Fishery Management Enforcement Gradients to Achieve Fishery Goals
Abstract
1. Introduction
2. Materials and Methods
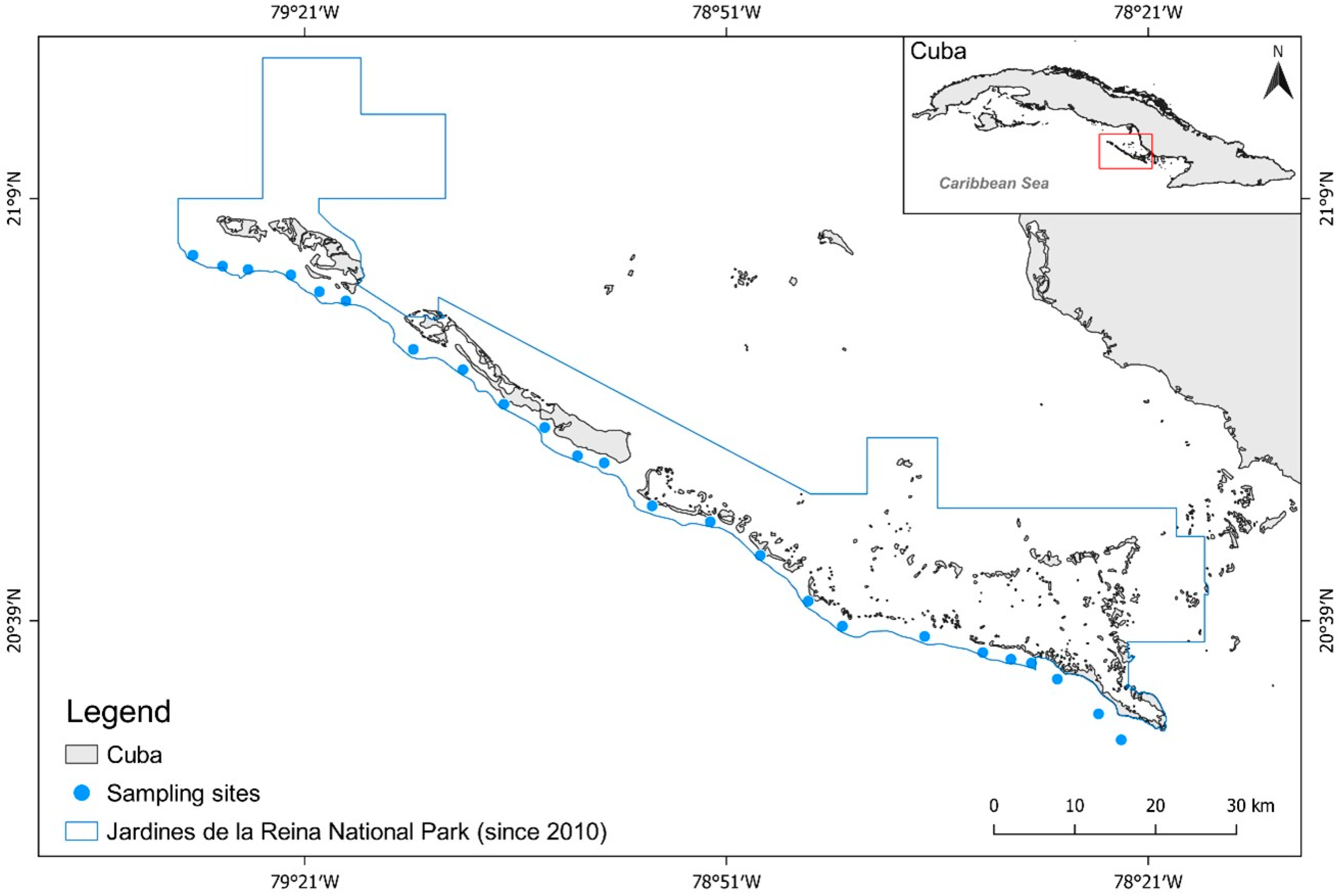
2.1. Case Study
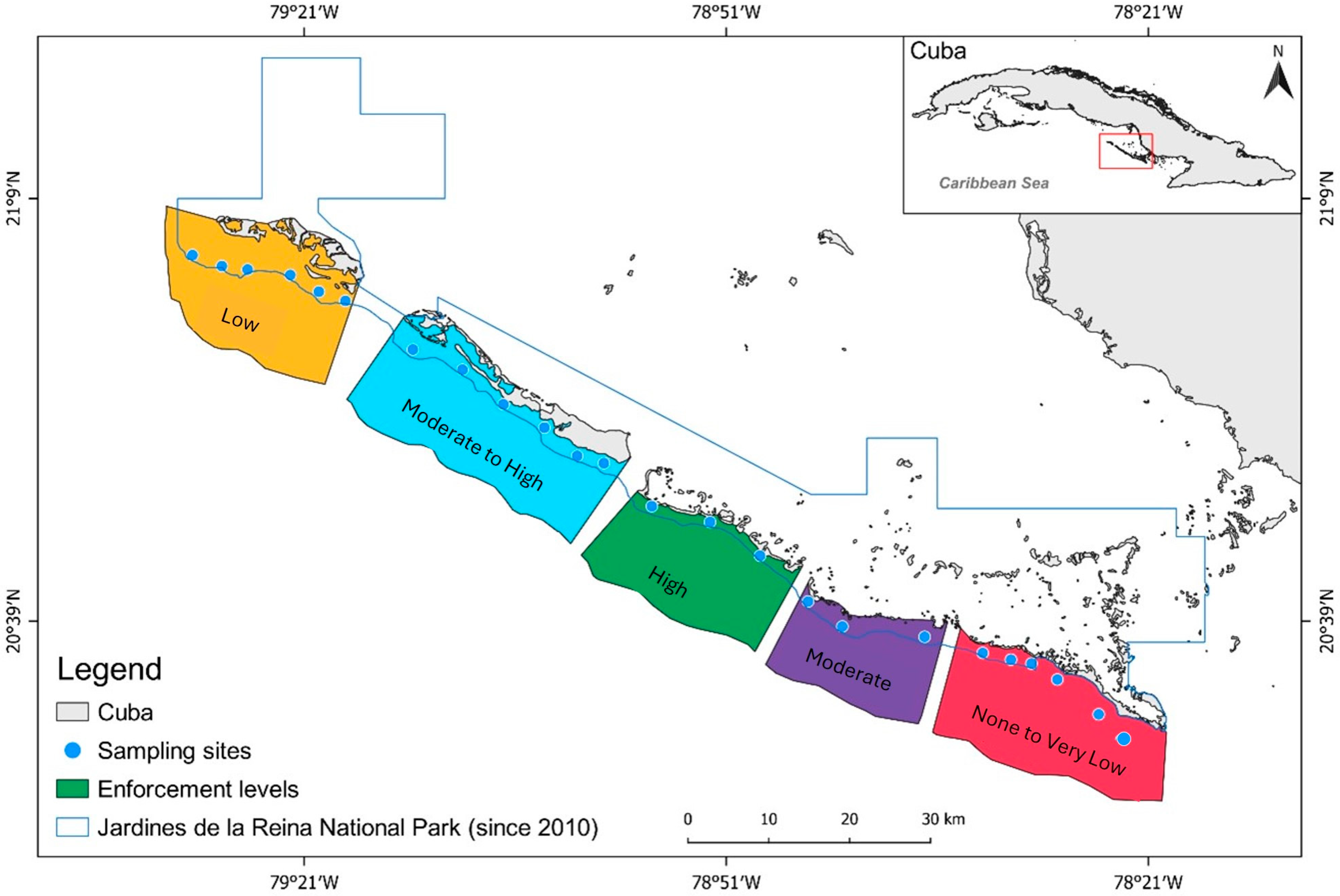
2.2. Enforcement Gradient
2.3. Field Sampling
2.4. Data Standardization
2.5. Assessing and Defining the Enforcement Gradient across JRNP
2.6. Modeling Variability in Fish Biomass in JRNP
2.7. Impact of the Enforcement Gradient on Target Fish Species in JRNP
3. Results
3.1. Assessing and Defining the Enforcement Gradient across JRNP
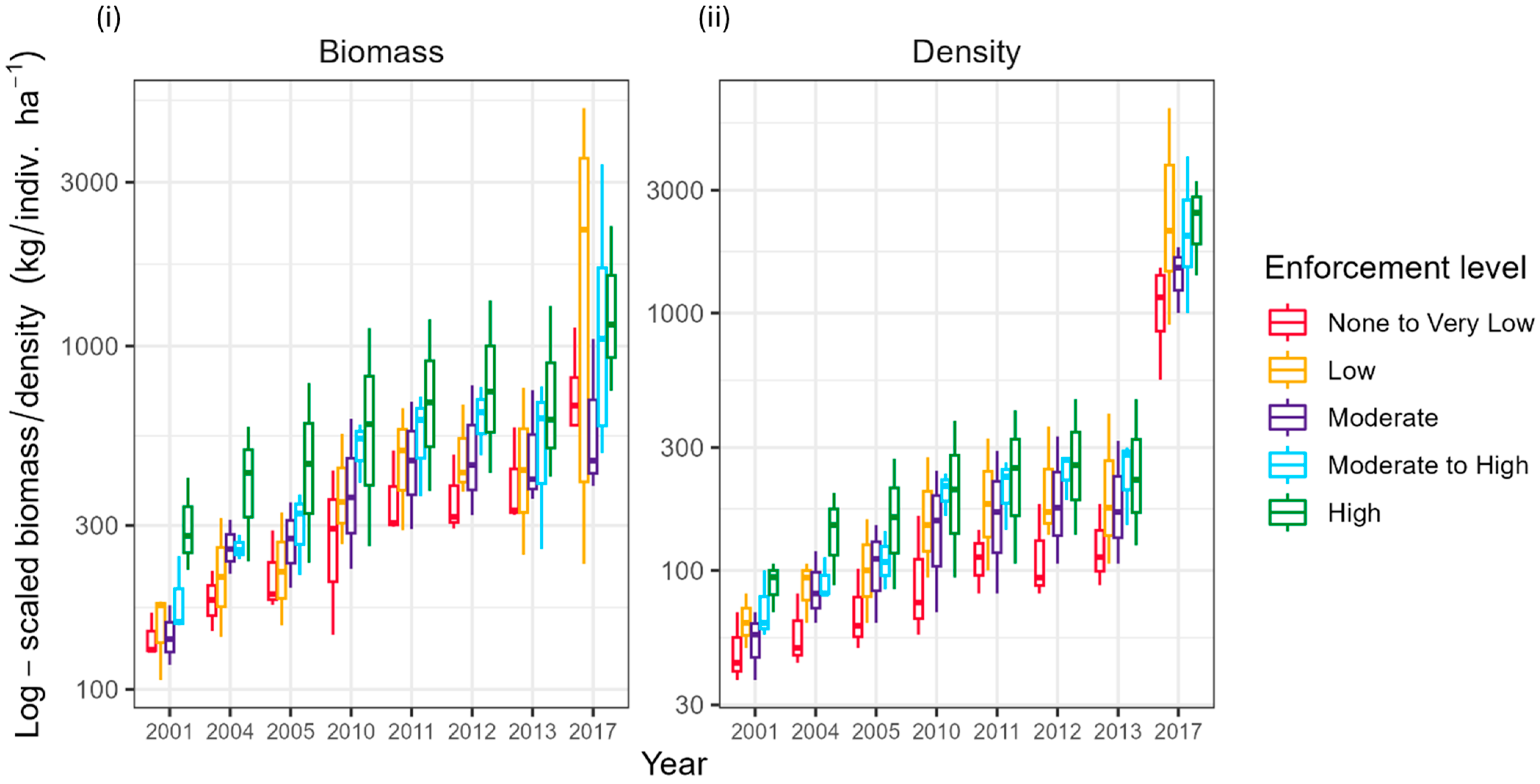
