Effect of Dietary β-Glucan on Growth Performance, Antioxidant Responses, and Immunological Parameters of Coral Trout (Plectropomus leopardus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Diet Preparation
2.3. Fish Rearing and Experimental Procedure
2.4. Sampling
2.5. Growth Performance
2.6. Diet Composition Analysis
2.7. Enzyme Activity Analysis
2.8. Gene Expression Analysis
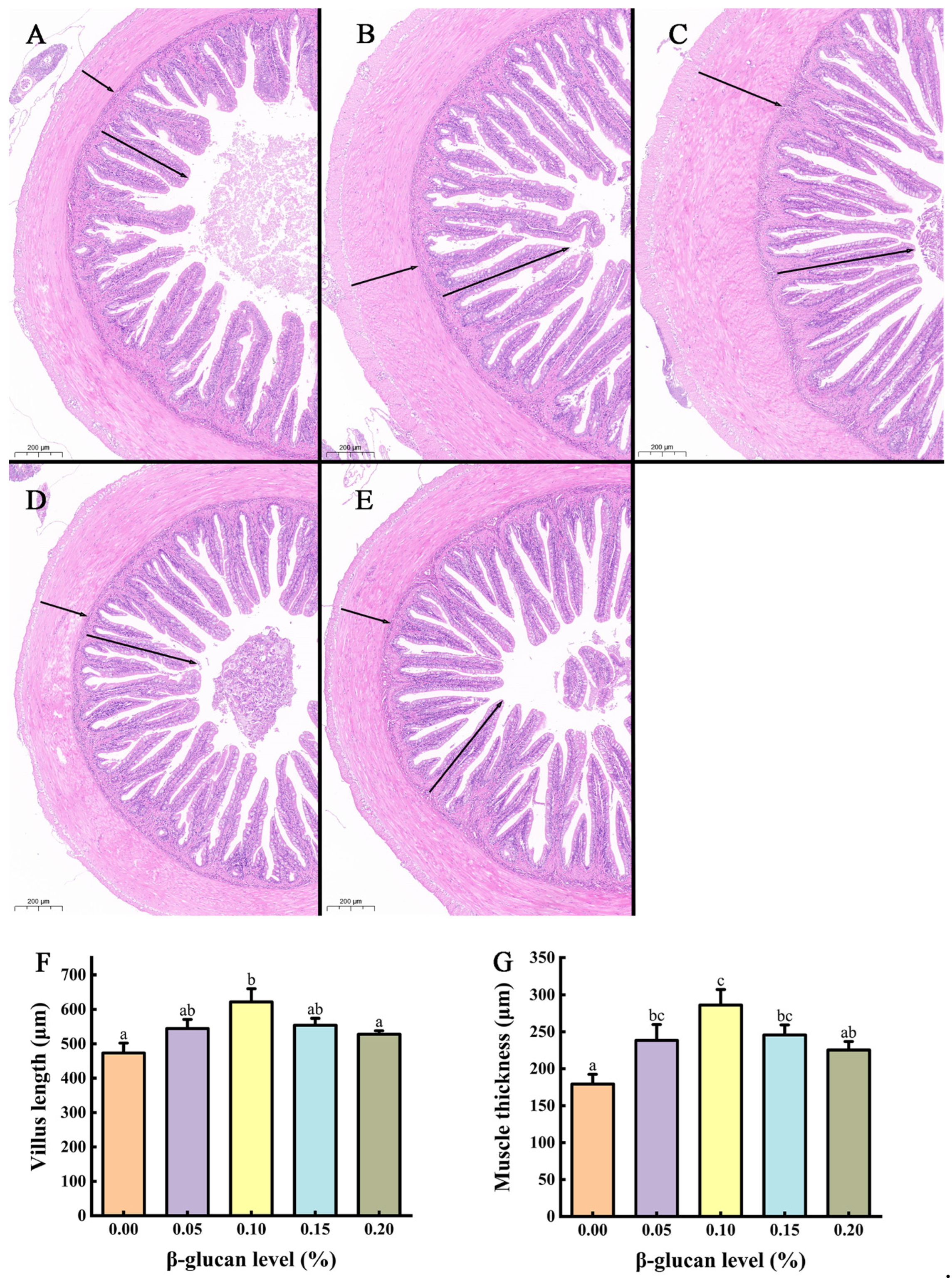
2.9. Mid-Gut Histological Observation
2.10. Statistical Analysis
3. Results
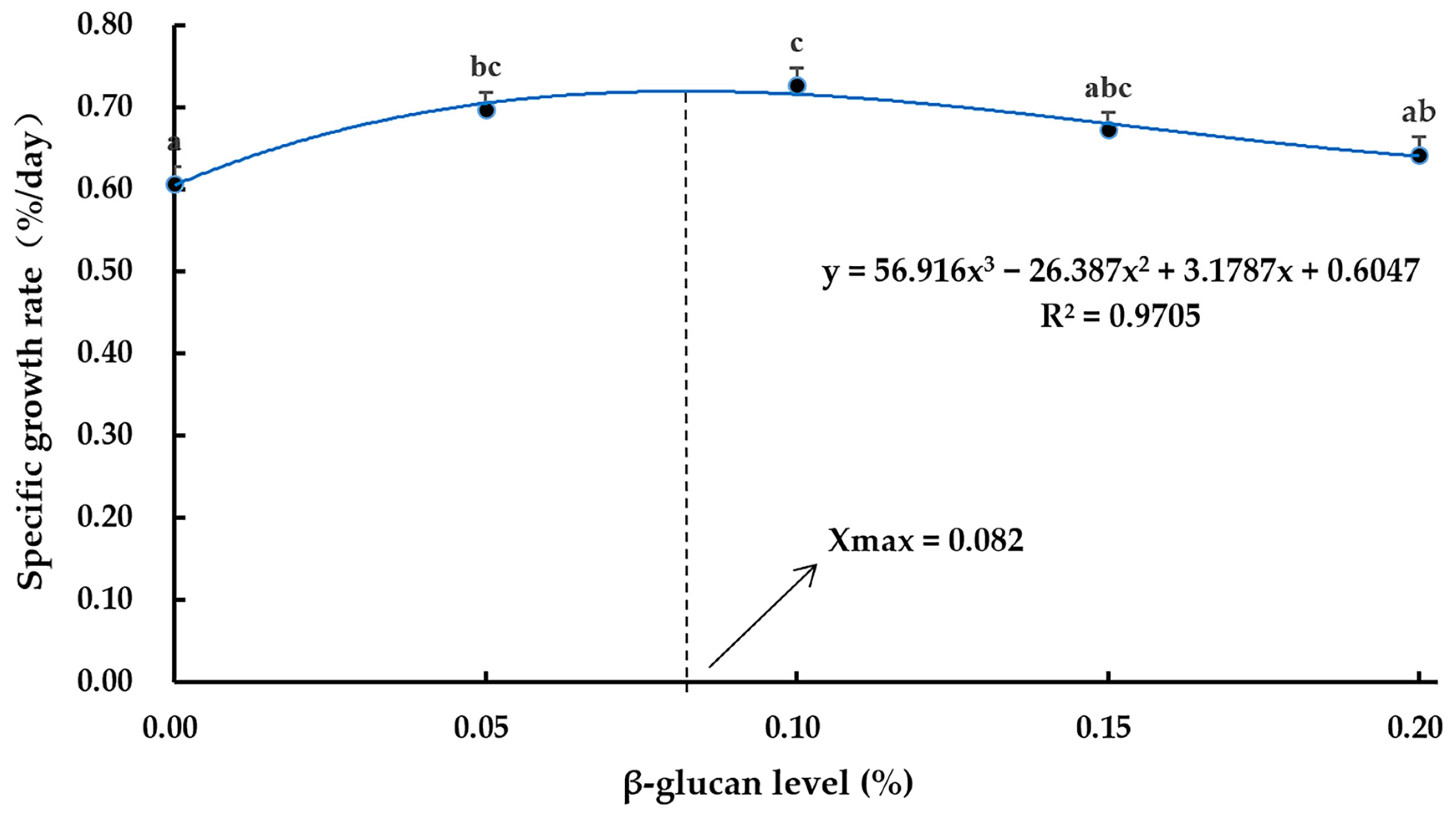
3.1. Growth Performance
3.2. Digestive Enzyme Activities
3.3. Mid-Gut Morphology
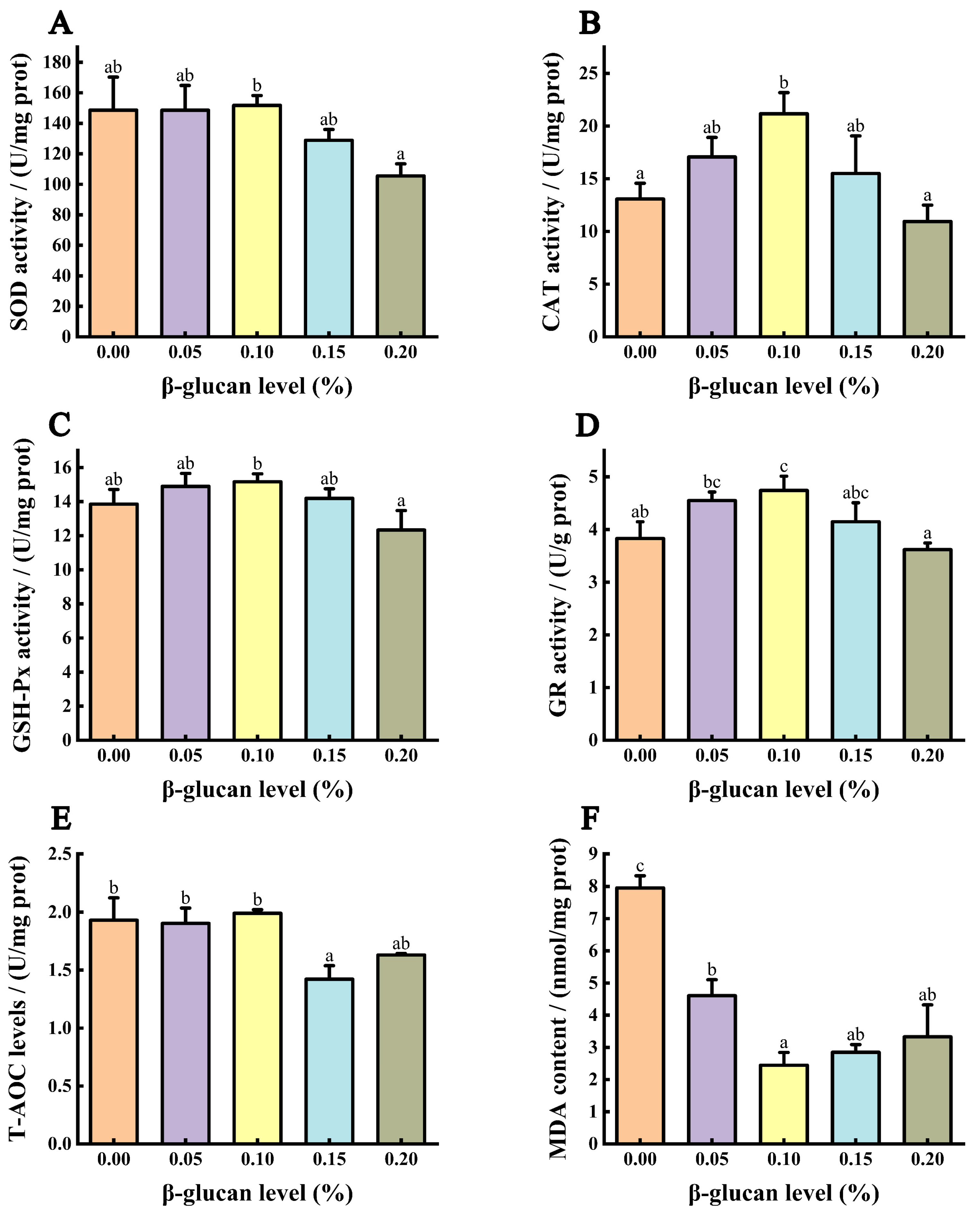
3.4. Antioxidant Capacity of the Liver
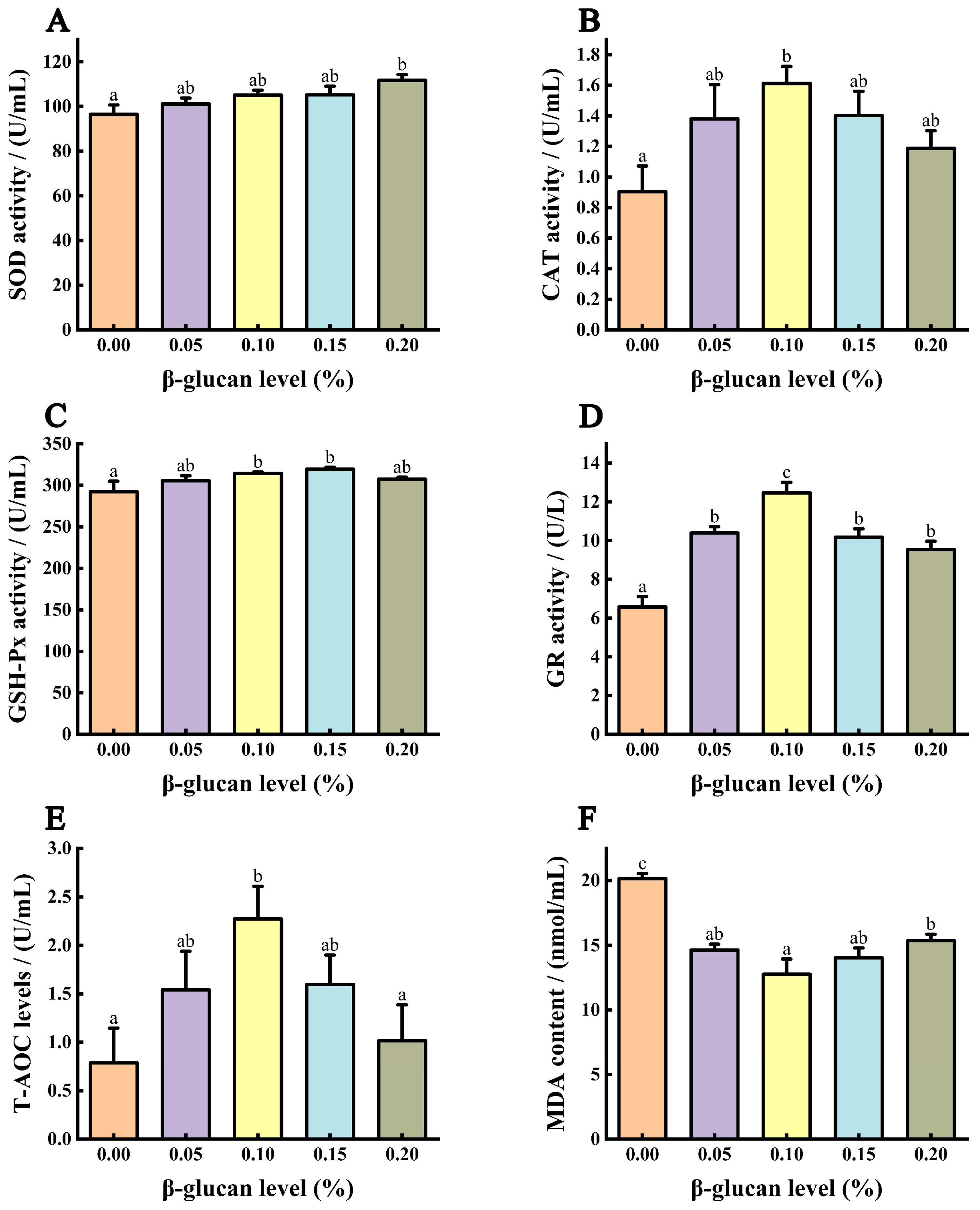
3.5. Antioxidant Capacity of Serum
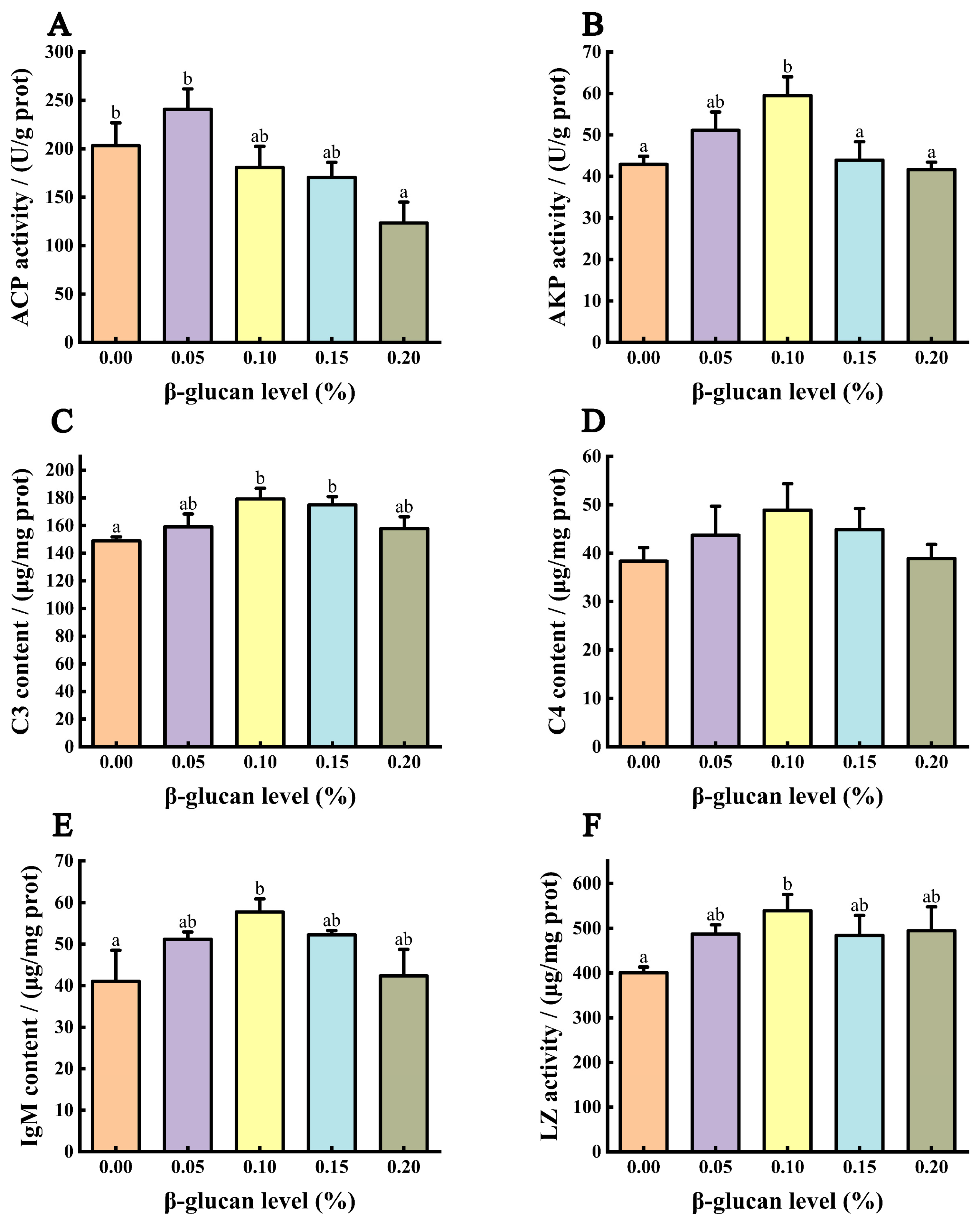
3.6. Immune Ability of Liver
3.7. Immune Ability of Serum
3.8. Relative mRNA Expression in Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Wu, L.N.; Chen, J.F.; Wu, X.; Xia, J.H.; Meng, Z.N.; Liu, X.C.; Lin, H.R. Whole-genome sequencing of leopard coral grouper (Plectropomus leopardus) and exploration of regulation mechanism of skin color and adaptive evolution. Zool. Res. 2020, 41, 328–340. [Google Scholar] [CrossRef]
- Hao, R.; Zhu, X.; Tian, C.; Jiang, M.; Huang, Y.; Li, G.; Zhu, C. LncRNA–miRNA–mRNA ceRNA network of different body colors in Plectropomus leopardus. Front. Mar. Sci. 2023, 10, 1170762. [Google Scholar] [CrossRef]
- Dou, X.; Huang, H.; Li, Y.; Deng, J.; Tan, B. Effects of dietary β-glucan on growth rate, antioxidant status, immune response, and resistance against Aeromonas hydrophila in genetic improvement of farmed tilapia (GIFT, Oreochromis niloticus). Aquac. Rep. 2023, 29, 101480. [Google Scholar] [CrossRef]
- Zhu, X. Regulation Mechanism of Astaxanthin and Vitamin C Improve the Growth and Immune Stress Incoral Trout (Plectropomus Leopardus). Doctoral Thesis, Guangdong Ocean University, Zhanjiang, China, 2022. [Google Scholar] [CrossRef]
