Frequency-Specific Responses: The Impact of an Acoustic Stimulus on Behavioral and Physiological Indices in Large Yellow Croaker
Abstract
1. Introduction
2. Materials and Methods
2.1. The Selection of Experimental Site and Subjects
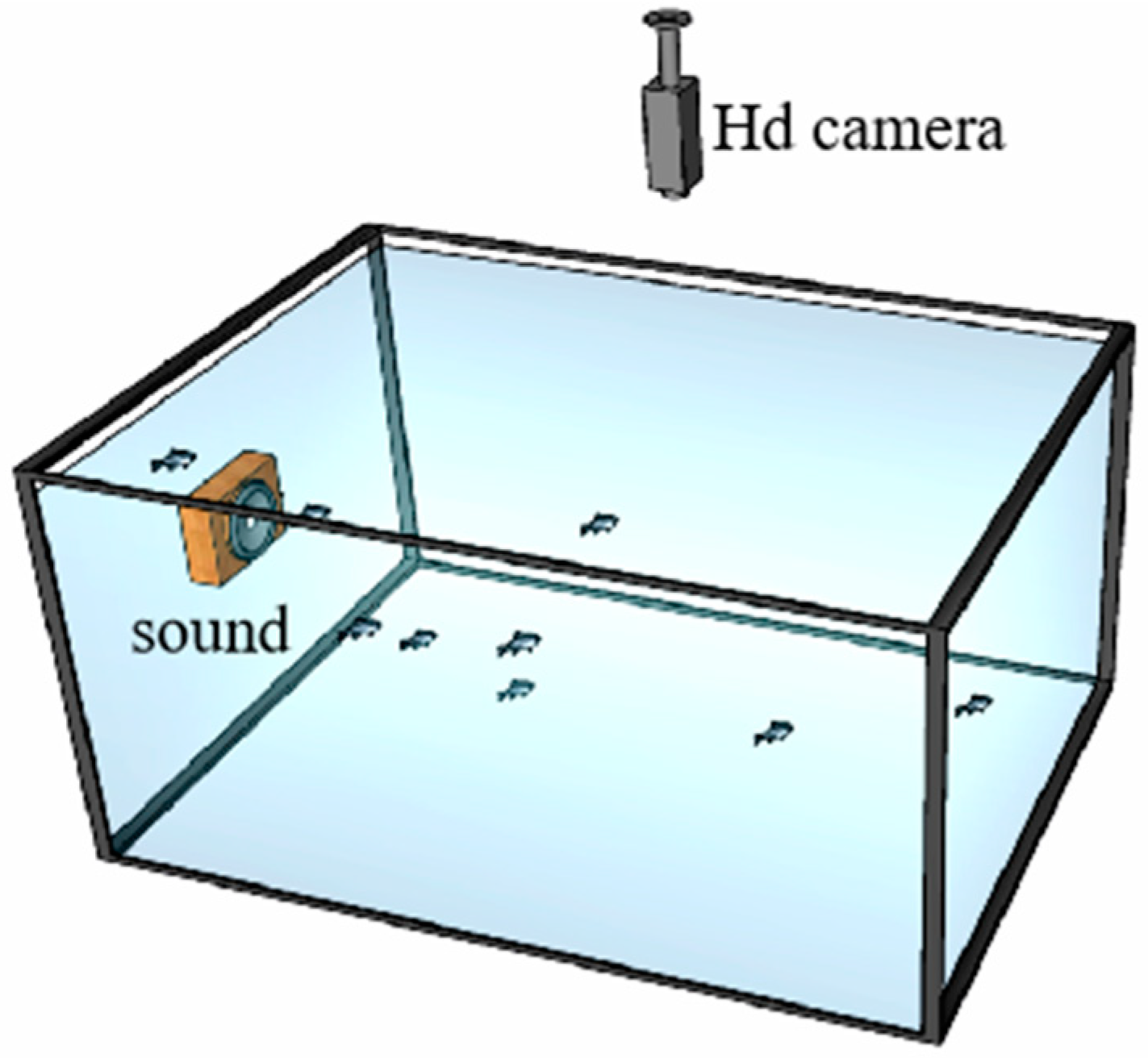
2.2. Behavioral Response to Sound Stimulation
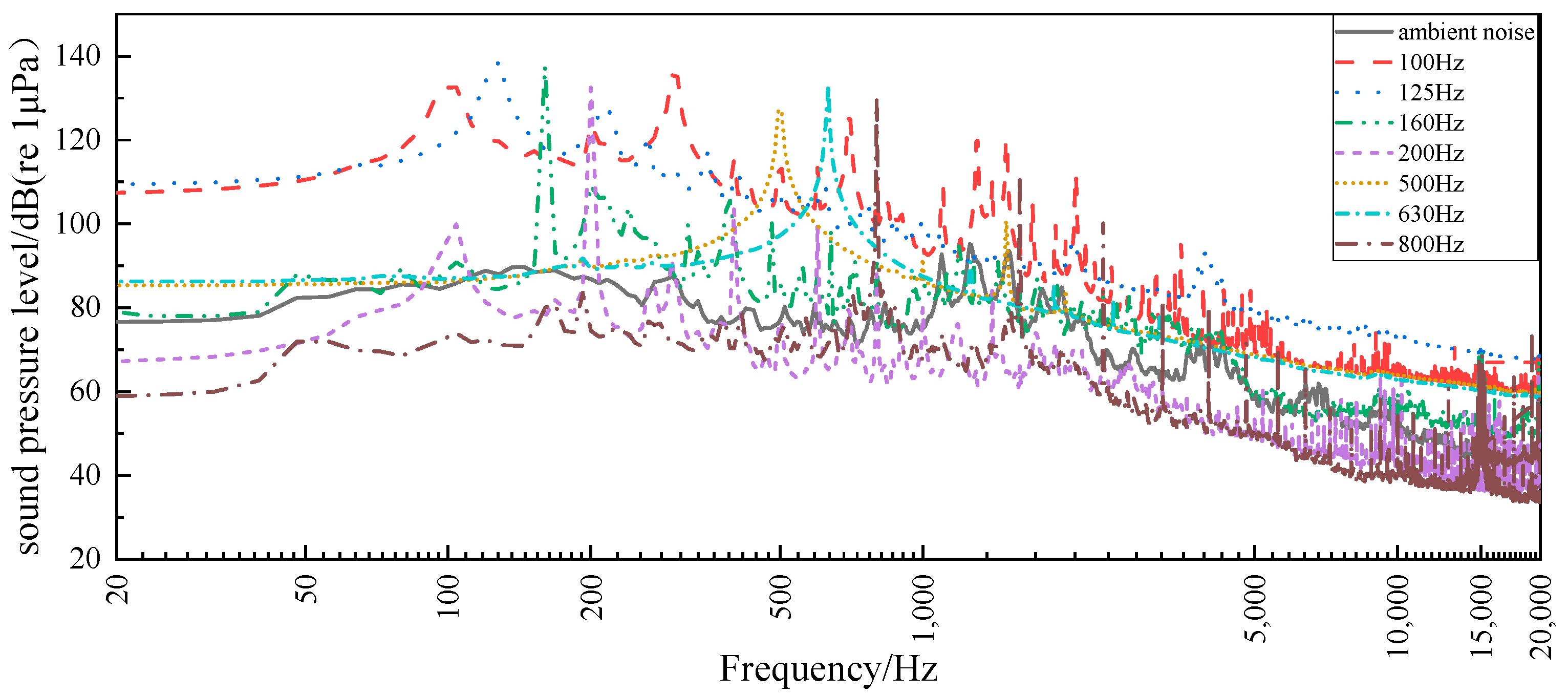
2.3. Physiological Response Stimulated by Sound
2.4. Data Analysis
3. Results
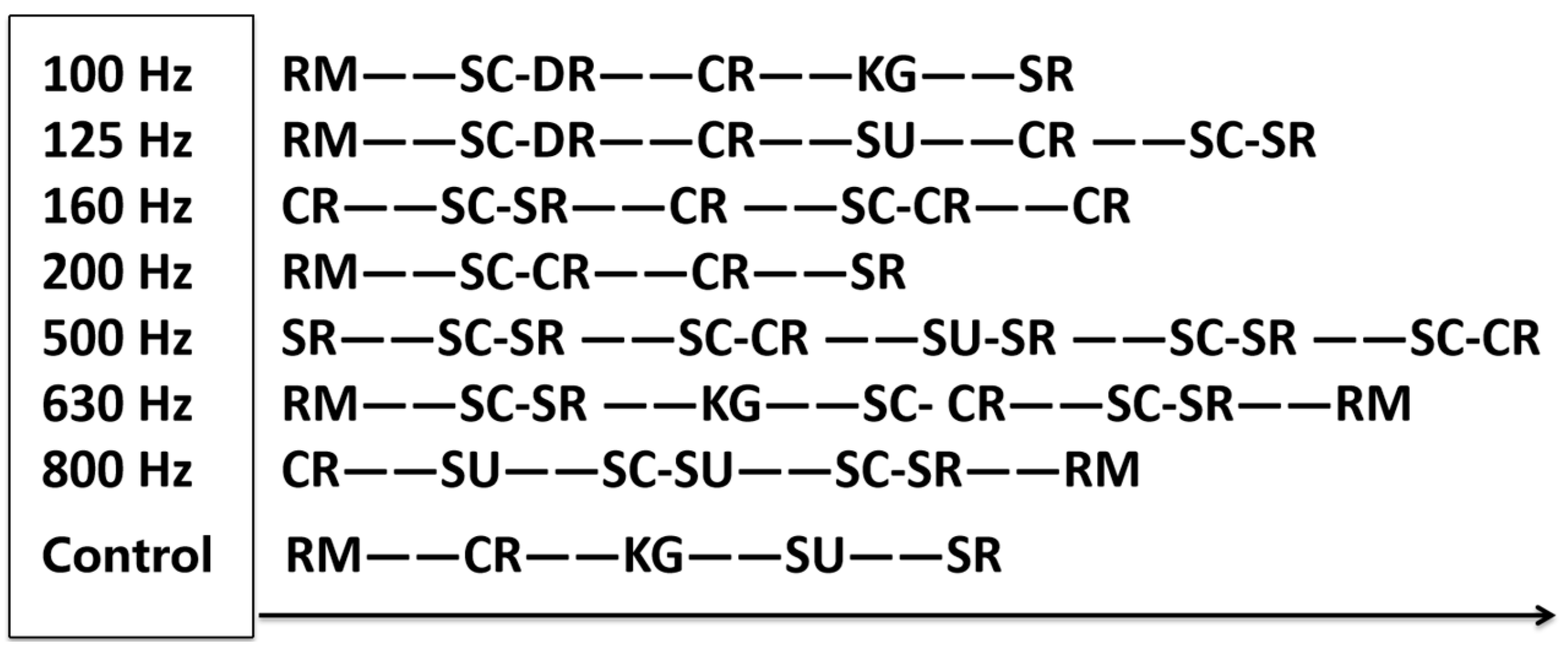
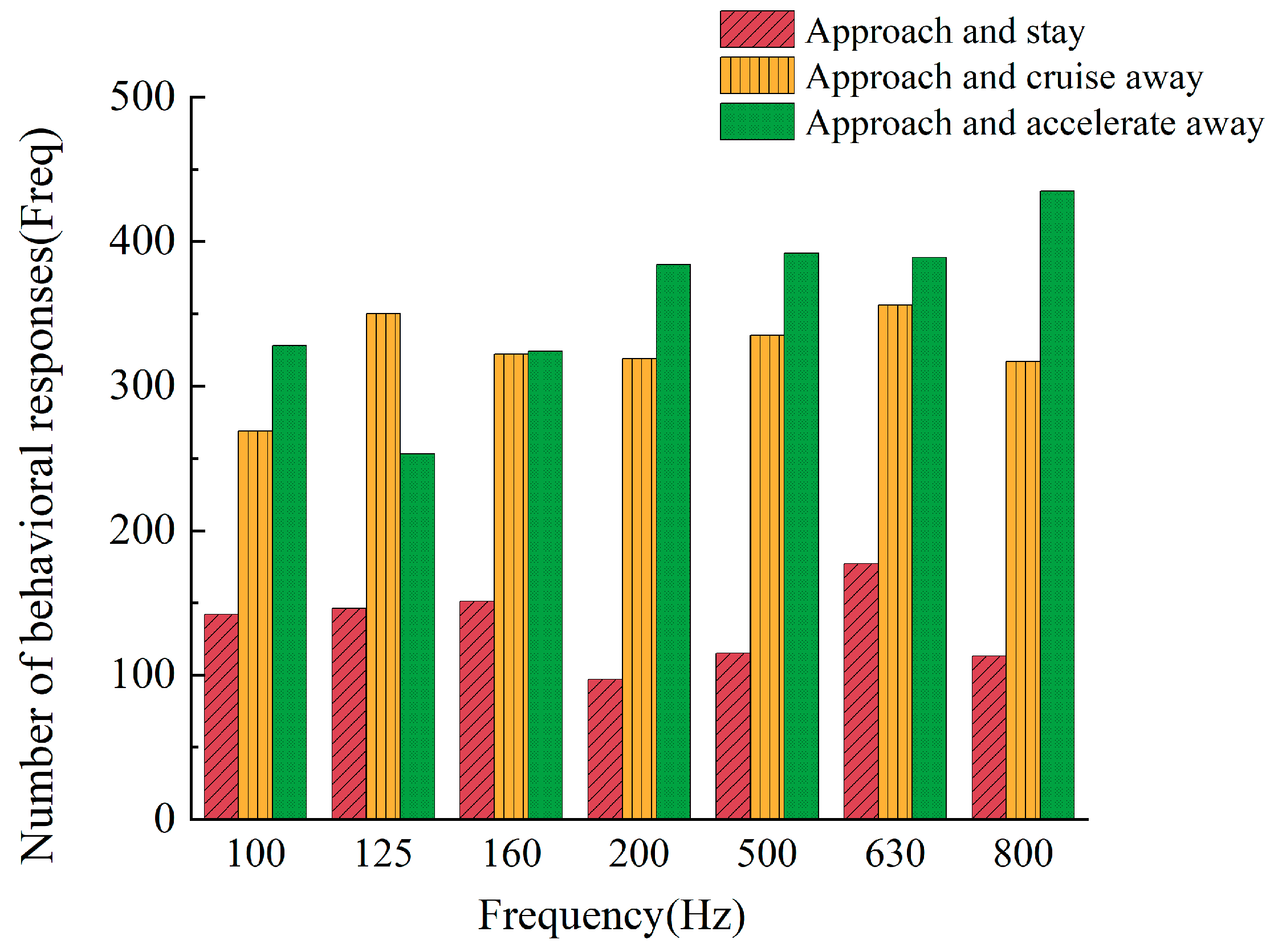
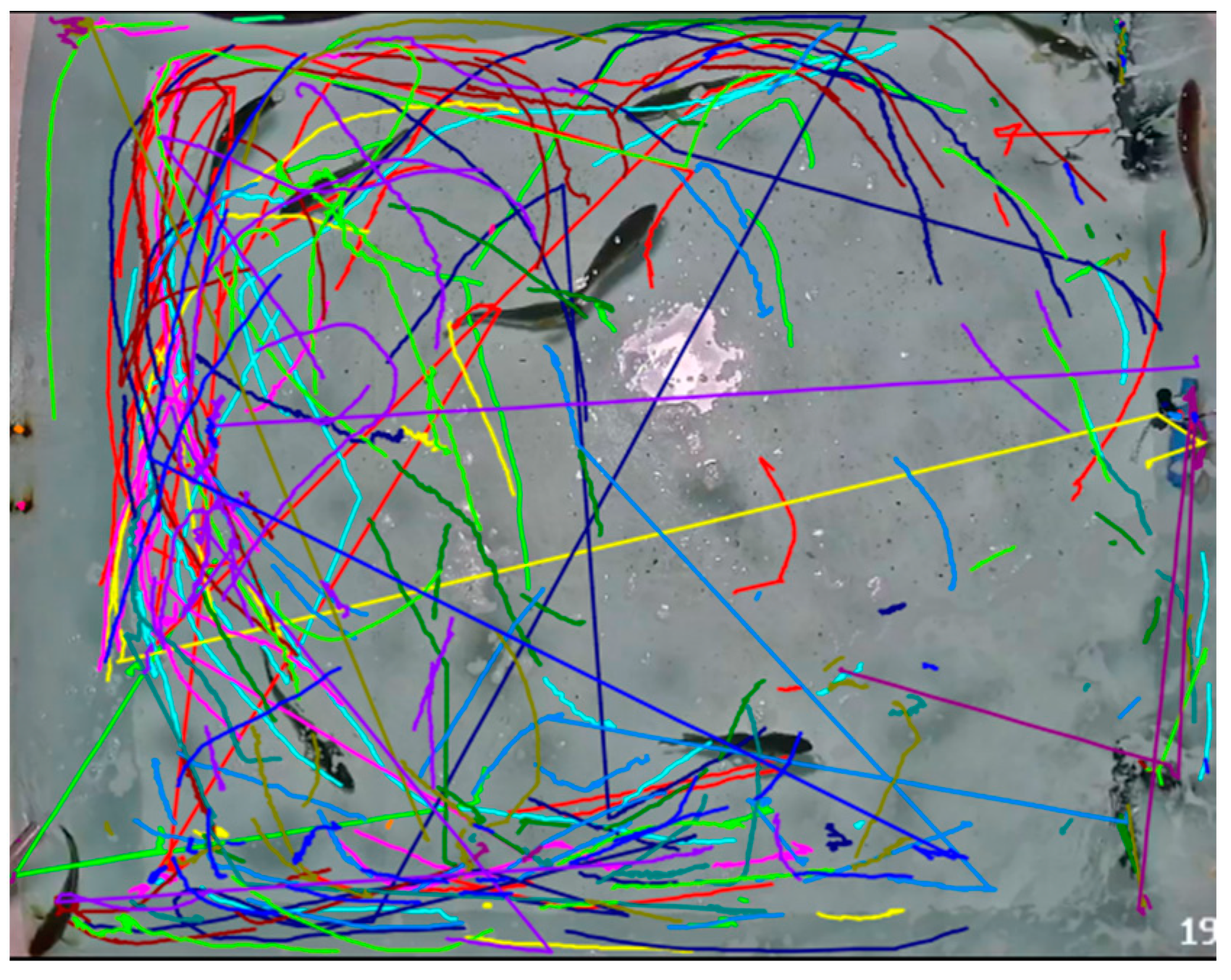
3.1. The Impact of Noise on the Behavior of the Large Yellow Croaker
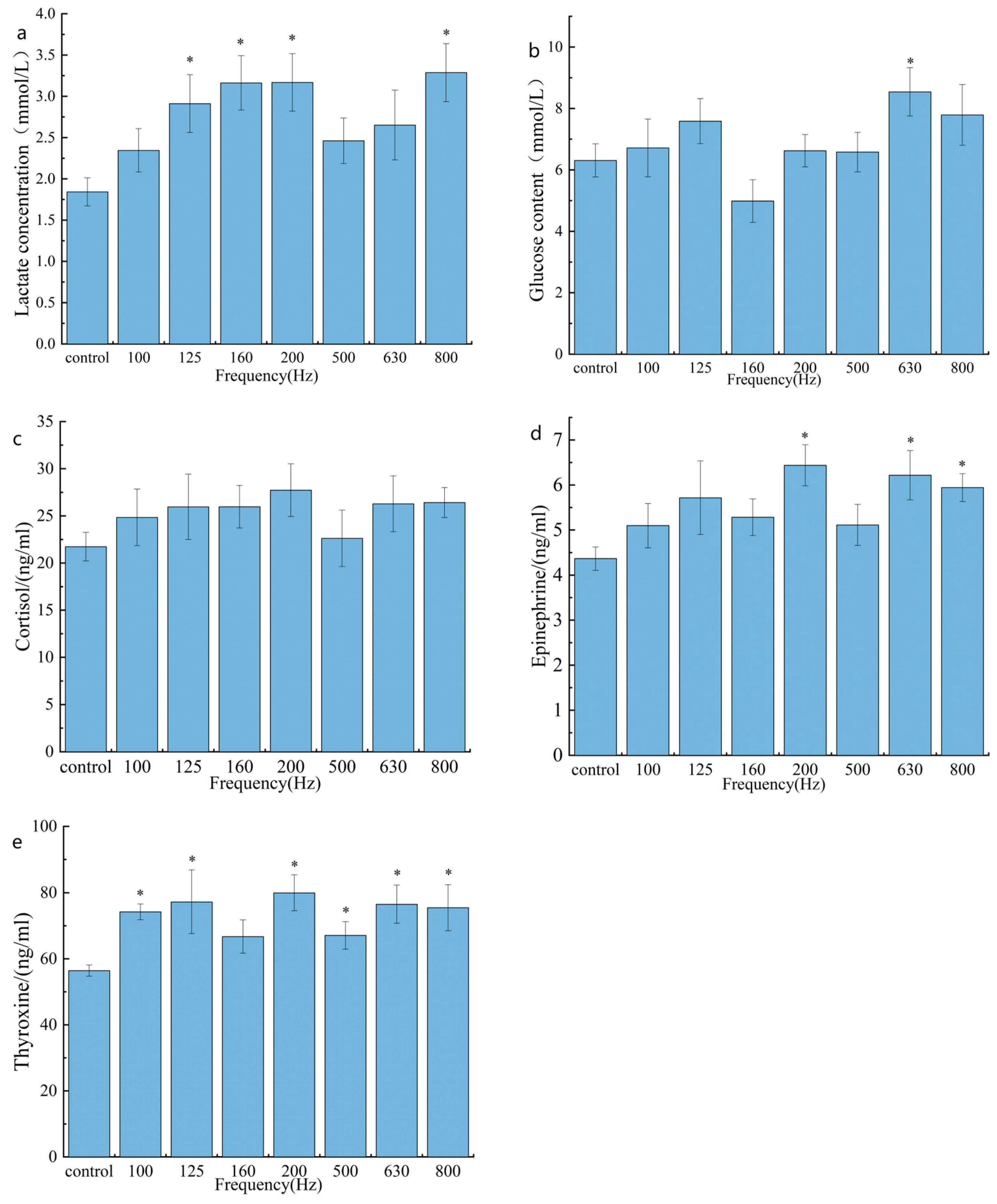
3.2. The Impact of Noise on the Philological Indexes of the Large Yellow Croaker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, H.Q.; Wang, Z.H. Advances in the Effects of Man-made Noise on Fish. J. Agric. 2017, 7, 46. (In Chinese) [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature Erratum in Nature 2021, 593, E12; Erratum in Nature 2021, 595, E36. 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Y.; Lin, D.Q.; Xing, B.B.; Gong, D.H.; Guo, J.L.; Liu, Y.T.; Li, Z.M.; Yin, L.M. Potential impact of noise from underwater tunnel during metros operation on aquatic organisms. J. Dalian Ocean. Univ. 2022, 37, 329–337. (In Chinese) [Google Scholar]
- Niu, F.Q.; Li, Z.; Xue, R.C.; Yang, Y.M.; Ma, L. Impact of pile driving underwater noise from offshore wind turbines on the large yellow croaker (Pseudosciaena crocea). Mar. Sci. 2021, 45, 9. (In Chinese) [Google Scholar]
