Effects of Various Photoperiods and Specific Wavelengths on Retinal Changes and Oxidative Stress in the Conch Tegula rustica
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Retinal Histology
2.3. Measurement of Oxidative-Stress-Related Substances
2.4. Rhodopsin Detected by Immunohistochemistry Staining
2.5. Statistical Analysis
3. Results
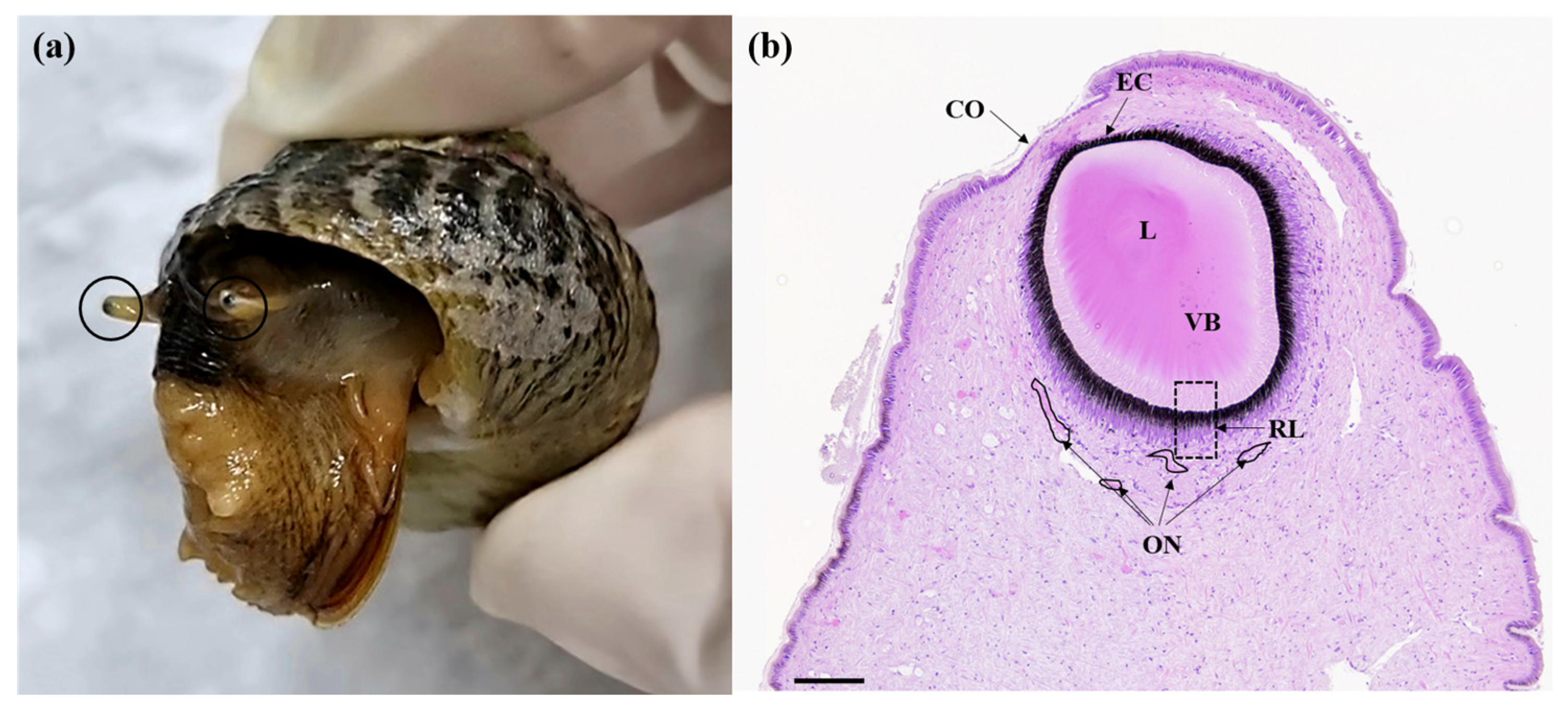
3.1. Eye Histology
3.2. Retinal Histology
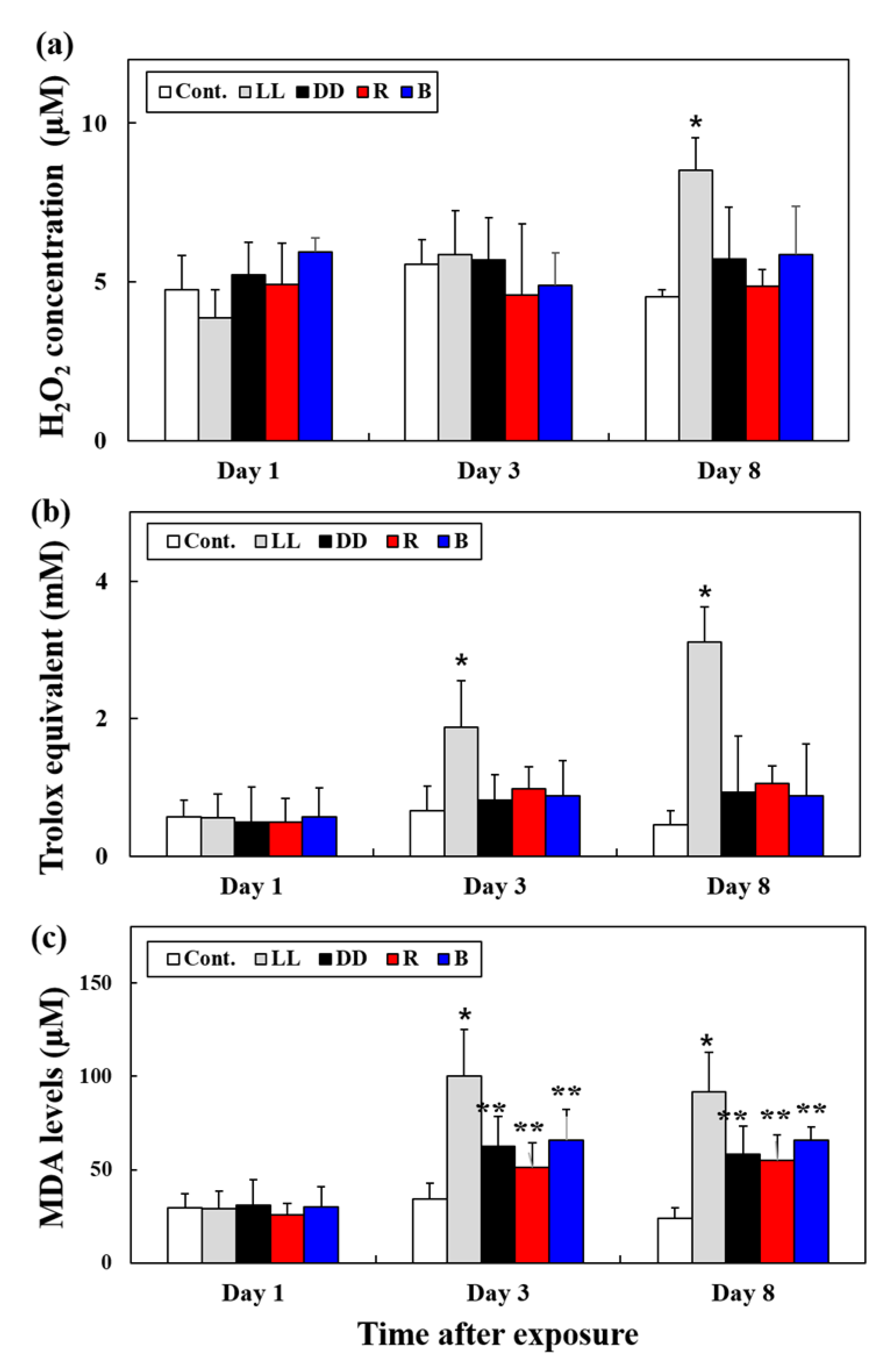
3.3. H2O2 Levels and TAC and MDA Contents
3.4. Immunohistochemical Staining of Rhodopsin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, L.P.; Denny, M.W. Importance of behavior and morphological traits for controlling body temperature in littorinid snails. Biol. Bull. 2011, 220, 209–223. [Google Scholar] [CrossRef]
- Beuchel, F.; Gulliksen, B.; Carroll, M.L. Long-term patterns of rocky bottom macrobenthic community structure in an Arctic fjord (Kongsfjorden, Svalbard) in relation to climate variability (1980–2003). J. Mar. Syst. 2006, 63, 35–48. [Google Scholar] [CrossRef]
- Kulikova, V.A.; Omel’yanenko, V.A. Reproduction and larval development of the gastropod mollusk Tegula rustica in Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 2000, 26, 128–130. [Google Scholar] [CrossRef]
- Serb, J.M. Toward developing models to study the disease, ecology, and evolution of the eye in Mollusca. Am. Malacol. Bull. 2008, 26, 3–18. [Google Scholar] [CrossRef]
- Young, J.Z. The Anatomy of the Nervous System of Octopus Vulgaris; Clarendon Press: Oxford, UK, 1971. [Google Scholar]
- Muntz, W.R.A.; Raj, U. On the visual system of Nautilus pompilius. J. Exp. Biol. 1984, 109, 253–263. [Google Scholar] [CrossRef]
- Hanlon, R.T.; Shashar, N. Aspects of the sensory ecology of cephalopods. In Sensory Processing in Aquatic Environments; Springer: New York, NY, USA, 2003; pp. 266–282. [Google Scholar]