- Cao, Q. Effect of Lactobacillus Salivarius GZPH2 on Gut Microbiota and Metabolome of Leopard Coral Grouper (Plectropomus Leopardus). Master’s Thesis, South China University of Technology, Guangzhou, China, 2020. [Google Scholar] [CrossRef]
- Boti, V.; Toli, V.; Efthymiou, C.; Albanis, T. Screening of commonly used antibiotics in fresh and saltwater samples impacted by aquacultures: Analytical methodology, occurrence and environmental risk assessment. Sustainability 2023, 15, 9199. [Google Scholar] [CrossRef]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: Risks, current concern, and future thinking. Environ. Sci. Pollut. Res. Int. 2022, 29, 11054–11075. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.K.; Das, P.; Kumar, S.; Mandal, S.C.; Prusty, A.K.; Singh, S.K.; Akhtar, M.S.; Behera, B.K.; Kumar, K.; Pal, A.K.; et al. Beta-glucan: An ideal immunostimulant in aquaculture (a review). Fish Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef] [PubMed]
- El-Boshy, M.E.; El-Ashram, A.M.; Abdelhamid, F.M.; Gadalla, H.A. Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 28, 802–808. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Hill Gaston, J.S.; Goodall, J.C. β-glucan size controls dectin-1-mediated immune responses in human dendritic cells by regulating IL-1β production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef]
- Mironczuk-Chodakowska, I.; Kujawowicz, K.; Witkowska, A.M. Beta-glucans from fungi: Biological and health-promoting potential in the COVID-19 pandemic era. Nutrients 2021, 13, 3960. [Google Scholar] [CrossRef]
- Atta-Allah, A.A.; Ahmed, R.F.; Shahin, A.A.M.; Hassan, E.A.; El-Bialy, H.A.; El-Fouly, M.Z. Optimizing the synthesis of yeast Beta-glucan via response surface methodology for nanotechnology application. BMC Microbiol. 2023, 23, 110. [Google Scholar] [CrossRef]