- Liu, D.; Li, W.J.; Du, H.B.; Wan, Y.; Wang, L.; Yang, S.F. Preliminary analysis on influence of channel noise on fish. Yangtze River 2021, 52, 58–64. (In Chinese) [Google Scholar]
- Scholik, A.R.; Yan, H.Y. Effects of underwater noise on auditory sensitivity of a cyprinid fish. Hear. Res. 2001, 152, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tavolga, W.N.E.; Poppera, N.E.; Fayr, R.E. Hearing and Sound Communication in Fishes; Springer Science & Business Media: Berlin, Germany, 1981. [Google Scholar]
- Hastings, M.C.; Popper, A.N.; Finneran, J.J.; Lanford, P.J. Effects of low-frequency underwater sound on hair cells of the inner ear and lateral line of the teleost fish Astronotus ocellatus. J. Acoust. Soc. Am. 1996, 99, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- McCauley, R.D.; Fewtrell, J.; Popper, A.N. High intensity anthropogenic sound damages fish ears. J. Acoust. Soc. Am. 2003, 113, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Pine, M.K.; Wilson, L.; Jeffs, A.G.; McWhinnie, L.; Juanes, F.; Scuderi, A.; Radford, C.A. A Gulf in lockdown: How an enforced ban on recreational vessels increased dolphin and fish communication ranges. Glob. Change Biol. 2021, 27, 4839–4848. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, R.W. Physiological Effects of Noise. Otolaryngol. Clin. N. Am. 1979, 12, 366. [Google Scholar] [CrossRef]
- National Research Council (US). Committee on Low-Frequency Sound and Marine Mammals. In Low-Frequency Sound and Marine Mammals; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Puig-Pons, V.; Soliveres, E.; Pérez-Arjona, I.; Espinosa, V.; Poveda-Martínez, P.; Ramis-Soriano, J.; Ordoñez-Cebrián, P.; Moszyński, M.; de la Gándara, F.; Bou-Cabo, M.; et