Oxidative Status of the Pyloric Caeca and Proximal Intestine in Gilthead Sea Bream Fed Diets Including Different Vegetable Oil Blends from Palm, Rapeseed and Linseed
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish, Feeding Trial and Sampling
2.3. Oxidative Stress Markers Analysis
2.4. RNA Extraction, cDNA Synthesis and Real-Time Quantitative-PCR (qPCR)
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, C.; Diógenes, A.F.; Coutinho, F.; Panserat, S.; Corraze, G.; Pérez-Jiménez, A.; Peres, H.; Oliva-Teles, A. Liver and Intestine Oxidative Status of Gilthead Sea Bream Fed Vegetable Oil and Carbohydrate Rich Diets. Aquaculture 2016, 464, 665–672. [Google Scholar] [CrossRef]
- Kiron, V.; Thawonsuwan, J.; Panigrahi, A.; Scharsack, J.P.; Satoh, S. Antioxidant and Immune Defences of Rainbow Trout (Oncorhynchus mykiss) Offered Plant Oils Differing in Fatty Acid Profiles from Early Stages. Aquac. Nutr. 2011, 17, 130–140. [Google Scholar] [CrossRef]
- Kjær, M.A.; Aursnes, I.A.; Berge, G.M.; Sørensen, M.; Marchenko, Y.; Gjøen, T.; Ruyter, B. The Influence of Different Dietary Oil Qualities on Growth Rate, Feed Utilization and Oxidative Stress in Atlantic Cod. Aquac. Nutr. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- García-Meilán, I.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I.; Gallardo, Á. Effects of Dietary Vegetable Oil Mixtures Including Soybean Oil on Intestinal Oxidative Stress in Gilthead Sea Bream (Sparus aurata). Animals 2023, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Xiao, W.; Jiang, W.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Zhang, Y.; Zhou, X. Effect of Dietary Enzyme-Treated Soy Protein on the Immunity and Antioxidant Status in the Intestine of Juvenile Jian Carp (Cyprinus carpio var. Jian). Aquac. Res. 2019, 50, 1411–1421. [Google Scholar] [CrossRef]
- Morais, S.; Edvardsen, R.B.; Tocher, D.R.; Bell, J.G. Transcriptomic Analyses of Intestinal Gene Expression of Juvenile Atlantic Cod (Gadus morhua) Fed Diets with Camelina Oil as Replacement for Fish Oil. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; da Cruz, I.B.M.; González-Gallego, J. Manganese Superoxide Dismutase and Oxidative Stress Modulation. Adv Clin Chem 2015, 68, 87–130. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Glencross, B.D. Exploring the Nutritional Demand for Essential Fatty Acids by Aquaculture Species. Aquac. Res. 2009, 48, 71–124. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; The Nacional Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish Oil Replacement in Finfish Nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty Acid Requirements in Ontogeny of Marine and Freshwater Fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Puangkaew, J.; Kiron, V.; Somamoto, T.; Okamoto, N.; Satoh, S.; Takeuchi, T.; Watanabe, T. Nonspecific Immune Response of Rainbow Trout (Oncorhynchus mykiss Walbaum) in Relation to Different Status of Vitamin E and Highly Unsaturated Fatty Acids. Fish. Shellfish Immunol. 2004, 16, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.K.; Kjær, M.A.; Rørå, A.M.B.; Torstensen, B.; Ruyter, B. High N-3 HUFA Levels in the Diet of Atlantic Salmon Affect Muscle and Mitochondrial Membrane Lipids and Their Susceptibility to Oxidative Stress. Aquac. Nutr. 2011, 17, 177–190. [Google Scholar] [CrossRef]
