Heterogeneous Weight Development of Lumpfish (Cyclopterus lumpus) Used as Cleaner Fish in Atlantic Salmon (Salmo salar) Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial Farm Data
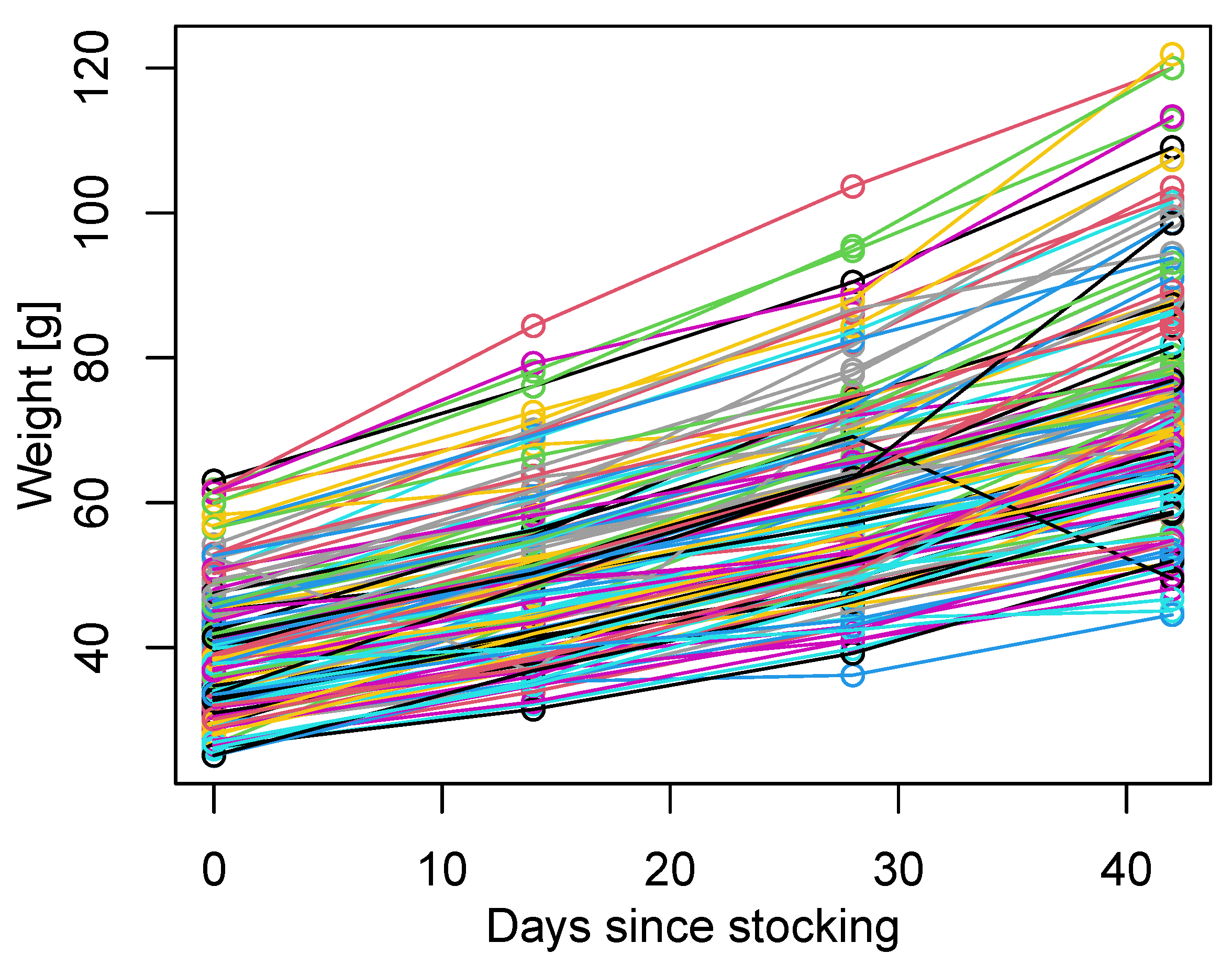
2.1.1. Lumpfish Weight from Commercial Farms
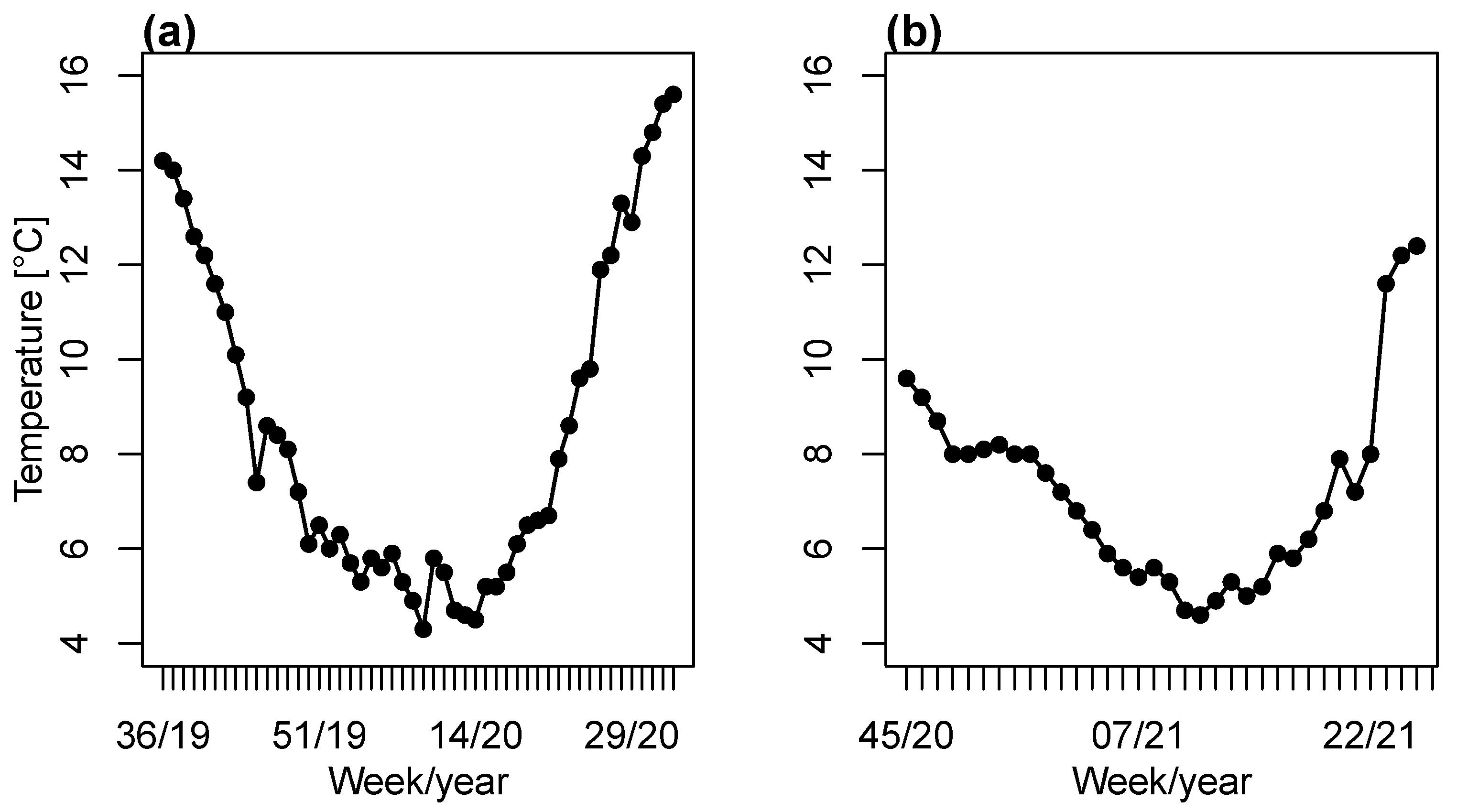
2.1.2. Sea Water Temperature from Commercial Farms
2.2. Lumpfish Weight Development in a Controlled Tank Experiment
2.3. Methods
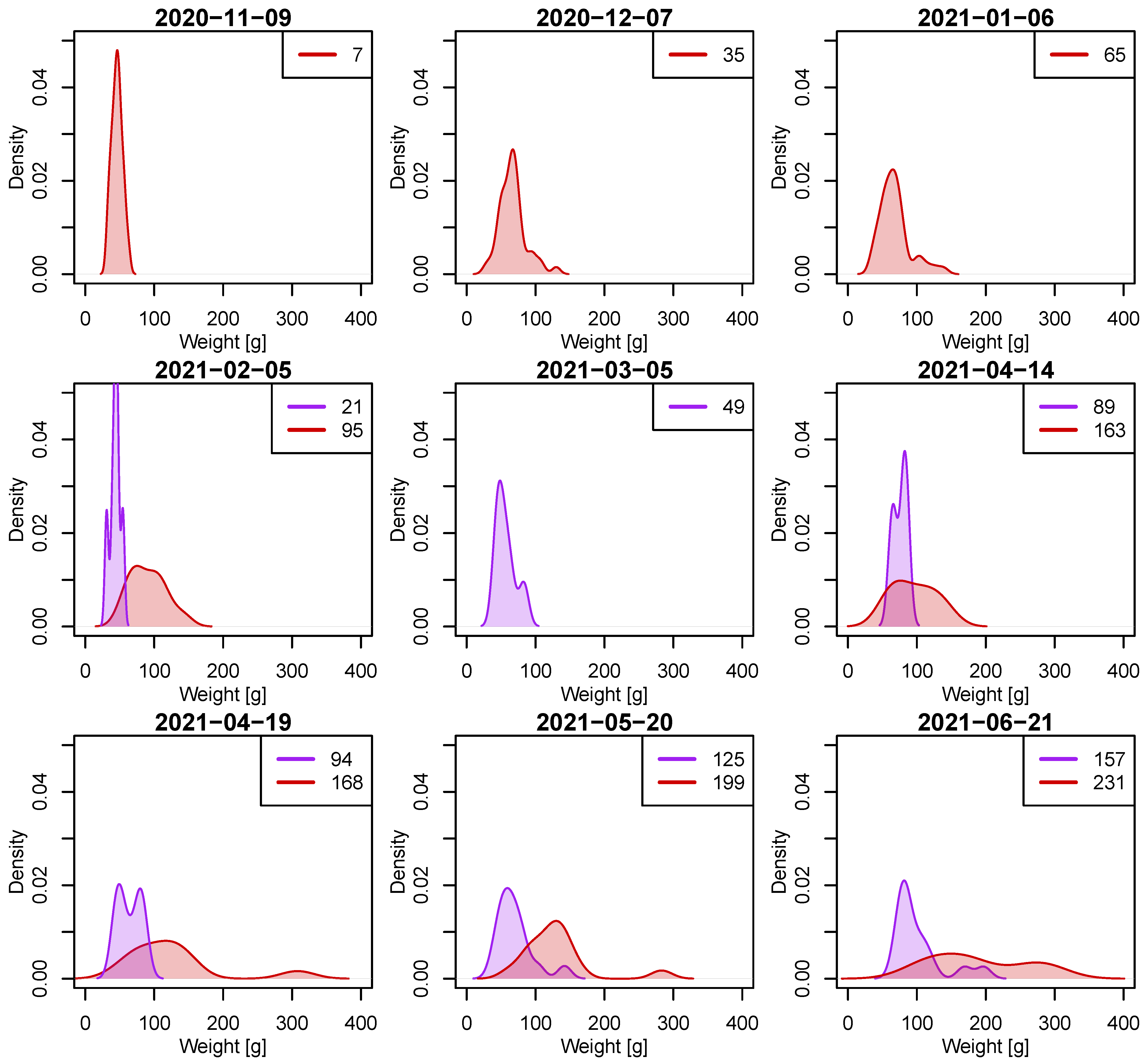
2.3.1. Probability Density of Weight over Time