3.2. Fish Biomass Variability in JRNP
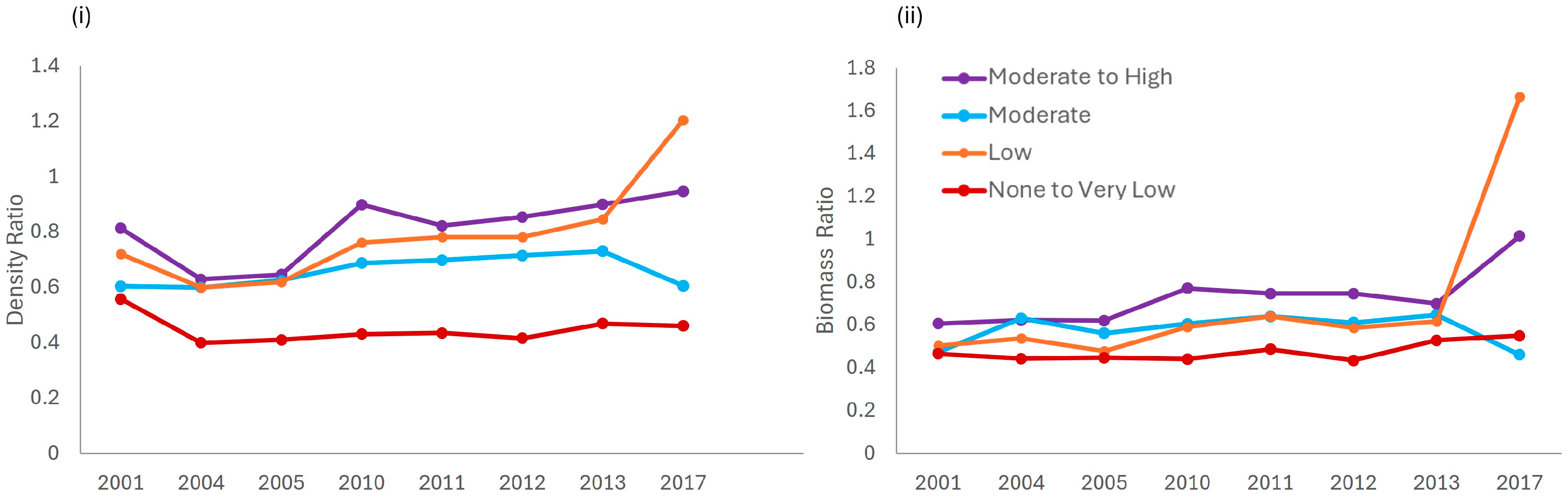
3.3. Impact of the Enforcement Gradient on Target Fish Species in JRNP
4. Discussion
4.1. Enforcement Gradient Informs Fishery Status
4.2. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barange, M.; Bahri, T.; Beveridge, M.C.M.; Cochrane, K.L.; Funge-Smith, S.; Poulain, F. (Eds.) Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options; FAO Fisheries and Aquaculture Technical Paper No. 627; FAO: Rome, Italy, 2018; 628p. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture—Meeting the Sustainable Development Goals (No. CC BY-NC-SA 3.0 IGO). 2018. Available online: https://www.fao.org/family-farming/detail/en/c/1145050/ (accessed on 1 April 2024).
- Bennett, A.; Patil, P.; Kleisner, K.; Rader, D.; Virdin, J.; Basurto, X. Contribution of Fisheries to Food and Nutrition Security: Current Knowledge, Policy, and Research; NI Report 18-02; Duke University: Durham, NC, USA, 2018; Available online: https://wfpc.sanford.duke.edu/reports/contribution-fisheries-food-nutrition-security-current-knowledge-policy-and-research/ (accessed on 1 April 2024).