- Singla, A.; Gupta, O.P.; Sagwal, V.; Kumar, A.; Patwa, N.; Mohan, N.; Ankush; Kumar, D.; Vir, O.; Singh, J.; et al. Beta-glucan as a soluble dietary fiber source: Origins, biosynthesis, extraction, purification, structural characteristics, bioavailability, biofunctional attributes, industrial utilization, and global trade. Nutrients 2024, 16, 900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Bogwald, J. β-glucans as conductors of immune symphonies. Fish Shellfish Immunol. 2008, 25, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Sharifinia, M.; Ghaedi, G. β-glucan as a promising food additive and immunostimulant in aquaculture industry. Ann. Anim. Sci. 2022, 22, 817–827. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Ghaedi, G.; Sharifinia, M. Effects of diets containing β-glucan on survival, growth performance, haematological, immunity and biochemical parameters of rainbow trout (Oncorhynchus mykiss ) fingerlings. Aquac. Res. 2021, 53, 1842–1850. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y.; Yu, J.; Fang, L.; Li, Y.; Wang, M.; Liu, J.; Yan, P.; Xia, J.; Liu, G.; et al. Effects of sulfated β-glucan from Saccharomyces cerevisiae on growth performance, antioxidant ability, nonspecific immunity, and intestinal flora of the red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2022, 127, 891–900. [Google Scholar] [CrossRef]
- Aramli, M.S.; Kamangar, B.; Nazari, R.M. Effects of dietary β-glucan on the growth and innate immune response of juvenile Persian sturgeon, Acipenser persicus. Fish Shellfish Immunol. 2015, 47, 606–610. [Google Scholar] [CrossRef]
- Talpur, A.D.; Munir, M.B.; Mary, A.; Hashim, R. Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead (Channa striata) fingerlings. Aquaculture 2014, 426–427, 14–20. [Google Scholar] [CrossRef]
- Lin, S.; Pan, Y.; Luo, L.; Luo, L. Effects of dietary β-1,3-glucan, chitosan or raffinose on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Fish Shellfish Immunol. 2011, 31, 788–794. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Van Dang, P.; Le, A.Q.; Nguyen, T.K.L.; Pham, D.H.; Van Nguyen, N.; Nguyen, Q.H. Effect of oligochitosan and oligo-β-glucan supplementation on growth, innate immunity, and disease resistance of striped catfish (Pangasianodon hypophthalmus). Biotechnol. Appl. Biochem. 2017, 64, 564–571. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhou, L.; Qu, Y.; Lu, K.; Han, F.; Li, E. Effects of different dietary β-glucan levels on antioxidant capacity and immunity, gut microbiota and transcriptome responses of white shrimp (Litopenaeus vannamei) under low salinity. Antioxidants 2022, 11, 2282. [Google Scholar] [CrossRef]
