Abstract
Fish health is of significant ecological and economic importance. In response to public observations of parasite-like structures in a popular edible fish, this study aimed to characterize nematode larvae commonly found in the muscle and body cavity of Saurida tumbil (Bloch, 1795), a commercially important fish species inhabiting the Persian Gulf and Oman Sea. This fish, locally known as Hasoom, holds substantial culinary importance, being a staple in the diets of millions residing in countries around the Persian Gulf. A total of 458 Saurida tumbil specimens were obtained from fish markets between June 2022 and May 2023. Subsequent examination revealed the presence of a total of 6132 nematode larvae. Nematodes found in the body cavity were identified as belonging to the genus Hysterothylacium sp., family Raphidascarididae, while those in the muscle were identified as Anisakis sp. larval type, family Anisakidae. Histopathology results suggested that these parasites may have adverse health impacts on their fish host. Notably, both nematode genera were found in the third larval stage, which is known to be the infective stage for anisakidosis. Given the reported cases of anisakidosis among people living in the study region, it is strongly recommended that fish be properly cooked before consumption to mitigate health risks.
Key Contribution:
This manuscript presents a significant finding regarding the health implications of consuming Saurida tumbil (Bloch, 1795) from the Persian Gulf and Oman Sea regions, as it reveals the presence of Anisakis sp. and Hysterothylacium sp. larval types, both in their infective stage. These findings underscore the potential risk of anisakidosis associated with consuming these fish, highlighting the importance of further research and awareness of the region’s fish and public health strategies.
1. Introduction
Fish health is important for several reasons, spanning ecological, economic, and public health perspectives [1,2]. Fish play a crucial role in aquatic ecosystems, serving as both predators and prey [3,4]. Robust fish populations are essential for preserving ecosystem equilibrium through regulating prey populations and facilitating nutrient cycling processes, thus safeguarding the overall health and resilience of aquatic habitats [5,6]. When fish populations are affected by diseases, it can disrupt these ecological processes, leading to imbalances in the ecosystem [7,8]. Fisheries and aquaculture industries contribute significantly to global food security and economic prosperity [9,10]. Healthy fish stocks support the livelihoods of millions worldwide, including fishermen, fish farmers, and those involved in related industries such as processing, transportation, and marketing [11,12,13,14]. Diseases in fish populations can result in reduced yields, economic losses, and even the collapse of fisheries, impacting food availability and economic stability [15,16].
The waters of the Persian Gulf and the Oman Sea are known for their richness and biodiversity, hosting thousands of species of fish, 20 species of whales and dolphins, and nearly 200 species of corals [17,18]. They also serve as vital sources of seafood for the countries surrounding them [9,19], sustaining millions of people who rely on marine fish to meet their population’s seafood needs. Seafood harvested from the Persian Gulf and the Oman Sea is not only consumed domestically but is also exported to various parts of the world, contributing to the seafood supply in distant regions [9,20]. Among the fish living in these waters, the greater lizardfish, scientifically identified as Saurida tumbil (Bloch, 1795), stands out [21,22]. This marine fish belongs to the family Synodontidae and holds substantial culinary importance, being a staple in the diets of millions in countries around the Persian Gulf. For example, in the Sistan and Baluchestan Province in Iran, the annual catch of Saurida tumbil surpasses 3000 tons [23], which shows a reliance on this particular fish species within the region.
Commonly referred to as Hasoom locally, this marine species is primarily found in oceanic environments and is seldom encountered in brackish waters. Its habitat spans the Pacific, Atlantic, and Indian Oceans, with additional presence noted in the southern regions of Iran, including the Persian Gulf and the Sea of Oman, where it is commercially viable [24,25,26,27]. Hasoom typically thrives in depths ranging from 10 to 60 m, predominantly in tropical areas. Exhibiting a common trait among fish, it displays a darker dorsal side and lighter ventral or belly side, showcasing hues of brown and gray in markets, each color garnering its own following [28]. Under optimal conditions, this species boasts an average lifespan of up to 7 years [29,30].
Renowned for its flavor and texture among local populations, the Hasoom fish is a delicacy. Its relatively delicate fins soften upon frying, contributing to dietary calcium intake. Abundant in protein, omega-3 fatty acids, and various essential nutrients, the Hasoom fish offers a nutritious option for consumption, providing essential minerals and vitamins for bodily functions [31,32]. Thus, its inclusion in dietary regimes is strongly advised, owing to its high protein content and nutritional profile.