- Zieger, M.V.; Meyer-Rochow, V.B. Understanding the cephalic eyes of pulmonate gastropods: A review. Am. Malacol. Bull. 2008, 26, 47–66. [Google Scholar] [CrossRef]
- Salvini-Plawen, L.V.; Mayr, E. On the evolution of photoreceptors and eyes. Evol. Biol. 1977, 10, 207–263. [Google Scholar]
- Serb, J.M.; Eernisse, D.J. Charting evolution’s trajectory: Using molluscan eye diversity to understand parallel and convergent evolution. Evol. Educ. Outreach. 2008, 1, 439–447. [Google Scholar] [CrossRef]
- Katagiri, N.; Terakita, A.; Shichida, Y.; Katagiri, Y. Demonstration of a rhodopsin-retinochrome system in the stalk eye of a marine gastropod, Onchidium, by immunohistochemistry. J. Comp. Neurol. 2001, 433, 380–389. [Google Scholar] [CrossRef]
- Ambekar, A.A.; Sivaperumal, P.; Kamala, K.; Kubal, P.; Prakash, C. Effect of temperature changes on antioxidant enzymes and oxidative stress in gastropod Nerita oryzarum collected along India’s first Tarapur Atomic Power Plant site. Environ. Res. 2023, 216, 114334. [Google Scholar] [CrossRef]
- Kingston, A.C.N.; Kuzirian, A.M.; Hanlon, R.T.; Cronin, T.W. Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. J. Exp. Biol. 2015, 218, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Kingston, A.C.N.; Wardill, T.J.; Hanlon, R.T.; Cronin, T.W. An Unexpected Diversity of Photoreceptor Classes in the Longfin Squid, Doryteuthis pealeii. PLoS ONE 2015, 10, e0135381. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M. Restoration of Visual Performance and Opsin Expression within the Retina during Eye Regeneration in the Florida Fighting Conch (Strombus alatus). Master’s Thesis, University of South Carolina, Columbia, SC, USA, 2018. [Google Scholar]