- Seiliez, I.; Panserat, S.; Corraze, G.; Kaushik, S.; Bergot, P. Cloning and Nutritional Regulation of a Δ6-Desaturase-like Enzyme in the Marine Teleost Gilthead Seabream (Sparus aurata). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Monroig, O.; Zheng, X.; Leaver, M.J.; Tocher, D.R. Highly Unsaturated Fatty Acid Synthesis in Atlantic Salmon: Characterization of ELOVL5- and ELOVL2-like Elongases. Mar. Biotechnol. 2009, 11, 627–639. [Google Scholar] [CrossRef]
- Betancor, M.B.; Howarth, F.J.E.; Glencross, B.D.; Tocher, D.R. Influence of Dietary Docosahexaenoic Acid in Combination with Other Long-Chain Polyunsaturated Fatty Acids on Expression of Biosynthesis Genes and Phospholipid Fatty Acid Compositions in Tissues of Post-Smolt Atlantic Salmon (Salmo salar). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 172–173, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of Different Dietary Vegetable Oils on Growth and Intestinal Performance, Lipid Metabolism and Flesh Quality in Gilthead Sea Bream. Aquaculture 2020, 519, 734881. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and Flaxseed Oil: An Ancient Medicine & Modern Functional Food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, J.N.; Rout-Pitt, N.; Bain, P.A.; Stone, D.A.J.; Schuller, K.A. Dietary Fish Oil Replacement with Canola Oil Up-Regulates Glutathione Peroxidase 1 Gene Expression in Yellowtail Kingfish (Seriola lalandi). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 162, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, C.; Rossi, A.S.; Cian, R.E.; Drago, S.R.; Cazenave, J. Dietary β-Carotene Improves Growth Performance and Antioxidant Status of Juvenile Piaractus mesopotamicus. Aquac. Nutr. 2019, 25, 761–769. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, D.; Zhou, X.Q.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.D.; Zhao, Y. Effects of Glutamate on Growth, Antioxidant Capacity, and Antioxidant-Related Signaling Molecule Expression in Primary Cultures of Fish Enterocytes. Fish Physiol. Biochem. 2015, 41, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wu, X.Y.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, Y. Effects of Dietary Curcumin Supplementation on Growth Performance, Intestinal Digestive Enzyme Activities and Antioxidant Capacity of Crucian Carp Carassius auratus. Aquaculture 2016, 463, 174–180. [Google Scholar] [CrossRef]
