Age, Growth, and Otolith Morphometrics of Trachinus draco (L., 1758) and Trachinus radiatus (Cuvier, 1829) in the Eastern Mediterranean
Abstract
1. Introduction
2. Material and Methods
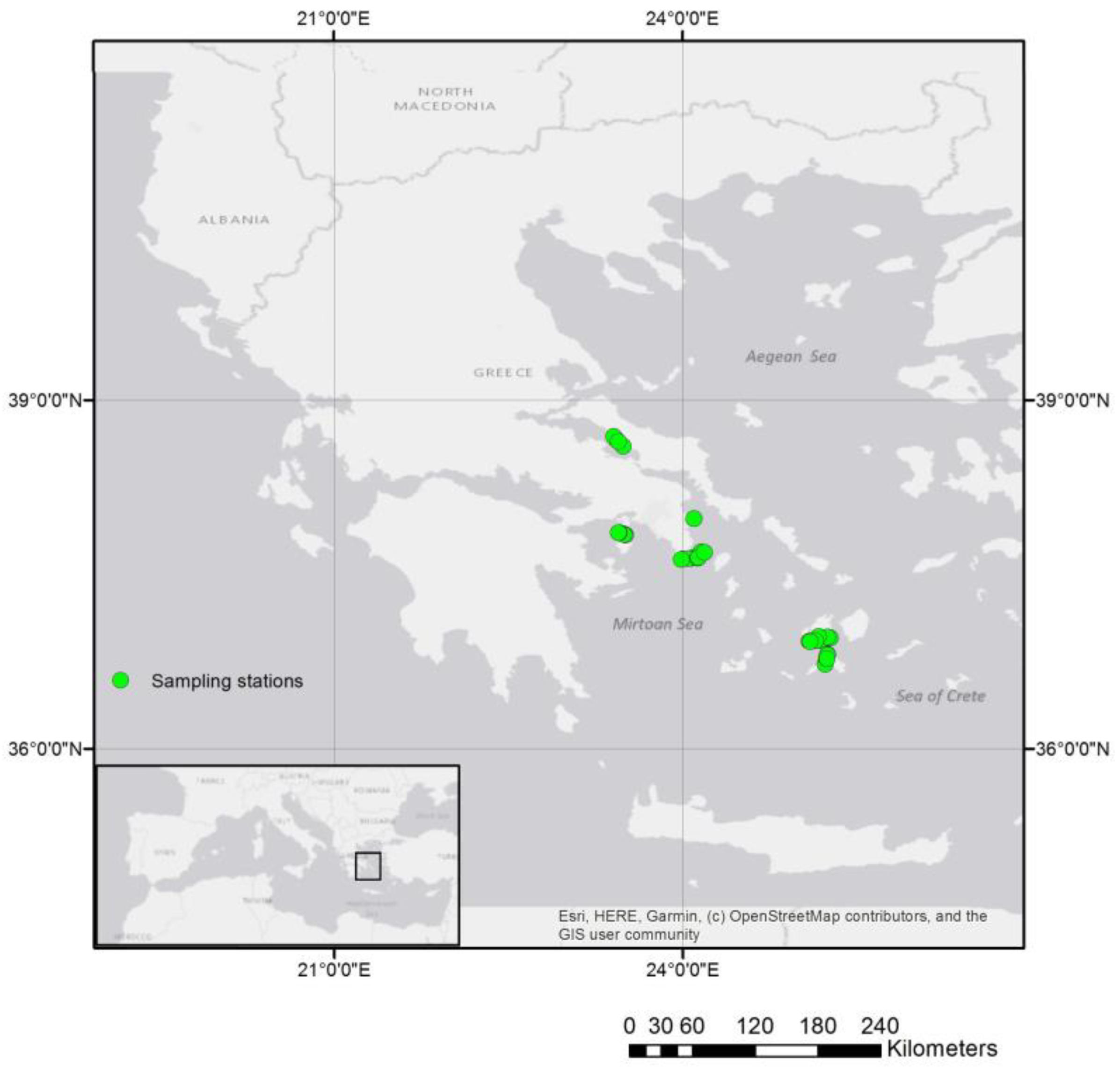
2.1. Study Area and Data Collection
2.2. Data Analysis
2.2.1. Growth
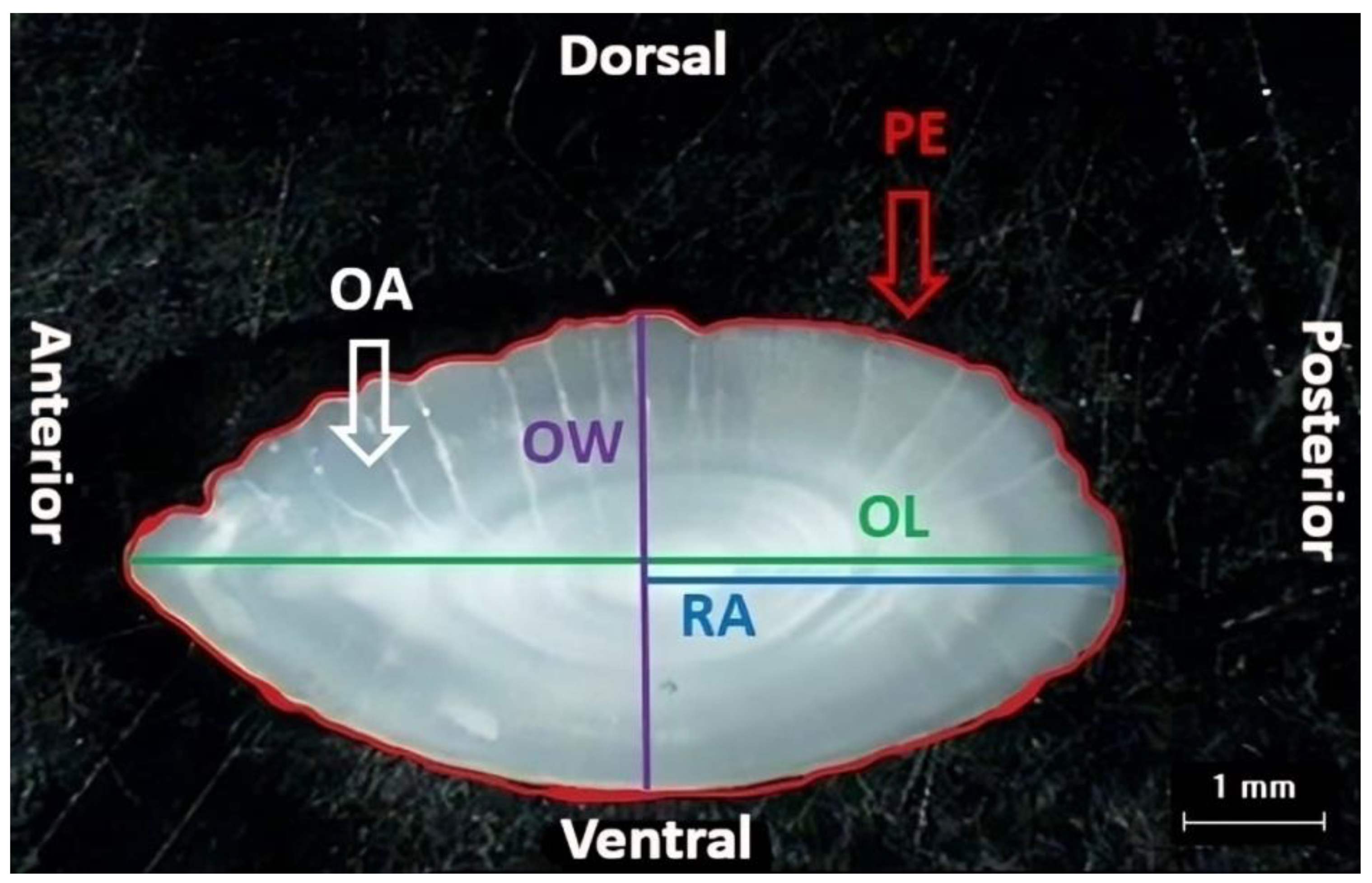
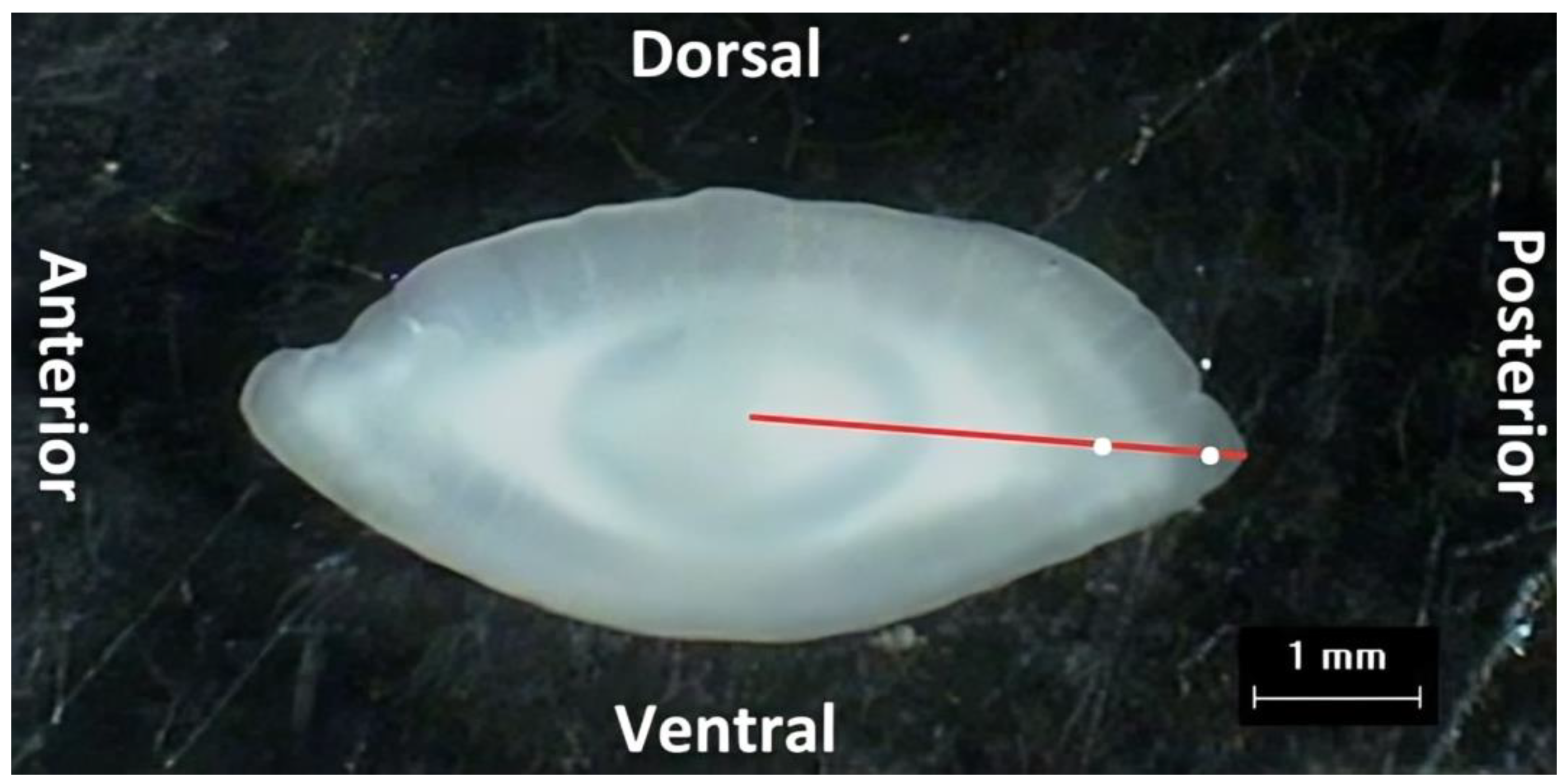
2.2.2. Otolith Morphometrics
3. Results
3.1. Growth
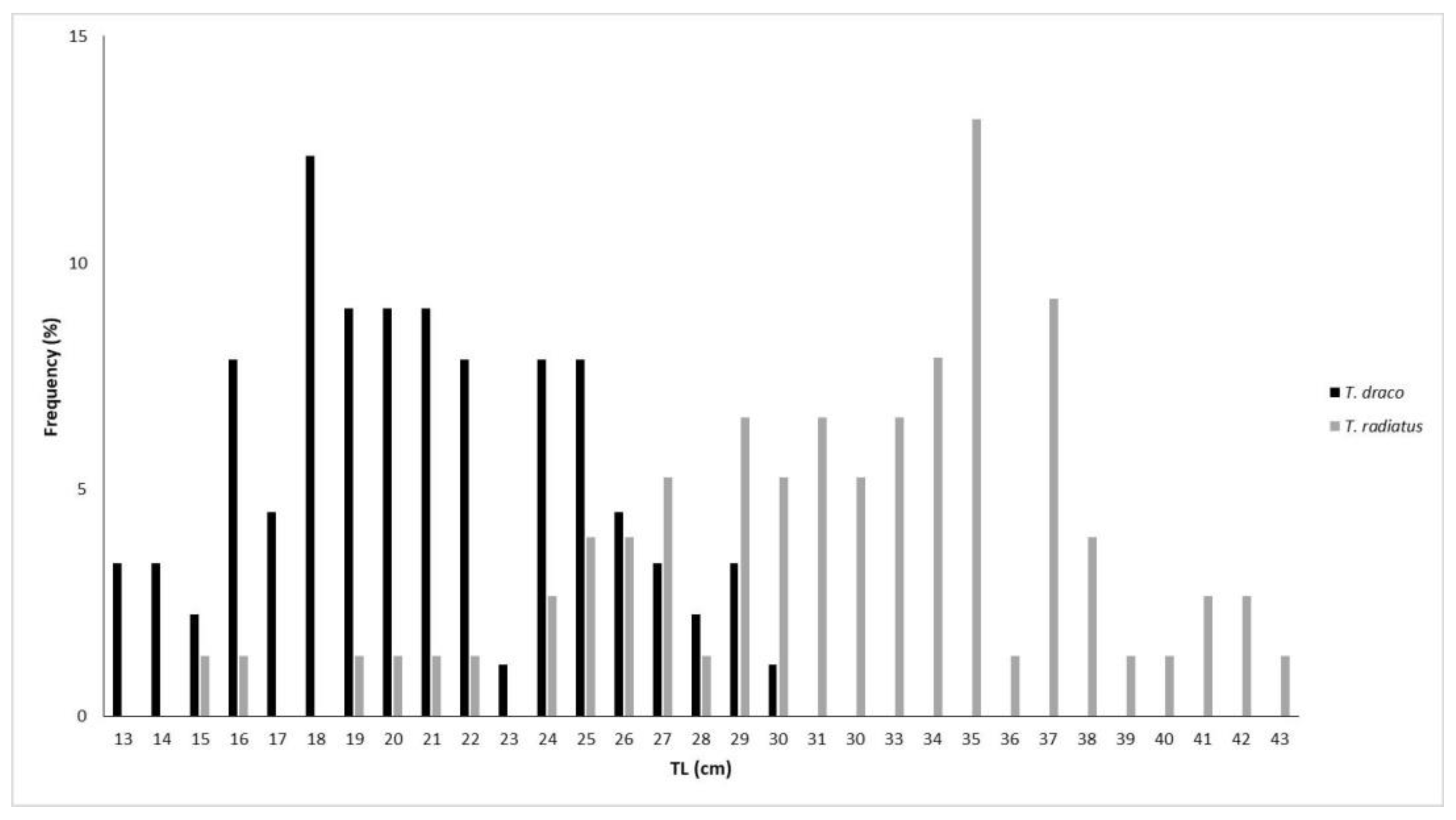
3.1.1. Length Distribution
3.1.2. Weight–Length Relationship
3.1.3. Age and Growth
3.2. Otolith Morphometrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadrin, S.X.; Friedland, K.D.; Waldman, J.R. Stock identification methods: An overview. In Stock Identification Methods: Applications in Fishery Science, 2nd ed.; Cadrin, S.X., Friedland, K.D., Waldman, J.R., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 3–6. [Google Scholar]
- Anastasopoulou, A.; Biandolino, F.; Chatzispyrou, A.; Hemida, F.; Guijarro, B.; Kousteni, V.; Prato, E. New Fisheries-related data from the Mediterranean Sea. Mediterr. Mar. Sci. 2016, 17, 822–827. [Google Scholar] [CrossRef][Green Version]
- FAO. The State of Mediterranean and Black Sea Fisheries; General Fisheries Commission for the Mediterranean: Rome, Italy, 2020; p. 172. [Google Scholar] [CrossRef]
- Fish Base. Available online: https://fishbase.mnhn.fr/summary/1363 (accessed on 13 March 2023).