- Fridovich, I. Superoxide dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, Z.; Wang, L.L.; Shi, X.W.; Wang, J.J.; Yue, F.; Yi, Q.L.; Yang, C.Y.; Song, L.S. The immunomodulation of inducible nitric oxide in scallop Chlamys farreri. Fish. Shellfish Immunol. 2013, 34, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.Q.; Ren, J.; Liu, J. Responses of antioxidant systems and LPO level to benzo[α]pyrene and benzo(k)uoranthene in the haemolymph of the scallop Chlamys ferrari. Environ. Pollut. 2006, 141, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, M.; Duan, L.; Qiu, Y.; Ma, T.; Chen, L.; Breitholtz, M.; Bergman, Å.; Zhao, J.; Hecker, M.; et al. Multiple biomarker responses in caged benthic gastropods Bellamya aeruginosa after in situ exposure to Taihu Lake in China. Environ. Sci. Eur. 2018, 30, 34. [Google Scholar] [CrossRef] [PubMed]
- Campoy-Diaz, A.D.; Malanga, G.; Giraud-Billoud, M.; Vega, I.A. Changes in the oxidative status and damage by non-essential elements in the digestive gland of the gastropod Pomacea canaliculata. Front. Physiol. 2023, 14, 1123977. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, J.; Choi, C.Y. Effects of LED light spectra on the growth of the yellowtail clownfish Amphiprion clarkii. Fish. Sci. 2012, 78, 549–556. [Google Scholar] [CrossRef]
- Song, J.A.; Lee, Y.S.; Choi, Y.U.; Choi, C.Y. Effects of light-emitting diodes on thermally-induced oxidative stress in the bay scallop Argopecten irradians. Molluscan Res. 2020, 40, 130–141. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.; Sun, Y.; Yang, Y.; Wang, A.; He, L.; Gu, Z. Effects of the daily light/dark cycle on photosynthetic performance, oxidative stress and illumination-related genes in boring giant clam Tridacna crocea. Mar. Biol. 2021, 168, 71. [Google Scholar] [CrossRef]
