acta Ecol. Sin. 2021, 41, 8159–8170. [Google Scholar]
- Wei, Y.Q.; Song, X.Q.; Zhao, Y.; Shi, Y.H. Interspecific Association of Woody Plants in Michelia shiluensis Community, Diaoluoshan Nature Reserve, Hainan Province. Chin. J. Trop. Crops 2022, 43, 2606–2613. [Google Scholar]
- Peng, S.Y.; Li, X.Z.; Wang, H.F.; Zhang, B.L. Niche analysis of dominant species of macrozoobenthic community in the southern Yellow Sea in spring. Acta Ecol. Sin. 2015, 35, 1917–1928. (In Chinese) [Google Scholar]
- Hu, C.Y.; Shui, Y.Y.; Du, X.; Shui, B.N. Niche and interspecific association of nekton in the south coastal waters of Cangnan, Zhejiang, China. Chin. J. Appl. Ecol. 2015, 26, 3195–3201. (In Chinese) [Google Scholar]
- Ge, B.M.; Zheng, X.; Cheng, H.Y.; Bao, Y.X.; Chen, L. The Macrobenthic Community and Niche Analysis of Creeks in Diked Tidal Flat at Lingkun Island, Wenzhou Bay. Acta Hydrobiol. Sin. 2007, 31, 675–681. (In Chinese) [Google Scholar] [CrossRef]
- Li, S.F.; Liu, W.D.; Su, J.R.; Lang, X.D.; Zhang, Z.J. Niche and Interspecific Association of Species of Taxus yunnanensis Communities in Northwest Yunnan Province. Plant Sci. J. 2012, 30, 568–576. (In Chinese) [Google Scholar] [CrossRef]
- Carscadden, K.A.; Emery, N.C.; Arnillas, C.A.; Cadotte, M.W.; Afkhami, M.E.; Gravel, D.; Livingstone, S.W.; Wiens, J.J. Niche breadth: Causes and consequences for ecology, evolution, and conservation. Q. Rev. Biol. 2020, 95, 179–214. [Google Scholar] [CrossRef]
- Xie, F.; Liang, J.; Xing, K. Distribution characteristics of zooplankton in summer and autumn in the coastal waters of Hainan Island. Coast. Eng. J. 2019, 38, 280–292. (In Chinese) [Google Scholar]
- Han, D.; Xue, Y.; Ji, Y.; Xu, B.; Liu, H.; Ma, Q. Trophic and spatial niche of five gobiid fishes in Jiaozhou Bay. J. Fish. Sci. China. 2013, 20, 148–156. (In Chinese) [Google Scholar] [CrossRef]
- Xu, C.Y.; Yu, Q.J.; Xu, F.J.; Hu, X.Q.; You, W.H. Niche analysis of phytoplankton’s dominant species in Dianshan Lake of East China. Chin. J. Appl. Ecol. 2012, 23, 2550–2558. (In Chinese) [Google Scholar]
- Liu, S.Y.; Yu, X.X.; Chen, L.H. Interspecific association and niche research of natural forest in Beijing mountainous area. J. Northeast For. Univ. 2009, 24, 26–30. (In Chinese) [Google Scholar]
- Deng, X.Y.; Liu, J.G.; Guo, P.J.; Yu, C.G.; Zhang, P.; Zheng, J.; Xie, X.; Miao, L. Niche and interspecific association of major nekton in Xiao Yangshan adjacent waters. Acta Oceanol. Sin. 2018, 40, 96–105. (In Chinese) [Google Scholar]
- Liao, B.W.; Li, M.; Zheng, S.F.; Chen, Y.J.; Zhong, C.R.; Huang, Z.Q. Niches of several mangrove species in Dongzhai harbor of Hainan island. Chin. J. Appl. Ecol. 2005, 16, 403–407. (In Chinese) [Google Scholar]
- Li, X.S.; Yu, Z.H.; Sun, S.; Jin, X.S. Ecological niche breadth and niche overlap of dominant species of fish assemblage in Yangtze River estuary and its adjacent waters. Chin. J. Appl. Ecol. 2013, 24, 2353–2359. (In Chinese) [Google Scholar]
- Pratchett, M.; Berumen, M. Interspecific variation in distributions and diets of coral reef butterflyfishes (Teleostei: Chaetodontidae). J. Fish Biol. 2008, 73, 1730–1747. [Google Scholar] [CrossRef]