The surge in affordable Japanese restaurants in the Middle East, including in the countries surrounding the Persian Gulf and the Oman Sea, where this fish is popular, raises concerns about food safety. Given that many Japanese dishes incorporate raw and undercooked seafood, there’s an inherent risk of parasitic infection among consumers.
In terms of its dietary habits, the greater lizardfish primarily feeds on fish, crustaceans, and squid [22,24]. It is adapted to preying on larger and faster prey, including finfishes and squids, and is known to target a wide range of prey groups [33]. Consequently, given its feeding behavior, it is conceivable that this fish species can host a variety of parasites [34,35].
Despite its significant role in the diets of people in the region, our knowledge of the parasites associated with the greater lizardfish and the safety aspects of consuming this fish is limited. Further research and investigation in this area would be valuable for a comprehensive understanding of the health and safety considerations related to the consumption of S. tumbil. The current research focuses on determining the prevalence and abundance of parasites commonly found in Saurida tumbil fish, sold in fish markets for human consumption, followed by characterizing of nematode larvae to determine their health significance for fish and consumers.
2. Materials and Methods
Following reports by members of the public about the presence of parasite-like organisms in greater lizard fish sold in fish markets, a total of 458 greater lizardfish (Saurida tumbil) were randomly sampled from various fish markets in Minab, Hormozgan Province, Iran (Figure 1) from June 2022 to May 2023. These fish were promptly placed on ice and transported to the laboratory for immediate examination after being weighed using a digital balance and measured using a ruler.
Figure 1.
The geographical location of the study area.
The fish species were identified using the available literature [36]. Total lengths (±0.01 cm) of the fish were taken from the tip of the snout (mouth closed) to the extended tip of the caudal fin using a measuring board. The body weights of the fish were measured with a top-loading Mettler balance and recorded to the nearest gram (±0.01). The fish were grouped into two distinct weight ranges (Supplementary Table S1): those weighing 470–800 g and those weighing 800–1030 g. Fish ranging from 30–40 cm in length were classified as small, while those measuring 41–50 cm were categorized as large. The prevalence of infection was analyzed with respect to body weight and fish length.
The entire digestive system of each fish, including the stomach, intestine, celomic cavity, and muscular tissues, was dissected to collect nematodes. This followed standard protocols previously published [37,38]. The isolated larvae were carefully quantified then rinsed with a physiological saline solution before being preserved in 70% ethanol. For morphological identification, the fixed nematodes were clarified in lactophenol, mounted on slides, and then observed under an optical microscope. Identification was based on morphological characteristics of the esophageal ventriculus, ventricular appendix, labia, the position of the excretory pore, and the tail [39,40,41,42]. Up to five representative larvae from each morphotype were then subjected to further molecular analysis using polymerase chain reaction (PCR)to identify the specific species.
Samples were sent to Ferdowsi University of Mashhad for scanning electron microscopy (SEM). SEM micrographs were taken from both the anterior and posterior ends of the worms. For the primary and secondary fixation, they were placed in glutaraldehyde and osmium tetroxide, respectively. Dehydration was carried out using a graded series of increasing ethanol concentrations, with each grade taking 15 min. The specimens were then mounted on copper stubs with double-sided adhesive tape and sputter coated with gold using an SC7620 fine coater. Following this preparation process, the stubs were examined using a LEO1450VP scanning electron microscope at a voltage of 20 kV, with the micrographs captured digitally.
Deoxyribonucleic Acid (DNA) extraction was carried out using a commercial DNA extraction tissue kit (Roche, Germany) following the manufacturer’s instructions. Polymerase chain reaction (PCR) was used to amplify the first and second internal transcribed spacers (ITS-1 and ITS-2, respectively) of ribosomal DNA (rDNA) regions, using primer sets, SS1 forward (5′-GTTTCCGTAGGTGAACCTGCG-3′) and NC13R reverse (5′-GCTGCGTTCTTCATCGAT-3′), respectively [43]. The PCR reaction was set up with a total volume of 20 μL, consisting of 4 μL of DNA template, 1.5 μL of each primer, 10 μL of the master mix, and 3 μL of distilled water, with the distilled water serving as the negative control. The PCR amplification process consisted of the following steps: initial denaturation at 95 °C for 3 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 45 s, extension at 72 °C for 90 s, and a final extension at 72 °C for 10 min. The resulting PCR products, representing the desired DNA fragments, were electrophoresed on a 1.5% agarose gel containing DNA Green Viewer with fluorescence dye and visualized and photographed under ultra violet (UV) light in a TBE buffer.
