Morphological Development and DNA Barcoding Identification of Pholis fangi Larvae and Juveniles in the Yellow Sea
Abstract
1. Introduction
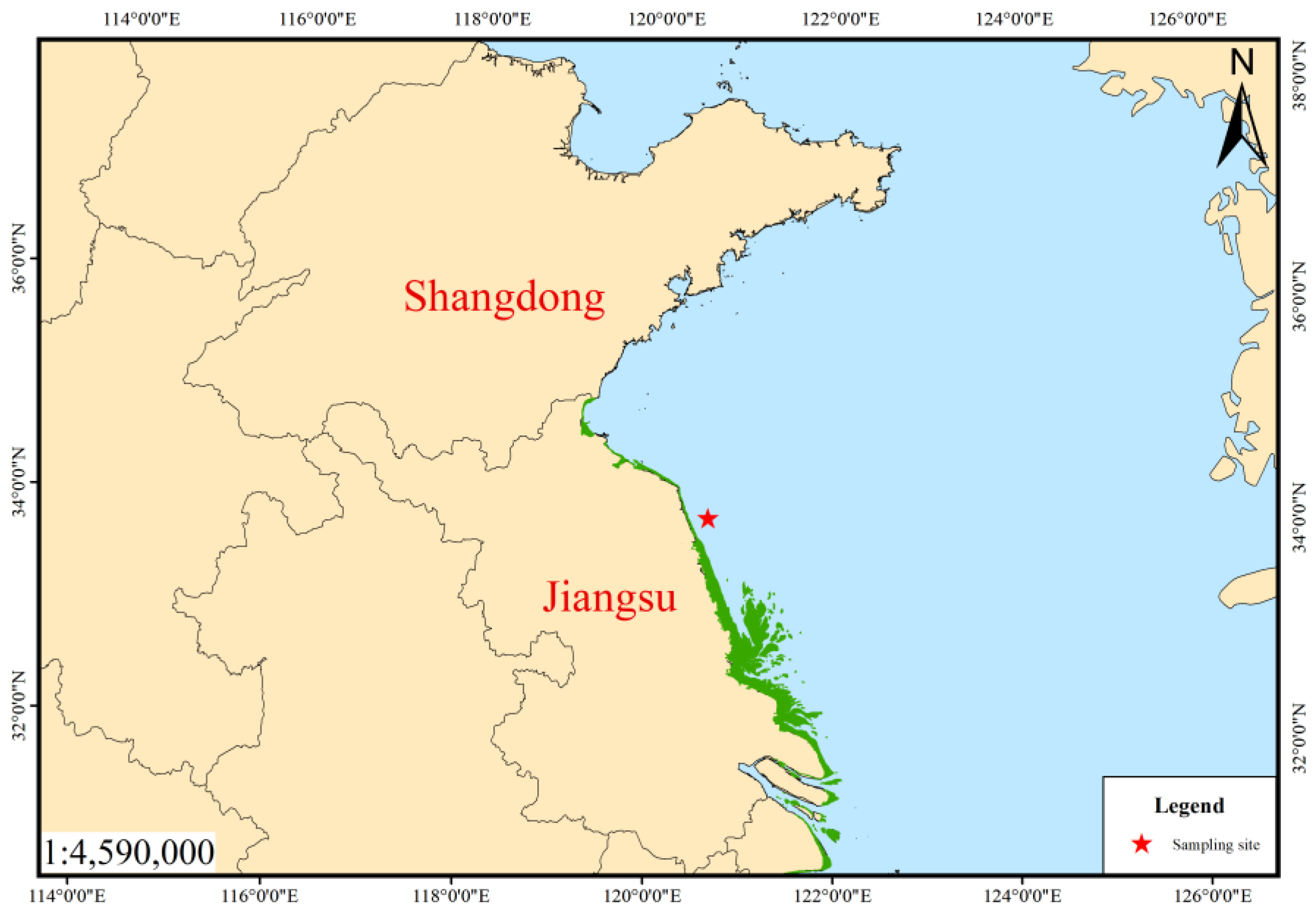
2. Materials and Methods
3. Results
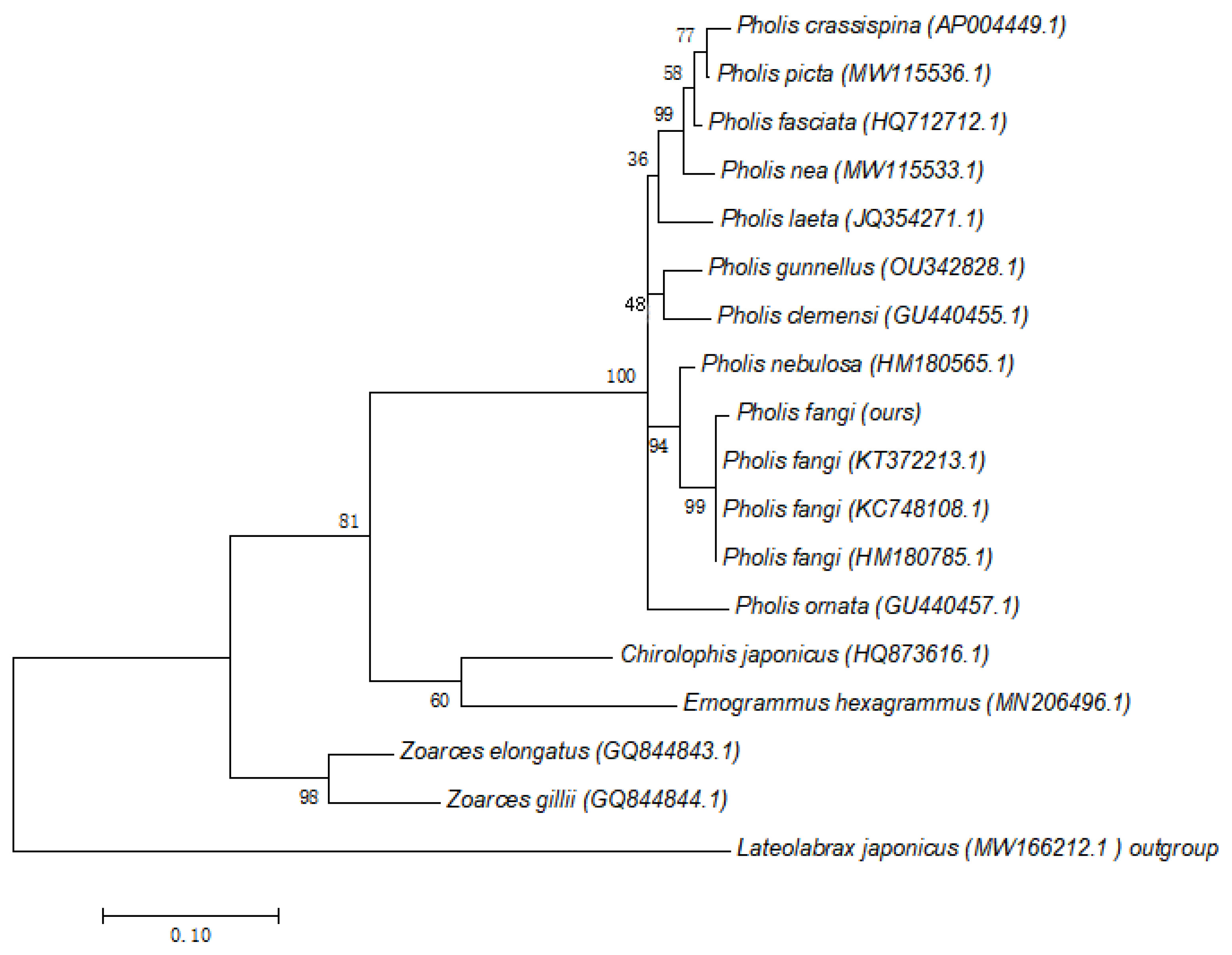
3.1. Molecular Identification
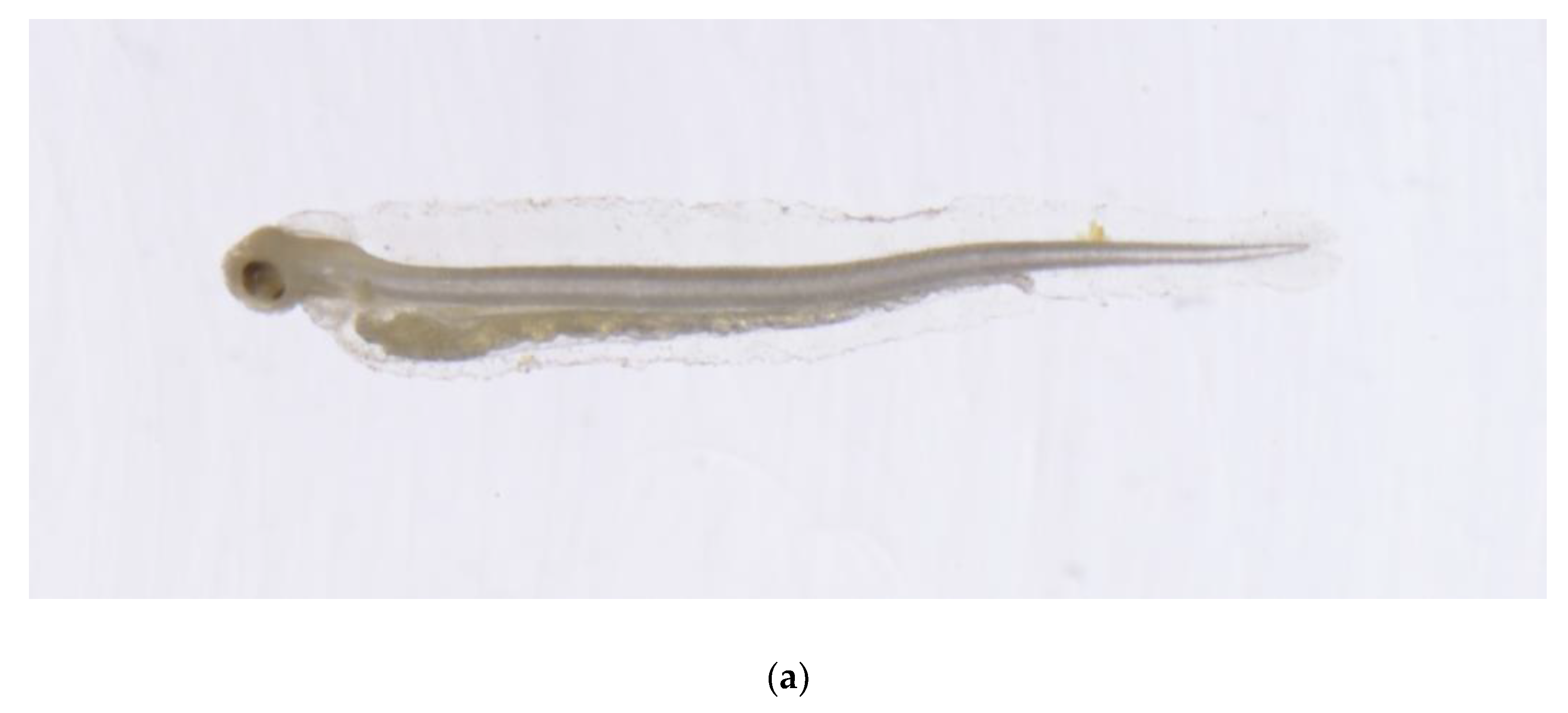
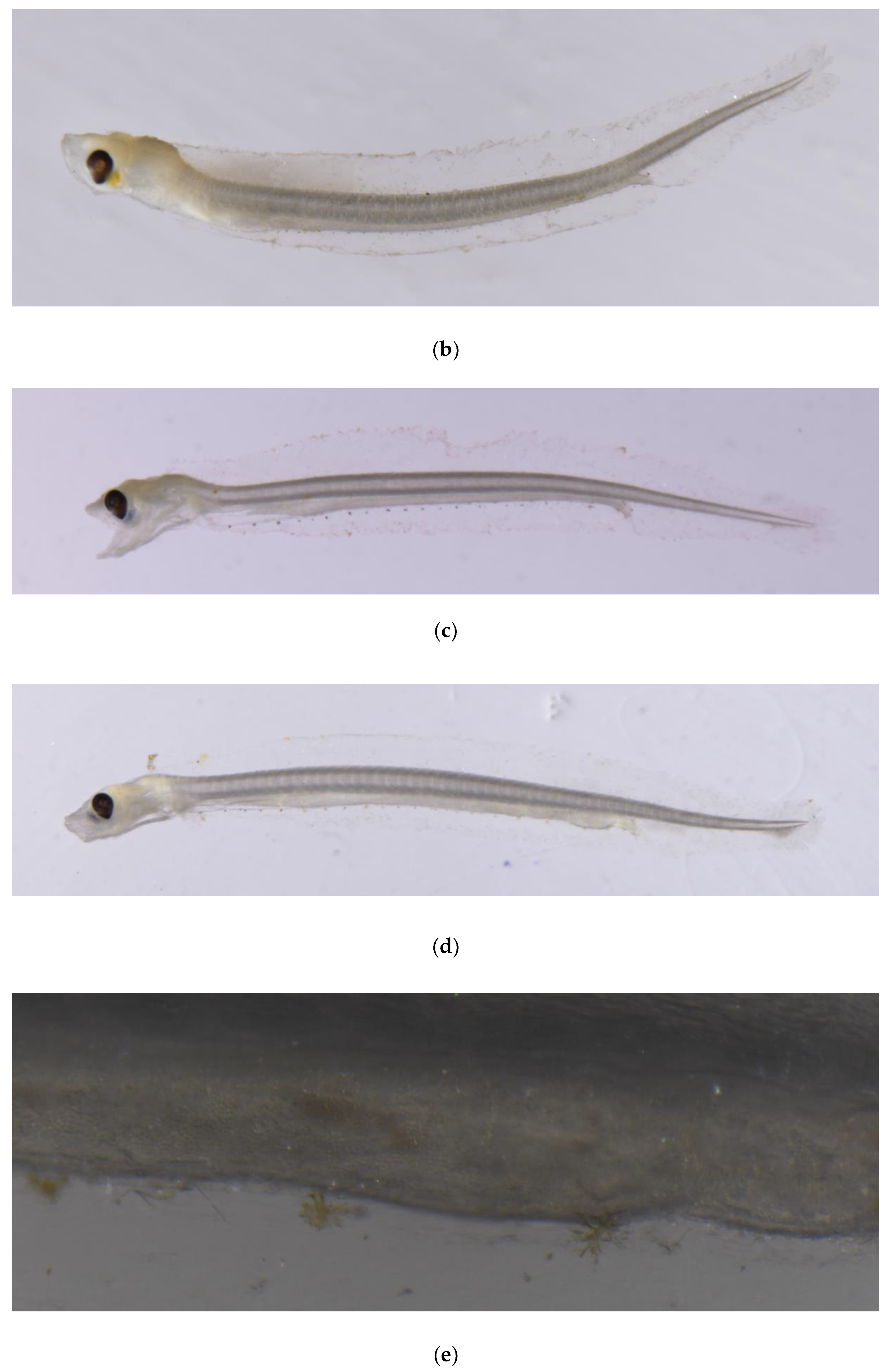
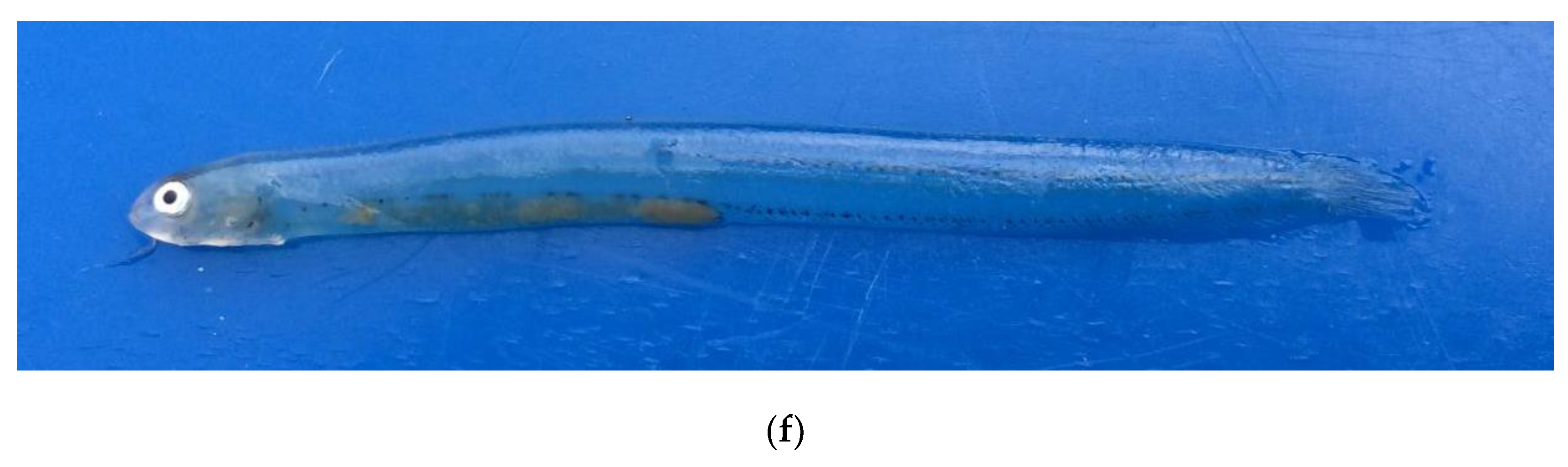
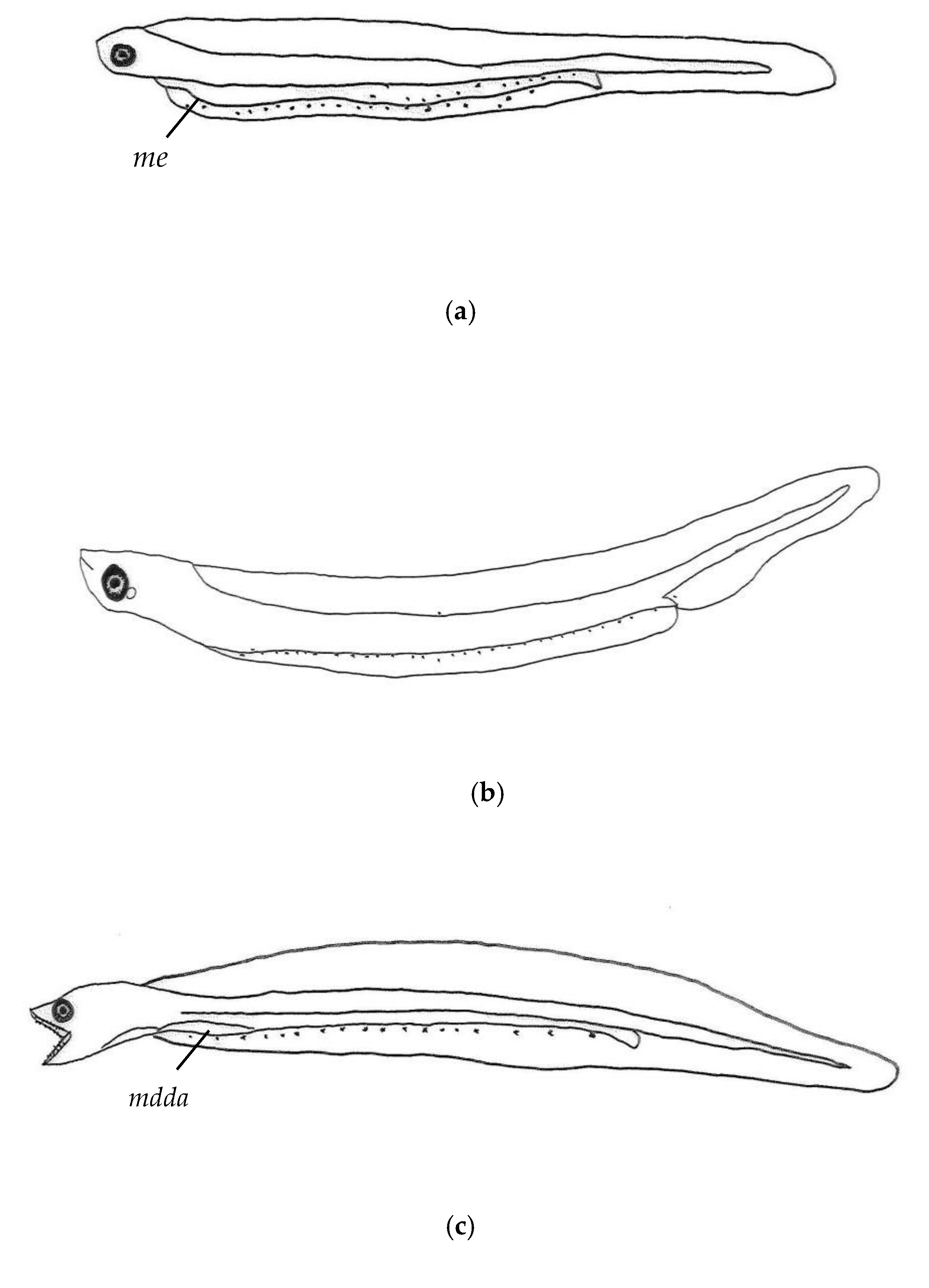
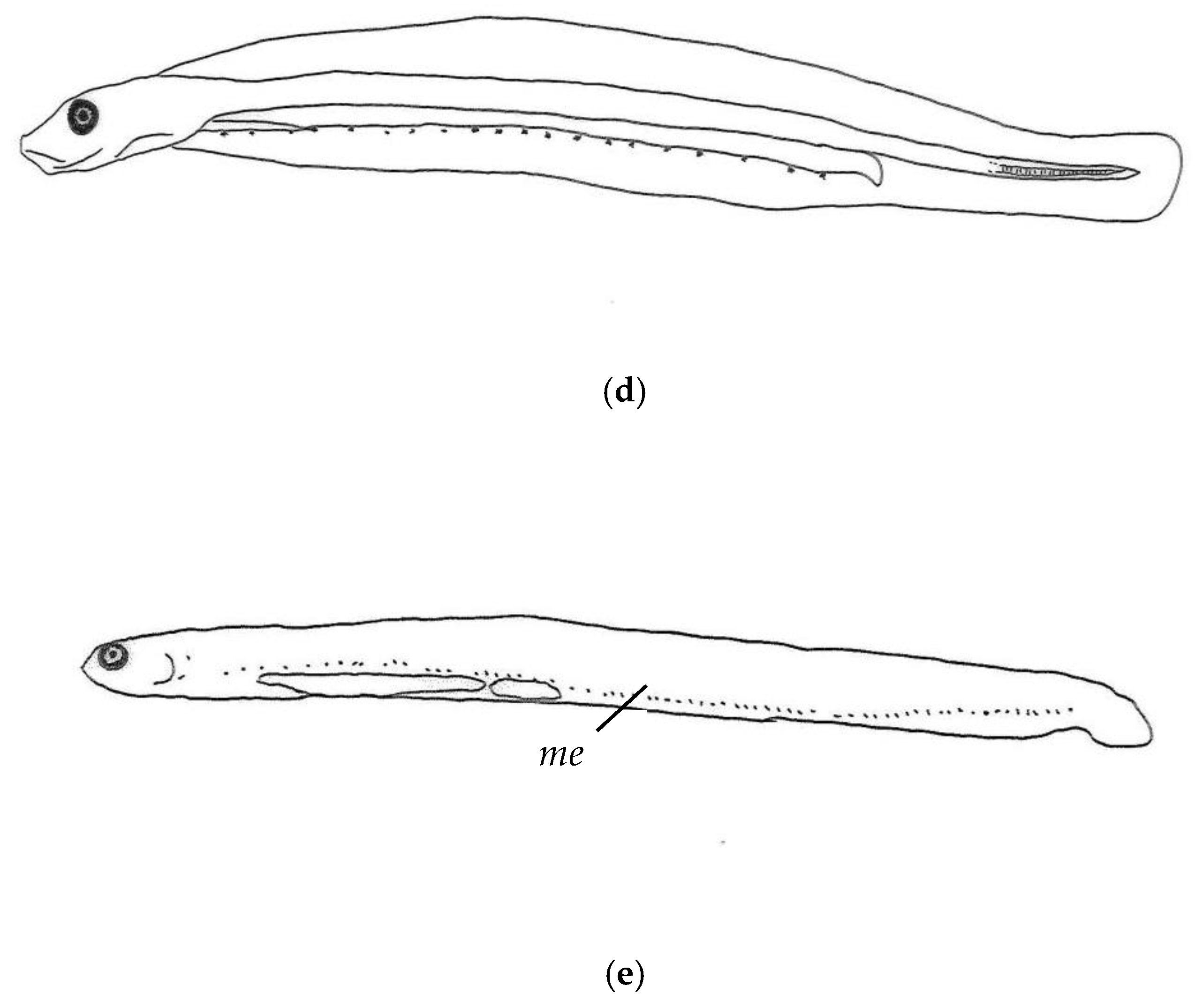
3.2. Morphological Description of P. fangi
3.3. Comparative Study of Pholis Larvae and Juveniles
- (2) Absence of melanophores at the posterior anal fin································Pholis fangi;
- (1) Presence of melanophores at the posterior anal fin;