- Ma, S.; Li, L.; Chen, X.; Chen, S.; Dong, Y.; Gao, Q.; Zhou, Y.; Dong, S. Influence of daily rhythmic light spectra and intensity changes on the growth and physiological status of juvenile steelhead trout (Oncorhynchus mykiss). Front. Mar. Sci. 2023, 10, 36. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, M.J.; Kim, J.H.; Choi, C.Y. Effects of Red LED Spectra and Different Photoperiods on the Circadian Rhythm of Abalones (Haliotis discus hannai). Ocean Polar Res. 2024, 46, 55–64. [Google Scholar]
- Gao, X.; Li, X.; Zhang, M.; Chi, L.; Song, C.; Liu, Y. Effects of LED light quality on the growth, survival and metamorphosis of Haliotis discus hannai Ino larvae. Aquac. Res. 2016, 47, 3705–3717. [Google Scholar] [CrossRef]
- Takata, T.; Zhao, M.; Uchida, T.; Kudo, Y.; Sato, S.; Nikai, H. Immunohistochemical demonstration of an enamel sheath protein, sheathlin, in odontogenic tumors. Virchows Arch. 2000, 436, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Seyer, J.O. Structure and optics of the eye of the hawk-wing conch, Strombus raninus (L.). J. Exp. Zool. 1994, 268, 200–207. [Google Scholar] [CrossRef]
- Irwin, A.R.; Williams, S.T.; Speiser, D.I.; Roberts, N.W. The marine gastropod Conomurex luhuanus (Strombidae) has high-resolution spatial vision and eyes with complex retinas. J. Exp. Biol. 2022, 225, jeb243927. [Google Scholar] [CrossRef] [PubMed]
- Migaud, H.; Cowan, M.; Taylor, J.; Ferguson, H.W. The effect of spectral composition and light intensity on melatonin, stress and retinal damage in post-smolt Atlantic salmon, Salmo salar. Aquaculture 2007, 270, 390–404. [Google Scholar] [CrossRef]
- Song, J.A.; Choi, C.Y. Effects of blue light spectra on retinal stress and damage in goldfish (Carassius auratus). Fish Physiol. Biochem. 2019, 45, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Bogerts, B. A brainstem atlas of catecholaminergic neurons in man, using melanin as a natural marker. J. Comp. Neurol. 1981, 197, 63–80. [Google Scholar] [CrossRef]