- Yu, X.B.; Yu, R.; Huang, J.; Yuan, Z.Y.; Yang, F.; Han, G.D. Analysis on interspecific associations among dominant populations in each layer of Loropetalum subcordatum community in Maolan National Nature Reserve of Guizhou. J. Plant Resour. Environ. 2018, 27, 24–32. (In Chinese) [Google Scholar]
- Su, S.J.; Liu, J.F.; He, Z.S.; Zheng, S.Q.; Hong, W.; Xu, D.W. Ecological species groups and interspecific association of dominant tree species in Daiyun Mountain National Nature Reserve. J. MT Sci.-Engl. 2015, 12, 637–646. (In Chinese) [Google Scholar] [CrossRef]
- Bao, J.J. Study on Topography and Geomorphologyof Xiamen Bay and its Adjacent Regions. Master’s Thesis, Third Institute of Oceangraphy, Ministry of Natutural Resources, Xiamen, China, 2021. (In Chinese). [Google Scholar]
- Zhu, X.J. The community structure and biodiversity of nekton in Xiamen sea area, Fujian Province. J. Fish. Res. 2024, 46, 69–84. (In Chinese) [Google Scholar]
- Sattler, T.; Bontadina, F.; Hirzel, A.H.; Arlettaz, R. Ecological niche modelling of two cryptic bat species calls for a reassessment of their conservation status. J. Appl. Ecol. 2007, 44, 1188–1199. [Google Scholar] [CrossRef]
- Zhang, L.N.; Zhu, G.Q.; Yang, K.; Liu, X.Y.; Gong, H.D.; Zheng, L. Niche and interspecific association of main woody plants in Myrica nana shrubland in central Yunnan, China. Chin. J. Plant Ecol. 2022, 46, 1400. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, J.; Shih, Y.J.; Huang, L.M.; Wang, X.R.; Chu, T.J. Sustainability perspective of Minjiang Estuary coastal fisheries management—Estimation of fish richness. Water 2023, 15, 2648. [Google Scholar] [CrossRef]
- Huang, L.M.; Wang, J.Q.; Shih, Y.J.; Li, J.; Chu, T.J. Revealing the effectiveness of fisheries policy: A biological observation of species Johnius belengerii in Xiamen Bay. J. Mar. Sci. Eng. 2022, 10, 732. [Google Scholar] [CrossRef]
| Species | Dominant Species | Spring | Autumn | ||
|---|---|---|---|---|---|
| IRI | Bi | IRI | Bi | ||
| S1 * | Johnius belengerii | 2763.268 | 2.321 | 3807.484 | 2.192 |
| S2 * | Chiloscyllium plagiosum | 2094.489 | 1.789 | 129.294 | 1.550 |
| S3 * | Trypauchen vagina | 1185.717 | 2.097 | 319.596 | 1.480 |
| S5 * | Leiognathus brevirostris | 350.257 | 0.928 | 1613.393 | 1.390 |
| S7 * | Dasyatis zugei | 252.140 | 1.034 | 129.894 | 1.386 |
| S8 * | Argyrosomus argentatus | 193.925 | 1.518 | 298.696 | 1.227 |
| S13 * | Daggertooth pike conger | 128.745 | 1.475 | 204.305 | 1.494 |
| S14 | Cynoglossus macrolepidotus | 118.364 | 1.165 | ||
| S15 | Pleuronichthys cornutus | 104.376 | 1.336 | ||
| S4 | Platyrhina limboonkengi | 761.224 | 1.750 | ||
| S6 | Sebastiscus marmoratus | 304.413 | 0.765 | ||
| S9 | Dasyatis akajei | 172.881 | 1.277 | ||
| S10 | Cynoglossus abbreviatus | 163.144 | 1.611 | ||
| S11 | Cociella crocodilla | 141.159 | 1.717 | ||
| S12 | Platyrhina sinensis | 134.611 | 0.796 | ||
| S16 | Coilia mystus | 119.271 | 1.691 | ||
| S17 | Harpodon nehereus | 703.351 | 1.676 | ||