Following PCR amplification, the amplicons were submitted to Pishgam Biotechnology Company for further processing. The sequences were subjected to purification and bidirectional sequencing, using the same primers that were initially used for the amplification of the ITS-1 and ITS-2 genes. The obtained sequences were compared and aligned with previously documented sequences available in the GenBank database, hosted by the National Center for Biotechnology Information (NCBI). This alignment was performed using the Clustal method [44], to identify similarities and differences between the obtained sequences and existing genetic data.
The statistical analysis of the collected data was carried out using SPSS 18 software. Two commonly used statistical tests, the Chi-squared test and Fisher’s exact test, were used to evaluate the statistical significance of the results. A 95% confidence interval and a p-value less than 0.05 were considered statistically significant. The prevalence and mean intensity of parasites were calculated in accordance with Bush et al. [45].
Tissue samples from the infected organs in 1 cm3 dimensions associated with attached larvae were taken and fixed in 10% neutral buffered formalin for histopathological investigation. The samples were dehydrated in graded ethanol and embedded in paraffin wax. Then, under an optical microscope, sections of 5 μm thicknesses were routinely stained with hematoxylin–eosin for histopathological and parasitological examinations.
3. Results
Nematode larvae were found in the body cavities of most fish and attached to various organs, including the liver, stomach, intestine, and beneath the visceral serosa and peri-intestinal fat. Fewer fish were infected with larvae in the muscle of fish (Figure 2). Larvae in the body cavity presented as white structures with lengths that varied from 10 to 40 mm. They were found in free forms, coiled and uncoiled, on the serosal surface of the viscera within the celomic cavity. Some of these larvae were tightly encapsulated within the peritoneum, mesentery, and inner muscular layers of the fish’s body wall, forming white nodules ranging in size from 3 to 5 mm. These larvae were identified as Hysterothylacium type XV, belonging to the family Raphidascarididae, based on the excretory pore being localized just beneath the nerve ring and the presence of both an intestinal cecum and ventral appendix. Sequences of the ITS-1 and ITS-2 regions obtained from the present study were identical to those in GenBank (accession numbers: LT576348 and LT576357) and demonstrated the presence of Hysterothylacium amoyense. Nematode larvae found in the muscle of the fish were identified morphologically as Anisakis sp. larval type I (Figure 3). However, we could not obtain a high-quality sequence for species identification.

Figure 2.
Hysterothylacium XV larvae in the celomic cavity (arrows). Scale bar: 1 mm.

Figure 3.
Scanning electron microscopy of the nematode larva found in the present study, showing the anterior end (A) and posterior end (B) of the parasite. Scale bars: 20 and 100 μm in image (A) and (B), respectively.
The analysis of the biometric data indicated that the highest prevalence of infection was observed in fish 30–40 cm long (95.7%) and 800–1030 g (94.41%) (Supplementary Table S1). However, no statistically significant correlation was found between the prevalence of infection and the length and weight of fish (p > 0.05) within each season. When comparing prevalence between different fish groups across seasons, it was consistently lower in autumn compared to other seasons among various fish groups (Figure 4). However, as indicated in Supplementary Table S1, the mean intensity of parasites in autumn was significantly higher (p < 0.05) than that in other seasons.

Figure 4.
The graph at the top displays the number of infected and examined fish in the present study. The graph at the bottom illustrates the number of nematode larvae found in various fish body parts throughout different seasons in the present study.
Histopathological examinations of infected fish (Figure 5) showed multiple granulomas containing sections of larvae surrounded by a fibrous capsule. The observed inflammation was characterized by a mild to moderate infiltration of mononuclear inflammatory cells, predominantly lymphocytes, macrophages, and eosinophils. A distinguishing feature in all infected tissues was a translucent space between the parasite’s cuticle and the host tissue. Additionally, some granulomas contained tunnels filled with eosinophilic and slightly basophilic materials, along with inflammatory reactions around the granuloma capsule. Some of these tunnels were empty, leaving only fibrotic capsules. Several solid nodules filled with homogenous eosinophilic substances and surrounded by inflammatory responses were located on the serosal surface of visceral organs and abdominal muscles. The muscular fibers near these parasitic nodules exhibited Zenker necrosis, rhabdomyolysis, and severe inflammation.