al. Monitoring of Caged Bluefin Tuna Reactions to Ship and Offshore Wind Farm Operational Noises. Sensors 2021, 21, 6998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leduc AO, H.C. Land-based noise pollution impairs reef fish behavior: A case study with a Brazilian carnival. Biol. Conserv. 2021, 253, 108910. [Google Scholar] [CrossRef]
- Liu, W.H. Research on Croaker Resisting Seismic Wave and Air Shock Wave of Blasting. Explos. Mater. 2001, 30, 3. (In Chinese) [Google Scholar]
- Jacobsen, L.; Baktoft, H.; Jepsen, N.; Aarestrup, K.; Berg, S.; Skov, C. Effect of boat noise and angling on lake fish behaviour. J. Fish Biol. 2014, 84, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, J.L.; Mccauley, R.D. Impact of air gun noise on the behaviour of marine fish and squid. Mar. Pollut. Bull. 2012, 64, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Hammar, L.; Perry, D.; Gullström, M. Offshore Wind Power for Marine Conservation. Open J. Mar. Sci. 2016, 6, 66–78. [Google Scholar] [CrossRef]
- Cox, K.; Brennan, L.P.; Gerwing, T.G.; Dudas, S.E.; Juanes, F. Sound the alarm: A meta-analysis on the effect of aquatic noise on fish behavior and physiology. Glob. Change Biol. 2018, 24, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.I.; Galhardo, L.; Noble, C.V.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.; Carter, T.J.; et al. Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Kane, A.S.; Popper, A.N. Noise-induced stress response and hearing loss in goldfish (Carassius auratus). J. Exp. Biol. 2004, 207, 427–435. [Google Scholar] [CrossRef]