| S18 | Plotosus anguillaris | 538.770 | 1.146 | ||
| S19 | Dendrophysa russelii | 192.623 | 1.954 | ||
| S20 | Sillago sihama | 190.976 | 2.091 | ||
| S21 | Ostorhinchus quadrifasciatus | 185.936 | 2.045 | ||
| S22 | Larimichthys crocea | 179.187 | 1.716 | ||
| S23 | Siganus canaliculatus | 131.650 | 1.246 | ||
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2 | 0.467 | |||||||||||||
| S3 | 0.516 | 0.364 | ||||||||||||
| S4 | 0.610 | 0.357 | 0.313 | |||||||||||
| S5 | 0.098 | 0.045 | 0.234 | 0.004 | ||||||||||
| S6 | 0.526 | 0.152 | 0.019 | 0.003 | 0.119 | |||||||||
| S7 | 0.309 | 0.418 | 0.316 | 0.524 | 0.003 | 0.003 | ||||||||
| S8 | 0.226 | 0.058 | 0.461 | 0.177 | 0.002 | 0.001 | 0.055 | |||||||
| S9 | 0.360 | 0.516 | 0.151 | 0.251 | 0.060 | 0.476 | 0.000 | 0.030 | ||||||
| S10 | 0.351 | 0.365 | 0.426 | 0.464 | 0.006 | 0.000 | 0.301 | 0.750 | 0.064 | |||||
| S11 | 0.473 | 0.172 | 0.209 | 0.302 | 0.070 | 0.627 | 0.055 | 0.459 | 0.457 | 0.236 | ||||
| S12 | 0.471 | 0.233 | 0.306 | 0.560 | 0.005 | 0.005 | 0.285 | 0.324 | 0.000 | 0.716 | 0.157 | |||
| S13 | 0.329 | 0.328 | 0.356 | 0.192 | 0.050 | 0.013 | 0.037 | 0.387 | 0.143 | 0.069 | 0.566 | 0.053 | ||
| S14 | 0.157 | 0.007 | 0.328 | 0.143 | 0.084 | 0.000 | 0.000 | 0.643 | 0.022 | 0.202 | 0.607 | 0.033 | 0.809 | |
| S15 | 0.444 | 0.000 | 0.148 | 0.060 | 0.052 | 0.305 | 0.000 | 0.544 | 0.000 | 0.301 | 0.307 | 0.083 | 0.385 | 0.506 |
| S1 | S4 | S17 | S18 | S3 | S6 | S7 | S19 | S20 | S21 | S22 | S23 | S5 | S2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S4 | 0.028 | |||||||||||||
| S17 | 0.561 | 0.000 | ||||||||||||
| S18 | 0.024 | 0.908 | 0.000 | |||||||||||
| S3 | 0.128 | 0.015 | 0.199 | 0.001 | ||||||||||
| S6 | 0.830 | 0.000 | 0.074 | 0.000 | 0.021 | |||||||||
| S7 | 0.295 | 0.180 | 0.381 | 0.394 | 0.169 | 0.018 | ||||||||
| S19 | 0.149 | 0.561 | 0.163 | 0.563 | 0.487 | 0.010 | 0.532 | |||||||
| S20 | 0.344 | 0.682 | 0.124 | 0.660 | 0.037 | 0.265 | 0.245 | 0.601 | ||||||
| S21 | 0.652 | 0.112 | 0.906 | 0.111 | 0.244 | 0.153 | 0.551 | 0.267 | 0.202 | |||||
| S22 | 0.687 | 0.000 | 0.857 | 0.000 | 0.110 | 0.281 | 0.217 | 0.074 | 0.181 | 0.775 | ||||
| S23 | 0.092 | 0.295 | 0.000 | 0.114 | 0.077 | 0.001 | 0.143 | 0.591 | 0.630 | 0.034 | 0.000 | |||
| S5 | 0.441 | 0.002 | 0.627 | 0.000 | 0.169 | 0.014 | 0.250 | 0.085 | 0.098 | 0.686 | 0.651 | 0.029 | ||
| S2 | 0.301 | 0.465 | 0.415 | 0.551 | 0.358 | 0.021 | 0.528 | 0.334 | 0.503 | 0.517 | 0.262 | 0.052 | 0.302 | |
| S16 | 0.915 | 0.000 | 0.391 | 0.000 | 0.053 | 0.815 | 0.209 | 0.024 | 0.321 | 0.542 | 0.544 | 0.000 | 0.314 | 0.252 |
| Season | VR | W | X2(x20.95(15), x20.05(15)) | Inspection Result | ||
|---|---|---|---|---|---|---|
| Spring | 3.10 | 4.51 | 1.45 | 21.79 | (7.26, 25.0) | Non-significant positive association |
| Autumn | 3.30 | 1.66 | 0.50 | 7.56 | (7.26, 25.0) | Non-significant negative association |
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2 | 11.25 ** | |||||||||||||
| S3 | 0.02 | 9.40 ** | ||||||||||||
| S4 | 3.36 | 0.02 | 11.25 ** | |||||||||||
| S5 | 0.1 | 0.16 | 1.89 | 11.12 ** | ||||||||||
