Impact of Starfish Predatory Pressure on the Immune and Antioxidant Functions of Sea Cucumber Apostichopus japonicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Materials
2.2. Experimental Design
2.3. Sample Collection
2.4. Enzyme Activity Assay
3. Results and Analysis
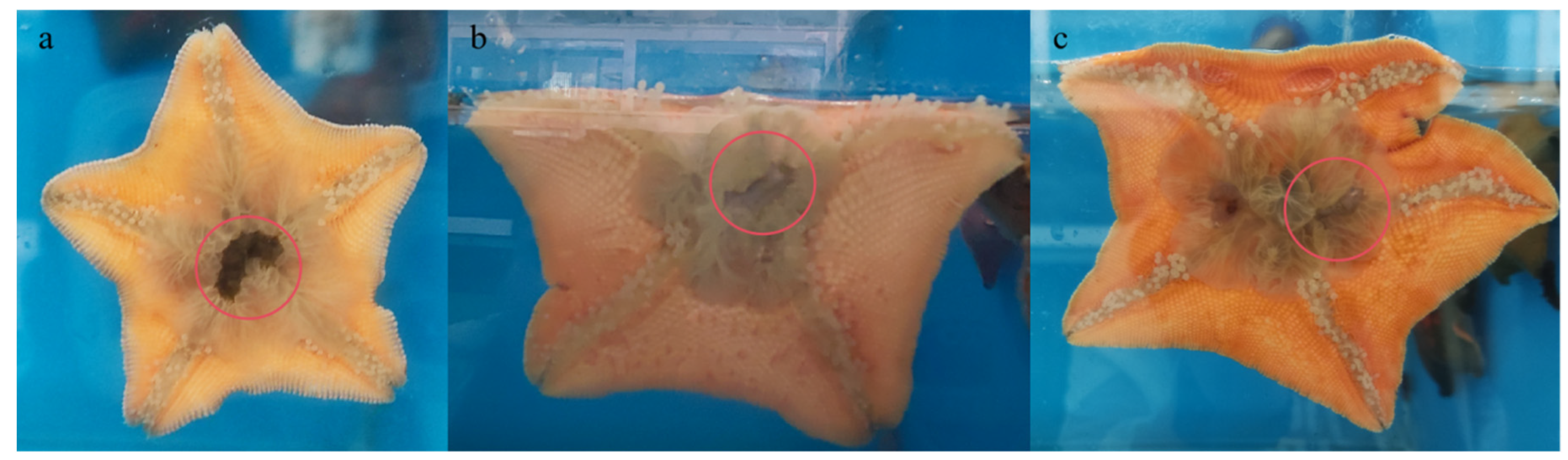
3.1. Direct Predation of Sea Cucumbers by A. pectinifera
3.2. Changes in the Activity of Immune Defense Enzymes in Sea Cucumbers under Different Predation Pressures
3.3. Changes in Antioxidant Stress Enzyme Activity in Sea Cucumbers under Different Predation Pressures
4. Discussion
4.1. Changes in Immune Defense-Related Functions of Sea Cucumbers under Different Predation Pressures
4.2. Changes in Antioxidant Defense Functions of Sea Cucumbers under Different Predation Pressures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, J.D.; Munro, J.L.; Nash, W.J.; Rothlisberg, P.C.; Loneragan, N.R.; Ward, R.D.; Andrew, N.L. Restocking and stock enhancement of marine invertebrate fisheries. Adv. Mar. Biol. 2005, 49, xi-374. [Google Scholar]
- Gosselin, L.A.; Qian, P.Y. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 1997, 146, 265–282. [Google Scholar] [CrossRef]
- Francour, P. Predation on holothurians: A literature review. Invertebr. Biol. 1997, 116, 52–60. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, H.; Hamel, J.F. Larval, juvenile, and adult predators. The Sea Cucumber Apostichopus japonicus: History, Biology and Aquaculture; Academic Press: Amsterdam, The Netherlands, 2015; pp. 243–256. [Google Scholar]
- Garm, A. Sensory biology of starfish-with emphasis on recent discoveries in their visual ecology. Integr. Comp. Biol. 2017, 57, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 2013, 27, 11–23. [Google Scholar] [CrossRef]
- Campbell, A.C.; Coppard, S.; Tudor-Tomas, C.D. Escape and aggregation responses of three echinoderms to conspecific stimuli. Biol. Bull. 2001, 201, 175–185. [Google Scholar] [CrossRef]
- Chi, X.; Hu, F.; Qin, C.; Huang, X.; Zhao, C. Conspecific alarm cues are a potential effective barrier to regulate foraging behavior of the sea urchin Mesocentrotus nudus. Mar. Environ. Res. 2021, 171, 105476. [Google Scholar] [CrossRef]
- Chi, X.; Yang, M.; Hu, F.; Huang, X.; Zhao, C. Foraging behavior of the sea urchin Mesocentrotus nudus exposed to conspecific alarm cues in various conditions. Sci. Rep. 2021, 11, 15654. [Google Scholar] [CrossRef]
- Zhadan, P.; Vaschenk, M. Long-term study of behaviors of two cohabiting sea urchin species, Mesocentrotus nudus and Strongylocentrotus intermedius, under conditions of high food quantity and predation risk in situ. PeerJ 2019, 7, e8087. [Google Scholar] [CrossRef]
- Hamel, J.F.; Jobson, S.; Caulier, G.; Mercier, A. Evidence of anticipatory immune and hormonal responses to predation risk in an echinoderm. Sci. Rep. 2020, 11, 10691. [Google Scholar] [CrossRef]
- Burnovicz, A.; Oliva, D.; Hermitte, G. The cardiac response of the crab Chasmagnathus granulatus as an index of sensory perception. J. Exp. Biol. 2009, 212, 313–324. [Google Scholar] [CrossRef]
- Zhang, F. Progresses in research on defence mechanism of echinodems. J. DaLian Fish. Univ. 2005, 20, 340–344. (In Chinese) [Google Scholar]
- Ye, H.B.; Fan, Y.; Li, T.B.; Li, L.; Wang, X.L. Study progress on immunity defense mechanism of Apostichopus japonicus. J. Anhui Agric. Sci. 2018, 46, 27–29. (In Chinese) [Google Scholar]
- Li, G.; Ren, L.; Sun, G.; Yang, J.; Wei, X.; Liu, Z.; Li, S.; Jiang, H. Effects of hypoxic stress on oxidative stress Indices in Apostichopus japonicus. Prog. Fish. Sci. 2016, 37, 133–139. (In Chinese) [Google Scholar]