2.3.2. Regression Model for Lumpfish Weight
2.3.3. Comparison with Individual Time–Series from the Controlled Tank Experiment
3. Results
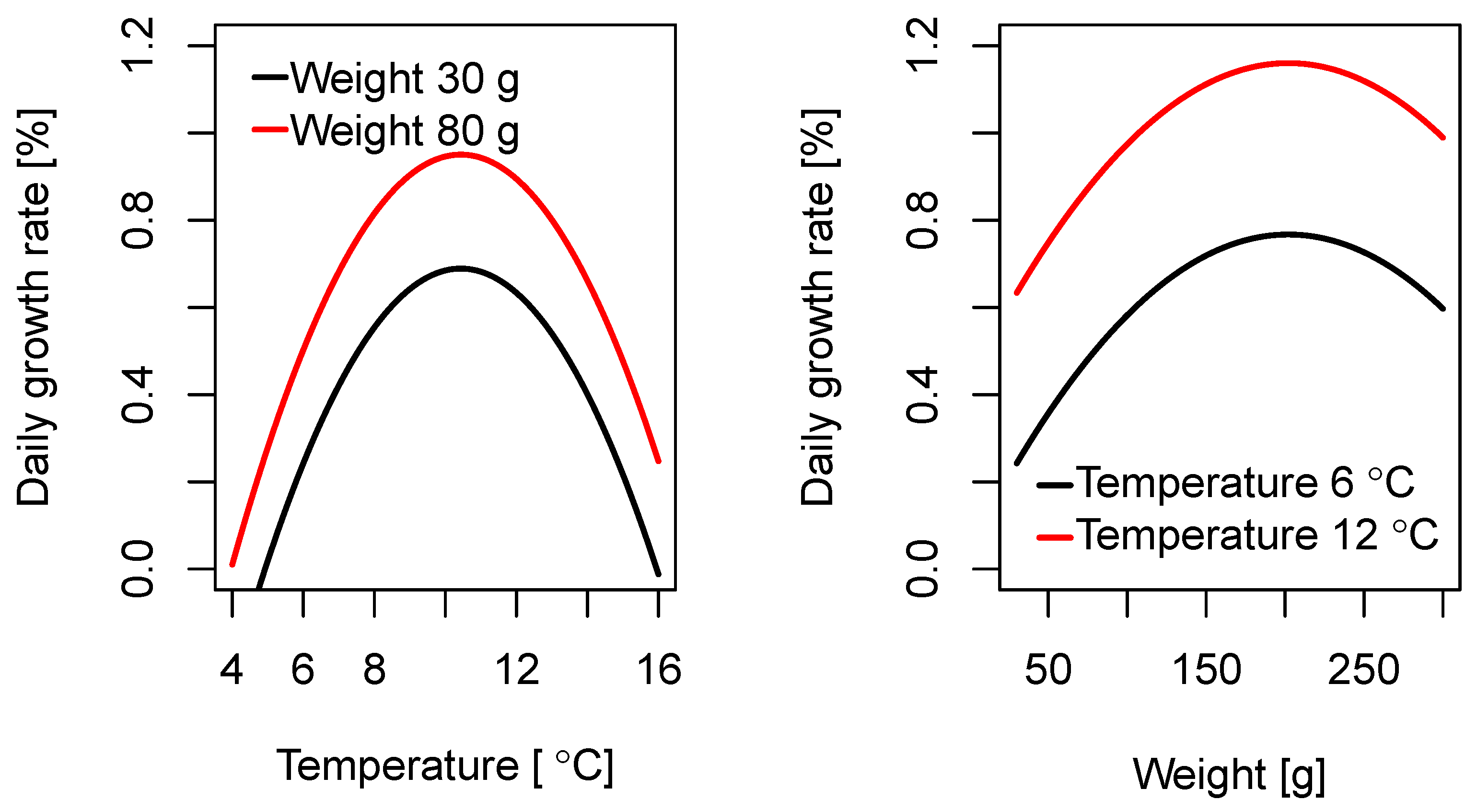
3.1. Regression Models for Daily Growth
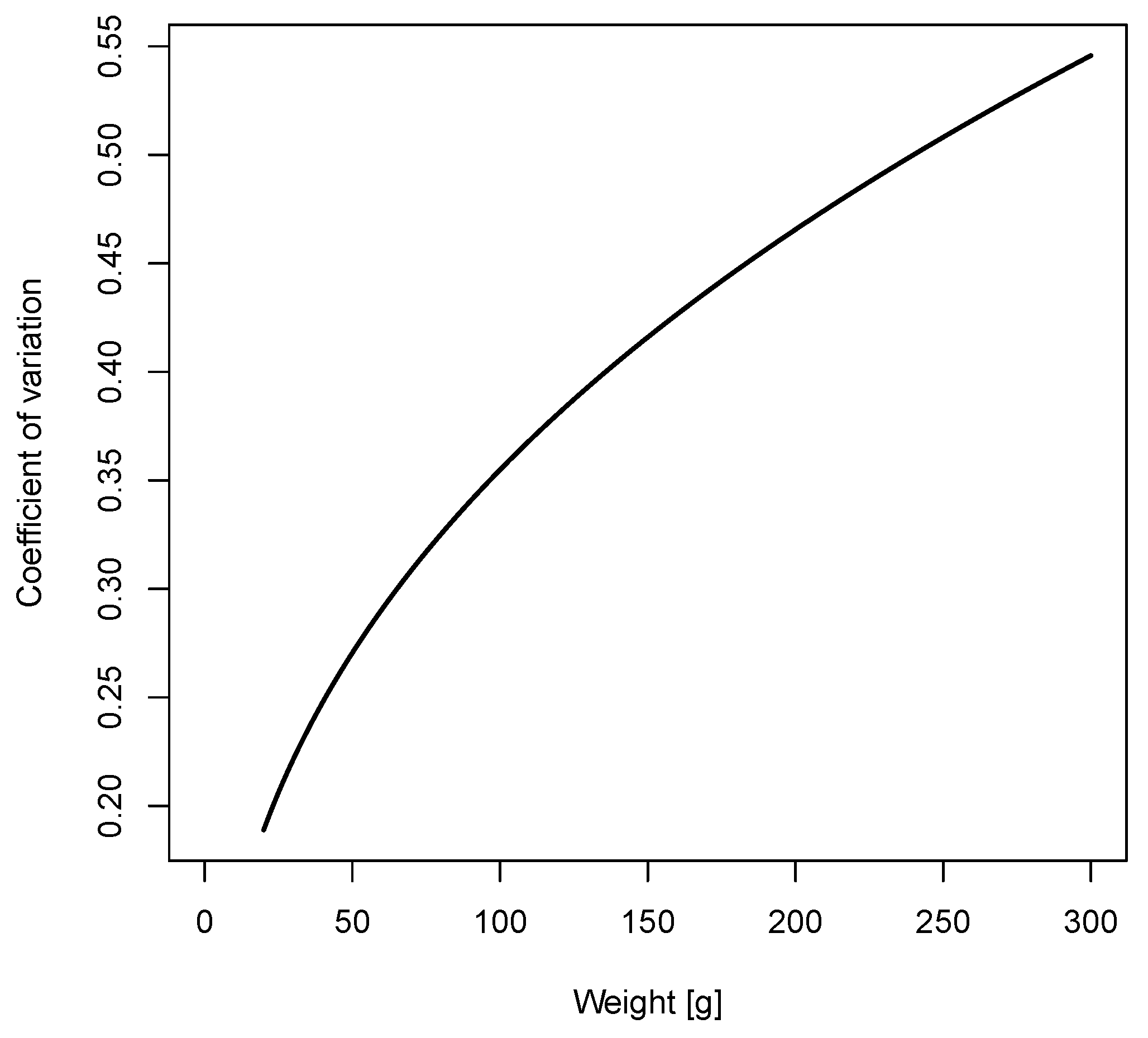
Coefficient of Variation
3.2. Individual Time–Series of Weight in the Controlled Tank Experiment
4. Discussion
4.1. Limitations
4.2. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powell, A.; Treasurer, J.W.; Pooley, C.L.; Keay, A.J.; Lloyd, R.; Imsland, A.K.; Garcia