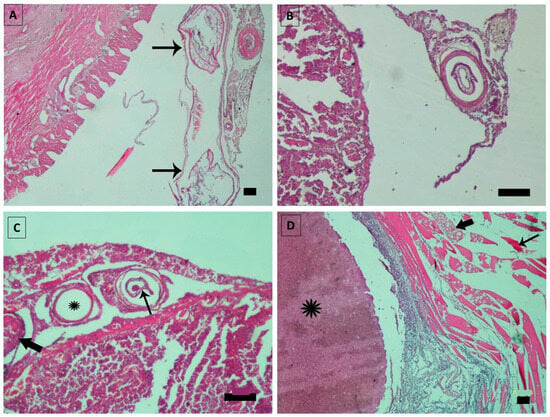
Figure 5.
(A) Longitudinal section of Hysterothylacium sp. larvae in the serous surface of the intestine (arrows); (B) cross-section of Hysterothylacium sp. larvae surrounded by a capsule of connective tissue in the fish spleen; (C) multiple granulomas in spleen composed of Hysterothylacium larvae (thin arrow) surrounded by a connective tissue capsule and inflammation around the capsule, empty granuloma (asterisk), also filled with degenerated larvae (thick arrow); (D) parasitic nodule containing homogenous eosinophilic materials (asterisk) in the abdominal muscles of fish. Muscular fibers show Zenker necrosis (thin arrow), rhabdomyolysis (thick arrow), and severe inflammation. Scale bars: 100 μm.
4. Discussion
The nematode larvae identified in the current study belong to the genera Anisakis and Hysterothylacium. The genus Anisakis belongs to the family Anisakidae [46]. The family Anisakidae comprises over one hundred species, with the genus Anisakis alone consisting of at least nine species [47]. The main definitive hosts for Anisakis spp. are marine mammals [48,49]. A wide range of invertebrates, such as crustaceans, are their first intermediate hosts [50,51,52]. Second/paratenic intermediate hosts include fish, cephalopods, fish-eating birds, and sea snakes [53,54,55,56]. From a medical perspective, the presence of Anisakis sp. larvae in fish muscle holds significant importance, as they represent the infective stage of the parasite, capable of inducing a severe illness known as anisakiasis upon consumption of infected seafood by humans [57]. Anisakis spp. have been associated with adverse effects on the immune system, gastrointestinal symptoms, and various other diseases [58]. Consequently, numerous studies have been conducted on the medical implications of these parasites, leading to the establishment of seafood safety protocols and guidelines regarding parasitic diseases transmitted through seafood in numerous countries [59]. However, these protocols and guidelines have not been established in Iran, despite the proven occurrence there of human infection [60]. Additionally, Anisakis spp. have veterinary significance due to their adverse effects on the health of their hosts. Examples include the induction of red vent syndrome in Atlantic salmon [61], gut hemorrhages in gilthead sea bream [62], hemorrhages and irregular neoformations within the coelomic cavity of European sea bass [63], and ulcerative lesions in cetaceans [64,65]. In yellowmouth barracuda (Sphyraena viridensis, Cuvier, 1829) granulomatous reactions composed of macrophages, epithelioid cells, some lymphocytes, and an external connective sheet were found to surround A. pegreffii [66].