- (4) Sparse melanophores on the abdominal back, not connected·······Pholis crassispina;
- (3) Dense distribution of melanophores on the abdominal back·······Pholis nebulosa.
- (2) Presence of melanophores on the abdomen··········································Pholis fangi;
- (1) Melanophores present on the abdomen;
- (4) Dense distribution of melanophores on the abdominal back·······Pholis nebulosa;
- (3) Sparse melanophores on the abdominal back, and a few melanophores on the abdominal back that are not connected;
- (6) Presence of melanophores on vertebrae················································Pholis ornate;
- (5) Absence of melanophores on vertebrae···········································Pholis crassispina.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J. Fauna Sinica: Scleroichthyes, Perciformes (IV); Science Press: Beijing, China, 2016. [Google Scholar]
- Meng, Q.W.; Su, J.X.; Miao, X.Z. Systematics of Fishes; Chinese Agricultural Press: Beijing, China, 1995. [Google Scholar]
- Kim, J.M.; Kim, D.Y.; Yoo, J.M.; Huh, H.T. Food of the Larval Gunnel Enedrias fangi. Bull. Korean Fish. Soc. 1985, 18, 484–490. [Google Scholar]
- Bi, Y.B. Biology and Its Fishery of Fang’s Blenny Enedrias fangi Wang et Wang in Offshore Liaoning Province. Fish. Sci. 2005, 24, 27–28. [Google Scholar]
- Hwang, S.D.; Lee, T.W.; Hwang, S.W. Age, Growth, and Life History of Gunnel, Pholis fangi, in the Yellow Sea. Fish. Res. 2008, 93, 72–76. [Google Scholar] [CrossRef]