- Tortonese, E. Trachinidae. In Checklist of the Fishes of the North-Eastern Atlantic and of the Mediterranean (CLOFNAM); Hureau, J.C., Monod, T., Eds.; UNESCO: Paris, France, 1986; Volume 2, pp. 951–954. ISBN 92-3-001100-2. [Google Scholar]
- Muus, B.J.; Nielsen, J.G. Sea Fish. Scandinavian Fishing Year Book; Narayana Press: Hedehusene, Denmark, 1999; p. 340. [Google Scholar]
- Hamed, O.; Chakroun-Marzouk, N. Aspects reproductifs de Trachinus draco Linnaeus, 1758 du golfe de Tunis. In Proceedings of the Actes des XVIèmes Journées Tunisiennes des Sciences de la Mer, Zarzis, Tunisia, 19–23 December 2015; p. 27. [Google Scholar]
- Betogian, L.; Mytilineou, C.; Anastasopoulou, A.; Batzakas, I. Diet composition of Trachinus draco and Trachinus radiatus from the South Aegean Sea (E. Mediterranean). Cah. Biol. Mar. 2019, 60, 461–464. [Google Scholar] [CrossRef]
- Hamed, O.; Dufour, J.L.; Chakroun-Marzouk, N.; Mahé, K. Age, growth and mortality of the starry weever Trachinus radiatus Cuvier, 1829 in the Tunisian waters. Cah. Biol. Mar. 2019, 60, 87–94. [Google Scholar] [CrossRef]
- Başusta, N.; Erdem, U. A Study on the pelagic and demersal fishes of Iskenderun Bay (in Turkish). Turk. J. Zool. 2000, 24, 1–19. [Google Scholar]
- Ak, O.; Genç, Y. Growth and reproduction of the greater weever (Trachinus draco L., 1758) along the eastern coast of the Black Sea. J. Black Sea/Mediterr. Environ. 2013, 19, 95–110. [Google Scholar]
- Dulčić, J.; Kraljević, M. Weight-length relationships for 40 fish species in the eastern Adriatic (Croatian waters). Fish. Res. 1996, 28, 243–251. [Google Scholar] [CrossRef]
- Merella, P.; Quetglas, A.; Alemany, F.; Carbonell, A. Weight-length relationships of fishes and cephalopods from the Balearic Islands (western Mediterranean). Naga ICLARM Q. 1997, 20, 66–68. [Google Scholar]
- Moutopoulos, D.K.; Stergiou, K.I. Length–weight and length–length relationships of fish species from the Aegean Sea (Greece). J. Appl. Ichthyol. 2002, 18, 200–203. [Google Scholar] [CrossRef]
- Özaydin, O.; Uckun, D.; Akalin, S.; Leblebici, S.; Tosunoglu, Z. Length-weight relationships of fishes captured from Izmir Bay, Central Aegean Sea. J. Appl. Ichthyol. 2007, 23, 695–696. [Google Scholar] [CrossRef]
- Ilkyaz, A.T.; Metin, G.; Soykan, O.; Kinacigil, H.T. Weight-length relationships of 62 fish species from the Central Aegean Sea, Turkey. J. Appl. Ichthyol. 2008, 24, 699–702. [Google Scholar] [CrossRef]
- Karachle, P.K.; Stergiou, K.I. Length-length and weight-length relationships of several fish species from the North Aegean Sea (Greece). J. Biol. Res. 2008, 10, 149–157. [Google Scholar]
- Giacalone, V.M.; D’Anna, G.; Badalamenti, F.; Pipitone, C. Weight-length relationships and condition factor trends for thirty-eight fish species in trawled and untrawled areas off the coast of northern Sicily (central Mediterranean Sea). J. Appl. Ichthyol. 2010, 26, 954–957. [Google Scholar] [CrossRef]
- Crec’hriou, R.; Neveu, R.; Lenfant, P. Length–weight relationship of main commercial fishes from the French Catalan coast. J. Appl. Ichthyol. 2012, 28, 861–862. [Google Scholar] [CrossRef]
- Ordines, F.; Farriols, M.; Lleonart, J.; Guijarro, B.; Quetglas, A.; Massutí, E. Biology and population dynamics of by-catch fish species of the bottom trawl fishery in the western Mediterranean. Mediterr. Mar. Sci. 2014, 15, 613–625. [Google Scholar] [CrossRef]
- Başusta, Ν.; Buz, K. The relationships between otolith dimensions and total length of the greater weever Trachinus draco from the North-eastern Mediterranean, Turkey. In New Fisheries-Related Data from the Mediterranean Sea; Karachle, P.K., Başusta, A., Başusta, N., Bostanci, D., Buz, K., Girgin, H., Chater, I., Kokokiris, L., Kontaş, S., Ktari, M.H., et al., Eds.; Mediterranean Marine Science: Athens, Greece, 2015; Volume 16, pp. 285–293. [Google Scholar] [CrossRef]
- Buz, K.; Başusta, N. Age and growth of the greater weever, Trachinus draco (Linnaeus, 1758) inhabiting Iskenderun Bay, North-eastern Mediterranean Sea. Cah. Biol. Mar. 2015, 56, 289–295. [Google Scholar]
- Hamed, O.; Mahé, K.; Chakroun-Marzouk, N. Somatic growth, condition and form factor of Trachinus draco (Linnaeus, 1758) in the gulf of Tunis (Tunisia). INSTM Bull. Mar. Freshw. Sci. 2017, 44, 1–11. [Google Scholar]
- Soldo, A. Length-weight relationships for the fifty littoral and coastal marine fish species from the Eastern Adriatic Sea. Acta Adriat. 2020, 61, 205–210. [Google Scholar] [CrossRef]
- Morey, G.; Moranta, J.; Massuti, E.; Grau, A.; Linde, M.; Riera, F.; Morales-Nin, B. Weight–length relationships of littoral to lower slope fishes from the western Mediterranean. Fish. Res. 2003, 62, 89–96. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Fournari-Konstantinidou, I. Length-weight relations for 20 fish species (Actinopterygii) from the southern Ionian Sea, Greece. Acta Ichthyol. Piscat. 2018, 48, 415–417. [Google Scholar] [CrossRef]
- ICES. Workshop on Age reading of Horse Mackerel, Mediterranean Horse Mackerel and Blue Jack Mackerel (Trachurus trachurus, T. mediterraneus and T. picturatus) (WKARHOM3). In Proceedings of the ICES CM 2018/EOSG:28, Livorno, Italy, 5–9 November 2018; p. 186. [Google Scholar]
- NOAA (National Oceanic and Atmospheric Administration Fisheries). Available online: https://www.fisheries.noaa.gov/national/science-data/age-and-growth (accessed on 25 October 2022).