Hysterothylacium spp. were previously categorized within the Anisakidae family, but they are now recognized as part of the Raphidascarididae family [67,68]. The life cycle of Hysterothylacium spp. includes invertebrates as their first intermediate hosts and various species of fish as their intermediate/paratenic/definitive hosts [43,69,70,71,72]. While there is a consensus among scientists regarding the zoonotic potential of Anisakis pegreffii and A. simplex, debates persist concerning the zoonotic potential of Hysterothylacium spp. and other Anisakis spp. [58,73]. Debates and conflicting findings have arisen among researchers regarding the pathogenicity and zoonotic significance of Hysterothylacium nematodes [73]. These have typically emerged from research based on animal models. Some authors proposed that Hysterothylacium aduncum larvae might not undergo evolution in homeothermic animals and, therefore, not in humans [74]. However, experimentally Hysterothylacium sp. larvae penetrated the stomach wall of a rhesus monkey (Macaca mulatta) and caused hemorrhage and attracted eosinophils [75]. There is also a report of naturally acquired human infection with Hysterothylacium sp. [76]. It is crucial to emphasize that many reports of human cases of anisakiasis/anisakidosis are based on the assumption that the nematode involved is Anisakis sp. larvae [58]. In their review, Shamsi and Barton [58] showed that, in over 90% of reported anisakidosis cases worldwide, there was a lack of concrete evidence identifying the causative agents. They suggested that a more thorough identification of anisakis species in humans could be achieved if healthcare professionals took appropriate steps in pathogen identification. Therefore, accurate identification of the parasite in human cases is essential for informing policies related to fisheries, food safety guidelines, and other relevant disciplines [77,78].
Although both nematode larval types found in the present study are known for their potential to cause infections in humans [58,73,76,79,80], there is less information available about their adverse health impact on fish hosts. Infection of fish with these nematodes is significant, not only due to the potential risk of anisakidosis in humans, but also because of the impact they have on the infected fish. These larvae can induce disease in fish, with symptoms and severity varying depending on factors such as the fish species, the species and intensity of the infecting parasite, and the specific organ invaded [81,82,83]. The disease is most severe when these larvae infect the liver, leading to fibrosis and atrophy of the organ, which results in a significant loss of body weight. Other symptoms may include granulomatous inflammation and necrosis of the muscularis externa of the pyloric caeca, gallbladder, intestine, and body cavity, potentially causing substantial mortality in fish [81].
Hence, the larvae of both Hysterothylacium sp. and Anisakis sp. found in this study may have zoonotic potential for consumers. Notably, both larvae were identified in the third stage of development, a known infectious stage for these parasites in humans. This shows the importance of considering the potential health risks associated with consuming seafood harboring these nematode larvae, necessitating vigilance and proper measures in food safety protocols to safeguard consumers from potential zoonotic infections [84].
The present study revealed the presence of Anisakis sp. larval types in the muscle of the examined fish, indicating an elevated risk. While Hysterothylacium sp. larvae were not detected in the fish muscle, it is noteworthy that they can migrate from internal organs and body cavities, posing a risk of contaminating the edible portions of the fish. For example, H. incurvum L3 larvae were found to migrate from the coelomic cavity of their fish host to the bloodstream, with consequent development to the adult stage within the heart [85]. The European Food Safety Authority [86] states that Anisakis sp. larvae found in fish muscle pose a potential risk of eliciting allergic reactions, including gastroenteritis and rheumatological and dermatological symptoms in consumers. It acknowledges that, based on current knowledge, no sea fishing areas for wild-caught fish can be considered entirely free of Anisakis sp. larvae. Therefore, prevention methods such as freezing at −15 °C for no less than 96 h, or −20 °C for 24 h or −35 °C for 15 h, and heating at more than 60 °C for at least 1 min, have been recommended.
The traditional cuisines in the study area and the countries surrounding the Persian Gulf and Oman Sea often involve the prolonged cooking of fish [87], a practice deeply embedded in the culinary heritage. However, in alignment with global trends [88], the consumption of raw seafood, exemplified by dishes like sushi and sashimi, is on the rise in these countries [89,90,91], as well as in regions of the world away from the sea [92]. A recent study conducted in Bushehr, located on the northern coast of the Persian Gulf, showed previously undisclosed cases of anisakiasis, a parasitic infection caused by Anisakis larvae [60]. The identification of three positive DNA cases of Anisakidae family parasites in gastric tissue biopsies of hospitalized patients serves as a stark indication of the potential health risks for residents in coastal areas of the Persian Gulf, where the consumption of infected fish is prevalent. As suggested by these authors, it is imperative to initiate a comprehensive population study in the region, focusing on the investigation of human sera for seropositive cases, and considering the allergic aspects of infection, which are common in regions where anisakiasis is prevalent. This underscores the need for increased awareness among physicians and researchers, emphasizing the potential health implications associated with consuming raw seafood in the region [60].
