Abstract
The bulbus arteriosus of goldfish, Carassius auratus, possesses unique structural features. The wall of the bulbus arteriosus is exceptionally thick, with an inner surface characterized by longitudinally arranged finger-like ridges, resulting in an uneven luminal appearance. These ridges are covered by endocardium and encased in an amorphous extracellular matrix. The inner surface of the bulbus arteriosus also contains rodlet cells at different developmental stages, often clustered beneath the endothelium lining the bulbar lumen. Ruptured rodlet cells release their contents via a holocrine secretion process. The high abundance of rodlet cells in the bulbus arteriosus suggests that this is the site of origin for these cells. Within the middle layer of the bulbus arteriosus, smooth muscle cells, branched telocytes (TCs), and collagen bundles coexist. TCs and their telopodes form complex connections within a dense collagen matrix, extending to rodlet cells and macrophages. Moreover, the endothelium makes direct contact with telopodes. The endocardial cells within the bulbus arteriosus display irregular, stellate shapes and numerous cell processes that establish direct contact with TCs. TEM reveals that they contain moderately dense bodies and membrane-bound vacuoles, suggesting a secretory activity. TCs exhibit robust secretory activity, evident from their telopodes containing numerous secretory vesicles. Furthermore, TCs release excretory vesicles containing bioactive molecules into the extracellular matrix, which strengthens evidence for telocytes as promising candidates for cellular therapies and regeneration in various heart pathologies.
Key Contribution:
Telocytes (TCs) play a crucial role in the bulbus arteriosus of goldfish, forming intricate connections with various cells and tissues and engaging in robust secretory activity. The findings of this study highlight the potential of TCs for cellular therapies and heart regeneration.
1. Introduction
Goldfish (Carassius auratus, Linnaeus 1758) is one of the most popular fish used as a biological model in many laboratories. It is a freshwater fish in the family Cyprinidae of the order Cypriniformes. It is one of the most popular aquarium fish and is frequently kept as a pet in indoor tanks. Breeds of goldfish differ significantly in terms of size, form of the body, arrangement of the fins, and color (different shades of white, orange, yellow, brown, red, and black) [1].
Fish only have one circuit in their circulatory system. Blood is oxygenated at the gill after exiting the heart and traveling through the outflow tract (OFT) to the ventral aorta. The aorta transports blood to the gills for oxygenation before it travels throughout the body to perfuse the tissues and return to the heart [2]. Strong ventricular contraction is necessary to pump out blood at a pressure high enough to circulate the body. Therefore, the OFT of teleost fish is very specialized and consists of a chamber known as the bulbus arteriosus to prevent injury to the delicate gill capillary network [3]. The bulbus arteriosus is the thick-walled chamber found in most teleost fish that sits between the ventral aorta and the single ventricle. It is an onion- or pear-shaped fibroelastic cylindrical structure [4]. Three layers make up the bulbus wall: the endocardium and endocardial ridges, which make up the inner layer, middle layer, and outer subepicardial layer [5]. As the ventricle pumps blood, the elastic bulbus acts as a “shock absorber”. According to Jones et al. [6], the enlargement of the heart during ventricular ejection stores a considerable amount of the heart stroke volume. Waveform recordings of both flow and pressure have been made synchronously to study the functional properties of this heart chamber.
A concept from cardiovascular physiology called the Windkessel theory models the elasticity and compliance of the major arteries and aorta, helping to explain how blood pressure and flow are regulated during the cardiac cycle. This hypothesis is very helpful for comprehending how fish and other vertebrates’ circulatory systems work [7]. According to the current limitations of the “Windkessel” theory [8], the dilatation of the bulbus during systolic ejection protects the branchial vasculature from abrupt increases in flow and abrupt changes in pressure. For effective gas exchange, constant blood flow through the gills is made possible by the bulbus wall’s elastic recoil during diastole. It is also crucial for the bulbus’s Windkessel function to lower myocardial tension and increase cardiac efficiency. Accordingly, the bulbus is essential to the teleost’s circulatory adaptations [6,8]. The bulbus wall probably controls bulbus compliance by limiting circumferential deformation because of its high elastin content and collagen fibers in its exterior (subepicardial) collagen layer [9]. Icardo et al. [10,11] found remarkable differences in bulbus morphology between two species similar in phylogeny and ecotype, the hemoglobin-less (icefish) Chionodraco hamatus, and the red-blooded Trematomus bernacchii.
However, detailed morphological analysis of the bulbus arteriosus in different teleost species may reveal unique structural characteristics that emphasize the importance of interspecific differences and their wider biological significance. This study aims to provide a detailed description of the cellular structure of C. auratus bulbous arteriosus, and to provide a morphological framework that can be used in further comparative studies on the morpho-functional design of the teleost bulbus.