- Cao, H.; Yu, R.; Zhang, Y.; Hu, B.; Jian, S.; Wen, C.; Kajbaf, K.; Kumar, V.; Yang, G. Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 2019, 508, 106–112. [Google Scholar] [CrossRef]
- Fath El-Bab, A.F.; Majrashi, K.A.; Sheikh, H.M.; Shafi, M.E.; El-Ratel, I.T.; Neamat-Allah, A.N.F.; El-Raghi, A.A.; Elazem, A.Y.A.; Abd-Elghany, M.F.; Abdelnour, S.A.; et al. Dietary supplementation of Nile tilapia (Oreochromis niloticus) with β-glucan and/or Bacillus coagulans: Synergistic impacts on performance, immune responses, redox status and expression of some related genes. Front. Vet. Sci. 2022, 9, 1011715. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, Y.; Chen, H.; Zhou, Q.; Zhang, Y.; Huang, X.; Huang, Z.; Li, T.; Zhou, C.; Ma, Z.; et al. Effects of dietary chitosan on the growth, health status and disease resistance of golden pompano (Trachinotus ovatus). Carbohydr. Polym. 2023, 300, 120237. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, Y.; Zhou, Q.; Huang, X.; Huang, Z.; Li, Y.; Wu, W.; Zhou, C.; Ma, Z.; Lin, H. Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 2022, 207, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Zhang, W.; Mai, K.; Wang, X.; Xu, W.; Ma, H. Effects of discontinuous administration of β-glucan and glycyrrhizin on the growth and immunity of white shrimp Litopenaeus vannamei. Aquaculture 2010, 306, 218–224. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Zanuzzo, F.S.; Koch, J.F.A.; de Oliveira, C.A.F.; Sima, P.; Vetvicka, V. Development of fish immunity and the role of β-glucan in immune responses. Molecules 2020, 25, 5378. [Google Scholar] [CrossRef]