- Ponton, D. Is geometric morphometrics efficient for comparing otolith shape of different fish species? J. Morphol. 2006, 267, 750–757. [Google Scholar] [CrossRef]
- Pothin, K.; Gonzalez-Salas, C.; Chabanet, P.; Lecomte-Finiger, R. Distinction between Mulloidichthys flavolineatus juveniles from Reunion Island and Mauritius Island (south-west Indian Ocean) based on otolith morphometrics. J. Fish Biol. 2006, 69, 38–53. [Google Scholar] [CrossRef]
- Tuset, V.M.; Lozano, I.J.; Gonzĺez, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Nikiforidou, V.; Anastasopoulou, A.; Betogian, L.; Mytilineou, C. Weight-Length Relationships of Trachinus draco (L., 1758) and Trachinus radiatus (C., 1829) in the Aegean Sea; Institute of Marine Biological Resources and Inland Waters, Hellenic Centre for Marine Research (HCMR): Argyroupoli, Greece; Attiki, Greece, 2024; submitted. [Google Scholar]
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Pauly, D.; Munro, J.L. Once more on the comparison of growth in fish and invertebrates. Fishbyte WorldFish Cent. 1984, 2, 1–21. [Google Scholar]
- İşmen, A.; Ozen, E.; Altınagaç, U.; Ozekinci, U.; Ayaz, A. Weight-length relationships of 63 fish species in Saros Bay, Turkey. J. Appl. Ichthyol. 2007, 23, 707–708. [Google Scholar] [CrossRef]
- Kinacigil, H.T.; Ilkyaz, A.T.; Metin, G.; Ulas, A.; Soykan, O.; Akyol, O.; Gurbet, R. Determining the first reproduction length, age and growth parameters of Aegean sea demersal fish for the regulation of fisheries management. Tubitak-Çaydag 2008, 327. (In Turkish) [Google Scholar] [CrossRef]
- Altın, A.; Ayyıldız, A.; Kale, S.; Alver, C. Length-weight relationships of forty-nine fish species from shallow waters of Gökçeada Island, northern Aegean Sea. Turk. J. Zool. 2015, 39, 971–975. [Google Scholar] [CrossRef]
- Öztekin, A.; Özekinci, U.; Daban, İ.B. Length-weight relationships of 26 fish species caught by longline from the Gallipoli peninsula, Turkey (northern Aegean Sea). Cah. Biol. Mar. 2016, 57, 335–342. [Google Scholar]
- Tesch, F.W. Age and Growth. In Methods for Assessments of Fish Production in Freshwaters, International Biological Programme; Ricker, W.E., Ed.; Blackwell Scientific Publications: Oxford, UK, 1971; Volume 54, pp. 101–313. [Google Scholar]
- Shepherd, G.; Grimes, C.B. Geographic and historic variations in growth of weakfish (Cynoscion regalis) in the middle Atlantic Bight. Fish. Bull. 1983, 81, 803–813. [Google Scholar]
- Weatherly, A.H.; Gill, H.S. The Biology of Fish Growth; Academic Press: London, UK, 1987. [Google Scholar]
- Wootton, R.J. Biotic interactions: II. Competition and mutualism. In Ecology of Teleost Fishes, Fish and Fisheries Series 1; Chapman and Hall, Ed.; Springer: Dordrecht, The Netherlands; London, UK, 1990; pp. 216–237. [Google Scholar]
- Garvey, J.E.; Devries, D.R.; Wright, R.A.; Miner, J.G. Energetic adaptations along a broad latitudinal gradient: Implications for widely distributed assemblages. Bioscience 2003, 53, 141–150. [Google Scholar] [CrossRef]
- Abdallah, M. Length-weight relationship of fishes caught by trawl off Alexandria, Egypt. Naga ICLARM Q. 2002, 25, 19–20. [Google Scholar]
- Karakulak, F.S.; Erk, H.; Bilgin, B. Length-weight relationships for 47 coastal fish species from the northern Aegean Sea, Turkey. J. Appl. Ichthyol. 2006, 22, 274–278. [Google Scholar] [CrossRef]