2. Materials and Methods
2.1. Sample Collection
The Ethics Committee of Assiut University in Egypt approved the study. A total of twelve healthy one-year-old goldfish (Carassius auratus, Linnaeus 1758) (20 to 30 g) were obtained from ornamental fish shops in Assiut City, Egypt. They were acclimated to their new environment (20 gal flowthrough aquaria with a water temperature of 20 ± 2 °C and a photoperiod of 16 h light and 8 h dark). The fish were kept in glass aquaria (60 L) capacity. About 25% of aquarium water (tap water free from chlorine) was exchanged daily. The aquaria were supplied with continuous aeration by air stone and fish were fed with a basal diet. After 2 weeks of acclimation, the fish were randomly selected from the aquariums and euthanized with an overdose of MS-222 (3% tricaine) (Merck KGaA, Darmstadt, Germany) [12]. Then, the hearts were extracted, flushed with PBS to clear the blood, and processed for histological analysis. All procedures were carried out in conformity with the applicable guidelines and ethical regulations; 06/2024/0191 is the ethical number.
2.2. Semithin Sections and TEM Preparations
The hearts were fixed by immersion in a combination of 2.5% paraformaldehyde–glutaraldehyde fixative for 24 h [13]. After fixation, the samples were washed in 0.1 Mol/L phosphate buffer and osmicated in 0.1 Mol/L sodium–cacodylate buffer at pH 7.3 with 1% osmium tetroxide. Then, the samples were dehydrated with ethanol 50%, 70%, 90%, 95%, and 100%, followed by propylene oxide, before being embedded in Araldite. Using Richert Ultracuts (Leica, Wetzlar, Germany), semithin sections (1 µm thick) were performed and stained with toluidine blue. Ultrotom VRV (LKB Bromma, Burladingen, Germany) was used to cut ultrathin sections. Sections measuring 70 nm were stained with a combination of lead citrate and uranyl acetate [14] and analyzed using JEOL 100CX II (JEOL, Tokyo, Japan) transmission electron microscope at Assiut University’s Electron Microscopy Unit; 5–10 fields of view were analyzed for each specimen.
3. Results
3.1. Light Microscopy
The wall of the goldfish bulbus arteriosus was thick and the inner surface of the bulbus was characterized by the presence of finger-like ridges in the form of longitudinal columns, giving the luminal surface an irregular appearance (Figure 1A,B). The endocardium covers the inside surface of the ridges and is in direct contact with the blood flow. The ridge endocardium was formed of squamous epithelium, surrounded by an amorphous extracellular matrix (Figure 1C,D). The middle layer of the bulbus contained smooth muscle cells, telocytes (TCs), and collagen bundles (Figure 1C). The bulbus opens to the aorta (Figure 1E).
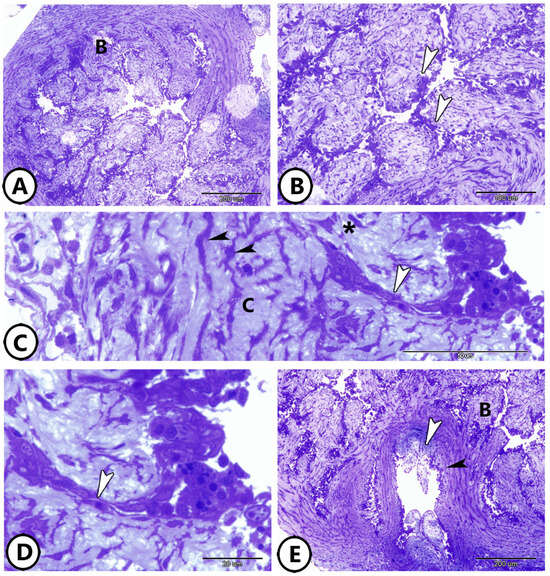
Figure 1.
Semithin sections of the wall of the bulbus arteriosus. (A,B) The wall of the bulbus (B) is characterized by the presence of ridges (arrowheads). (C,D) The inner surface of the ridges is covered by endocardium (white arrowheads). The middle layer of the bulbus contains branched telocytes (black arrowheads), smooth muscle fibers (asterisk), and collagen fibers (C). (E) The bulbus (B) opens to the aorta (black arrowhead). Note the presence of aortic valves (white arrowhead).
The inner surfaces of the bulbous contained various stages of rodlet cells. These cells were frequently found in groups, under the endothelium facing the bulbar lumen. The rodlet cells contained many cytoplasmic rodlet inclusions ranging from rounded to rod-like (Figure 2A–E). Different stages of the rodlet cells could be observed in the endocardial region, ranging from the vesicular stages that contained small vesicles and granules to mature stages that were filled with rodlet inclusions and contained an eccentric nucleus surrounded by a thick capsule (Figure 2F).
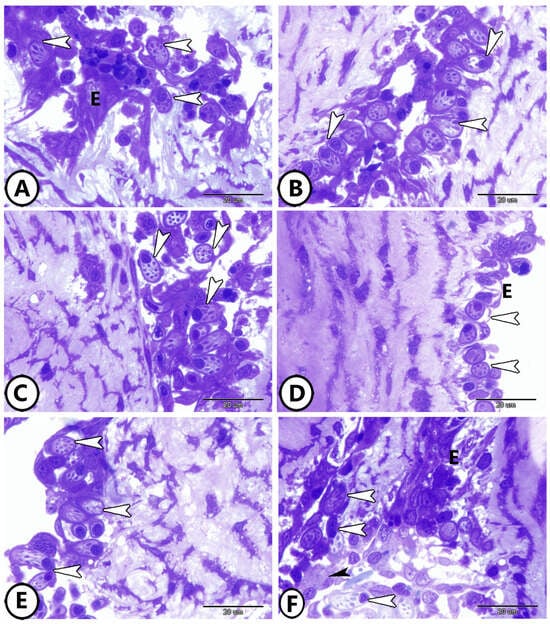
Figure 2.