- Dieterich, A.; Troschinski, S.; Schwarz, S.; Di Lellis, M.A.; Henneberg, A.; Fischbach, U.; Ludwig, M.; Gärtner, U.; Triebskorn, R.; Köhler, H.R. Hsp70 and lipid peroxide levels following heat stress in Xeropicta derbentina (Krynicki 1836) (Gastropoda, Pulmonata) with regard to different colour morphs. Cell Stress Chaperones 2015, 20, 159–168. [Google Scholar] [CrossRef]
- Sandt, V.J.; Stoner, A.W. Ontogenic shift in habitat by early juvenile queen conch, strombus-gigas-patterns and potential mechanisms. Fish. Bull. 1993, 91, 516–525. [Google Scholar]
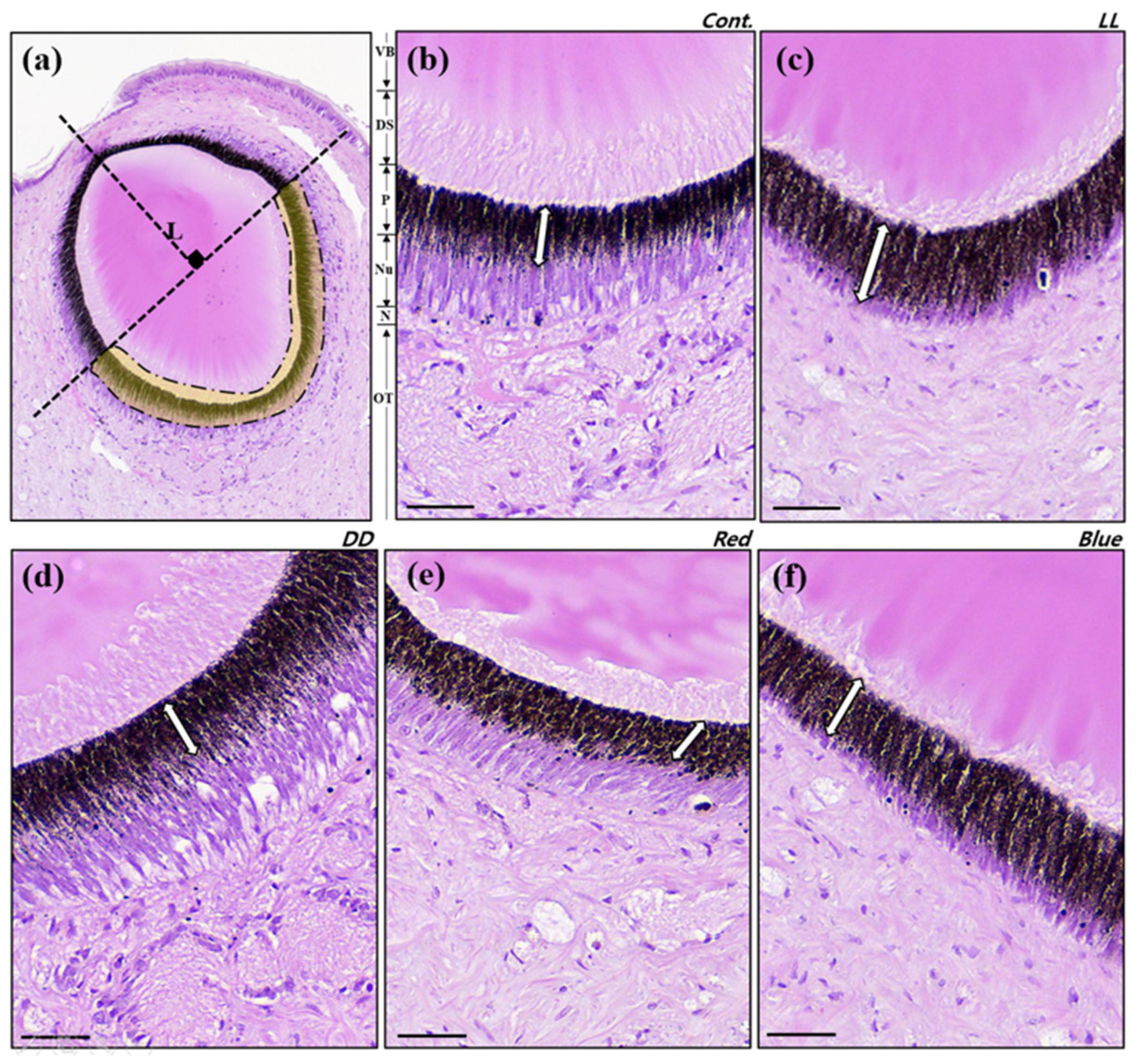

| Cont. | LL | DD | Red | Blue | |
|---|---|---|---|---|---|
| Thickness of pigment layer (μm, TPL) | 21.65 ± 4.66 | 29.38 ± 7.03 * | 22.02 ± 7.40 | 22.01 ± 6.85 | 25.10 ± 4.85 |
| TPL⁄(lens length) | 0.18 ± 0.04 | 0.24 ± 0.06 * | 0.18 ± 0.06 | 0.18 ± 0.05 | 0.20 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.A.; Park, H.-S.; Jung, Y.-H.; Choi, D.M.; Choi, C.Y.; Lee, D.-W. Effects of Various Photoperiods and Specific Wavelengths on Retinal Changes and Oxidative Stress in the Conch Tegula rustica. Fishes 2024, 9, 226. https://doi.org/10.3390/fishes9060226
Song JA, Park H-S, Jung Y-H, Choi DM, Choi CY, Lee D-W. Effects of Various Photoperiods and Specific Wavelengths on Retinal Changes and Oxidative Stress in the Conch Tegula rustica. Fishes. 2024; 9(6):226. https://doi.org/10.3390/fishes9060226
Chicago/Turabian StyleSong, Jin Ah, Heung-Sik Park, Yun-Hwan Jung, Dong Mun Choi, Cheol Young Choi, and Dae-Won Lee. 2024. "Effects of Various Photoperiods and Specific Wavelengths on Retinal Changes and Oxidative Stress in the Conch Tegula rustica" Fishes 9, no. 6: 226. https://doi.org/10.3390/fishes9060226
APA StyleSong, J. A., Park, H.-S., Jung, Y.-H., Choi, D. M., Choi, C. Y., & Lee, D.-W. (2024). Effects of Various Photoperiods and Specific Wavelengths on Retinal Changes and Oxidative Stress in the Conch Tegula rustica. Fishes, 9(6), 226. https://doi.org/10.3390/fishes9060226