| S6 | 0.18 | 1.04 | 0.18 | 5.13 * | 10.32 | |||||||||
| S7 | 2.56 | 0.19 | 3.65 | 0.01 | 0.33 | 10.32 ** | ||||||||
| S8 | 0.1 | 0.85 | 0.55 | 0.94 | 0.01 | 0.01 | 11.12 ** | |||||||
| S9 | 2.56 | 0.19 | 0.18 | 0.01 | 0.33 | 0.56 | 0.01 | 10.32 ** | ||||||
| S10 | 0.1 | 0.16 | 0.1 | 0.94 | 1.72 | 1.15 | 0.01 | 0.01 | 11.12 ** | |||||
| S11 | 0.06 | 0.02 | 0.06 | 1.89 | 0.18 | 0.18 | 3.23 | 0.18 | 0.55 | 11.25 ** | ||||
| S12 | 0.02 | 0.03 | 0.02 | 0.16 | 0.19 | 1.04 | 2.93 | 0.19 | 0.16 | 0.02 | 9.40 ** | |||
| S13 | 0.03 | 0.47 | 0.03 | 0.31 | 0.04 | 0.04 | 0.31 | 0.04 | 0.31 | 0.03 | 0.47 | 10.84 ** | ||
| S14 | 1.64 | 0.47 | 0.03 | 0.31 | 1.07 | 1.07 | 2.81 | 0.04 | 0.31 | 1.64 | 0.47 | 0.04 | 10.84 ** | |
| S15 | 5.66 * | 0.47 | 0.84 | 0.31 | 0.04 | 1.07 | 0.31 | 1.07 | 0.31 | 0.03 | 0.47 | 0.04 | 0.94 | 10.84 |
| S1 | S5 | S17 | S18 | S3 | S8 | S13 | S19 | S20 | S21 | S22 | S23 | S7 | S2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S5 | 11.25 ** | |||||||||||||
| S17 | 11.25 | 11.25 | ||||||||||||
| S18 | 5.66 * | 5.66 * | 10.84 | |||||||||||
| S3 | 0.06 | 0.06 | 1.64 | 11.25 ** | ||||||||||
| S8 | 1.89 | 1.89 | 2.81 | 0.1 | 11.12 ** | |||||||||
| S13 | 0.03 | 0.03 | 0.04 | 0.03 | 0.31 | 10.84 ** | ||||||||
| S19 | 3.36 | 3.36 | 0.84 | 0.06 | 0.55 | 0.84 | 11.25 ** | |||||||
| S20 | 0.18 | 0.18 | 1.07 | 2.56 | 1.15 | 0.04 | 0.18 | 10.32 ** | ||||||
| S21 | 1.64 | 1.64 | 0.04 | 0.03 | 0.31 | 0.04 | 0.84 | 0.04 | 10.84 ** | |||||
| S22 | 5.90 * | 5.90 * | 2.81 | 0.1 | 0.01 | 0.31 | 3.23 | 0.01 | 0.31 | 11.12 | ||||
| S23 | 8.14 ** | 8.14 ** | 2.81 | 0.1 | 0.94 | 0.31 | 1.89 | 0.01 | 2.81 | 4.18 * | 11.12 | |||
| S7 | 0.18 | 0.18 | 1.07 | 2.56 | 0.01 | 0.04 | 0.55 | 0.33 | 0.04 | 1.15 | 0.01 | 10.32 ** | ||
| S2 | 0.03 | 0.03 | 0.04 | 0.03 | 0.31 | 0.94 | 0.84 | 0.04 | 0.04 | 0.31 | 0.31 | 0.04 | 10.84 ** | |
| S16 | 5.90 * | 5.90 * | 2.81 | 0.1 | 1.4 | 0.31 | 3.23 | 0.01 | 0.31 | 5.10 * | 4.18 * | 0.01 | 0.31 | 11.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-M.; Xu, H.-Q.; Yu, J.-Y.; Chen, Y.-H.; Wang, J.-Q.; Ji, F.-F.; Li, J.; Cai, J.-D.; Chu, T.-J. The Ecological Niches and Interspecific Associations of the Dominant Fishes in the Xiamen Seas, China. Fishes 2024, 9, 354. https://doi.org/10.3390/fishes9090354
Huang L-M, Xu H-Q, Yu J-Y, Chen Y-H, Wang J-Q, Ji F-F, Li J, Cai J-D, Chu T-J. The Ecological Niches and Interspecific Associations of the Dominant Fishes in the Xiamen Seas, China. Fishes. 2024; 9(9):354. https://doi.org/10.3390/fishes9090354
Chicago/Turabian StyleHuang, Liang-Min, Hao-Qi Xu, Jia-Yue Yu, Yong-He Chen, Jia-Qiao Wang, Fen-Fen Ji, Jun Li, Jian-Di Cai, and Ta-Jen Chu. 2024. "The Ecological Niches and Interspecific Associations of the Dominant Fishes in the Xiamen Seas, China" Fishes 9, no. 9: 354. https://doi.org/10.3390/fishes9090354
APA StyleHuang, L.-M., Xu, H.-Q., Yu, J.-Y., Chen, Y.-H., Wang, J.-Q., Ji, F.-F., Li, J., Cai, J.-D., & Chu, T.-J. (2024). The Ecological Niches and Interspecific Associations of the Dominant Fishes in the Xiamen Seas, China. Fishes, 9(9), 354. https://doi.org/10.3390/fishes9090354