- Santulli, A.; Modica, A.; Messina, C.; Ceffa, L.; Curatolo, A.; Rivas, G.; Fabi, G.; D’Amelio, V. Biochemical Responses of European Sea Bass (Dicentrarchus labrax L.) to the Stress Induced by Off Shore Experimental Seismic Prospecting. Mar. Pollut. Bull. 1999, 38, 1105–1114. [Google Scholar] [CrossRef]
- Alves, D.; Vieira, M.; Amorim, M.C.P.; Fonseca, P.J. Boat noise interferes with Lusitanian toadfish acoustic communication. J. Exp. Biol. 2021, 224, jeb234849. [Google Scholar] [CrossRef] [PubMed]
- Celi, M.; Filiciotto, F.; Maricchiolo, G.; Genovese, L.; Quinci, E.M.; Maccarrone, V.; Mazzola, S.; Vazzana, M.; Buscaino, G. Vessel noise pollution as a human threat to fish: Assessment of the stress response in gilthead sea bream (Sparus aurata, Linnaeus 1758). Fish Physiol. Biochem. 2016, 42, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tao, Y.; Zhou, Y.; Tang, L.; Liu, M.; Xu, X. Acoustic properties of the otolith of the large yellow croaker large yellow croaker (Perciformes: Sciaenidae). Zool. Stud. 2021, 60, e64. (In Chinese) [Google Scholar] [PubMed]
- Blamey, P.J. Sine Waves and Simple Acoustic Phenomena in Experimental Music-with Special Reference to the Work of La Monte Young and Alvin Lucier. Ph.D. Thesis, University of Western Sydney, Penrith, Australia, 2008. [Google Scholar]
- Liu, Z.W.; Xu, X.M.; Huang, E.H.; Yang, Y.M. Study on behavior of sound stimulation for large yellow croaker (Pseudosciaena crocea). J. Appl. Oceanogr. 2014, 33, 105–110. (In Chinese) [Google Scholar]