- Thompson, R.F.; Spencer, W.A. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 1966, 73, 16–43. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Hill, L. Nearshore Marine Resources of the South Pacific: Information for Fisheries Development, Management; Institute of Pacific Studies Forum Fisheries Agency International Centre for Ocean Development: Suva, Fiji, 1993; pp. 371–401. [Google Scholar]
- Hatanaka, H.; Uwaoku, H.; Yasuda, T. Experimental studies on the predation of juvenile sea cucumber, Apostichopus japonicus by sea star, Asterina pectinifera. Suisanzoshoku 1994, 42, 563–566. [Google Scholar]
- Sui, X. The status and prospects for artificial breeding and enhancement of sea cucumber, Apostichopus japonicus (Selenka). Mod. Fish. Inf. 1996, 1–4. (In Chinese) [Google Scholar]
- Liao, Y. Zoology of China: Echinodermata: Sea Cucumbers; Science Press: Beijing, China, 1997. [Google Scholar]
- Liu, S. Handbook of Aquaculture Seedling Breeding Techniques; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Tanaka, M. Diminution of sea cucumber Stichopus japonicus juveniles released on artificial reefs. Bull. Ishikawa Prefect. Fish. Res. Cent. 2000, 2, 19–29. [Google Scholar]
- Kang, K.H.; Kim, J.M. The predation of trumpet shell, Charonia sp., on eight different marine invertebrate species. Aquac. Res. 2004, 35, 1202–1206. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Z.; Wan, C.; Xin, M.; Lu, B. Factors affecting the survival rate of Apostichopus japonicus in pond culture and their solutions. Chin. Fish. 2007, 10, 30–31. (In Chinese) [Google Scholar]
- Uekusa, R.; Yoshida, N.; Kashio, S.; Tokaji, H. Low discovery rate of sea cucumber Apostichopus japonicus juveniles after seed release in the field. Bull. Fish. Sci. 2012, 62, 43–49. [Google Scholar]
- Jiang, H.; Liu, Y.; Xin, X.; Yu, L.; Zhang, X. Starfish (Asterina pectinifera), an enemy of sea cucumbers. Shandong Fish. 2008, 25, 22–23. (In Chinese) [Google Scholar]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Öhman, A. Fear and anxiety: Overlaps and dissociations. In Handbook of Emotions; The Guilford Press: New York, NY, USA, 2008; pp. 709–728. [Google Scholar]
- Li, L.; Tian, X.; Yu, X.; Dong, S. Effects of acute and chronic heavy metal (Cu, Cd, and Zn) exposure on sea cucumbers (Apostichopus japonicus). BioMed Res. Int. 2016, 2016, 4532697. [Google Scholar] [CrossRef] [PubMed]
- Canicatti, C.R.; Parrinello, N. Studies on Holothuria polii (Echinodermata) antibacterial proteins. I. Evidence for and activity of a coelomocyte lysozyme. Cell Mol. Life Sci. 1989, 45, 756–759. [Google Scholar] [CrossRef]
- Zheng, H.; Li, B.; Rong, X.; Liao, M.; Chen, G.; Zhang, Z.; Wang, L.; Wang, Y.; Zou, A. Effects of salinity and dissolved oxygen variation on the nonspecific immune response of Apostichopus japonicus. Prog. Fish. Sci. 2014, 30, 118–124. (In Chinese) [Google Scholar]
- Han, S.; Zhao, B.; Li, C.; Hu, W.; Liu, Z. Effeets of acute pH stress on immunity enzyme of sea cucumber, Apostichopus japonicus. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2020, 49, 666–670. (In Chinese) [Google Scholar] [CrossRef]
- Xing, J.; Lin, T.; Zhan, W. Variations of enzyme activities in the haemocytes of scallop Chlamys farreri after infection with the acute virus necrobiotic virus (AVNV). Fish. Shellfish Immunol. 2008, 25, 847–852. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Mohandas, A. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicol. Environ. Saf. 2005, 62, 140–143. [Google Scholar] [CrossRef]
- Song, X.; Zhang, L.; Gao, W.; Pan, B. Effect of Vibrio anguillarum on activity of phosphatase in Cyclina sinensis. Oceanol. Limnol. Sin. 2010, 41, 254–258. (In Chinese) [Google Scholar]