Semithin sections of rodlet cells in the bulbous. (A–E) The inner surfaces of the bulbous contain various stages of rodlet cells (arrowheads) that occur between the endocardial cells (E). (F) Different stages of the rodlet cells could be observed in the endocardial region (E), ranging from the vesicular stages (black arrowhead) to mature stages (white arrowheads) that were filled with rodlet inclusions.
The middle layer consisted of smooth muscle cells intermingled between many branched TCs with expanded telopodes (Figure 3A,B). The subepicardium is a thin layer, rich in collagen. Also, it contained lymphocytes, dendritic cells, plasma cells, telocytes, nerve fibers, and blood capillaries (Figure 3C–F). The lymphocytes were characterized by their small size, dark nucleus, and high nuclear-to-cytoplasmic ratio. The dendritic cells were similar to lymphocytes but possessed dendrite-like processes. Plasma cells displayed eccentric clock-face nucleus and basophilic cytoplasm. The telocytes were characterized by oval-shaped cell bodies with distended processes called telopodes.
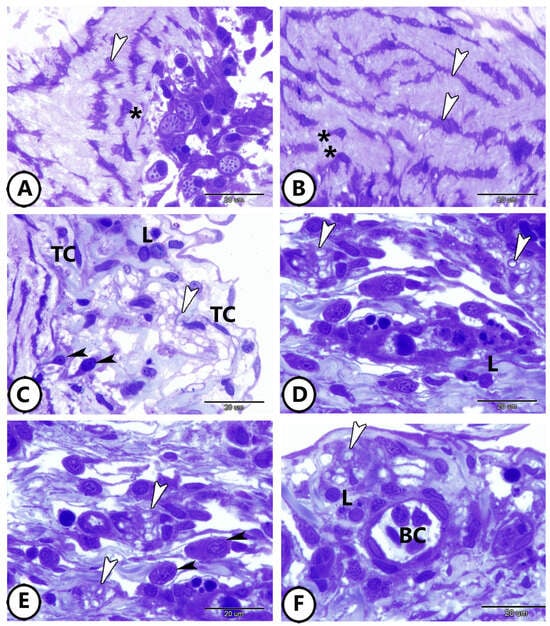
Figure 3.
Semithin sections of the middle layer and subepicardium of the bulbous. (A,B) The middle layer consisted of smooth muscle cells (asterisks) intermingled between many branched TCs (arrowheads). (C,D) The subepicardium contained lymphocytes (L), dendritic cells (black arrowheads), nerve fibers (white arrowheads), and telocytes (TC). (E,F) The subepicardium contains blood capillaries (BC), nerve fibers (white arrowheads), lymphocytes (L), and plasma cells (black arrowheads).
3.2. Electron Microscopy
TCs established direct heterocellular contact with rodlet cells both with their cell bodies and telopodes (Figure 4A–C). TCs and rodlet cells showed a direct connection with the simple squamous endocardial cells (Figure 4A,B) and underlying smooth muscle cells (Figure 4D). The rodlet cells were characterized by eccentric heterochromatic nucleus and cytoplasmic rounded to club-shaped inclusions of moderate electron density, vesicles, and ribosomes, and surrounded by a thick capsule (Figure 4A–C).
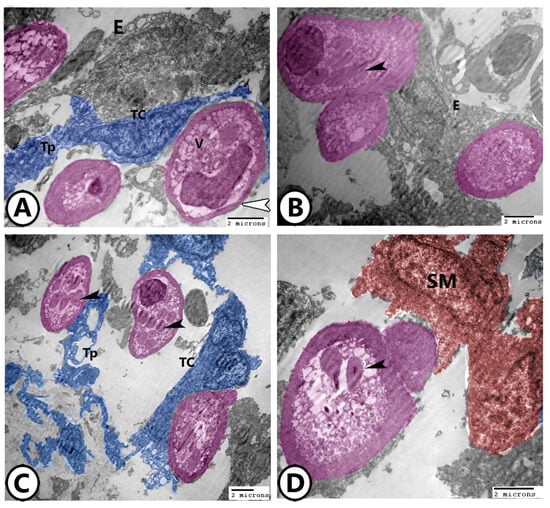
Figure 4.
Digitally colored TEM images of rodlets cells and telocytes (TCs). (A–C) TCs (blue) and their telopodes (Tps) established direct heterocellular contact with rodlet cells (pink). Note their relationship to the endocardium (E). The rodlet cells are characterized by cytoplasmic rounded to rodlet-like inclusions (black arrowheads) and surrounded by a thick capsule (white arrowhead). (D) Mature rodlet cells with club-shaped inclusions (arrowhead) connected with smooth muscle fibers (SM, red).