- Xu, L.Y.; Qi, M.E. Yellow, bohai sea two kinds of noise spectrum of underwater observation. J. Mar. Sci. 1999, 4, 2. (In Chinese) [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac: A fast and robust software for tracking organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, M. ToxId: An efficient algorithm to solve occlusions when tracking multiple animals. Sci. Rep. 2017, 7, 14774. [Google Scholar] [CrossRef]
- He, D.R. Fish Ethology; Fish Ethology: Faro, Portugal, 1998. [Google Scholar]
- Zhao, M.J.; Zhou, X.Q. The Effect of Dietary Tryptophan on the Stress-Induced Secretion of Cortisol. Fish. Sci. 2007, 26, 420–422. (In Chinese) [Google Scholar]
- Shi, H.X.; Jiao, H.F.; You, Z.J. The effect of ship noise on the secretion of cortisol in Lateolabrax japonicus and Pseudosciaena crocea. Acta Ecol. Sin. 2010, 30, 3760–3765. (In Chinese) [Google Scholar]
- Mai, K.S.; Ai, Q.H.; Xu, W. Stress in Aquaculture and Its Prevention with Emphasis on Nutritional Methods. Period. Ocean Univ. China 2004, 34, 767–774. [Google Scholar]
- Yarahmadi, P.; Miandare, H.K.; Fayaz, S.; Caipang, C.M. Increased stocking density causes changes in expression of selected stress-and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 48, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Leach, G.J.; Taylor, M.H. The role of cortisol in stress-induced metabolic changes in Fundulus heteroclitus. Gen. Comp. Endocrinol. 1980, 42, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hemre, G.I.; Mommsen, T.P.; Krogdahl, Å. Carbohydrates in fish nutrition: Effects on growth, glucose metabolism and hepatic enzymes. Aquac. Nutr. 2002, 8, 175–194. [Google Scholar] [CrossRef]
- Lu, Y.B.; You, C.H.; Wang, S.Q.; Li, Y.Y. Physiological chances in siganus Canaliculatus after shallow water stress and the anti-stress effects of taurine. Acta Hydrobiol. Sin. 2014, 38, 68–74. (In Chinese) [Google Scholar]