- Huang, X.X.; Zeng, X.Q.; Zhang, C. The Reproductive Biology of Enedrias fangi in the Inshore Waters of Qingdao. Period. Ocean Univ. China 2010, 40, 55–59. [Google Scholar]
- Li, S.Y. Feed Habits of Enedrias fangi in Jiaozhou Bay Based on Stomach Contents Analysis and Stable Isotope Analysis. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2015. [Google Scholar]
- Li, L.; Zhang, H.; Sun, D.R.; Gao, T.X. Structure of Mitochondrial DNA Control Region of Pholis fangi and Its Phylogenetic Implication. J. Ocean Univ. China 2014, 13, 491–496. [Google Scholar] [CrossRef]
- Gao, T.X.; Li, L.; Fang, R.D.; Liu, G.H.; Wang, L.; Xu, H.X.; Song, N. Shallow Genetic Structure of Pholis fangi in Bohai Sea and Yellow Sea Inferred from mtDNA Control Region. J. Ocean Univ. China 2019, 18, 947–952. [Google Scholar] [CrossRef]
- Wan, R.J.; Zhang, R.Z. (Eds.) Fish Eggs, Larvae and Juveniles in the Offshore Waters of China and Their Adjacent Waters; Shanghai Science and Technology Press: Shanghai, China, 2016. [Google Scholar]
- Kunihiko, T.; Kunio, A. Studies on larval and juvenile blennies in the coastal waters of the southern Hokkaido (Pisces: Blennioidei). Bull. Fac. Fish. 1980, 31, 16–49. [Google Scholar]
- Okiyama, M. An Atlas of the Early Stage Fishes in Japan, 2nd ed.; Takai University Press: Tokyo, Japan, 2014. [Google Scholar]