- Lubchenco, J.; Palumbi, S.R.; Gaines, S.D.; Andelman, S. Plugging a hole in the ocean: The emerging science of marine reserves. Ecol. Appl. 2003, 13, S3–S7. [Google Scholar] [CrossRef]
- Lubchenco, J.; Gaines, S.D. A new narrative for ocean science. Science 2019, 364, 911. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S.; et al. Rebuilding global fisheries. Science 2009, 325, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.L.; Andrew, N.L.; Parma, A.M. Primary fisheries management: A minimum requirement for provision of sustainable human benefits in small-scale fisheries. Fish Fish. 2011, 12, 275–288. [Google Scholar] [CrossRef]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef]
- Mace, P.M. Relationships between common biological reference points used as thresholds and targets of fisheries management strategies. Can. J. Fish. Aquat. Sci. 1994, 51, 110–122. [Google Scholar] [CrossRef]
- Dowling, N.A.; Wilson, J.R.; Cope, J.M.; Dougherty, D.T.; Lomonico, S.; Revenga, C.; Snouffer, B.J.; Salinas, N.G.; Torres-Cañete, F.; Chick, R.C.; et al. The FishPath approach for fisheries management in a data- and capacity-limited world. Fish Fish. 2023, 24, 212–230. [Google Scholar] [CrossRef]
- Karr, K.A.; Fujita, R.; Carcamo, R.; Epstein, L.; Foley, J.R.; Fraire-Cervantes, J.A.; Gongora, M.; Gonzalez-Cuellar, O.T.; Granados-Dieseldorff, P.; Guirjen, J.; et al. Integrating Science-Based Co-Management, Partnerships, Participatory Processes and Stewardship Incentives to Improve the Performance of Small-Scale Fisheries. Front. Mar. Sci. 2017, 4, 345. [Google Scholar] [CrossRef]
- Fujita, R.; Karr, K.; Battista, W.; Rader, D. A Framework for Developing Scientific Management Guidance for Data-Limited Fisheries. In Proceedings of the 66th Gulf and Caribbean Fisheries Institute, Corpus Christi, TX, USA, 4–8 November 2013; p. 66. [Google Scholar]
- Ovando, D.; Caselle, J.E.; Costello, C.; Deschenes, O.; Gaines, S.D.; Hilborn, R.; Liu, O. Assessing the population-level conservation effects of marine protected areas. Conserv. Biol. 2021, 35, 1861–1870. [Google Scholar] [CrossRef]
- Wilson, J.R.; Bradley, D.; Phipps, K.; Gleason, M.G. Beyond protection: Fisheries co-benefits of no-take marine reserves. Mar. Policy 2020, 122, 104224. [Google Scholar] [CrossRef]
- Gill, D.A.; Lester, S.E.; Free, C.M.; Pfaff, A.; Iversen, E.; Reich, B.J.; Yang, S.; Ahmadia, G.; Andradi-Brown, D.A.; Darling, E.S.; et al. A diverse portfolio of marine protected areas can better advance global conservation and equity. Proc. Natl. Acad. Sci. USA 2024, 121, e2313205121. [Google Scholar] [CrossRef]
- MPA Atlas. 2023. Available online: https://mpatlas.org/ (accessed on 13 January 2024).
- Turnbull, J.W.; Johnston, E.L.; Clark, G.F. Evaluating the social and ecological effectiveness of partially protected marine areas. Conserv. Biol. 2021, 35, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Lubchenco, J.; Grorud-Colvert, K.; Novelli, C.; Roberts, C.; Sumaila, U.R. Assessing real progress towards effective ocean protection. Mar Policy 2018, 91, 11–13. [Google Scholar] [CrossRef]
- Zupan, M.; Fragkopoulou, E.; Claudet, J.; Erzini, K.; Horta e Costa, B.; Gonçalves, E.J. Marine partially protected areas: Drivers of ecological effectiveness. Front. Ecol. Environ. 2018, 16, 381–387. [Google Scholar] [CrossRef]
- Ban, N.C.; Darling, E.S.; Gurney, G.G.; Friedman, W.; Jupiter, S.D.; Lestari, W.P.; Yulianto, I.; Pardede, S.; Tarigan, S.A.R.; Prihatiningsih, P.; et al. Effects of management objectives and rules on marine conservation outcomes. Conserv. Biol. 2023, 37, e14156. [Google Scholar] [CrossRef]
- Babcock, E.A.; MacCall, A.D. How useful is the ratio of fish density outside versus inside no-take marine reserves as a metric for fishery management control rules? Can. J. Fish. Aquat. Sci. 2014, 68, 343–359. [Google Scholar] [CrossRef]
- McGilliard, C.R.; Hilborn, R.; MacCall, A.; Punt, A.E.; Field, J.C. Can information from marine protected areas be used to inform control-rule-based management of small-scale, data-poor stocks? ICES J. Mar. Sci. 2011, 68, 201–211. [Google Scholar] [CrossRef]
- Bohnsack, J.A. Incorporating no-take marine reserves into precautionary management and stock assessment. In Proceedings of the 5th National NMFS Stock Assessment Workshop, Key Largo, FL, USA, 24–26 February 1998; pp. 8–16. Available online: www.st.nmfs.noaa.gov/StockAssessment/workshop_documents/nsaw5/bohnsack.pdf (accessed on 1 March 2023).