In the mature stages, the cytoplasm of rodlet cells contained an electron-dense line in the center of rodlet bodies and numerous ribosomes (Figure 5A). The rodlet cells showed signs of secretory activity (Figure 5A,B). Furthermore, ruptured rodlet cells could be seen with the holocrine mode of secretion (Figure 5C,D). Macrophages with distinct kidney-shaped nuclei and cytoplasmic vacuoles were seen in association with the ruptured rodlet cells (Figure 5D). Also, TCs extended their telopodes around the ruptured rodlet cells and macrophages (Figure 5D).
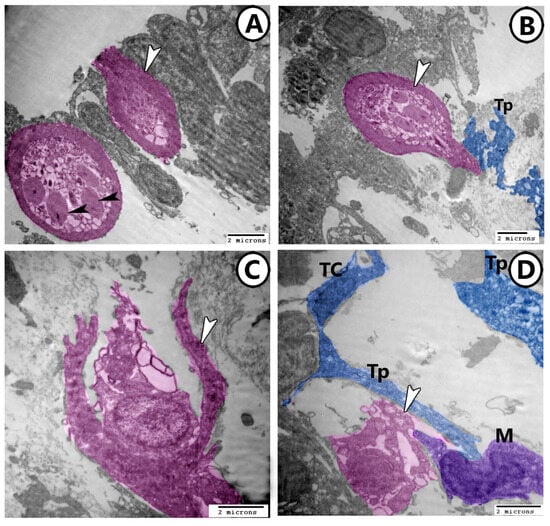
Figure 5.
Digitally colored TEM images of mature and ruptured rodlets cells. (A,B) In the mature stages, the cytoplasm of rodlet cells (pink) contained an electron-dense line in the center of rodlet bodies (black arrowheads). The rodlet cells showed signs of secretory activity (white arrowheads). Note their connections with telopodes (Tp). (C,D) Ruptured rodlet cells could be seen with the holocrine mode of secretion (pink, arrowheads). Macrophage (M, violet) and telocytes (TC, blue) with their telopodes (Tp, blue) are associated with ruptured rodlet cells.
TCs and their telopodes were embedded in a dense collagenous matrix and showed 3D connections with rodlet cells, smooth muscle fibers, and macrophages (Figure 6A–D). The endocardium showed direct contact with telopodes (Figure 6C). Macrophages could be observed in association with the endothelium (Figure 6C).
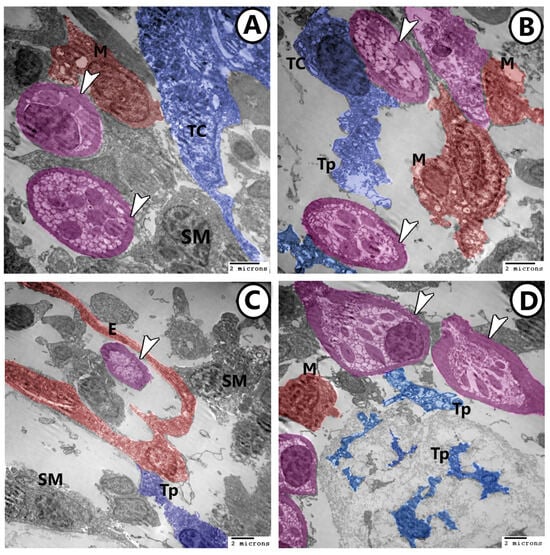
Figure 6.
Digitally colored TEM images of rodlets cells, TCs, and macrophages. (A,B) TCs (blue) and their telopodes (Tps) showed 3D connections with rodlet cells (pink, arrowheads) and macrophages (red, M). Note the presence of the underlying smooth muscle (SM) fibers. (C) The endocardium (E, red) showed a direct contact with telopodes (Tp, blue) and smooth muscle (SM) fibers. Macrophages (red, M) could be observed in association with the endothelium. Note the presence of rodlet cell (pink, arrowhead). (D) Numerous rodlet cells (pink, arrowheads) were seen in connection with Tps and macrophages (red, M).
The endocardial cells were irregular, stellate in shape, and showed many cell processes that established direct contact with TCs (Figure 7A). The endocardial cells showed irregular-shaped nuclei with peripheral heterochromatin and contained moderately dense bodies as well as membrane-bound vacuoles (Figure 7B). TCs showed high secretory activity in the bulbous arteriosus as their telopodes showed many secretory vesicles. The telopodes showed heterocellular contact with smooth muscle fibers (Figure 7C). TCs shed some excretory vesicles to the extracellular matrix (Figure 7D).