de Leaniz, C. Use of lumpfish for sea-lice control in salmon farming: Challenges and opportunities. Rev. Aquac. 2018, 10, 683–702. [Google Scholar] [CrossRef]
- Brooker, A.J.; Papadopoulou, A.; Gutierrez, C.; Rey, S.; Davie, A.; Migaud, H. Sustainable production and use of cleaner fish for the biological control of sea lice: Recent advances and current challenges. Vet. Rec. 2018, 183, 383. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.T.; Overton, K.; Stien, L.H.; Oppedal, F.; Dempster, T. Effect of cleaner fish on sea lice in Norwegian salmon aquaculture: A national scale data analysis. Int. J. Parasitol. 2020, 50, 787–796. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Foss, A.; Vikingstad, E.; Elvegård, T.A. The use of lumpfish (Cyclopterus lumpus L.) to control sea lice (Lepeophtheirus salmonis Krøyer) infestations in intensively farmed Atlantic salmon (Salmo salar L.). Aquaculture 2014, 424, 18–23. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Jónsdóttir, O.D.; Elvegård, T.A.; Lemmens, S.C.; Rydland, R.; Nytrø, A.V. Investigation of behavioural interactions between lumpfish (Cyclopterus lumpus) and goldsinny wrasse (Ctenolabrus rupestris) under controlled conditions. Aquac. Int. 2016, 24, 1509–1521. [Google Scholar] [CrossRef]
- Fiskeridirektoratet. Utsett av Rensefisk 1998–2021-Publ. 13 October 2022. Available online: https://www.fiskeridir.no/Akvakultur/Tall-og-analyse/Akvakulturstatistikk-tidsserier/Rensefisk/ (accessed on 14 February 2023).