- Claro, R.; Sadovy de Mitchenson, I.; Lindeman, K.C.; García–Cagide, A. Historical analysis of Cuban commercial fishing effort and the effects of management interventions on important reef fishes from 1960–2005. Fish Res. 2009, 99, 7–16. [Google Scholar] [CrossRef]
- Chollett, I.; Mumby, P.J.; Muller–Karger, F.E.; Chuanmin, H. Physical environments of the Caribbean Sea. Limnol. Oceanogr. 2012, 57, 1233–1244. [Google Scholar] [CrossRef]
- Pina–Amargós, F.; Hernández–Fernández, L.; Clero–Alonso, L.; González–Sansón, G. Características de hábitats coralinos en Jardines de la Reina. Cuba Rev. Investig. Mar. 2008, 29, 225–237. [Google Scholar]
- Ministerio de la Industria Pesquera. Resolución N° 562/96—Declarar Como Zona Bajo Régimen Especial de uso y Protección las Aguas Marítimas Comprendidas en los Tramos Delimitados por Coordenadas Geográficas Específicas. Havana, Cuba, 1996. Available online: https://www.gacetaoficial.gob.cu/es/gaceta-oficial-no046-ordinaria-de-1996 (accessed on 5 September 2024).
- CNAP (Centro Nacional de Áreas Protegidas). Plan del Sistema Nacional de Áreas Protegidas de Cuba: Período 2014–2020; Ministerio de Ciencia, Tecnología y Medio Ambiente: Havana, Cuba, 2013; p. 336. [Google Scholar]
- Perera-Valderrama, S.; Hernández-Ávila, A.; González-Méndez, J.; Moreno-Martínez, O.; Cobián-Roja, S.D.; Ferro-Azcona, H.; Aragón, H.C.; Alcolado, P.M.; Pina-Amargós, F.; Hernández González, Z. Marine protected areas in Cuba. Bull. Mar. Sci. 2018, 94, 423–442. [Google Scholar] [CrossRef]
- Angulo-Valdés, J.A.; Navarro-Martínez, Z.; López-Castañeda, L.; Frazer, T.; Adams, A.J. Collaborating on a new vision for Cuba’s coastal fisheries. Bonefish Tarpon Trust J. 2017, 40–44. Available online: https://www.bonefishtarpontrust.org/wp-content/uploads/2018/12/2017-journal-fall.pdf (accessed on 5 September 2024).
- Hernández-Fernández, L.; Bustamante López, C.; Dulce Sotolongo, L.B.; Pina Amargós, F.; Figueredo Martín, T. Influencia del Gradiente de Protección Sobre el Estado de las Comunidades de Corales y Algas Coralinas Costrosas en el Parque Nacional Jardines de la Reina, Cuba. J. Mar. Res. 2018, 38, 83–99. Available online: https://revistas.uh.cu/rim/article/view/4212 (accessed on 5 September 2024).
- Appeldoorn, R.S.; Lindeman, K.C.A. Caribbean-Wide Survey of Marine Reserves: Spatial Coverage and Attributes of Effectiveness. Gulf Cari Res. 2003, 14, 139–154. [Google Scholar] [CrossRef]
- Pina-Amargós, F.; González–Sansón, G.; Martín–Blanco, F.; Valdivia, A. Evidence for protection of targeted reef fish on the largest marine reserve in the Caribbean. PeerJ 2014, 2, e274. [Google Scholar] [CrossRef]
- Puga, R.; Valle, S.; Kritzer, J.P.; Delgado, G.; Estela de León, M.; Giménez, E.; Ramos, I.; Moreno, O.; Karr, K.A. Vulnerability of nearshore tropical finfish in Cuba: Implications for scientific and management planning. Bull. Mar. Sci. 2018, 94, 377–392. [Google Scholar] [CrossRef]
- Pina-Amargós, F.; González-Díaz, P.; González-Sansón, G.; Aguilar-Betancourt, C.; Rodríguez-Cueto, Y.; Olivera-Espinosa, Y.; Figueredo-Martín, T.; Rey-Villiers, N.; Arias Barreto, R.; Cobián-Rojas, D.; et al. Chapter 15: Status of Cuban Coral Reefs. In Coral Reefs of Cuba, Coral Reefs of the World; Zlatarski, V., Reed, J.K., Pomponi, S.A., Brooks, S., Farrington, S., Eds.; Springer Nature: Cham, Switzerland, 2024; Volume 18, pp. 283–308. [Google Scholar] [CrossRef]
- Claro, R. (Ed.) Ecología de los Peces Marinos de Cuba; Instituto de Oceanología Academia de Ciencias de Cuba: Quintana Roo, México, 1994; p. 525. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. 2013. Available online: https://www.fishbase.org (accessed on 5 September 2024).
- Ranjan, C.; Najari, V. nlcor: Nonlinear Correlation. Res. Gate 2019. [Google Scholar] [CrossRef]
- Pardy, C. mpmi: Mixed-Pair Mutual Information Estimators, R Package Version 0.43.2.1. 2023. Available online: https://r-forge.r-project.org/projects/mpmi/ (accessed on 5 September 2024).