- Wu, N.; Li, L.; Hou, J.; Long, X.P.; Xu, M.S.; Su, Y.J.; Lin, W. Stress reponse of grass carp after passing the jet fish pump. J. Huazhong Agric. Univ. 2016, 35, 75–83. (In Chinese) [Google Scholar]
- Li, Q.; Deng, L.R.; Diao, X.M. The effects of fasting on cortisol,metabolism of glucose in juveniles of Pelteobagrus vachelli. Freshw. Fish. 2014, 44, 36–40. (In Chinese) [Google Scholar]
- Xiao, F.; Jie, Z.G.; Zhuang, J.Z. The effects of hyperthermia on immunocompetence and energy consumption in Bufo bufo gargarizans. Acta Ecol. Sin. 2015, 35, 3087–3092. (In Chinese) [Google Scholar]
- Iversen, M.; Finstad, B.; McKinley, R.S.; Eliassen, R.A.; Carlsen, K.T.; Evjen, T. Stress responses in Atlantic salmon (Salmo salar L.) smolts during commercial well boat transports, and effects on survival after transfer to sea. Aquaculture 2005, 243, 373–382. [Google Scholar] [CrossRef]
- Ottolenghi, C.; Ac, P.; Ricci, D.; Brighenti, L.; Morsiani, E. The effect of high temperature on blood glucose level in two teleost fish (Ictalurus melas and Ictalurus punctatus). Comp. Biochem. Physiol. Part A Physiol. 1995, 111, 229–235. [Google Scholar] [CrossRef]
- West, T.G.; Arthur, P.G.; Suarez, R.K.; Doll, C.J.; Hochachka, P.W. In vivo utilization of glucose by heart and locomotory muscles of exercising rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1993, 177, 63–79. [Google Scholar] [CrossRef]
- Buscaino, G.; Filiciotto, F.; Buffa, G.; Bellante, A.; Di Stefano, V.; Assenza, A.; Fazio, F.; Caola, G.; Mazzola, S. Impact of an acoustic stimulus on the motility and blood parameters of European sea bass (Dicentrarchus labrax L.) and gilthead sea bream (Sparus aurata L.). Mar. Environ. Res. 2010, 69, 136–142. [Google Scholar] [CrossRef]