- Magalhães, R.; Guerreiro, I.; Santos, R.A.; Coutinho, F.; Couto, A.; Serra, C.R.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Oxidative Status and Intestinal Health of Gilthead Sea Bream (Sparus aurata) Juveniles Fed Diets with Different ARA/EPA/DHA Ratios. Sci. Rep. 2020, 10, 13824. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, H.; Mokrani, A.; Ji, K.; Yu, H.; Ge, X.; Ren, M.; Xie, J.; Pan, L.; Sun, A. Dietary Histidine Affects Intestinal Antioxidant Enzyme Activities, Antioxidant Gene Expressions and Inflammatory Factors in Juvenile Blunt Snout Bream (Megalobrama amblycephala). Aquac. Nutr. 2019, 25, 249–259. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Jiang, W.D.; Duan, X.D.; Feng, L.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Soybean Glycinin Caused NADPH-Oxidase-Regulated ROS Overproduction and Decreased ROS Elimination Capacity in the Mid and Distal Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Aquaculture 2020, 516, 734651. [Google Scholar] [CrossRef]
- Magalhães, R.; Martins, N.; Fontinha, F.; Couto, A.; Serra, C.R.; Santos, R.A.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Effects of Dietary ARA, DHA, and Carbohydrates Levels on Gilthead Sea Bream Liver and Intestine Oxidative Stress, Tissue Histomorphology, and Gut Microbiota. Aquaculture 2022, 552, 738014. [Google Scholar] [CrossRef]
- García-Meilán, I.; Valentín, J.M.; Fontanillas, R.; Gallardo, M.A. Different Protein to Energy Ratio Diets for Gilthead Sea Bream (Sparus aurata): Effects on Digestive and Absorptive Processes. Aquaculture 2013, 412–413, 1–7. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Gallardo, M.A. Meal Timing Affects Protein-Sparing Effect by Carbohydrates in Sea Bream: Effects on Digestive and Absorptive Processes. Aquaculture 2014, 434, 121–128. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Machahua, C.; Buenestado, S.; Fontanillas, R.; Gallardo, M.A. Effects of Dietary Protein-to-Lipid Ratio on Digestive and Absorptive Processes in Sea Bass Fingerlings. Aquaculture 2016, 463, 163–173. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Valentín, J.M.; Hernández, M.D.; García, B.; Fontanillas, R.; Gallardo, M.A. Modulation of Digestive and Absorptive Processes with Age and/or after a Dietary Change in Gilthead Sea Bream. Aquaculture 2016, 459, 54–64. [Google Scholar] [CrossRef]
- Santigosa, E.; García-Meilán, I.; Valentín, J.M.; Navarro, I.; Pérez-Sánchez, J.; Gallardo, M.Á. Plant Oils’ Inclusion in High Fish Meal-Substituted Diets: Effect on Digestion and Nutrient Absorption in Gilthead Sea Bream (Sparus aurata L.). Aquac. Res. 2011, 42, 962–974. [Google Scholar] [CrossRef]
- Santigosa, E.; García-Meilán, I.; Valentin, J.M.; Pérez-Sánchez, J.; Médale, F.; Kaushik, S.; Gallardo, M.A. Modifications of Intestinal Nutrient Absorption in Response to Dietary Fish Meal Replacement by Plant Protein Sources in Sea Bream (Sparus aurata) and Rainbow Trout (Onchorynchus mykiss). Aquaculture 2011, 317, 146–154. [Google Scholar] [CrossRef]