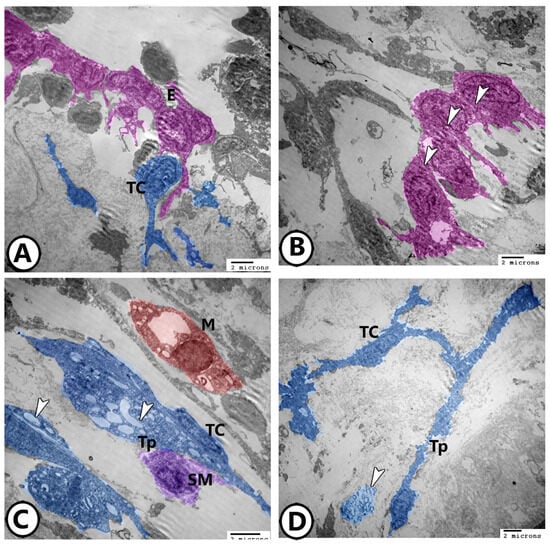
Figure 7.
Digitally colored TEM images of the endocardial cells and TCs. (A) The endocardial cells (violet) established direct contact with TCs (blue). (B) The endocardial cells contained dense bodies (arrowheads). (C) The telopodes (Tp, blue) of TCs showed many secretory vesicles (arrowheads). They showed a direct homocellular contact with smooth muscle fibers (SM violet). Note the presence of macrophages (red, M). (D) TCs shed some excretory vesicles to the extracellular matrix (arrowhead).
4. Discussion
In more evolved teleosts, a structurally significant component of the outflow tract (OFT) is the bulbus arteriosus. It performs the role of an elastic reservoir, “Windkessel”, extending to accommodate a significant portion of the stroke volume during ventricular contraction [15]. To prevent gill damage from high arterial systolic blood pressure and to provide a more equal perfusion of gill lamellae for gas exchange during each cardiac cycle, subsequent elastic recoil progressively releases this volume [16]. The proximity of teleost’s heart and gills, as well as their low systolic pressures, may be linked to numerous characteristics of the bulbus arteriosus. As a result, the bulbus can expand more than the human aorta and needs to be positioned inside the pericardial cavity to allow for its larger excursions. The bulbus contains elastic fibers instead of lamellae, possibly due to its great distensibility [17]. According to Licht and Harris [18], the distensibility of the carp bulbus is nearly 30 times greater than that of the human thoracic aorta. Serafini-Fracassini et al. [19] report that the elastic tissue in the bulbus is different from mammalian protein in composition and does not include the form of lamellae.
Like other teleosts, the goldfish has multiple different layers in its bulbus wall. The continuous endothelium that is in contact with blood flow is known as the endocardium. Secretory vacuoles are seen by TEM in many endocardial cells. In agreement with our study, Icardo et al. [10,11] mentioned that the endocardium invaginates the ridge tissue to create structures that resemble epithelial cells and have secretory function.
The current study showed that the endocardial cells contained dense bodies that were implicated in many functions, such as the secretory function as in the Antarctic teleosts [10,11] and eel bulbus arteriosus [17], where they are involved in the production of anti-freeze mucins. Many dense bodies are composed of homogeneous material and are thought to be the storage site for glycosaminoglycans (GAGs) [20,21]. In other heart chambers, the endocardial cells show scavenger functions [22], bind natriuretic peptides [23], or act as a source of endogenous nitric oxide [24], or may be involved in the autocrine/paracrine regulation of the subjacent tissue [25].
Collagen bundles, blood vessels, and nerves may be present in the middle layer of the bulbous, as in tuna [16], or it may have a few collagen layers interspersed with elastin material, as in the common eel [26]. But like the Antarctic teleosts and the goldfish under study, it might be devoid of elastin. A fibrillar network, most likely composed of glycosaminoglycans (GAGs), replaces the elastin material in these species [10,11]. According to Priede [27], the bulbus arteriosus could still function as an elastic reservoir in the absence of elastic fibers, preserving steady aortic flow throughout ventricular diastole. A matrix rich in GAGs can withstand cyclical pressure fluctuations since GAGs are instantly compressible molecules [28]. It is debatable whether smooth muscle cells contribute to the bulbus wall’s elastic qualities [9,18,27]. These cells may be able to contract based on the existence of directed bundles of microfilaments and irregular nuclei. The bulbus matrix appears to receive this tension, based on the direction of the matrix filaments.
The present study showed that the outer layer of the bulbus is made of connective tissue and comprises blood vessels, immune cells, and unmyelinated nerve fibers. The immune cells include lymphocytes, dendritic cells, and mast cells. In contrast to the tunica adventitia of an elastic artery, the epicardium is made up of flattened cells (mesothelium) with numerous pinocytotic vesicles, suggesting an active exchange of solutes with the pericardial cavity [17,26]. The subepicardium of the sturgeon contains thymus-like tissue [29], which has been implicated in the establishment and maintenance of the cellular immune responses. Plasma cells and dendritic cells were described in the bulbus arteriosus of the Antarctic teleosts [10]. These cells are involved in presenting antigens to B lymphocytes to stimulate the humoral response. In this context, the presence of macrophages and granulocytes is important since these cells have been suggested to play important roles in the induction of the immune response in fish by stimulating the differentiation of plasma cells and the secretion of cytokines [30].