Our study indicates a considerable increase in the infection rate of this fish compared to previous research. In a study in the same region [93], out of 120 fish examined, only 5 were found to be infected with Anisakis (one larva per fish), and 30 were infected with Hysterothylacium larval type XV infections, ranging from one to fifteen larvae per fish. In contrast, our study revealed a substantial rise in infection rates, with 430 out of 458 fish (93.9%) found to be infected with the same nematode larvae. The Persian Gulf, recognized as the warmest sea globally, is experiencing elevated temperatures attributed to climate change. Consequently, it is undergoing increased temperatures, heightened salinity, sea level rise, and decreased oxygen levels [94,95], factors known to positively influence the abundance and prevalence of anisakis nematodes. The hatching duration of these nematodes inversely correlates with temperature, while light exposure diminishes hatching time. Moreover, the viability of newly hatched larvae is enhanced under higher salinity levels [96]. These factors may account for the disparities observed between our current study and the previous investigation conducted approximately a decade ago.
In our study, we observed that heavier fish displayed more parasitic infections. It is noteworthy to consider that this might be attributed to the weight of the parasite itself, rather than establishing a direct link between the size of the fish and susceptibility to nematode larvae infestation [97]. The notable increase in mean intensity during autumn warrants further investigation to gain a deeper understanding of the biology of these parasites. It is plausible that the warmer months during late spring and summer contribute to a higher number of egg and larval stage productions, resulting in the observed high intensity during autumn. Additionally, while statistically insignificant, smaller fish exhibited a greater prevalence of parasites than larger fish. This phenomenon could be attributed to the substantial health impact of parasites on fish, potentially hindering the growth of infected individuals [98,99], supporting the histopathological changes observed in the present study. The formation of a connective tissue capsule, the inflammation, and the sequestration of the parasite is one of the host’s reactions to the presence of the parasite [100].
5. Conclusions
In conclusion, this study documents a heightened occurrence of potentially zoonotic nematodes in commonly consumed fish within the Persian Gulf region, with no statistically significant correlation between the prevalence of infection and the length and weight of fish within each season. It was found that, in autumn, the prevalence of infection was lower, but the mean intensity of parasites was significantly higher. A limitation of the study pertains to the unknown provenance of the samples, which hinders the precise determination of the true infection status in the studied area.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9040143/s1, Table S1: Correlation between the morphology of Saurida tumbil and the rate of Hysterothylacium sp. infection in different seasons; mean intensity was calculated for total number of larvae in infected fish.
Author Contributions
Conceptualization, Y.G., M.M. and S.S.; methodology, Y.G., S.A. and S.H.; validation, S.S.; formal analysis, Y.G., S.A. and S.H.; investigation, Y.G., S.A. and S.H.; resources, M.M.; data curation, Y.G., S.A. and S.H.; writing—original draft preparation, Y.G., S.A. and S.H.; writing—review and editing, S.S.; visualization, S.S.; supervision, M.M. and S.S.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Kerman and had partial support from CSU.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Genetic data are available publicly through GenBank database.
Acknowledgments
The authors are grateful to Craig Poynter from CSU for preparing the map of the study area and Mark Filmer for editing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brunner, E.J.; Jones, P.J.; Friel, S.; Bartley, M. Fish, human health and marine ecosystem health: Policies in collision. Int. J. Epidemiol. 2009, 38, 93–100. [Google Scholar] [CrossRef]
- Pawde, E.D.; Thaware, V.H.; Paul, K. Conservation strategies for fish biodiversity to maintain healthy ecosystem. Int. J. Fish. Aquat. Stud. 2023, 11, 147–149. [Google Scholar] [CrossRef]
- Ortuño Crespo, G.; Dunn, D.C. A review of the impacts of fisheries on open-ocean ecosystems. ICES J. Mar. Sci. 2017, 74, 2283–2297. [Google Scholar] [CrossRef]
- Sommer, U.; Charalampous, E.; Scotti, M.; Moustaka-Gouni, M. Big fish eat small fish: Implications for food chain length? Community Ecol. 2018, 19, 107–115. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Radinger, J.; Matern, S.; Klefoth, T.; Wolter, C.; Feldhege, F.; Monk, C.T.; Arlinghaus, R. Ecosystem-based management outperforms species-focused stocking for enhancing fish populations. Science 2023, 379, 946–951. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef]
- Riley, S.C.; Munkittrick, K.R.; Evans, A.N.; Krueger, C.C. Understanding the ecology of disease in Great Lakes fish populations. Aquat. Ecosyst. Health Manag. 2008, 11, 321–334. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Country Profiles. Iran, 2015. Country Profile Fact Sheets. Fisheries and Aquaculture Division [Online]. Rome. 2023. Updated 1 April 2016. Available online: https://www.fao.org/fishery/en/facp/irn (accessed on 15 December 2023).