- Pipe, R.K.; Porte, C.; Livingstone, D.R. Antioxidant enzymes associated with the blood cells and hemolymph of the mussel. Mytilus edulis. Fish. Shellfish Immunol. 1993, 3, 221–233. [Google Scholar] [CrossRef]
- Sun, J.; Bai, Y. Predator-induced stress influences fall armyworm immune response to inoculating bacteria. Invertebr. Pathol. 2020, 172, 107352. [Google Scholar] [CrossRef] [PubMed]
- Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M.K.; Ryter, S.W.; Kim, H.P. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Elstner, E.F. Oxygen activation and oxygen toxicity. Annu. Rev. Plant Physiol. 1982, 33, 73–96. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, H. Researh and functon of catalase in organism. Food Sci. Technol. 2007, 8–11. [Google Scholar]
- Del, R.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as a toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 115–121. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Carvalho, C.; Vanessa, A.B.; Heloísa, S.S.A.; Evaldo, L.G.E.; Marisa, N.F. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere 2012, 89, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, D.; Zhang, H.; Tian, B.; Ma, S.; Chen, X.; Wang, Q.; Du, X.; Xu, Y.; Javed, M.T. Dietary supplement of Antheraea pernyi cecropin enhances the growth rate and disease resistance of the Yesso scallop, Patinopecten yessoensis. Aquac. Rep. 2023, 31, 101634. [Google Scholar] [CrossRef]
- Xu, Y.J.; Sun, B. Effect of salinity stress on the growth, body composition and enzyme activities of juvenile Hippocsampus kuda. Oceanol. Limnol. Sin. 2012, 43, 1079–1285. (In Chinese) [Google Scholar]
- Zang, Y.; Tian, X.; Dong, S.; Dong, Y. Growth, metabolism and immune responses to evisceration and the regeneration of viscera in sea cucumber, Apostichopus japonicus. Aquaculture 2012, 358–359, 50–60. [Google Scholar] [CrossRef]
- Sha, F.; Chang, Y.Q.; Ding, J. Effects of two cooling modes of low temperature stress on antioxidant enzyme activities and malondiadehyde level in sea cucumber Apostichopus japonicus. J. Dalian Ocean Univ. 2015, 30, 25–29. (In Chinese) [Google Scholar]






| Enzymes | Abbreviation | Test Method (Kit Cargo No.) |
|---|---|---|
| Lysozyme | LZM | A050, turbidimetry |
| Acid phosphatase | ACP | A060-1, spectrophotometry |
| Alkaline phosphatase | AKP | A059-1, visible-light colorimetry |
| Catalase | CAT | A007-1, visible-light method |
| Superoxide dismutase | SOD | A001-1, hydroxylamine method |
| Malondialdehyde | MDA | A003-1, TBA method |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, S.; Wang, C.; Wang, M.; Kang, W.; Qu, L.; Song, J.; Zhao, C.; Wang, Q. Impact of Starfish Predatory Pressure on the Immune and Antioxidant Functions of Sea Cucumber Apostichopus japonicus. Fishes 2024, 9, 337. https://doi.org/10.3390/fishes9090337
Sun Y, Wang S, Wang C, Wang M, Kang W, Qu L, Song J, Zhao C, Wang Q. Impact of Starfish Predatory Pressure on the Immune and Antioxidant Functions of Sea Cucumber Apostichopus japonicus. Fishes. 2024; 9(9):337. https://doi.org/10.3390/fishes9090337
Chicago/Turabian StyleSun, Yongxin, Shuo Wang, Chong Wang, Meng Wang, Wenbin Kang, Liang Qu, Jian Song, Chong Zhao, and Qingzhi Wang. 2024. "Impact of Starfish Predatory Pressure on the Immune and Antioxidant Functions of Sea Cucumber Apostichopus japonicus" Fishes 9, no. 9: 337. https://doi.org/10.3390/fishes9090337
APA StyleSun, Y., Wang, S., Wang, C., Wang, M., Kang, W., Qu, L., Song, J., Zhao, C., & Wang, Q. (2024). Impact of Starfish Predatory Pressure on the Immune and Antioxidant Functions of Sea Cucumber Apostichopus japonicus. Fishes, 9(9), 337. https://doi.org/10.3390/fishes9090337