The present study clearly shows that rodlet cells are abundant throughout the luminal region of the bulbus arteriosus. Vascular endothelium was found to be connected to the rodlet cells. However, rodlet cells were consistently observed beneath or in between endothelial cells in the circulatory system of cyprinids, particularly in the bulbus arteriosus, but they were never linked to the vascular endothelia of gadids, salmonids, or labrids [31,32]. The contents of ruptured rodlet cells are secreted through apical pores and extruded to the bulbus arteriosus lumen (holocrine mode of secretion); this was demonstrated by the TEM analysis of the current work. There are several roles that these cells have been reported to play, one of which is a defensive secretory function [33], particularly against parasite helminths [34]. Their possible functions include pH regulation, lubrication, and the movement of water or electrolytes [35]. The presence of numerous rodlet cells at different developmental stages with their secretory activities along the luminal part of the bulbous indicated that the bulbous arteriosus is the origin of these cells.
In this study, macrophages were observed both in the endocardium and in the middle layer, they were in direct contact with rodlet cells and TCs. Macrophages were also seen in association with the ruptured rodlet cells, indicating their role in degradation and phagocytosis. These cells play a major role in the immune response involving mechanisms as phagocytosis, the breakdown of foreign antigens, tissue remodeling, and the synthesis of growth factors, cytokines, and chemokines [36]. According to a recent study by Huang et al. [37] TCs are thought to be involved in the apoptosis, mitochondrial pathway, apoptosis, and macrophages differentiation.
Telocytes are interstitial cells recorded in many organs, including the heart [38,39]. Their functions include cell-to-cell signaling, stem-cell nursing, mechanical support, and immunoregulation [40,41]. Based on their localization and cell interactions during heart development, telocytes are thought to have a role in establishing the intricate three-dimensional structure of the organ and guiding tissue organization throughout morphogenesis [42]. TCs possibly perform significant tasks during cardiogenesis, including the organization of the cytoarchitecture of the myocardial constituents, mechanical support, and supervision of the correct sequence of stem-cell differentiation [43]. Moreover, the proximity of TC processes to heart endothelial cells raises the possibility that they are involved in the development of the blood–heart or blood–myocardial barrier [44]. TCs were shown to “nurse” cardiac progenitor cells in the epicardium’s stem-cell niches.
The present study showed that TCs shed extracellular vesicles containing bioactive molecules to the extracellular matrix, through which TCs may influence their surroundings in a paracrine manner [45]. TCs may coordinate the differentiation of stem cells and use paracrine signaling to regulate all the surrounding interstitial compartment [46]. A study of frogs concluded that TC renewal might be the first essential step in any further fruitful regeneration of the tissue architecture [47].
In this study, we were unable to perform immunohistochemistry due to several limitations, including the lack of specific antibodies for fish tissue, technical challenges with optimizing protocols, and limited access to specialized equipment and reagents. Despite these issues, our research underscores the importance of studying the bulbus arteriosus in fish, as it plays a vital role in regulating blood flow from the heart to the gills. Understanding its structure and function can provide valuable insights into cardiovascular adaptations in aquatic environments. Future studies should focus on developing fish-specific antibodies and optimized protocols to enable deeper investigation into the cellular and molecular mechanisms of the bulbus arteriosus.
5. Conclusions
The bulbous arteriosus (Figure 8) possesses unique characteristics, suggesting that the bulbus arteriosus is the origin of rodlet cells. Telocytes (TCs) play a crucial role in this structure, forming intricate connections with various cells and tissues and engaging in robust secretory activity. The direct contact between endothelium and telopodes points to important interactions within the bulbus. These findings highlight the potential of TCs for cellular therapies and heart regeneration, offering promising avenues for future research and treatment strategies in cardiovascular health.
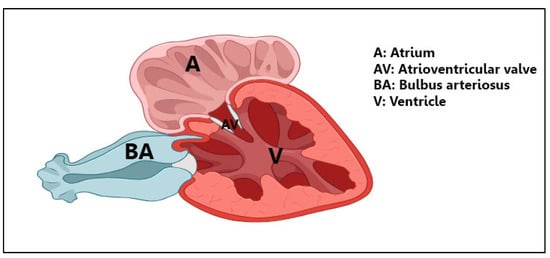
Figure 8.
A schematic diagram of the anatomy of the heart of the goldfish.