- Douxfils, J.; Fierro-Castro, C.; Mandiki, S.N.; Emile, W.; Tort, L.; Kestemont, P. Dietary β-glucans differentially modulate immune and stress-related gene expression in lymphoid organs from healthy and Aeromonas hydrophila-infected rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2017, 63, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lin, H.; Yang, Y.; Zhou, Q.; Chen, H.; Huang, X.; Zhou, C.; Huang, Z.; Li, T. Effects of supplemental dietary Haematococcus pluvialison growth performance, antioxidant capacity, immune responses and resistance to Vibrio harveyi challenge of spotted sea bass Lateolabrax maculatus. Aquac. Nutr. 2020, 27, 355–365. [Google Scholar] [CrossRef]
- AOAC. Official Methods for Analysis, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xie, J.; Niu, J. Evaluation of four macro-algae on growth performance, anti-oxidant capacity and non-specific immunity in golden pompano (Trachinotus ovatus). Aquaculture 2022, 548, 737690. [Google Scholar] [CrossRef]
- Do Huu, H.; Sang, H.M.; Thanh Thuy, N.T. Dietary β-glucan improved growth performance, Vibrio counts, haematological parameters and stress resistance of pompano fish, Trachinotus ovatus Linnaeus, 1758. Fish Shellfish Immunol. 2016, 54, 402–410. [Google Scholar] [CrossRef]
- Guzman-Villanueva, L.T.; Ascencio-Valle, F.; Macias-Rodriguez, M.E.; Tovar-Ramirez, D. Effects of dietary β-1,3/1,6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol. Biochem. 2013, 40, 827–837. [Google Scholar] [CrossRef]
- Del Rio-Zaragoza, O.B.; Fajer-Avila, E.J.; Almazan-Rueda, P. Influence of β-glucan on innate immunity and resistance of Lutjanus guttatus to an experimental infection of dactylogyrid monogeneans. Parasite Immunol. 2011, 33, 483–494. [Google Scholar] [CrossRef]
- Welker, T.L.; Lim, C.; Yildirim-Aksoy, M.; Klesius, P.H. Use of diet crossover to determine the effects of β-glucan supplementation on immunity and growth of Nile tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2012, 43, 335–348. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Nieves-Rodríguez, K.; Álvarez-González, C.; Peña-Marín, E.; Vega-Villasante, F.; Martínez-García, R.; Camarillo-Coop, S.; Tovar-Ramírez, D.; Guzmán-Villanueva, L.; Andree, K.; Gisbert, E. Effect of β-glucans in diets on growth, survival, digestive enzyme activity, and immune system and intestinal barrier gene expression for tropical gar (Atractosteus tropicus) juveniles. Fishes 2018, 3, 27. [Google Scholar] [CrossRef]