- Bennett, A.F.; Licht, P. Anaerobic metabolism during activity in lizards. J. Comp. Physiol. 1972, 81, 277–288. [Google Scholar] [CrossRef]
- Goolish, E.M. Aerobic and anaerobic scaling in fish. Biol. Rev. 1991, 66, 33–56. [Google Scholar] [CrossRef]
- Lu, S.W.; Liu, Z.P.; Yu, Y. Effects of density stress on growth and metabolism of juvenile Epinephelus malabaricus. J. Fish. Sci. China 2011, 18, 322–328. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.J.; Li, W.M.; Lv, W.Q. Effects of transportation density and salinity on cortisol,glycogen and lactate of large yellow croaker(large yellow croaker) juveniles. J. Fish. China 2014, 38, 973–980. (In Chinese) [Google Scholar]
- Lagardcre, J.P. Effects of noise on growth and reproduction of Crangon crangon in rearing tanks. Mar. Biol. 1982, 71, 177–185. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; Malarkey, W.B.; Neri, E.; McEwen, B.S. Stress-induced redistribution of immune cells—From barracks to boulevards to battlefields: A tale of three hormones–Curt Richter Award Winner. Psychoneuroendocrinology 2012, 37, 1345–1368. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.R.; Usami, T.; Suzuki, Y. Seasonal changes in the immune activities of common carp (Cyprinus carpio). Fish Physiol. Biochem. 2002, 26, 379–387. [Google Scholar] [CrossRef]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef]