- Béné, C.; Arthur, R.; Norbury, H.; Allison, E.H.; Beveridge, M.; Bush, S.; Campling, L.; Leschen, W.; Little, D.; Squires, D. Contribution of fisheries and aquaculture to food security and poverty reduction: Assessing the current evidence. World Dev. 2016, 79, 177–196. [Google Scholar] [CrossRef]
- Rabo, P.; Zarmai, D.; Jwanya, B.; Dikwahal, S. The role of fisheries resources in national development: A review. Int. Lett. Nat. Sci. 2014, 18, 20–28. [Google Scholar] [CrossRef]
- Hamerlynck, O.; Nyingi, W.D.; Paul, J.-L.; Duvail, S. The fish-based farming system: Maintaining ecosystem health and flexible livelihood portfolios. In Farming Systems and Food Security in Africa; Routledge: London, UK, 2019; pp. 354–392. [Google Scholar]
- Funge-Smith, S.; Bennett, A. A fresh look at inland fisheries and their role in food security and livelihoods. Fish Fish. 2019, 20, 1176–1195. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Bellmann, C.; Tipping, A. Fishing for the future: An overview of challenges and opportunities. Mar. Policy 2016, 69, 173–180. [Google Scholar] [CrossRef]
- Johnson, P.T.; Paull, S.H. The ecology and emergence of diseases in fresh waters. Freshw. Biol. 2011, 56, 638–657. [Google Scholar] [CrossRef]
- Preston, D.L.; Mischler, J.A.; Townsend, A.R.; Johnson, P.T. Disease ecology meets ecosystem science. Ecosystems 2016, 19, 737–748. [Google Scholar] [CrossRef]
- Saud, M.; Jawad, L.A.; Park, J.M.; Al Sariri, T.S.; Al Balushi, B.Y. Fish diversity of mangrove ecosystems in Sultanate of Oman. Cah. Biol. Mar 2021, 62, 235–249. [Google Scholar]
- Asadi, H.; Dehghani, R. Atlas of Marine Fishes Persian Gulf and Oman Sea; Iranian Fisheries Research and Training Organization: Tehran, Iran, 1997; p. 226. ISBN 9789645513151. [Google Scholar]
- Ben-Hasan, A.; Daliri, M. Persian Gulf artisanal fisheries: Magnitude, threats, and opportunities. Rev. Fish Biol. Fish. 2023, 33, 541–559. [Google Scholar] [CrossRef]
- Al-Abdulrazzak, D.; Zeller, D.; Belhabib, D.; Tesfamichael, D.; Pauly, D. Total marine fisheries catches in the Persian Gulf from 1950 to 2010. Reg. Stud. Mar. Sci. 2015, 2, 28–34. [Google Scholar] [CrossRef]
- Eagderi, S.; Fricke, R.; Esmaeili, H.R.; Jalili, P. Annotated checklist of the fishes of the Persian Gulf: Diversity and conservation status. Iran. J. Ichthyol. 2019, 6, 1–171. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication, Version (10/2023). Available online: www.fishbase.org (accessed on 12 December 2023).