Author Contributions
Conceptualization, methodology, formal analysis, data curation, D.M.M. and M.T.H.; resources, E.A.A.-E. and M.A.; writing—original draft preparation, D.M.M. and M.T.H.; writing—review and editing, M.A. and G.Z.; validation, M.A., E.A.A.-E. and G.Z.; investigation—supervision, D.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol and all the procedures in this study were approved by the National Ethics Committee of the Faculty of Veterinary Medicine, Assiut University, Egypt. N° 06/2024/0191.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the technical support provided from the department of cell and tissues, Faculty of Veterinary medicine, and Electron Microscopy unit in Assiut University, Egypt.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Simmons, D.B.D.; McCallum, E.S.; Balshine, S.; Chandramouli, B.; Cosgrove, J.; Sherry, J.P. Reduced anxiety is associated with the accumulation of six serotonin reuptake inhibitors in wastewater treatment effluent exposed goldfish Carassius auratus. Sci. Rep. 2017, 7, 17001. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.P.; Jones, D.R. The Heart. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 12, pp. 1–88. [Google Scholar] [CrossRef]
- Icardo, J.M. Conus arteriosus of the teleost heart: Dismissed, but not missed. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Grimes, A.C.; Kirby, M.L. The outflow tract of the heart in fishes: Anatomy, genes and evolution. J. Fish Biol. 2009, 74, 983–1036. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J.M. The Teleost Heart: A Morphological Approach. In Ontogeny and Phylogeny of the Vertebrate Heart; Springer: New York, NY, USA, 2012; pp. 35–53. ISBN 9781461433873. [Google Scholar] [CrossRef]
- Jones, D.R.; Brill, R.W.; Bushnell, P.G. Ventricular and Arterial Dynamics of Anaesthetised and Swimming Tuna. J. Exp. Biol. 1993, 182, 97–112. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ito, F.; Takeda, H.; Yano, T.; Okabe, M.; Kuraku, S.; Keeley, F.W.; Koshiba-Takeuchi, K. Evolution of the fish heart by sub/neofunctionalization of an elastin gene. Nat. Commun. 2016, 7, 10397. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, P.G.; Jones, D.R.; Farrell, A.P. The Arterial System. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 12, pp. 89–139. ISBN 1546-5098. [Google Scholar]
- Watson, A.D.; Cobb, J.L.S. A comparative study on the innervation and the vascularization of the bulbus arteriosus in teleost fish. Cell Tissue Res. 1979, 196, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Bulbus arteriosus of the antarctic teleosts. I. The white-blooded Chionodraco hamatus. Anat. Rec. 1998, 254, 396–407. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Bulbus Arteriosus of the Antarctic Teleosts. II. The Red-Blooded Trematomus bernacchii. Anat. Rec. 1999, 256, 116–126. [Google Scholar] [CrossRef]
- Posner, L.P.; Scott, G.N.; Law, J.M. Repeated Exposure of Goldfish (Carassius auratus) to Tricaine Methanesulfonate (MS-222). J. Zoo Wildl. Med. 2013, 44, 340–347. [Google Scholar] [CrossRef]
- Karnovsky, M.; Karnovsky, M.; Karnovsky, M.; Karnovsky, M.L.; Karnovsky, M. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron-Microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Reynolds, E.S. The Use of Lead Citrate at High pH as an Electron-Opaque Stain in Electron Microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.P. The Wind-Kessel effect of the bulbus arteriosus in trout. J. Exp. Zoöl. 1979, 209, 169–173. [Google Scholar] [CrossRef]
- Braun, M.H.; Brill, R.W.; Gosline, J.M.; Jones, D.R. Form and function of the bulbus arteriosus in yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus) and blue marlin (Makaira nigricans): Static properties. J. Exp. Biol. 2003, 206, 3311–3326. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Norman, D.; Santer, R.M.; Scarborough, D. Histological, histochemical and ultrastructural studies on the bulbus arteriosus of the sticklebacks, Gasterosteus aculeatus and Pungitius pungitius (Pisces: Teleostei). J. Zoöl. 1983, 200, 325–346. [Google Scholar] [CrossRef]
- Licht, J.; Harris, W.S. The structure, composition and elastic properties of the teleost bulbus arteriosus in the carp, Cyprinus carpio. Comp. Biochem. Physiol. Part A Physiol. 1973, 46, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Serafini-Fracassini, A.; Field, J.; Spina, M.; Garbisa, S.; Stuart, R. The morphological organization and ultrastructure of elastin in the arterial wall of trout (Salmo gairdneri) and salmon (Salmo salar). J. Ultrastruct. Res. 1978, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Norman, D.; Scarborough, D.; Santer, R.M. Carbohydrate-containing endothelial cells lining the bulbus arteriosus of teleosts and the conus arteriosus of elasmobranchs (Pisces). J. Zoöl. 1984, 202, 383–392. [Google Scholar] [CrossRef]
- Walker, M.G.; Santer, R.M.; Benjamin, M.; Norman, D. Heart structure of some deep-sea fish (Teleostei: Macrouridae). J. Zoöl. 1985, 205, 75–89. [Google Scholar] [CrossRef]
- Seternes, T.; Sørensen, K.; Smedsrød, B. Scavenger endothelial cells of vertebrates: A nonperipheral leukocyte system for high-capacity elimination of waste macromolecules. Proc. Natl. Acad. Sci. USA 2002, 99, 7594–7597. [Google Scholar] [CrossRef]