- Whittaker, B.A.; Consuegra, S.; de Leaniz, C.G. Genetic and phenotypic differentiation of lumpfish (Cyclopterus lumpus) across the North Atlantic: Implications for conservation and aquaculture. PeerJ 2018, 6, e5974. [Google Scholar] [CrossRef]
- Davenport, J.; Thorsteinsson, V. Sucker action in the lumpsucker cyclopterus Lumpus L. Sarsia 1990, 75, 33–42. [Google Scholar] [CrossRef]
- Ingólfsson, A.; Kristjánsson, B.K. Diet of juvenile lumpsucker Cyclopterus lumpus (Cyclopteridae) in floating seaweed: Effects of ontogeny and prey availability. Copeia 2002, 2002, 472–476. [Google Scholar] [CrossRef]
- Eriksen, E.; Durif, C.M.; Prozorkevich, D. Lumpfish (Cyclopterus lumpus) in the Barents Sea: Development of biomass and abundance indices, and spatial distribution. ICES J. Mar. Sci. 2014, 71, 2398–2402. [Google Scholar] [CrossRef]
- Davenport, J. Synopsis of Biological Data on the Lumpsucker, Cyclopterus lumpus (Linnaeus, 1758); Number 147; Food & Agriculture Org.: Rome, Italy, 1985. [Google Scholar]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Nytrø, A.V.; Foss, A.; Vikingstad, E.; Elvegård, T.A. Notes on the behaviour of lumpfish in sea pens with and without Atlantic salmon present. J. Ethol. 2014, 32, 117–122. [Google Scholar] [CrossRef]
- Staven, F.R.; Gesto, M.; Iversen, M.H.; Andersen, P.; Patel, D.M.; Nordeide, J.T.; Kristensen, T. Cohabitation with Atlantic Salmon (Salmo salar) Affects Brain Neuromodulators but Not Welfare Indicators in Lumpfish (Cyclopterus lumpus). Front. Physiol. 2022, 13, 781519. [Google Scholar] [CrossRef] [PubMed]
- Walde, C.S.; Stormoen, M.; Pettersen, J.M.; Persson, D.; Røsæg, M.V.; Jensen, B.B. How delousing affects the short-term growth of Atlantic salmon (Salmo salar). Aquaculture 2022, 561, 738720. [Google Scholar] [CrossRef]
- Parsons, A.E.; Escobar-Lux, R.H.; Sævik, P.N.; Samuelsen, O.B.; Agnalt, A.L. The impact of anti-sea lice pesticides, azamethiphos and deltamethrin, on European lobster (Homarus gammarus) larvae in the Norwegian marine environment. Environ. Pollut. 2020, 264, 114725. [Google Scholar] [CrossRef]
- Boissonnot, L.; Karlsen, C.; Reynolds, P.; Austad, M.; Stensby-Skjærvik, S.; Remen, M.; Imsland, A.K. Welfare and survival of lumpfish (Cyclopterus lumpus) in Norwegian commercial Atlantic salmon (Salmo salar) production. Aquaculture 2023, 572, 739496. [Google Scholar] [CrossRef]
- Haugland, G.T.; Olsen, A.B.; Rønneseth, A.; Andersen, L. Lumpfish (Cyclopterus lumpus L.) develop amoebic gill disease (AGD) after experimental challenge with Paramoeba perurans and can transfer amoebae to Atlantic salmon (Salmo salar L.). Aquaculture 2017, 478, 48–55. [Google Scholar] [CrossRef]
- Sandlund, N.; Rønneseth, A.; Ellul, R.M.; Nylund, S.; Sandlund, L. Pasteurella spp. Infections in Atlantic salmon and lumpsucker. J. Fish Dis. 2021, 44, 1201–1214. [Google Scholar] [CrossRef]
- Garcia de Leaniz, C.; Gutierrez Rabadan, C.; Barrento, S.I.; Stringwell, R.; Howes, P.N.; Whittaker, B.A.; Minett, J.F.; Smith, R.G.; Pooley, C.L.; Overland, B.J.; et al. Addressing the welfare needs of farmed lumpfish: Knowledge gaps, challenges and solutions. Rev. Aquac. 2022, 14, 139–155. [Google Scholar] [CrossRef]
- Gutierrez-Rabadan, C.; Spreadbury, C.; Consuegra, S.; de Leaniz, C.G. Development, validation and testing of an Operational Welfare Score Index for farmed lumpfish Cyclopterus lumpus L. Aquaculture 2021, 531, 735777. [Google Scholar] [CrossRef]
- Eliasen, K.; Patursson, E.J.; McAdam, B.J.; Pino, E.; Morro, B.; Betancor, M.; Baily, J.; Rey, S. Liver colour scoring index, carotenoids and lipid content assessment as a proxy for lumpfish (Cyclopterus lumpus L.) health and welfare condition. Sci. Rep. 2020, 10, 8927. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Jonassen, T.M.; Hangstad, T.A.; Elvegård, T.A.; Urskog, T.C.; Mikalsen, B. Effects of three commercial diets on growth, cataract development and histopathology of lumpfish (Cyclopterus lumpus L.). Aquac. Res. 2018, 49, 3131–3141. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Jonassen, T.M.; Hangstad, T.A.; Elvegård, T.A.; Urskog, T.C.; Hanssen, A.; Mikalsen, B. Effects of different feeding frequencies on growth, cataract development and histopathology of lumpfish (Cyclopterus lumpus L.). Aquaculture 2019, 501, 161–168. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Hangstad, T.A.; Jónsdóttir, Ó.D.; Noble, T.; Wilson, M.; Mackie, J.A.; Elvegård, T.A.; Urskog, T.C.; Mikalsen, B. Feeding behaviour and growth of lumpfish (Cyclopterus lumpus L.) fed with feed blocks. Aquac. Res. 2018, 49, 2006–2012. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Lorentzen, M.; Eilertsen, R.A.; Micallef, G.; Tvenning, R. Improving survival and health of lumpfish (Cyclopterus lumpus L.) by the use of feed blocks and operational welfare indicators (OWIs) in commercial Atlantic salmon cages. Aquaculture 2020, 527, 735476. [Google Scholar] [CrossRef]
- Klakegg, Ø.; Myhren, S.; Juell, R.A.; Aase, M.; Salonius, K.; Sørum, H. Improved health and better survival of farmed lumpfish (Cyclopterus lumpus) after a probiotic bath with two probiotic strains of Aliivibrio. Aquaculture 2020, 518, 734810. [Google Scholar] [CrossRef]
- Blackwell, B.G.; Brown, M.L.; Willis, D.W. Relative weight (Wr) status and current use in fisheries assessment and management. Rev. Fish. Sci. 2000, 8, 1–44. [Google Scholar] [CrossRef]
- Bolger, T.; Connolly, P. The selection of suitable indices for the measurement and analysis of fish condition. J. Fish Biol. 1989, 34, 171–182. [Google Scholar] [CrossRef]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Nytrø, A.V.; Eliassen, G.; Hangstad, T.A.; Jónsdóttir, Ó.D.; Emaus, P.A.; Elvegård, T.A.; Lemmens, S.C.; Rydland, R.; et al. Effects of lumpfish size on foraging behaviour and co-existence with sea lice infected Atlantic salmon in sea cages. Aquaculture 2016, 465, 19–27. [Google Scholar] [CrossRef]
- Eliasen, K.; Danielsen, E.; Johannesen, Á.; Joensen, L.L.; Patursson, E.J. The cleaning efficacy of lumpfish (Cyclopterus lumpus L.) Faroese salmon (Salmo salar L.) farming pens in relation to lumpfish size and seasonality. Aquaculture 2018, 488, 61–65. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Reynolds, P.; Hangstad, T.A.; Kapari, L.; Maduna, S.N.; Hagen, S.B.; Jónsdóttir, Ó.D.B.; Spetland, F.; Lindberg, K.S. Quantification of grazing efficacy, growth and health score of different lumpfish (Cyclopterus lumpus L.) families: Possible size and gender effects. Aquaculture 2021, 530, 735925. [Google Scholar] [CrossRef]
- Engebretsen, S.; Aldrin, M.; Qviller, L.; Stige, L.C.; Rafoss, T.; Danielsen, O.R.; Lindhom, A.; Jansen, P.A. Salmon lice (Lepeophtheirus salmonis) in the stomach contents of lumpfish (Cyclopterus lumpus) sampled from Norwegian fish farms: Relationship between lice grazing and operational conditions. Aquaculture 2023, 563, 738967. [Google Scholar] [CrossRef]
- Aldrin, M.; Huseby, R.B.; Stien, A.; Grøntvedt, R.N.; Viljugrein, H.; Jansen, P.A. A stage-structured Bayesian hierarchical model for salmon lice populations at individual salmon farms–Estimated from multiple farm data sets. Ecol. Model. 2017, 359, 333–348. [Google Scholar] [CrossRef]
- Aldrin, M.; Jansen, P.; Stryhn, H. A partly stage-structured model for the abundance of salmon lice in salmonid farms. Epidemics 2019, 26, 9–22. [Google Scholar] [CrossRef]
- BarentsWatch. Norwegian Fish Health. 2023. Available online: https://www.barentswatch.no/en (accessed on 15 September 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- W, S.B. Density Estimation; Chapman and Hall: Boca Raton, FL, USA, 1986. [Google Scholar]
- Nytrø, A.V.; Vikingstad, E.; Foss, A.; Hangstad, T.A.; Reynolds, P.; Eliassen, G.; Elvegård, T.A.; Falk-Petersen, I.B.; Imsland, A.K. The effect of temperature and fish size on growth of juvenile lumpfish (Cyclopterus lumpus L.). Aquaculture 2014, 434, 296–302. [Google Scholar] [CrossRef]
- Claeskens, G.; Hjort, N.L. Model Selection and Model Averaging; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Roy, J.L.; Boulding, E.G. Comparison of DNA metabarcoding and morphological diet analysis of lumpfish (Cyclopterus lumpus) as methods of estimating sea lice cleaning efficacy inside commercial Atlantic salmon (Salmo salar) sea cages. Aquaculture 2024, 586, 740817. [Google Scholar] [CrossRef]