- Hoang, D.-H.; Thi Thanh Thuy, N.; Ky, P.X. A synergistic effect of dietary β-glucan and mannan oligosaccharide on growth performance, haematology, body composition, nutrient utilisation, and intestinal morphology in pompano, Trachinotus ovatus. Reg. Stud. Mar. Sci. 2024, 73, 103494. [Google Scholar] [CrossRef]
- Fu, H.; Qi, M.; Yang, Q.; Li, M.; Yao, G.; Bu, W.; Zheng, T.; Pi, X. Effects of dietary chito-oligosaccharide and β-glucan on the water quality and gut microbiota, intestinal morphology, immune response, and meat quality of Chinese soft-shell turtle (Pelodiscus sinensis). Front. Immunol. 2023, 14, 1266997. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Cao, J.; Lu, Y. Long-and short-term dietary beta-glucan improves intestinal health and disease resistance in pearl gentian grouper (Epinephelus lanceolatus♂ x Epinephelus fuscoguttatus♀). Fish Physiol. Biochem. 2024, 50, 973–988. [Google Scholar] [CrossRef]
- Talpur, A.D.; Ikhwanuddin, M. Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 2012, 364–365, 6–12. [Google Scholar] [CrossRef]
- Ai, Q.; Mai, K.; Zhang, L.; Tan, B.; Zhang, W.; Xu, W.; Li, H. Effects of dietary beta-1, 3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2007, 22, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, M.; Li, N.; Dong, Z.; Cai, L.; Wu, B.; Xie, J.; Liu, L.; Ren, L.; Shi, B. New insights into β-glucan-enhanced immunity in largemouth bass Micropterus salmoides by transcriptome and intestinal microbial composition. Front. Immunol. 2022, 13, 1086103. [Google Scholar] [CrossRef]
- Deng, J.; Mai, K.; Chen, L.; Mi, H.; Zhang, L. Effects of replacing soybean meal with rubber seed meal on growth, antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in tilapia (Oreochromis niloticus x O. Aureus). Fish Shellfish Immunol. 2015, 44, 436–444. [Google Scholar] [CrossRef]