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R Package Version 0.7.2. 2023. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 5 September 2024).
- Christensen, R. Ordinal-Regression Models for Ordinal Data, R Package Version 2023.12-4. 2023. Available online: https://cran.r-project.org/web/packages/ordinal/ordinal.pdf (accessed on 5 September 2024).
- Ugba, E. gofcat: Goodness-of-Fit Measures for Categorical Response Models, R Package Version 0.1.2. 2023. Available online: https://cran.r-project.org/web/packages/gofcat/gofcat.pdf (accessed on 5 September 2024).
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 623–637. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Sólymos, P.; Stevens, M.; et al. vegan: Community Ecology Package, R Package Version 2.6-4; 2023. Available online: https://cran.r-project.org/web/packages/plm/index.html (accessed on 5 September 2024).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M. Cluster: Cluster Analysis Basics and Extensions, R Package Version 2.1.4. 2022. Available online: https://cran.r-project.org/web/packages/cluster/citation.html (accessed on 5 September 2024).
- Mair, P.; De Leeuw, J. Gifi: Multivariate Analysis with Optimal Scaling, R Package Version 0.4-0. 2022. Available online: https://cran.r-project.org/web/packages/Gifi/Gifi.pdf (accessed on 5 September 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Ranjan, C.; Banerjee, D. Nlcor: Compute Nonlinear Correlations, R Package Version 2.3. 2023. Available online: https://rdrr.io/github/ProcessMiner/nlcor/ (accessed on 5 September 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Shono, H. Application of the Tweedie distribution to zero-catch data in CPUE analysis. Fish Res. 2008, 93, 154–162. [Google Scholar] [CrossRef]
- Gaines, S.D.; White, C.; Carr, M.H.; Palumbi, S.R. Designing marine reserve networks for both conservation and fisheries management. Proc. Natl. Acad. Sci. USA 2010, 107, 18286–18293. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, A.; Thiriet, P.; Di Carlo, G.; Dimitriadis, C.; Francour, P.; Gutiérrez, N.L.; Jeudy de Grissac, A.; Koutsoubas, D.; Milazzo, M.; del Mar Otero, M.; et al. Five key attributes can increase marine protected areas performance for small-scale fisheries management. Sci. Rep. 2016, 6, 38135. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.L.; Brooks, R.O.; Bellquist, L.F.; Caselle, J.E.; Morgan, S.G.; Mulligan, T.J.; Ruttenberg, B.I.; Semmens, B.X.; Starr, R.M.; Tyburczy, J.; et al. Collaborative fisheries research reveals reserve size and age determine efficacy across a network of marine protected areas. Conserv. Lett. 2024, 17, e13000. [Google Scholar] [CrossRef]
- Pina-Amargós, F.; Olivera-Espinosa, Y.; Ruiz-Abierno, A.; Graham, R.; Hueter, R.; Márquez-Farías, J.F.; Hernández-Betancourt, A.; Borroto-Vejerano, R.; Figueredo-Martín, T.; Briones, A.; et al. Chapter 13: Sharks and Rays in Cuban Coral Reefs: Ecology, Fisheries, and Conservation. In Coral Reefs of Cuba, Coral Reefs of the World; Zlatarski, V., Reed, J.K., Pomponi., S.A., Brooks, S., Farrington, S., Eds.; Springer Nature: Cham, Switzerland, 2024; Volume 18, pp. 283–308. [Google Scholar] [CrossRef]
- Hilborn, R. Pretty Good Yield and exploited fishes. Mar. Policy 2010, 34, 193–196. [Google Scholar] [CrossRef]
- Lubchenco, J.; Grorud-Colvert, K. Making waves: The science and politics of ocean protection. Science 2015, 350, 382–383. [Google Scholar] [CrossRef]
- Goñi, R.; Adlerstein, S.; Alvarez-Berastegui, D.; Forcada, A.; Reñones, O.; Criquet, G.; Polti, S.; Cadiou, G.; Valle, C.; Lenfant, P.; et al. Spillover from six western Mediterranean marine protected areas: Evidence from artisanal fisheries. Mar. Ecol. Prog. Ser. 2008, 366, 159–174. [Google Scholar] [CrossRef]
- Buxton, C.D.; Hartmann, K.; Kearney, R.; Gardner, C. When is spillover from marine reserves likely to benefit fisheries? PLoS ONE 2014, 9, e107032. [Google Scholar] [CrossRef]
- McClanahan, T.R. Marine reserve more sustainable than gear restriction in maintaining long-term coral reef fisheries yields. Mar. Policy 2021, 128, 104478. [Google Scholar] [CrossRef]