- Zhang, R.Z.; Lu, S.F.; Zhao, C.Y.; Chen, L.F.; Zang, Z.J. (Eds.) Fish Eggs and Larvae in the Offshore Waters of China; Shanghai Science and Technology Press: Shanghai, China, 1985. [Google Scholar]
- Zhou, M.Y.; Chen, X.; Yang, S.Y. Identification of several fish eggs and larvae by DNA barcoding in Xiamen water. Mar. Environ. Sci. 2015, 34, 120–125, 135. [Google Scholar]
- Liu, S.H.; Qin, Y.T.; Liu, C.C.; Ji, X.; Zhang, H.F. Molecular identification of fish larvae and juveniles based on DNA barcoding. Ocean Dev. Manag. 2017, 34, 92–95. [Google Scholar]
- Liu, S.H.; Yang, Y.Y.; He, Y.L.; Ji, X.; Wang, Y.T.; Zhang, H.J.; Mao, R.Y.; Jiang, X.S.; Cheng, X.S. Morphological classification of ichthyoplankton in the Changjiang River Estuary based on DNA barcoding. Haiyang Xuebao 2021, 43, 93–104. [Google Scholar]
- Hanahara, N.; Miyamoto, K.; Oka, S. Morphological and genetic identification of formalin–fixed gobioid larvae and description of postflexion larvae of Paragunnellichthys sp. and Ctenogobiops feroculus. Ichthyol. Res. 2021, 68, 182–190. [Google Scholar] [CrossRef]
- Endo, S.; Hibino, Y.; Mochioka, N. Identification of first recorded ophichthid larvae of Ophichthus celebicus and O. macrochir (Anguilliformes; Ophichthidae) from Japan, based on morphometric and genetic evidence. Ichthyol. Res. 2022, 69, 393–398. [Google Scholar] [CrossRef]
- GB/T 12763.6-2007; Specification for Oceanographic Survey Part 6: Marine Biology Survey. China Standards Press: Beijing, China, 2007; pp. 38–41.