- Pogue, R.; Murphy, E.J.; Fehrenbach, G.W.; Rezoagli, E.; Rowan, N.J. Exploiting immunomodulatory properties of β-glucans derived from natural products for improving health and sustainability in aquaculture-farmed organisms: Concise review of existing knowledge, innovation and future opportunities. Curr. Opin. Environ. Sci. Health 2021, 21, 100248. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, W.N.; Wang, L.J.; Liu, Y.F.; Wang, A.L. Oxidative stress, DNA damage and osmolality in the Pacific white shrimp, Litopenaeus vannamei exposed to acute low temperature stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 154, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, L.; Lu, T.; Zhang, Y.; Wang, Y.; Yin, J. Protective effects of lentinan on lipopolysaccharide induced inflammatory response in intestine of juvenile taimen (Hucho taimen, Pallas). Int. J. Biol. Macromol. 2019, 121, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, X.F.; Yang, Z.Y.; Zhu, R.; Li, D.L.; Shang, G.J.; Wang, H.T.; Meng, S.T.; Wang, Y.T.; Liu, S.Y.; et al. Alleviative effect of poly-β-hydroxybutyrate on lipopolysaccharide-induced oxidative stress, inflammation and cell apoptosis in Cyprinus carpio. Int. J. Biol. Macromol. 2023, 253, 126784. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, R.; Zhang, J.; Tian, C.; Hong, Y.; Zhu, C.; Li, G. Improved growth performance, digestive ability, antioxidant capacity, immunity and Vibrio harveyi resistance in coral trout (Plectropomus leopardus) with dietary vitamin C. Aquac. Rep. 2022, 24, 101111. [Google Scholar] [CrossRef]


| Index | Diets (β-Glucan %) | ||||
|---|---|---|---|---|---|
| 0 | 0.05 | 0.10 | 0.15 | 0.20 | |
| IBW (g) | 75.93 ± 0.27 | 75.75 ± 0.08 | 75.86 ± 0.10 | 75.80 ± 0.32 | 75.70 ± 0.42 |
| FBW (g) | 106.65 ± 1.13 a | 111.93 ± 1.11 bc | 114.00 ± 0.99 c | 110.45 ± 1.28 abc | 108.50 ± 2.27 ab |
| WGR (%) | 40.48 ± 1.80 a | 47.76 ± 1.33 bc | 50.27 ± 1.21 c | 45.72 ± 1.54 abc | 43.35 ± 3.34 ab |
| SGR (%) | 0.61 ± 0.02 a | 0.70 ± 0.02 bc | 0.73 ± 0.01 c | 0.67 ± 0.02 abc | 0.64 ± 0.04 ab |