- Kurokawa, T.; Suzuki, T. Structure of the Exocrine Pancreas of Flounder (Paralichthys olivaceus): Immunological Localization of Zymogen Granules in the Digestive Tract Using Anti-Trypsinogen Antibody. J. Fish Biol. 1995, 46, 292–301. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Sundby, A. Characteristics of Pancreatic Function in Fish. In Biology of the Pancreas in Growing Animals; Pierzynowski, S.G., Zabielski, R., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1999; pp. 437–458. [Google Scholar]
- Morrison, C.M.; Pohajdak, B.; Tam, J.; Wright, J.R., Jr. Development of the Islets, Exocrine Pancreas, and Related Ducts in the Nile Tilapia, Oreochromis Niloticus (Pisces: Cichlidae). J. Morphol. 2004, 261, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.A.; Castro, R.; Abos, B.; Rodríguez Saint-Jean, S.S.; Pérez-Prieto, S.I.; Tafalla, C. The Pyloric Caeca Area Is a Major Site for IgM+ and IgT+ B Cell Recruitment in Response to Oral Vaccination in Rainbow Trout. PLoS ONE 2013, 8, e66118. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jami, E.; Harpaz, S.; Mizrahi, I. Involvement of Dietary Salt in Shaping Bacterial Communities in European Sea Bass (Dicentrarchus labrax). Sci. Rep. 2013, 3, srep01558. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Diamond, J.M. Pyloric Ceca of Fish: A “New” Absorptive Organ. Am. J. Physiol. Gastrointest. Liver Physiol. 1987, 252, G65–G76. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.E.M.; Pirie, B.J.S.; Sargent, J.R. An Electron Microscopic Study of Lipid Absorption in the Pyloric Caeca of Rainbow Trout (Salmo gairdnerii) Fed Wax Ester—Rich Zooplankton. Cell Tissue Res. 1979, 200, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Sire, M.-F.; Lutton, C.; Vernier, J.-M. New Views on Intestinal Absorption of Lipids in Teleostean Fishes: An Ultrastructural and Biochemical Study in the Rainbow Trout. J. Lipid Res. 1981, 22, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Bakke, A.M.; Glover, C.; Krogdahl, A. Feeding, Digestion and Absorption of Nutrients. In Multifunctional Gut of Fish; Grosell, M., Farrell, A., Brauner, C., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 57–75. [Google Scholar]
- Buddington, R.K.; Krogdahl, A.; Bakke-Mckellep, A.M. The Intestines of Carnivorous Fish: Structure and Functions and the Relations with Diet. Acta Physiol. Scand. Suppl. 1997, 638, 67–80. [Google Scholar] [PubMed]
- Mccords, J.M.; Fridovich, I. Superoxide Dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Aebi, H. [13] Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.G.; Pirie, B.J.S.; Adron, J.W.; Cowey, C.B. Some Effects of Selenium Deficiency on Glutathione Peroxidase (EC 1.11.1.9) Activity and Tissue Pathology in Rainbow Trout (Salmo gairdneri). Br. J. Nutr. 1986, 55, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Fleischner, G.; Gatmaitan, Z.; Arias, I.M.; Jakoby, W.B. The Identity of Glutathione S Transferase B with Ligandin, a Major Binding Protein of Liver. Proc. Natl. Acad. Sci. USA 1974, 71, 3879–3882. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mannervik, B. Purification and Characterization of the Flavoenzyme Glutathione Reductase from Rat Liver. J. Biol. Chem. 1975, 260, 5475–5480. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, I.; Couto, A.; Pérez-Jiménez, A.; Oliva-Teles, A.; Enes, P. Gut Morphology and Hepatic Oxidative Status of European Sea Bass (Dicentrarchus labrax) Juveniles Fed Plant Feedstuffs or Fishmeal-Based Diets Supplemented with Short-Chain Fructo-Oligosaccharides and Xylo-Oligosaccharides. Br. J. Nutr. 2015, 114, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Coutinho, F.; Iglesias, P.; Oliva-Teles, A.; Couto, A. Chlorella Sp. and Nannochloropsis Sp. Inclusion in Plant-Based Diets Modulate the Intestine and Liver Antioxidant Mechanisms of European Sea Bass Juveniles. Front. Vet. Sci. 2020, 7, 607575. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, F.; Castro, C.; Rufino-Palomares, E.; Ordóñez-Grande, B.; Gallardo, M.A.; Oliva-Teles, A.; Peres, H. Dietary Glutamine Supplementation Effects on Amino Acid Metabolism, Intestinal Nutrient Absorption Capacity and Antioxidant Response of Gilthead Sea Bream (Sparus aurata) Juveniles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Zhao, K.; Whiteman, M. The Gastrointestinal Tract: A Major Site of Antioxidant Action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Trenzado, C.; Hidalgo, M.C.; García-Gallego, M.; Morales, A.E.; Furné, M.; Domezain, A.; Domezain, J.; Sanz, A. Antioxidant Enzymes and Lipid Peroxidation in Sturgeon Acipenser naccarii and Trout Oncorhynchus mykiss. A Comparative Study. Aquaculture 2006, 254, 758–767. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Sun, X.; Liu, Y. The Mathematical Prediction Model for the Oxidative Stability of Vegetable Oils by the Main Fatty Acids Composition and Thermogravimetric Analysis. LWT 2018, 96, 51–57. [Google Scholar] [CrossRef]
- Tan, P.; Dong, X.; Xu, H.; Mai, K.; Ai, Q. Dietary Vegetable Oil Suppressed Non-Specific Immunity and Liver Antioxidant Capacity but Induced Inflammatory Response in Japanese Sea Bass (Lateolabrax japonicus). Fish Shellfish Immunol. 2017, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Box, A.; Sureda, A.; Galgani, F.; Pons, A.; Deudero, S. Assessment of Environmental Pollution at Balearic Islands Applying Oxidative Stress Biomarkers in the Mussel Mytilus galloprovincialis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Saera-Vila, A.; Benedito-Palos, L.; Sitjà-Bobadilla, A.; Nácher-Mestre, J.; Serrano, R.; Kaushik, S.; Pérez-Sánchez, J. Assessment of the Health and Antioxidant Trade-off in Gilthead Sea Bream (Sparus aurata L.) Fed Alternative Diets with Low Levels of Contaminants. Aquaculture 2009, 296, 87–95. [Google Scholar] [CrossRef]
- Rønnestad, I.; Rojas-Garcia, C.R.; Skadal, J. Retrograde Peristalsis; a Possible Mechanism for Filling the Pyloric Caeca? J. Fish Biol. 2000, 56, 216–218. [Google Scholar] [CrossRef]