- Peter, M.C. The role of thyroid hormones in stress response of fish. Gen. Comp. Endocrinol. 2011, 172, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Bau, F.; Parent, J. Seasonal variations of thyroid hormone levels in wild fish. Comptes Rendus de L’académie des Sci.-Ser. III-Sci. de la Vie 2000, 323, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Gardner, C.; Braithwaite, V.A. Differential stress responses in fish from areas of high and low-predation pressure. J. Comp. Physiol. B 2005, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Xu, P.; Tian, T.; Song, M.; Qin, X.; Gong, D.; Wang, Y.; Zhang, X.; Xing, B.; Li, M.; et al. Frequency-Specific Responses: The Impact of an Acoustic Stimulus on Behavioral and Physiological Indices in Large Yellow Croaker. Fishes 2024, 9, 217. https://doi.org/10.3390/fishes9060217
Cui X, Xu P, Tian T, Song M, Qin X, Gong D, Wang Y, Zhang X, Xing B, Li M, et al. Frequency-Specific Responses: The Impact of an Acoustic Stimulus on Behavioral and Physiological Indices in Large Yellow Croaker. Fishes. 2024; 9(6):217. https://doi.org/10.3390/fishes9060217
Chicago/Turabian StyleCui, Xiaojie, Pengxiang Xu, Tao Tian, Mingyuan Song, Xuyang Qin, Dehua Gong, Yan Wang, Xuguang Zhang, Binbin Xing, Mingzhi Li, and et al. 2024. "Frequency-Specific Responses: The Impact of an Acoustic Stimulus on Behavioral and Physiological Indices in Large Yellow Croaker" Fishes 9, no. 6: 217. https://doi.org/10.3390/fishes9060217
APA StyleCui, X., Xu, P., Tian, T., Song, M., Qin, X., Gong, D., Wang, Y., Zhang, X., Xing, B., Li, M., & Yin, L. (2024). Frequency-Specific Responses: The Impact of an Acoustic Stimulus on Behavioral and Physiological Indices in Large Yellow Croaker. Fishes, 9(6), 217. https://doi.org/10.3390/fishes9060217