- Kerwath, S.; Winker, H.; Götz, A.; Attwood, C.G. Marine protected area improves yield without disadvantaging fishers. Nat. Commun. 2013, 4, 2347. [Google Scholar] [CrossRef]
- Russ, G.R.; Cheal, A.J.; Dolman, A.M.; Emslie, M.J.; Evans, R.D.; Miller, I.; Sweatman, H.; Williamson, D.H. Rapid increase in fish numbers follows the creation of the world's largest marine reserve network. Curr. Biol. 2008, 18, R514–R515. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Ainsworth, T.D.; Baird, A.H.; Ban, N.C.; Bay, L.K.; Cinner, J.E.; De Freitas, D.M.; Diaz-Pulido, G.; Dornelas, M.; Dunn, S.R.; et al. From microbes to people: Tractable benefits of no-take areas for coral reefs. Oceanogr. Mar. Biol. Ann. Rev. 2011, 49, 105–136. [Google Scholar]
- McClanahan, T.R.; Graham, N.A.J.; Calnan, J.M.; MacNeil, M.A. Toward pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecol. Appl. 2007, 17, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Botsford, L.W.; Micheli, F.; Hastings, A. Principles for the design of marine reserves. Ecol. Appl. 2003, 13, S25–S31. [Google Scholar] [CrossRef]
- Russ, G.R.; Alcala, A.C. Marine reserves: Long-term protection is required for full recovery of predatory fish populations. Oecologia 2004, 138, 622–627. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, T.R.; Graham, N.A.J. Marine reserve recovery rates towards a baseline are slower for reef fish community life histories than biomass. Proc. R. Soc. B 2015, 282, 20151938. [Google Scholar] [CrossRef]
- Halpern, B.S. The impact of marine reserves: Do reserves work and does reserve size matter? Ecol. Appl. 2003, 13, 117–137. [Google Scholar] [CrossRef]
- Guidetti, P.; Milazzo, M.; Bussotti, S.; Molinari, A.; Murenu, M.; Pais, A.; Spanò, N.; Balzano, R.; Agardy, T.; Boero, F.; et al. Italian marine reserve effectiveness: Does enforcement matter? Biol. Conserv. 2008, 141, 699–709. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The Structure of Mediterranean Rocky Reef Ecosystems across Environmental and Human Gradients, and Conservation Implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef]
- Edgar, G.J.; Ward, T.J.; Stuart-Smith, R.D. Rapid declines across Australian fishery stocks indicate global sustainability targets will not be achieved without an expanded network of ‘no-fishing’ reserves. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1337–1350. [Google Scholar] [CrossRef]
- Thresher, R.E.; Gunn, J.S. Comparative analysis of visual census techniques for highly mobile, reef-associated piscivores (Carangidae). Environ. Biol. Fish. 1986, 17, 93–116. [Google Scholar] [CrossRef]
- Díaz-Asencio, L.; Clausing, R.J.; Vandersea, M.; Chamero-Lago, D.; Gómez-Batista, M.; Hernández-Albernas, J.I.; Chomérat, N.; Rojas-Abrahantes, G.; Litaker, R.W.; Tester, P.; et al. Ciguatoxin Occurrence in Food-Web Components of a Cuban Coral Reef Ecosystem: Risk-Assessment Implications. Toxins 2019, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Karr, K.A.; Fujita, R.; Halpern, B.S.; Kappel, C.V.; Crowder, L.; Selkoe, K.A.; Alcolado, P.M.; Rader, D. Thresholds in Caribbean coral reefs: Implications for ecosystem-based fishery management. J. Appl. Ecol. 2015, 52, 402–412. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Graham, N.A.; MacNeil, M.A.; Muthiga, N.A.; Cinner, J.E.; Bruggemann, J.H.; Wilson, S.K. Critical thresholds and tangible targets for ecosystem-based management of coral reef fisheries. Proc. Natl. Acad. Sci. USA 2011, 108, 17230–17233. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Stokes, K.; Maguire, J.J.; Smith, T.; Botsford, L.W.; Mangel, M.; Orensanz, J.; Parma, A.; Rice, J.; Bell, J.; et al. When can marine reserves improve fisheries management? Ocean. Coast. Manag. 2004, 47, 197–205. [Google Scholar] [CrossRef]
- Duarte, C.M.; Agusti, S.; Barbier, E.; Britten, G.L.; Castilla, J.C.; Gattuso, J.P.; Fulweiler, R.W.; Hughes, T.P.; Knowlton, N.; Lovelock, C.E.; et al. Rebuilding marine life. Nature 2020, 580, 39–51. [Google Scholar] [CrossRef]
- Karr, K.; Miller, V.; Coronad, E.; Olivares-Banuelos, N.C.; Rosales, M.; Naretto, J.; Hiriart-Bertrand, L.; Vargas-Fernandez, C.; Alzugaray, R.; Puga, R.; et al. Identifying Pathways for Climate-Resilient Multispecies Fisheries. Front. Mar. Sci. 2021, 8, 721883. [Google Scholar] [CrossRef]