- Jones, B.S.; Keightley, L.J.; Harris, J.O.; Wiklendt, L.; Spencer, N.J.; Dinning, P.G. Identification of Neurogenic Intestinal Motility Patterns in Silver Perch (Bidyanus bidyanus) That Persist over Wide Temperature Ranges. Neurogastroenterol. Motil. 2021, 33, e14037. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Noh, H.; Numayama-Tsuruta, K.; Ishikawa, T. Mechanical Roles of Anterograde and Retrograde Intestinal Peristalses after Feeding in a Larval Fish (Danio rerio). Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, 1013–1021. [Google Scholar] [CrossRef]
- Verdile, N.; Pasquariello, R.; Scolari, M.; Scirè, G.; Brevini, T.A.L.; Gandolfi, F. A Detailed Study of Rainbow Trout (Onchorhynchus mykiss) Intestine Revealed That Digestive and Absorptive Functions Are Not Linearly Distributed along Its Length. Animals 2020, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.A.; Lee, R.F. The Role of Wax in Oceanic Food Chains. Sci. Am. 1975, 232, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Grenne, C.W. The Fat Absorving Function of the Alimentary Tract of the King Salmon. Bull. Bur. Fish. 1914, 33, 149–175. [Google Scholar]
- Jansson, B.-O.; Olsson, R. The Cytology of the Caecal Epithelial Cells of Perca. Acta Zool. 1960, 41, 267–276. [Google Scholar] [CrossRef]

| (A) | |||||
| Diets | |||||
| Ingredients (%) | P | R | PL | RL | RPL |
| Palm oil | 13.88 | - | 9.87 | - | 5.03 |
| Linseed oil | - | - | 3.94 | 2.02 | 3.00 |
| Rapeseed oil | - | 13.58 | - | 11.57 | 5.67 |
| Vit/Min premix | 1.53 | 1.83 | 1.60 | 1.82 | 1.71 |
| (B) | |||||
| Diets | |||||
| Fatty acid (%) | P | R | PL | RL | RPL |
| C14:0 | 2.63 | 2.20 | 2.47 | 2.21 | 2.34 |
| C16:0 | 30.81 | 9.32 | 24.83 | 9.36 | 17.41 |
| C16:1n-7 | 2.31 | 2.50 | 2.30 | 2.48 | 2.36 |
| C16:2n-6 | 0.27 | 0.31 | 0.27 | 0.30 | 0.28 |
| C18:0 | 3.73 | 2.13 | 3.82 | 2.47 | 3.19 |
| C18:1n-9 | 30.57 | 38.67 | 26.79 | 36.19 | 31.73 |
| C18:1n-7 | 1.43 | 2.84 | 1.41 | 2.67 | 2.04 |
| C18:2n-6 LA | 10.89 | 17.47 | 12.08 | 16.52 | 14.12 |
| C18:3n-3 ALA | 0.98 | 6.52 | 9.75 | 10.31 | 10.07 |
| C18:4n-3 | 0.70 | 0.75 | 0.72 | 0.74 | 0.73 |
| C20:1 sum. isomers | 1.72 | 2.59 | 1.72 | 2.47 | 2.03 |
| C20:4n-6 ARA | 0.24 | 0.25 | 0.24 | 0.23 | 0.21 |
| C20:4n-3 | 0.23 | 0.24 | 0.24 | 0.24 | 0.24 |
| C20:5n-3 EPA | 3.04 | 3.27 | 3.07 | 3.24 | 3.12 |
| C22:1 sum. isomers | 2.10 | 2.40 | 2.07 | 2.32 | 2.12 |
| C22:5n-3 | 0.45 | 0.48 | 0.46 | 0.52 | 0.43 |
| C22:6n-3 DHA | 2.89 | 3.07 | 2.95 | 2.98 | 2.90 |
| C24:1n-9 | 0.24 | 0.34 | 0.24 | 0.33 | 0.29 |
| SFA not listed | 0.81 | 1.08 | 0.76 | 1.01 | 0.90 |
| Monoenes not listed | 0.11 | 0.13 | 0.11 | 0.11 | 0.11 |
| n-6 FA not listed | 0.22 | 0.29 | 0.27 | 0.29 | 0.24 |
| n-3 FA not listed | 0.16 | 0.20 | 0.16 | 0.20 | 0.18 |
| Others | 0.36 | 0.37 | 0.33 | 0.38 | 0.37 |
| Sum. SFA | 37.98 | 14.73 | 31.88 | 15.05 | 23.84 |
| Sum. MUFA | 38.48 | 49.47 | 34.64 | 46.57 | 40.68 |
| Sum. n-6 FA | 11.62 | 18.32 | 12.86 | 17.34 | 14.85 |
| Sum. n-3 FA | 8.45 | 14.53 | 17.35 | 18.23 | 17.67 |
| UFA/SFA | 1.54 | 5.59 | 2.03 | 5.46 | 3.07 |