- Jobling, M. Temperature and growth: Modulation of growth rate via temperature. In Global Warming: Implication for Freshwater and Marine Fish; Cambridge University Press: Cambridge, UK, 1997; pp. 225–253. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Hanssen, A.; Nytrø, A.V.; Reynolds, P.; Jonassen, T.M.; Hangstad, T.A.; Elvegård, T.A.; Urskog, T.C.; Mikalsen, B. It works! Lumpfish can significantly lower sea lice infestation in large-scale salmon farming. Biol. Open 2018, 7, bio036301. [Google Scholar] [CrossRef] [PubMed]
- Hvas, M.; Folkedal, O.; Imsland, A.; Oppedal, F. Metabolic rates, swimming capabilities, thermal niche and stress response of the lumpfish, Cyclopterus lumpus. Biol. Open 2018, 7, bio036079. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, R.M.; Sandrelli, R.M.; Gamperl, A.K. Comparing methods for determining the metabolic capacity of lumpfish (Cyclopterus lumpus Linnaeus 1758). J. Fish Biol. 2024, 104, 787–796. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Nytrø, A.V.; Foss, A.; Vikingstad, E.; Elvegård, T.A. Assessment of growth and sea lice infection levels in Atlantic salmon stocked in small-scale cages with lumpfish. Aquaculture 2014, 433, 137–142. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Reynolds, P. In lumpfish We Trust? The Efficacy of Lumpfish Cyclopterus lumpus to Control Lepeophtheirus salmonis Infestations on Farmed Atlantic Salmon: A Review. Fishes 2022, 7, 220. [Google Scholar] [CrossRef]
- Boissonnot, L.; Engebretsen, S.; Aldrin, M. Kondisjonsfaktor hos rognkjeks. Nor. Fiskeoppdrett 2022, 4, 62–65. [Google Scholar]
- Overton, K.; Barrett, L.T.; Oppedal, F.; Kristiansen, T.S.; Dempster, T. Sea lice removal by cleaner fish in salmon aquaculture: A review of the evidence base. Aquac. Environ. Interact. 2020, 12, 31–44. [Google Scholar] [CrossRef]



| T | T2 | W | W2 | WT | CV | AIC |
|---|---|---|---|---|---|---|
| x | x | x | x | - | Non-constant | 0 |
| x | x | x | x | x | Non-constant | 0.4 |
| x | - | x | x | - | Non-constant | 2.2 |
| x | - | x | x | x | Non-constant | 2.5 |
| x | x | x | - | - | Non-constant | 9.0 |
| x | - | x | - | - | Non-constant | 11.2 |
| x | x | x | x | - | Constant | 84.2 |
| Covariate | Parameter | Estimate | Standard Error | p-Value |
|---|---|---|---|---|
| Start weight stock 1 | 77.2 | 9.35 | - | |
| Start weight stock 2 | 60.8 | 2.24 | - | |
| Start weight stock 3 | 85.6 | 3.62 | - | |
| Start weight stock 4 | 44.8 | 1.84 | - | |
| Start weight stock 5 | 48.5 | 3.92 | - | |
| Intercept | −0.0197 | 0.00325 | - | |
| Temperature | 0.00472 | 0.000629 | <0.001 | |
| Weight | 0.00997 | |||
| Temperature2 | <0.001 | |||
| Weight2 | 0.00592 | |||
| Variance parameter | 0.00342 | 0.00155 | - | |
| Variance parameter | 0.783 | 0.0925 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engebretsen, S.; Aldrin, M.; Staven, F.R.; Bendiksen, E.; Stige, L.C.; Jansen, P.A. Heterogeneous Weight Development of Lumpfish (Cyclopterus lumpus) Used as Cleaner Fish in Atlantic Salmon (Salmo salar) Farming. Fishes 2024, 9, 336. https://doi.org/10.3390/fishes9090336
Engebretsen S, Aldrin M, Staven FR, Bendiksen E, Stige LC, Jansen PA. Heterogeneous Weight Development of Lumpfish (Cyclopterus lumpus) Used as Cleaner Fish in Atlantic Salmon (Salmo salar) Farming. Fishes. 2024; 9(9):336. https://doi.org/10.3390/fishes9090336
Chicago/Turabian StyleEngebretsen, Solveig, Magne Aldrin, Fredrik R. Staven, Eskil Bendiksen, Leif Christian Stige, and Peder A. Jansen. 2024. "Heterogeneous Weight Development of Lumpfish (Cyclopterus lumpus) Used as Cleaner Fish in Atlantic Salmon (Salmo salar) Farming" Fishes 9, no. 9: 336. https://doi.org/10.3390/fishes9090336
APA StyleEngebretsen, S., Aldrin, M., Staven, F. R., Bendiksen, E., Stige, L. C., & Jansen, P. A. (2024). Heterogeneous Weight Development of Lumpfish (Cyclopterus lumpus) Used as Cleaner Fish in Atlantic Salmon (Salmo salar) Farming. Fishes, 9(9), 336. https://doi.org/10.3390/fishes9090336