- Sangün, L.; Akamca, E.; Akar, M. Weight-length relationships for 39 fish species from the north-eastern Mediterranean coast of Turkey. Turk. J. Fish. Aquat. Sci. 2007, 7, 37–40. [Google Scholar]
- Ak, O.; Kutlu, S.; Aydin, İ. Weight-length relationships for 16 fish species from the Eastern Black Sea, Türkiye. Turk. J. Fish. Aquat. Sci. 2009, 9, 125–126. [Google Scholar]
- Gözler, A.M.; Baytaşoğlu, H. Length-Weight Relationship For 14 Fish Species From The South-Western Black Sea, Turkey. J. Anatol. Environ. Anim. Sci. 2022, 7, 138–144. [Google Scholar]
- Heneish, R.; Rizkalla, S.; Abdallah, M. Age Composition, Growth and Mortality of Spotted Weever (Trachinus araneus- Cuvier, 1829) and Greater Weever (Trachinus draco- Linnaeus, 1758) from the Western Egyptian Mediterranean Sea. Egypt. J. Aquat. Biol. Fish. 2022, 26, 1039–1051. [Google Scholar] [CrossRef]
- Karadurmuş, U. Length–Weight Relationship and Condition Factor of Sixteen Demersal Fish Species from the Southern part of the Marmara Sea, Turkey. J. Ichthyol. 2022, 62, 543–551. [Google Scholar] [CrossRef]
- Lombarte, A.; Lleonart, J. Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fishes 1993, 37, 297–306. [Google Scholar] [CrossRef]
- Tuset, V.M.; Rosin, P.L.; Lombarte, A. Sagittal otolith shape used in the identification of fishes of the genus Serranus. Fish. Res. 2006, 81, 316–325. [Google Scholar] [CrossRef]
- Škeljo, F.; Ferri, J. The use of otolith shape and morphometry for identification and size-estimation of five wrasse species in predator-prey studies. J. Appl. Ichthyol. 2011, 28, 524–530. [Google Scholar] [CrossRef]
- Disspain, M.C.; Ulm, S.; Gillanders, B.M. Otoliths in archaeology: Methods, applications and future prospects. J. Archaeol. Sci. Rep. 2016, 6, 623–632. [Google Scholar] [CrossRef]
- Jawad, L.; Hoedemakers, K.; Ibáñez, A.; Ahmed, Y.; Abu El-Regal, M.; Mehanna, S. Morphology study of the otoliths of the parrotfish, Chlorurus sordidus (Forsskål, 1775) and Hipposcarus harid (Forsskål, 1775) from the Red Sea coast of Egypt (Family: Scaridae). J. Mar. Biol. Assoc. 2018, 98, 819–828. [Google Scholar] [CrossRef]

| Trachinus draco | Trachinus radiatus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length Classes (TL, cm) | Age Classes | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 13.0–13.9 | 2 | ||||||||||||
| 14.0–14.9 | 2 | 1 | |||||||||||
| 15.0–15.9 | 3 | ||||||||||||
| 16.0–16.9 | 5 | 1 | |||||||||||
| 17.0–17.9 | 4 | ||||||||||||
| 18.0–18.9 | 10 | ||||||||||||
| 19.0–19.9 | 8 | 2 | 1 | ||||||||||
| 20.0–20.9 | 7 | ||||||||||||
| 21.0–21.9 | 7 | 2 | |||||||||||
| 22.0–22.9 | 9 | ||||||||||||
| 23.0–23.9 | 1 | 1 | |||||||||||
| 24.0–24.9 | 6 | 2 | |||||||||||
| 25.0–25.9 | 5 | 2 | |||||||||||
| 26.0–26.9 | 5 | 1 | 4 | ||||||||||
| 27.0–27.9 | 2 | 4 | |||||||||||
| 28.0–28.9 | 2 | ||||||||||||
| 29.0–29.9 | 3 | 5 | |||||||||||
| 30.0–30.9 | 1 | 4 | |||||||||||
| 31.0–31.9 | 6 | ||||||||||||
| 32.0–32.9 | 1 | 2 | |||||||||||
| 33.0–33.9 | 1 | 3 | |||||||||||
| 34.0–34.9 | 2 | ||||||||||||
| 35.0–35.9 | 3 | 7 | |||||||||||
| 36.0–36.9 | 1 | 1 | 1 | ||||||||||
| 37.0–37.9 | 2 | 5 | |||||||||||
| 38.0–38.9 | 1 | 1 | |||||||||||
| 39.0–39.9 | 1 | ||||||||||||
| 40.0–40.9 | 2 | ||||||||||||
| 41.0–41.9 | 1 | ||||||||||||
| 42.0–42.9 | |||||||||||||
| 43.0–43.9 | 1 | ||||||||||||
| N | 4 | 31 | 25 | 17 | 5 | 4 | 1 | 3 | 13 | 21 | 18 | 11 | 1 |
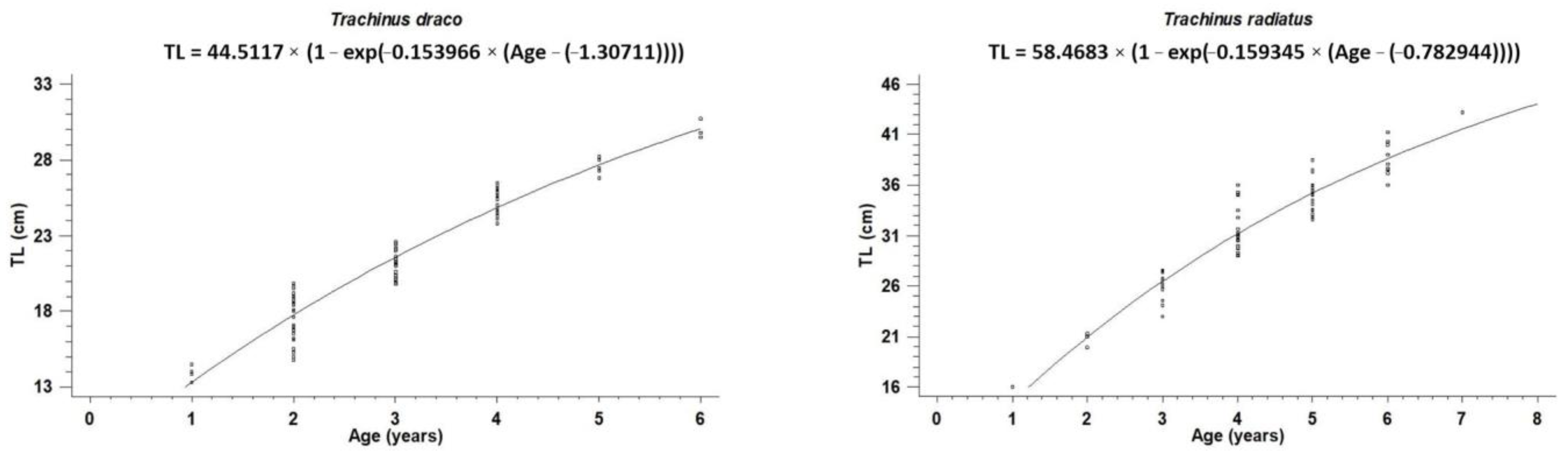
| Species | N | L∞ ± S.E (cm) | k ± S.E (Year−1) | t0 ± S.E (Year) | Φ′ |