- Makushok, V.M. Morphological Foundations of Stichaeoidae and Closely Related to Them Fish Families (Blennioidei, Pisces). Tr. Zool. Inst. Akad. Nauk SSSR 1958, 25, 3–129. [Google Scholar]
- Yatsu, A. A Revision of the Gunnel Family Pholididae (Pisces, Blennioidei). Bull. Nat. Sci. Mus. Tokyo Ser. A 1981, 7, 165–190. [Google Scholar]
- Yatsu, A. Phylogeny of the Family Pholididae (Blenniodei) with a Redescription of Pholis scopoli. J. Ichthyol. 1985, 12, 273–282. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World, 3rd ed.; Wiley: NewYork, NY, USA, 1994. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; Wiley: NewYork, NY, USA, 2006. [Google Scholar]
- Mecklenburg, C.W. Family Pholidae Gill, 1893 Gunnels. Calif. Acad. Sci. Annot. Checkl. Fish. 2003, 6, 15–20. [Google Scholar]
- Fedorov, V.V. Annotated Checklist of Fishes and Fishlike Organisms Living in Seas of Russia and Adjacent Countries. Part 6. Suborder Zoarcoidei. J. Ichthyol. 2004, 44, 73–128. [Google Scholar]
- Radchenko, O.A.; Chereshnev, I.A.; Petrovskaya, A.V. Phylogenetic relations in the family Pholidae (Perciformes: Zoarcoidei) based on genetic and morphological data. J. Ichthyol. 2010, 50, 728–739. [Google Scholar] [CrossRef]
- Wu, H.L.; Shao, G.Z.; Lai, C.F.; Zhuang, D.H.; Lin, P.L. (Eds.) Latin–Chinese Dictionary of Fishes Names by Classification System; The Shuichan Press: Keelung, China, 1999. [Google Scholar]
- Zhu, Y.D.; Zhang, C.L.; Cheng, Q.T. The Fishes of the East China Sea; Science Press: Beijing, China, 1963. [Google Scholar]
- Cheng, Q.T.; Zheng, B.S. Systematic Synopsis of Chinese Fishes; Science Press: Beijing, China, 1987. [Google Scholar]
- Liu, C.X.; Qin, K.J.; Ding, G.W.; Wang, X.Y.; Mou, X.L.; Piao, X.L.; Chen, J.K.; Shi, Y.R.; Qin, Y.J.; Qin, K.J. (Eds.) Fauna in Liaoning Province (Fish); Liaoning Science and Technology Press: Shenyang, China, 1987. [Google Scholar]
- Cheng, Q.T.; Zhou, C.W. The Fishes of Shandong Province; Shandong Science and Technology Press: Jinan, China, 1997. [Google Scholar]
- Wang, S.A.; Wang, Z.M.; Li, G.L.; Cao, Y.P.; Zhang, Z.W.; Xu, X.J.; Sun, J.H. Fauna in Hebei Province (Fish); Hebei Science and Technology Press: Shijiazhuang, China, 2001. [Google Scholar]
- Ni, Y.; Wu, H.L. Fishes of Jiangsu; Chinese Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Zhao, S.L.; Xu, H.X.; Zhong, J.S.; Chen, J. Fauna of Marine Fishes in Zhejiang; Zhejiang Science and Technology Press: Hangzhou, China, 2016. [Google Scholar]
- Zhuang, P.; Zhang, T.; Li, S.F.; Ni, Y.; Wang, Y.H.; Deng, S.M.; Zhang, L.Z.; Ling, J.Z.; Hu, F.; Yang, G. Fishes of the Yangtze Estuary, 2nd ed.; Shanghai Science and Technology Press: Shanghai, China, 2019. [Google Scholar]
- Fishes of Fujian Province Editorial Subcommittee. The Fishes of Fujian Province; Fujian Science and Technology Press: Fuzhou, China, 1984. [Google Scholar]
- Liu, M.; Chen, X.; Yang, S.Y. Marine Fishes of Southern Fujian, China; China Ocean Press: Beijing, China, 2014; Volume 2. [Google Scholar]
- Shao, K.T. Taiwan Fish Database. WWW Web Electronic Publication. 2023. Available online: http://fishdb.sinica.edu.tw (accessed on 26 January 2024).
- Zheng, C.Y. Fishes of the Zhujiang River; Science Press: Beijing, China, 1989. [Google Scholar]
- Institute of Zoology, Chinese Academy of Sciences; Institute of Oceanography, Chinese Academy of Sciences; Shanghai Fisheries University. The Fishes of the South China Sea; Science Press: Beijing, China, 1962. [Google Scholar]
- South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. The Fishes of the Islands in the South China Sea; Science Press: Beijing, China, 1979. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, H.; Ji, X.; Peng, X.; Qin, Y.; Yao, W. Morphological Development and DNA Barcoding Identification of Pholis fangi Larvae and Juveniles in the Yellow Sea. Fishes 2024, 9, 213. https://doi.org/10.3390/fishes9060213
Liu S, Zhang H, Ji X, Peng X, Qin Y, Yao W. Morphological Development and DNA Barcoding Identification of Pholis fangi Larvae and Juveniles in the Yellow Sea. Fishes. 2024; 9(6):213. https://doi.org/10.3390/fishes9060213
Chicago/Turabian StyleLiu, Shouhai, Haijing Zhang, Xiao Ji, Xiaojia Peng, Yutao Qin, and Weimin Yao. 2024. "Morphological Development and DNA Barcoding Identification of Pholis fangi Larvae and Juveniles in the Yellow Sea" Fishes 9, no. 6: 213. https://doi.org/10.3390/fishes9060213
APA StyleLiu, S., Zhang, H., Ji, X., Peng, X., Qin, Y., & Yao, W. (2024). Morphological Development and DNA Barcoding Identification of Pholis fangi Larvae and Juveniles in the Yellow Sea. Fishes, 9(6), 213. https://doi.org/10.3390/fishes9060213