| FCR | 1.53 ± 0.07 c | 1.37 ± 0.04 ab | 1.24 ± 0.02 a | 1.47 ± 0.02 bc | 1.50 ± 0.04 bc |
| SR (%) | 90.00 ± 2.89 a | 100.00 ± 0.00 b | 100.00 ± 0.00 b | 96.67 ± 3.33 b | 98.33 ± 1.67 b |
| VSI (%) | 4.74 ± 0.29 | 4.29 ± 0.34 | 4.18 ± 0.12 | 4.49 ± 0.23 | 4.12 ± 0.16 |
| HSI (%) | 1.16 ± 0.13 | 1.03 ± 0.06 | 1.09 ± 0.06 | 1.31 ± 0.04 | 1.03 ± 0.09 |
| CF (%) | 2.20 ± 0.02 | 2.18 ± 0.05 | 2.03 ± 0.09 | 2.06 ± 0.08 | 2.08 ± 0.04 |
| Index | Diets (β-Glucan %) | ||||
|---|---|---|---|---|---|
| 0 | 0.05 | 0.10 | 0.15 | 0.20 | |
| α-Amylase (U/mg prot) | 1.17 ± 0.05 a | 1.39 ± 0.07 b | 1.47 ± 0.03 b | 1.40 ± 0.05 b | 1.27 ± 0.09 ab |
| Lipase (U/g prot) | 0.82 ± 0.07 | 0.97 ± 0.06 | 1.12 ± 0.12 | 1.09 ± 0.14 | 0.95 ± 0.08 |
| Chymotrypsin (U/mg prot) | 1.19 ± 0.07 a | 1.60 ± 0.09 b | 1.73 ± 0.09 b | 1.67 ± 0.10 b | 1.47 ± 0.13 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Lin, Z.; Ma, Z.; Yang, Y.; Zhou, C.; Hu, J.; Yu, W.; Lin, H. Effect of Dietary β-Glucan on Growth Performance, Antioxidant Responses, and Immunological Parameters of Coral Trout (Plectropomus leopardus). Fishes 2024, 9, 298. https://doi.org/10.3390/fishes9080298
Hao X, Lin Z, Ma Z, Yang Y, Zhou C, Hu J, Yu W, Lin H. Effect of Dietary β-Glucan on Growth Performance, Antioxidant Responses, and Immunological Parameters of Coral Trout (Plectropomus leopardus). Fishes. 2024; 9(8):298. https://doi.org/10.3390/fishes9080298
Chicago/Turabian StyleHao, Xiaoqi, Ziyang Lin, Zhenhua Ma, Yukai Yang, Chuanpeng Zhou, Jing Hu, Wei Yu, and Heizhao Lin. 2024. "Effect of Dietary β-Glucan on Growth Performance, Antioxidant Responses, and Immunological Parameters of Coral Trout (Plectropomus leopardus)" Fishes 9, no. 8: 298. https://doi.org/10.3390/fishes9080298
APA StyleHao, X., Lin, Z., Ma, Z., Yang, Y., Zhou, C., Hu, J., Yu, W., & Lin, H. (2024). Effect of Dietary β-Glucan on Growth Performance, Antioxidant Responses, and Immunological Parameters of Coral Trout (Plectropomus leopardus). Fishes, 9(8), 298. https://doi.org/10.3390/fishes9080298