|---|---|---|---|---|---|
| Trachinus draco | 86 | 44.51 ± 5.48 | 0.15 ± 0.04 | −1.31 ± 0.37 | 2.47 |
| Trachinus radiatus | 68 | 58.47 ± 10.22 | 0.16 ± 0.06 | −0.78 ± 0.63 | 2.74 |
| Morphometric Variable | Trachinus draco | Trachinus radiatus | ANOVA (p-Value) |
|---|---|---|---|
| RA (mm) | 3.60 ± 0.06 (2.75–4.97) | 5.61 ± 0.09 (3.75–6.84) | <0.05 * |
| OL (mm) | 7.87 ± 0.14 (5.78–10.83) | 11.73 ± 0.19 (7.53–14.81) | <0.05 * |
| OW (mm) | 3.62 ± 0.05 (2.61–4.52) | 4.89 ± 0.08 (3.14–6.24) | <0.05 * |
| OA (mm2) | 20.85 ± 8.42 (11.28–39.48) | 44.70 ± 1.36 (16.95–67.42) | <0.05 * |
| PE (mm) | 18.62 ± 0.33 (13.54–26.15) | 28.06 ± 0.48 (17.16–34.95) | <0.05 * |
| RD | 1.29 ± 0.00 (1.21–1.43) | 1.42 ± 0.01 (1.30–1.56) | <0.05 * |
| CI | 16.23 ± 0.06 (15.18–17.92) | 17.90 ± 0.10 (16.34–19.84) | <0.05 * |
| FF | 0.25 ± 0.00 (0.22–0.26) | 0.22 ± 0.00 (0.20–0.24) | <0.05 * |
| RC | 0.75 ± 0.03 (0.69–0.83) | 0.77 ± 0.00 (0.71–0.83) | <0.05 * |
| EL | 0.37 ± 0.00 (0.31–0.44) | 0.41 ± 0.00 (0.33–0.47) | <0.05 * |
| Variables | Species | A | B | R2 | r | Regression p-Value | ANCOVA p-Value for b |
|---|---|---|---|---|---|---|---|
| TL/RA | T. draco | 0.11 | 0.66 | 0.82 | 0.90 | <0.01 * | 0.88 |
| T. radiatus | 0.14 | 0.65 | 0.82 | 0.91 | <0.01 * | ||
| TL/OL | T. draco | 0.14 | 0.76 | 0.92 | 0.96 | <0.01 * | 0.14 |
| T. radiatus | 0.21 | 0.70 | 0.89 | 0.94 | <0.01 * | ||
| TL/OW | T. draco | 0.13 | 0.63 | 0.84 | 0.92 | <0.01 * | 0.59 |
| T. radiatus | 0.11 | 0.66 | 0.80 | 0.89 | <0.01 * | ||
| TL/OA | T. draco | 0.01 | 1.49 | 0.93 | 0.97 | <0.01 * | 0.22 |
| T. radiatus | 0.01 | 1.40 | 0.92 | 0.96 | <0.01 * | ||
| TL/PE | T. draco | 0.29 | 0.78 | 0.94 | 0.97 | <0.01 * | 0.37 |
| T. radiatus | 0.38 | 0.75 | 0.92 | 0.96 | <0.01 * | ||
| TL/RD | T. draco | 0.90 | 0.07 | 0.18 | 0.42 | <0.01 * | 0.48 |
| T. radiatus | 0.85 | 0.09 | 0.18 | 0.43 | <0.01 * | ||
| TL/CI | T. draco | 11.26 | 0.07 | 0.18 | 0.42 | <0.01 * | 0.37 |
| T. radiatus | 10.34 | 0.10 | 0.19 | 0.43 | <0.01 * | ||
| TL/FF | T. draco | 0.36 | − 0.07 | 0.18 | −0.42 | <0.01 * | 0.37 |
| T. radiatus | 0.39 | −0.10 | 0.19 | −0.43 | <0.01 * | ||
| TL/RC | T. draco | 0.43 | 0.10 | 0.35 | 0.59 | <0.01 * | 0.04 * |
| T. radiatus | 0.59 | 0.05 | 0.08 | 0.28 | 0.03 * | ||
| TL/EL | T. draco | 0.17 | 0.15 | 0.19 | 0.44 | <0.01 * | - |
| T. radiatus | 0.34 | 0.03 | 0.01 | 0.10 | 0.44 |
| Reference | Area | TL Range (cm) | α | b |
|---|---|---|---|---|
| [12] | Eastern Adriatic | 9.2–26.8 | 0.00002 | 2.934 |
| [13] | Balearic Islands | 14.0–34.0 | 0.00740 | 2.930 |
| [14] | Kyklades, Aegean Sea | 14.5–32.0 | 0.00441 | 3.120 |
| [44] | Alexandria, Egypt | 10.0–23.0 | 0.01400 | 2.800 |
| [25] | Balearic Islands and eastern coast of the Iberian Peninsula | 6.2–26.5 | 0.01000 | 2.835 |
| [45] | Gökceada Island, northern Aegean Sea (coastal waters of Turkey) | 4.4–35.2 | 0.02430 | 2.578 |
| [35] | Saros bay, northern Aegean Sea (Turkey waters) | 15.0–37.0 | 0.00366 | 3.202 |
| [15] | Izmir bay, Central Aegean Sea, (Turkey waters) | 17.2–34.0 | 0.00400 | 3.178 |
| [46] | North-Eastern Mediterranean Coast of Turkey | 9.0–20.0 | 0.00520 | 3.090 |
| [16] | Izmir Bay, Central Aegean Sea, (Turkey waters) | 15.3–36.6 | 0.00520 | 3.100 |
| [17] | North Aegean Sea | 15.0–30.5 | 0.00540 | 3.062 |
| [36] | Izmir Bay, Aegean Sea (Turkey waters) | 15.3–36.6 | 0.00500 | 3.137 |
| [47] | Eastern Black Sea | 5.0–35.0 | 0.00400 | 3.433 |