| MUFA/PUFA | 1.92 | 1.51 | 1.15 | 1.31 | 1.25 |
| n-6/n-3 | 1.38 | 1.26 | 0.74 | 0.95 | 0.84 |
| Unknown | 3.10 | 2.60 | 3.0 | 2.40 | 2.60 |
| Gene | Sequence (5′-3′) | Ta (°C) | Accession Number | |
|---|---|---|---|---|
| β-actin | F | TCCTGCGGAATCCATGAGA | 60 | X89920 |
| R | GACGTCGCACTTCATGATGCT | |||
| ef1α | F | CTTCAACGCTCAGGTCATCAT | 60 | AF184170 |
| R | GCACAGCGAAACGACCAAGGGGA | |||
| rps18 | F | GGGTGTTGGCAGACGTTAC | 60 | AM490061.1 |
| R | CTTCTGCCTGTTGAGGAACCA | |||
| cat | F | TTCCCGTCCTTCCATTCACTC | 60 | FG264808 |
| R | CTCCAGAAGTCCCACACCAT | |||
| gpx1 | F | GAAGGTGGATGTGAATGGAAAAGATG | 60 | DQ524992 |
| R | CTGACGGGACTCCAAATGATGG | |||
| gpx4 | F | TGCGTCTGATAGGGTCCACTGTC | 60 | AM977818 |
| R | GTCTGCCAGTCCTCTGTCGG | |||
| gr | F | CAAAGCGCAGTGTGATTGTGG | 60 | AJ937873 |
| R | CCACTCCGGAGTTTTGCATTTC | |||
| gst3 | F | CCAGATGATCAGTACGTGAAGACCGTC | 60 | JQ308828 |
| R | TGCTGATGTGAGGAATGTACCGTAAC | |||
| sod1 | F | CCATGGTAAGAATCATGGCGG | 60 | AJ937872 |
| R | CGTGGATCACCATGGTTCTG | |||
| sod2 | F | CCTGACCTGACCTACGACTATGG | 60 | JQ308832 |
| R | AGTGCCTCCTGATATTTCTCCTCTG |
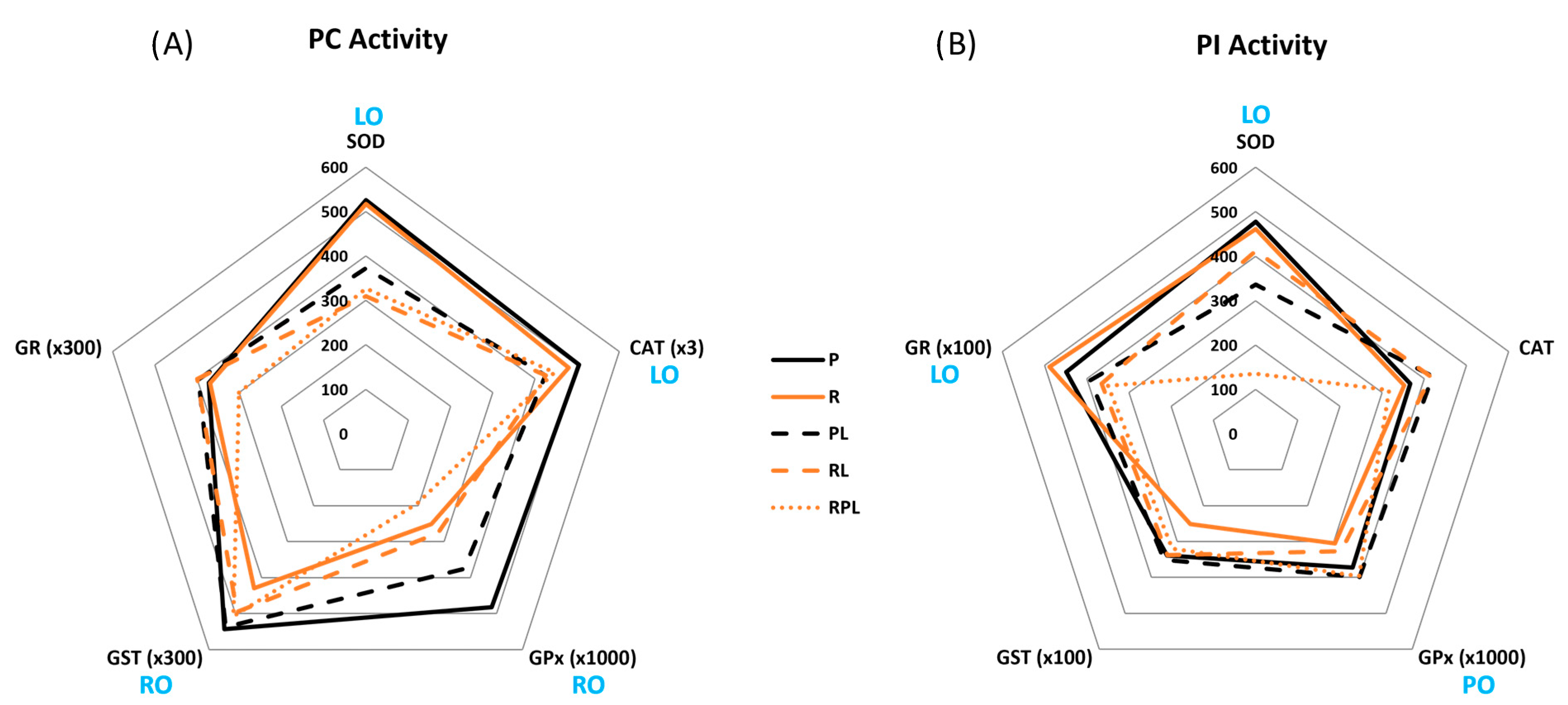
| Pyloric Caeca | |||||
| Dietary Treatment | SOD | CAT | GPx | GST | GR |
| P | 525.2 ± 67.3 a | 168.0 ± 9.7 a | 0.482 ± 0.078 a | 1.81 ± 0.10 a | 1.24 ± 0.07 |
| R | 516.4 ± 54.8 a | 159.8 ± 7.3 ab | 0.251 ± 0.037 b | 1.43 ± 0.10 b | 1.23 ± 0.09 |
| PL | 372.5 ± 26.6 ab | 141.6 ± 6.7 b | 0.376 ± 0.048 a | 1.79 ± 0.16 ab | 1.32 ± 0.12 |
| RL | 309.9 ± 36.0 b | 142.0 ± 7.8 ab | 0.276 ± 0.037 b | 1.65 ± 0.15 ab | 1.33 ± 0.13 |
| RPL | 327.3 ± 50.0 b * | 148.9 ± 11.3 ab | 0.195 ± 0.010 c | 1.69 ± 0.06 ab | 1.00 ± 0.10 |
| Proximal Intestine | |||||
| Dietary Treatment | SOD | CAT | GPx | GST | GR |
| P | 477.9 ± 42.8 m | 366.6 ± 17.7 n * | 0.372 ± 0.035 mn | 3.39 ± 0.24 m * | 4.49 ± 0.44 mn * |
| R | 461.3 ± 50.2 m | 352.3 ± 21.0 no * | 0.305 ± 0.032 n | 2.51 ± 0.14 n * | 4.88 ± 0.49 m * |
| PL | 335.9 ± 26.7 n | 420.0 ± 25.5 m * | 0.399 ± 0.034 m | 3.51 ± 0.20 m * | 3.91 ± 0.37 mn * |
| RL | 411.5 ± 43.7 mn | 416.0 ± 14.1 m * | 0.327 ± 0.040 mn | 3.38 ± 0.15 m * | 3.65 ± 0.31 n * |
| RPL | 135.0 ± 16.9 o | 317.0 ± 18.1 o * | 0.397 ± 0.039 m * | 3.20 ± 0.13 m * | 3.50 ± 0.48 n * |
| Pyloric Caeca | |||||||
| Dietary Treatment | sod1 | sod2 | cat | gpx1 | gpx4 | gr | gst |
| P | 1.32 ± 0.28 | 2.05 ± 0.53 | 2.19 ± 0.47 | 3.16 ± 0.84 | 1.10 ± 0.14 | 1.46 ± 0.44 | 1.36 ± 0.37 |
| R | 1.35 ± 0.29 | 1.49 ± 0.26 | 0.92 ± 0.10 | 1.28 ± 0.33 | 0.98 ± 0.26 | 0.60 ± 0.07 | 1.12 ± 0.19 |
| PL | 1.42 ± 0.23 | 1.73 ± 0.25 | 1.27 ± 0.28 | 1.01 ± 0.29 | 1.06 ± 0.18 | 1.06 ± 0.31 | 1.43 ± 0.35 |
| RL | 2.05 ± 0.49 | 1.89 ± 0.31 | 1.22 ± 0.17 | 1.00 ± 0.23 | 1.15 ± 0.17 | 1.56 ± 0.29 | 1.29 ± 0.20 |
| RPL | 1.39 ± 0.31 | 1.97 ± 0.39 | 1.20 ± 0.27 | 0.89 ± 0.21 | 0.98 ± 0.18 | 1.05 ± 0.32 | 1.31 ± 0.27 |
| Proximal Intestine | |||||||
| Dietary Treatment | sod1 | sod2 | cat | gpx1 | gpx4 | gr | gst |