- Lynham, J.; Nikolaev, A.; Raynor, J.; Vilela, T.; Villaseñor-Derbez, J.C. Impact of two of the world’s largest protected areas on longline fishery catch rates. Nat. Commun. 2020, 11, 979. [Google Scholar] [CrossRef]
- Pina-Amargós, F.; González-Sansón, G.; Cabrera-Paez, Y. Effects of fishing activity reduction in Jardines de la Reina Marine Reserve, Cuba. In Proceedings of the 61st Gulf and Caribbean Fisheries Institute, Gosier, Guadeloupe, 10–14 November 2008; pp. 334–348. [Google Scholar]
- McDonald, G.; Campbell, S.J.; Karr, K.; Clemence, M.; Granados-Dieseldorff, P.; Jakub, R.; Kartawijaya, T.; Mueller, J.C.; Prihatinningsih, P.; Siegel, K.; et al. An adaptive assessment and management toolkit for data-limited fisheries. Ocean Coast Manag. 2018, 152, 100–119. [Google Scholar] [CrossRef]


| Terms | Estimate | S.E. | t | p | Signif. |
|---|---|---|---|---|---|
| Protection | |||||
| None to Very Low|Low | −2.46 | 0.29 | −8.63 | <0.001 | *** |
| Low|Moderate | −0.71 | 0.18 | −3.93 | <0.001 | *** |
| Moderate|Moderate to High | −0.01 | 0.17 | −0.07 | 0.940 | |
| Moderate to High|High | 1.6 | 0.22 | 7.15 | <0.001 | *** |
| Protection—No | −3.19 | 0.39 | −8.22 | <0.001 | *** |
| Patrolling effort | |||||
| None to Very Low|Low | −0.59 | 0.18 | −3.23 | 0.001 | ** |
| Low|Moderate | 0.6 | 0.17 | 3.54 | <0.001 | *** |
| Moderate|Moderate to High | 1.25 | 0.19 | 6.58 | <0.001 | *** |
| Moderate to High|High | 3.52 | 0.38 | 9.27 | <0.001 | *** |
| Patrolling hours | 0.07 | 0.01 | 7.29 | <0.001 | *** |
| Fishing effort | |||||
| None to Very Low|Low | −2.21 | 0.26 | −8.56 | <0.001 | *** |
| Low|Moderate | −0.85 | 0.20 | −4.23 | <0.001 | *** |
| Moderate|Moderate to High | −0.23 | 0.19 | −1.22 | 0.223 | |
| Moderate to High|High | 1.32 | 0.24 | 5.55 | <0.001 | *** |
| Fishing effort | −0.01 | 0.001 | −6.20 | <0.001 | *** |
| Fishing intensity | |||||
| None to Very Low|Low | −3.44 | 0.48 | −7.10 | <0.001 | *** |
| Low|Moderate | −0.12 | 0.28 | −0.43 | 0.666 | |
| Moderate|Moderate to High | 0.89 | 0.28 | 3.21 | 0.001 | ** |
| Moderate to High|High | 2.94 | 0.31 | 9.54 | <0.001 | *** |
| Fishing intensity—Low | −4.85 | 0.51 | −9.48 | <0.001 | *** |
| Fishing intensity—Moderate | −2.37 | 0.47 | −5.04 | <0.001 | *** |
| Fishing intensity—High | 0.29 | 0.49 | 0.59 | 0.556 | |
| Distance to the nearest port | |||||
| None to Very Low|Low | 14.59 | 4.60 | 3.18 | 0.001 | ** |
| Low|Moderate | 17.54 | 5.21 | 3.37 | <0.001 | *** |
| Moderate|Moderate to High | 18.3 | 5.26 | 3.48 | <0.001 | *** |
| Moderate to High|High | 20.46 | 5.52 | 3.71 | <0.001 | *** |
| Distance to the nearest port | 0.22 | 0.06 | 3.47 | <0.001 | *** |
| Estimate | S.E. | t | p | |
|---|---|---|---|---|
| (Intercept) | −191.625 *** | 12.292 | −15.589 | <0.001 |
| Year | 0.098 *** | 0.006 | 16.094 | <0.001 |
| Low Enforcement | 23.255 | 27.805 | 0.836 | 0.405 |
| Moderate Enforcement | 26.629 | 27.725 | 0.960 | 0.339 |
| Moderate to High Enforcement | −13.013 | 27.147 | −0.479 | 0.633 |
| High Enforcement | 62.397 * | 27.265 | 2.289 | 0.024 |
| Year × Low Enforcement | −0.011 | 0.014 | −0.823 | 0.412 |
| Year × Moderate Enforcement | −0.013 | 0.014 | −0.957 | 0.341 |
| Year × Moderate to High Enforcement | 0.007 | 0.013 | 0.486 | 0.628 |
| Year × High Enforcement | −0.031 * | 0.014 | −2.295 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karr, K.A.; Pina-Amargós, F.; Figueredo-Martín, T.; Olivera-Espinosa, Y. Fishery Management Enforcement Gradients to Achieve Fishery Goals. Fishes 2024, 9, 355. https://doi.org/10.3390/fishes9090355
Karr KA, Pina-Amargós F, Figueredo-Martín T, Olivera-Espinosa Y. Fishery Management Enforcement Gradients to Achieve Fishery Goals. Fishes. 2024; 9(9):355. https://doi.org/10.3390/fishes9090355
Chicago/Turabian StyleKarr, Kendra A., Fabián Pina-Amargós, Tamara Figueredo-Martín, and Yunier Olivera-Espinosa. 2024. "Fishery Management Enforcement Gradients to Achieve Fishery Goals" Fishes 9, no. 9: 355. https://doi.org/10.3390/fishes9090355
APA StyleKarr, K. A., Pina-Amargós, F., Figueredo-Martín, T., & Olivera-Espinosa, Y. (2024). Fishery Management Enforcement Gradients to Achieve Fishery Goals. Fishes, 9(9), 355. https://doi.org/10.3390/fishes9090355