| [18] | Gulf of Castellammare (north-western Sicily) | 16.0–29.5 | 0.00540 | 3.059 |
| [19] | French Catalan coast | 15.0–38.5 | 0.04300 | 3.070 |
| [11] | Eastern Black Sea | 5.0–25.8 | 0.00690 | 3.005 |
| [20] | Mallorca, Spain | 9.0–29.0 | 0.00500 | 3.075 |
| [37] | Gökçeada Island, northern Aegean Sea, (Turkey waters) | 2.4–29.3 | 0.00900 | 2.847 |
| [22] | Iskenderun Bay, North-eastern Levantine Sea | 13.5–32.0 | 0.00770 | 2.950 |
| [38] | Gallipoli peninsula, northern Aegean Sea, (Turkey waters) | 13.0–36.4 | 0.00800 | 2.972 |
| [23] | Gulf of Tunis | 10.0–32.0 | 0.00600 | 3.040 |
| [24] | Eastern Adriatic Sea | 10.4–32.2 | 0.00820 | 2.906 |
| [48] | Western Black Sea | 5.6–21.5 | 0.01740 | 2.657 |
| [49] | Western Egyptian Mediterranean Sea | 11.8–27.6 | 0.00680 | 2.963 |
| [50] | Sea of Marmara | 6.7–24.6 | 0.00760 | 2.971 |
| This study | SW Aegean Sea, Eastern Mediterranean Sea | 13.3–30.7 | 0.00242 | 3.357 |
| Reference | Area | TL Range (cm) | α | b |
|---|---|---|---|---|
| [14] | Kyklades, Aegean Sea | 15.4–40.4 | 0.01271 | 2.897 |
| [25] | Balearic Islands and eastern coast of the Iberian Peninsula | 16.5–47.0 | 0.00520 | 3.206 |
| [26] | Zakynthos Island, Ionian Sea | 16.0–25.4 | 0.01600 | 2.923 |
| [9] | Gulf of Tunis | 11.0–50.7 | 0.00900 | 3.035 |
| [24] | Eastern Adriatic Sea | 13.8–33.5 | 0.00850 | 3.029 |
| This study | SW Aegean Sea | 16.0–43.2 | 0.00758 | 3.095 |
| Species | Reference | Area | TL Range (cm) | Age Groups N (Range) | L∞ (cm) | k (Year−1) | t0 (Year) | Φ′ |
|---|---|---|---|---|---|---|---|---|
| Trachinus draco | [11] | Eastern Black Sea | 5.0–25.8 | 6 (1–6) | 28.62 | 0.28 | −0.89 | 2.36 |
| [20] | Mallorca, Spain | 9.0–29.0 | 10 (0–9) | 42.3 | 0.10 | −1.83 | 2.25 | |
| [22] | Iskenderun Bay, North-eastern Levantine Sea | 13.5–32.0 | 10 (2–11) | 46.45 | 0.08 | −3.29 | 2.24 | |
| [49] | Western Egyptian Mediterranean Sea | 11.8–27.6 | 5(1–5) | 27.3 | 0.5 | 13.29 | 2.27 | |
| This study | SW Aegean Sea, Eastern Mediterranean Sea | 13.3–30.7 | 6 (1–6) | 44.51 | 0.15 | −1.31 | 2.47 | |
| Trachinus radiatus | [9] | Gulf of Tunis | 11.0–50.7 | 15 (1–15) | 41.54 | 0.26 | - | 2.65 |
| This study | SW Aegean Sea, Eastern Mediterranean Sea | 16.0–43.2 | 7 (1–7) | 58.47 | 0.16 | −0.78 | 2.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforidou, V.; Mytilineou, C.; Alexandropoulos, A.; Anastasopoulou, A. Age, Growth, and Otolith Morphometrics of Trachinus draco (L., 1758) and Trachinus radiatus (Cuvier, 1829) in the Eastern Mediterranean. Fishes 2024, 9, 152. https://doi.org/10.3390/fishes9050152
Nikiforidou V, Mytilineou C, Alexandropoulos A, Anastasopoulou A. Age, Growth, and Otolith Morphometrics of Trachinus draco (L., 1758) and Trachinus radiatus (Cuvier, 1829) in the Eastern Mediterranean. Fishes. 2024; 9(5):152. https://doi.org/10.3390/fishes9050152
Chicago/Turabian StyleNikiforidou, Vasiliki, Chryssi Mytilineou, Athanasios Alexandropoulos, and Aikaterini Anastasopoulou. 2024. "Age, Growth, and Otolith Morphometrics of Trachinus draco (L., 1758) and Trachinus radiatus (Cuvier, 1829) in the Eastern Mediterranean" Fishes 9, no. 5: 152. https://doi.org/10.3390/fishes9050152
APA StyleNikiforidou, V., Mytilineou, C., Alexandropoulos, A., & Anastasopoulou, A. (2024). Age, Growth, and Otolith Morphometrics of Trachinus draco (L., 1758) and Trachinus radiatus (Cuvier, 1829) in the Eastern Mediterranean. Fishes, 9(5), 152. https://doi.org/10.3390/fishes9050152