- Cerra, M.C.; Canonaco, M.; Acierno, R.; Tota, B. Different binding activities of A- and B-type natriuretic hormones in the heart of two Antarctic teleosts, the red-blooded Trematomus bernacchii and the hemoglobinless Chionodraco hamatus. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 993–999. [Google Scholar] [CrossRef]
- Imbrogno, S.; Cerra, M.C.; Tota, B. Angiotensin II-induced inotropism requires an endocardial endothelium-nitric oxide mechanism in the in-vitro heart of Anguilla anguilla. J. Exp. Biol. 2003, 206, 2675–2684. [Google Scholar] [CrossRef]
- Icardo, J.M. The Fish Endocardium: A Review on the Teleost Heart. In Endothelial Biomedicine; Cambridge University Press: Cambridge, UK, 2007; pp. 79–84. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Light and Electron Microscopy of the Bulbus arteriosus of the European Eel (Anguilla anguilla). Cells Tissues Organs 2000, 167, 184–198. [Google Scholar] [CrossRef]
- Priede, I.G. Functional morphology of the bulbus arteriosus of rainbow trout (Salmo gairdneri Richardson). J. Fish Biol. 1976, 9, 209–216. [Google Scholar] [CrossRef]
- Hascall, V.C.; Hascall, G.K. Proteoglycans. In Cell Biology of Extracellular Matrix; Springer: Boston, MA, USA, 1981; pp. 39–63. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. The structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart: II. The myocardium, the subepicardium, and the conus-aorta transition. Anat. Rec. 2002, 268, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Picchietti, S.; Taverne-Thiele, J.J.; Taverne, N.; Abelli, L.; Mastrolia, L.; Kemenade, B.M.L.V.-V.; Rombout, J.H.W.M. Distribution of macrophages during fish development: An immunohistochemical study in carp (Cyprinus carpio L.). Anat. Embryol. 1998, 198, 31–41. [Google Scholar] [CrossRef]
- Leknes, I.L. Fine structure and cytochemistry of the endothelial cells and rodlet cells in the bulbus arteriosus in species of Cichlidae (Teleostei). J. Fish Biol. 1986, 28, 29–36. [Google Scholar] [CrossRef]
- Reite, O.B. The rodlet cells of teleostean fish: Their potential role in host defence in relation to the role of mast cells/eosinophilic granule cells. Fish Shellfish Immunol. 2005, 19, 253–267. [Google Scholar] [CrossRef]
- Mendonça, I.; Matos, E.; Rodrigues, G.; Matos, P.; Casal, G.; Azevedo, C. Rodlet Cells from the Gills and Kidneys of two Brazilian Freshwater Fishes: An Ultrastructural Study. Braz. J. Morphol. Sci. 2005, 22, 187–192. [Google Scholar]
- Secombes, C.J.; Wang, T. The Innate and Adaptive Immune System of Fish. In Infectious Disease in Aquaculture: Prevention and Control; Woodhead Publishing: Sawston, UK, 2012; pp. 3–68. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdelhafez, E.A. An Overview of the Structural and Functional Aspects of Immune Cells in Teleosts. Histol. Histopathol. 2021, 36, 16. [Google Scholar]
- Mokhtar, D.M.; Hussein, M.M. Microanalysis of Fish Ovarian Follicular Atresia: A Possible Synergic Action of Somatic and Immune Cells. Microsc. Microanal. 2020, 26, 599–608. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Zhang, F.-L.; Tang, X.-L.; Yang, X.-J. Telocytes Enhances M1 Differentiation and Phagocytosis While Inhibits Mitochondria-Mediated Apoptosis Via Activation of NF-κB in Macrophages. Cell Transplant. 2021, 30. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Curici, A.; Wang, E.; Zhang, H.; Hu, S.; Gherghiceanu, M. Telocytes and putative stem cells in ageing human heart. J. Cell. Mol. Med. 2015, 19, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Shim, W. Myocardial Telocytes: A New Player in Electric Circuitry of the Heart. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2016; Volume 913, pp. 241–251. [Google Scholar] [CrossRef]
- Klein, M.; Csöbönyeiová, M.; Žiaran, S.; Danišovič, L’.; Varga, I. Cardiac Telocytes 16 Years on—What Have We Learned So Far, and How Close Are We to Routine Application of the Knowledge in Cardiovascular Regenerative Medicine? Int. J. Mol. Sci. 2021, 22, 10942. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Tay, H.; Vandecasteele, T.; Broeck, W.V.D. Identification of telocytes in the porcine heart. Anat. Histol. Embryol. 2017, 46, 519–527. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.-S.; Bani, D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J. Cell. Mol. Med. 2010, 14, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Popescu, L.M. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: Electron microscope images. J. Cell. Mol. Med. 2010, 14, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Manole, C.G.; Gherghiceanu, M.; Ardelean, A.; Nicolescu, M.I.; Hinescu, M.E.; Kostin, S. Telocytes in human epicardium. J. Cell. Mol. Med. 2010, 14, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Sukhacheva, T.; Nizyaeva, N.; Samsonova, M.; Chernyaev, A.L.; Shchegolev, A.; Serov, R.A. Telocytes in the Myocardium of Children with Congenital Heart Disease Tetralogy of Fallot. Bull. Exp. Biol. Med. 2020, 169, 137–146. [Google Scholar] [CrossRef]
- Lv, L.; Liao, Z.; Luo, J.; Chen, H.; Guo, H.; Yang, J.; Huang, R.; Pu, Q.; Zhao, H.; Yuan, Z.; et al. Cardiac telocytes exist in the adult Xenopus tropicalis heart. J. Cell. Mol. Med. 2020, 24, 2531–2541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).