| P | 0.76 ± 0.13 | 1.35 ± 0.24 | 0.95 ± 0.12 | 1.75 ± 0.23 | 0.55 ± 0.07 n | 1.92 ± 0.34 | 1.13 ± 0.32 |
| R | 0.83 ± 0.06 | 1.84 ± 0.23 | 0.99 ± 0.08 | 2.15 ± 0.23 | 1.07 ± 0.08 m | 1.89 ± 0.23 | 1.32 ± 0.17 |
| PL | 0.83 ± 0.03 | 2.03 ± 0.26 | 1.27 ± 0.28 | 2.34 ± 0.49 | 1.35 ± 0.51 mn | 2.00 ± 0.21 | 1.39 ± 0.24 |
| RL | 0.80 ± 0.13 | 2.02 ± 0.36 | 0.82 ± 0.14 | 1.85 ± 0.19 | 0.66 ± 0.09 n | 2.11 ± 0.26 | 0.81 ± 0.11 |
| RPL | 0.73 ± 0.07 | 1.71 ± 0.17 | 0.81 ± 0.05 | 1.73 ± 0.14 | 0.67 ± 0.08 n | 2.21 ± 0.26 | 1.02 ± 0.15 |
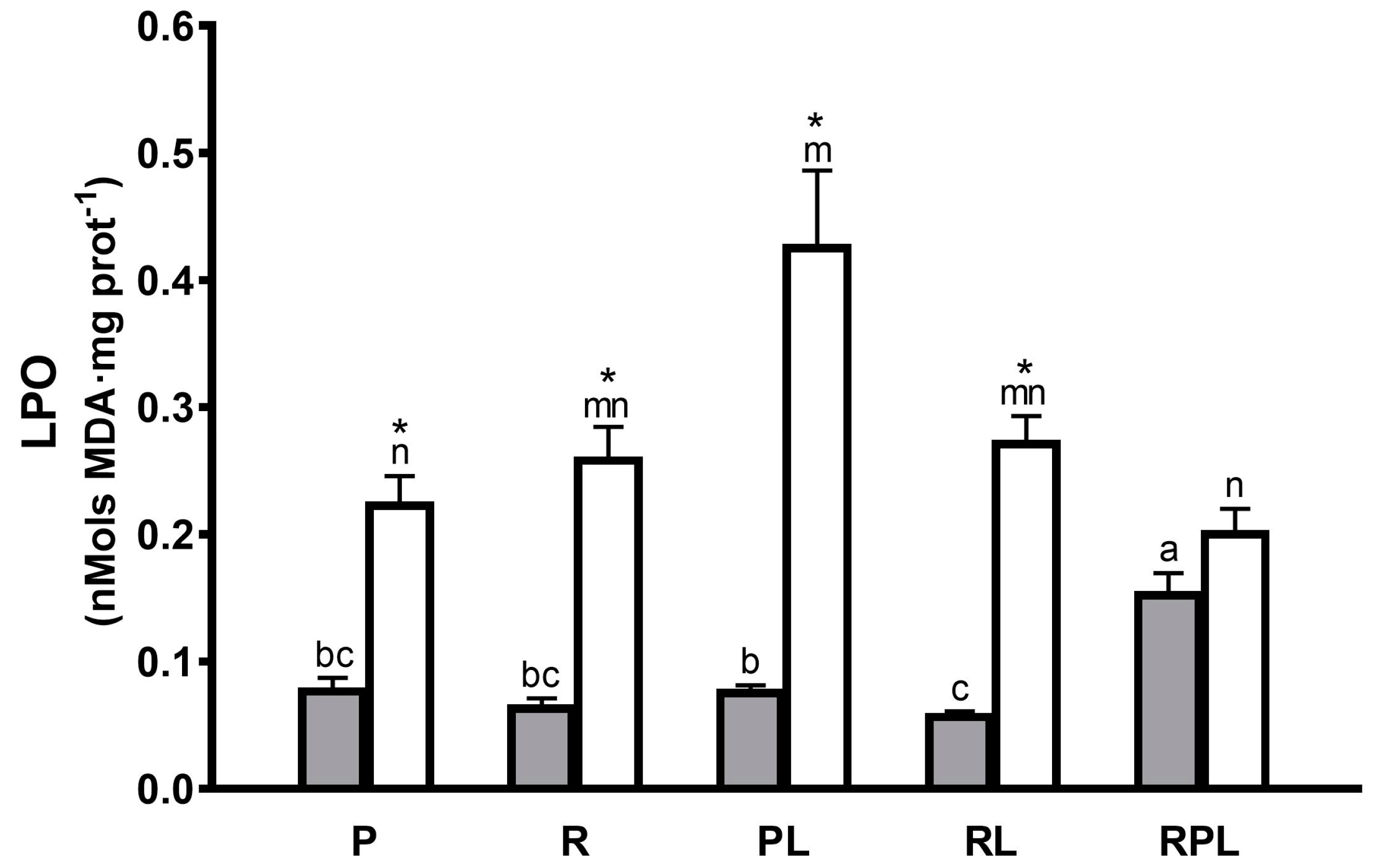
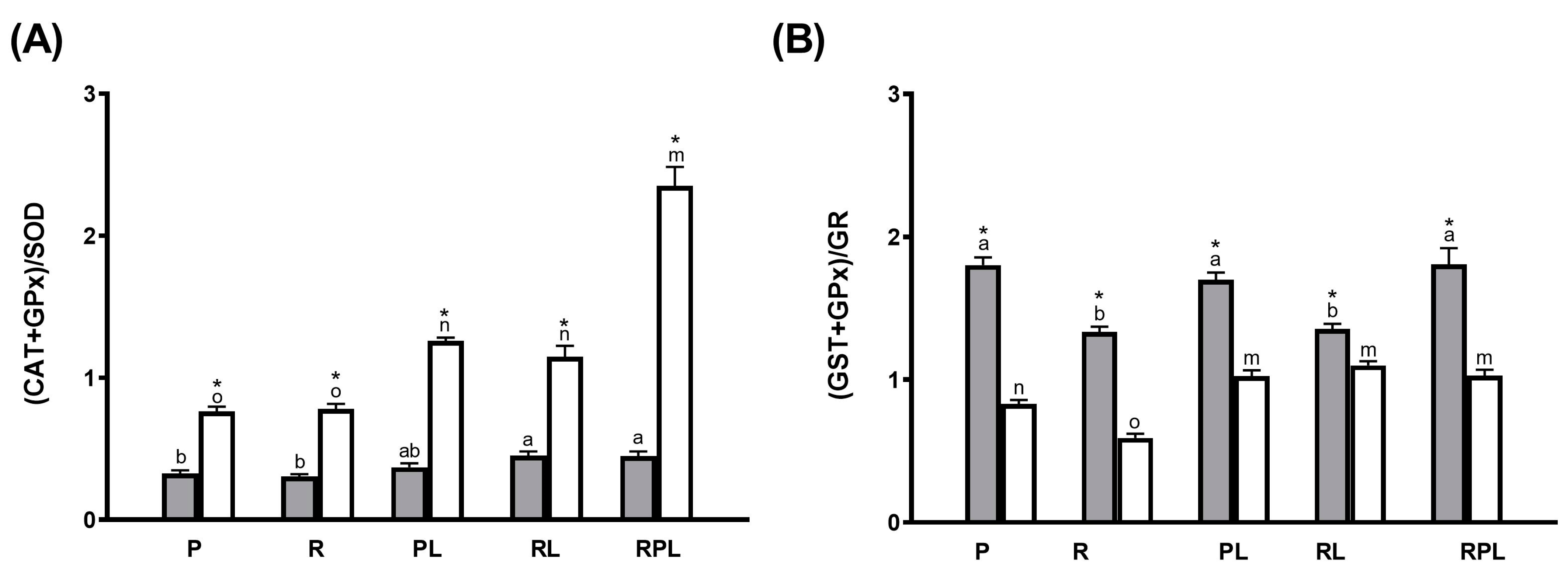
| Pyloric Caeca | ||||
|---|---|---|---|---|
| Dietary Treatment | SOD/LPO | CAT/LPO | GPx × 1000/LPO | GST × 100/LPO |
| P | 6.48 ± 0.95 a * | 2.49 ± 0.25 ab * | 4.60 ± 0.49 a * | 2.38 ± 0.28 a * |
| R | 7.89 ± 1.26 a * | 2.61 ± 0.41 ab * | 3.84 ± 1.10 a * | 2.58 ± 0.43 a * |
| PL | 5.00 ± 0.38 a * | 2.18 ± 0.15 b * | 5.78 ± 0.87 a * | 2.60 ± 0.25 a * |
| RL | 5.70 ± 0.58 a * | 2.86 ± 0.17 a * | 5.13 ± 0.66 a * | 2.84 ± 0.21 a * |
| RPL | 2.70 ± 0.25 b * | 1.11 ± 0.09 c | 1.41 ± 0.14 b | 1.23 ± 0.12 b |
| Proximal Intestine | ||||
| Dietary Treatment | SOD/LPO | CAT/LPO | GPx × 1000/LPO | GST × 100/LPO |
| P | 2.12 ± 0.36 m | 1.76 ± 0.21 m | 1.66 ± 0.20 mn | 1.19 ± 0.07 n |
| R | 1.99 ± 0.29 m | 1.60 ± 0.16 mn | 1.39 ± 0.24 no | 1.07 ± 0.07 n |
| PL | 1.32 ± 0.21 m | 1.26 ± 0.13 n | 1.30 ± 0.23 no | 1.14 ± 0.14 n |
| RL | 1.68 ± 0.19 m | 1.54 ± 0.11 mn | 1.17 ± 0.18 o | 1.31 ± 0.12 n |
| RPL | 0.552 ± 0.05 n | 1.60 ± 0.12 mn * | 1.95 ± 0.14 m * | 1.70 ± 0.14 m * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Meilán, I.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I.; Gallardo, Á. Oxidative Status of the Pyloric Caeca and Proximal Intestine in Gilthead Sea Bream Fed Diets Including Different Vegetable Oil Blends from Palm, Rapeseed and Linseed. Fishes 2024, 9, 228. https://doi.org/10.3390/fishes9060228
García-Meilán I, Fontanillas R, Gutiérrez J, Capilla E, Navarro I, Gallardo Á. Oxidative Status of the Pyloric Caeca and Proximal Intestine in Gilthead Sea Bream Fed Diets Including Different Vegetable Oil Blends from Palm, Rapeseed and Linseed. Fishes. 2024; 9(6):228. https://doi.org/10.3390/fishes9060228
Chicago/Turabian StyleGarcía-Meilán, Irene, Ramon Fontanillas, Joaquim Gutiérrez, Encarnación Capilla, Isabel Navarro, and Ángeles Gallardo. 2024. "Oxidative Status of the Pyloric Caeca and Proximal Intestine in Gilthead Sea Bream Fed Diets Including Different Vegetable Oil Blends from Palm, Rapeseed and Linseed" Fishes 9, no. 6: 228. https://doi.org/10.3390/fishes9060228
APA StyleGarcía-Meilán, I., Fontanillas, R., Gutiérrez, J., Capilla, E., Navarro, I., & Gallardo, Á. (2024). Oxidative Status of the Pyloric Caeca and Proximal Intestine in Gilthead Sea Bream Fed Diets Including Different Vegetable Oil Blends from Palm, Rapeseed and Linseed. Fishes, 9(6), 228. https://doi.org/10.3390/fishes9060228