- Doustdar, M.; Hashemi, S.; Rahmati, R. The feeding and reproductive habits of Saurida tumbil and Rastrelliger kanagurata in the northern Oman Sea. Iran. J. Fish. Sci. 2022, 21, 816–828. [Google Scholar]
- Yang, X.; Deng, Y.; Qin, J.; Luo, K.; Kang, B.; He, X.; Yan, Y. Dietary shifts in the adaptation to changing marine resources: Insights from a decadal study on greater lizardfish (Saurida tumbil) in the Beibu Gulf, South China Sea. Animals 2024, 14, 798. [Google Scholar] [CrossRef]
- SriHari, M.; Silpa, S.; Pavan-Kumar, A.; Bhushan, S.; Nayak, B.B.; Abidi, Z.J. Stock characterization of Greater Lizardfish, Saurida tumbil (Bloch, 1795) along the west coast of India using morphological and molecular markers. Mar. Biol. Res. 2021, 17, 107–119. [Google Scholar] [CrossRef]
- Zellibooriabadi, M.; Gorgin, S.; Fujimori, Y.; Zare, P.; Niri, A.S.; Susanto, A. Estimation of gillnets selectivity for greater lizardfish, Saurida tumbil (Bloch, 1795) in coastal waters of the Oman Sea. Int. J. Aquat. Biol. 2023, 11, 230–241. [Google Scholar]
- Kalhoro, M.A.; Liu, Q.; Memon, K.H.; Waryani, B.; Soomro, S.H. Maximum sustainable yield of Greater lizardfish Saurida tumbil fishery in Pakistan using the CEDA and ASPIC packages. Acta Oceanol. Sin. 2015, 34, 68–73. [Google Scholar] [CrossRef]
- Ahmadi, M.; Akbarzadeh, A. Phenotypic variation of greater lizard fish (Saurida tumbil) in Northern Persian Gulf (Hormozgan waters). J. Mar. Sci. Technol. 2021, 20, 96–109. [Google Scholar]
- Saberi, M.; Jabaleh, A.; Paighambari, S.Y.; Pormozaffar, S. Determination of Growth, Mortality, and Exploitation Parameters for Saurida tumbil in Coastal Waters off Bandar-e-Jask, Hormozgan Province. Sci. Res. J. Anim. Environ. 2020, 12, 435–442. [Google Scholar] [CrossRef]
- Kalhoro, M.A.; Liu Qun, L.Q.; Tooraj Valinassab, T.V.; Baradi Waryani, B.W.; Abbasi, A.R.; Memon, K.H. Population dynamics of greater lizardfish, Saurida tumbil from Pakistani waters. Pak. J. Zool. 2015, 47, 921–931. [Google Scholar]
- Bahram, S.; Khezri, M.; Javadian, S.R. Evaluation of antioxidant and antimicrobial properties of hydrolyzed protein of Saurida tumbil. Exp. Anim. Biol. 2020, 9, 23–35. [Google Scholar]
- Jaziri, A.A.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Microstructural and physicochemical analysis of collagens from the skin of lizardfish (Saurida tumbil Bloch, 1795) extracted with different organic acids. Molecules 2022, 27, 2452. [Google Scholar] [CrossRef]
- Vivekanandan, E. Predatory diversity of two demersal finfish species in the trawling grounds off Veraval. Indian J. Fish. 2001, 48, 133–143. [Google Scholar]
- Abd El-Ghany, A.M.; Nada, M.S.; Nadler, S.A. Morphological and molecular characterization of larval Echinocephalus sp. (Spirurida: Gnathostomatidae), a parasite of the greater lizard fish (Saurida undosquamis) and red porgy or common seabream (Pagrus pagrus). Parasitol. Res. 2023, 122, 2405–2411. [Google Scholar] [CrossRef]
- Morsy, K.; Bashtar, A.-R.; Mostafa, N.; El Deeb, S.; Thabet, S. New host records of three juvenile nematodes in Egypt: Anisakis sp. (Type II), Hysterothylacium patagonense (Anisakidae), and Echinocephalus overstreeti (Gnathostomatidae) from the greater lizard fish Saurida undosquamis of the Red Sea. Parasitol. Res. 2015, 114, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- FAO Union Nations. FAO Species Fact Sheets: Saurida Tumbil (Bloch, 1795). FAO Fisheries and Aquaculture Department. Available online: https://www.fao.org/figis/pdf/fishery/species/2984/en%3Ftitle%3DFAO%2520Fisheries%2520%2526amp%253B%2520Aquaculture%2520-%2520Aquatic%2520speciessighted04/04/2024 (accessed on 12 December 2023).
- Fernando, C.H.; Furtado, J.I.; Gussev, A.V.; Hanek, G.; Kakonge, S.A. Methods for the Study of Freshwater Fish Parasites; University of Waterloo Publishing: Waterloo, ON, Canada, 1972; Volume 12, p. 76. [Google Scholar]
- Bron, J.E.; Wiegertjes, G.; Piazzon, M.C.; Bobadilla, A. Fish Parasites: A Handbook of Protocols for Their Isolation, Culture and Transmission; 5m Books Ltd.: Great Easton, UK, 2021. [Google Scholar]
- Shamsi, S.; Poupa, A.; Justine, J.L. Characterisation of Ascaridoid larvae from marine fish off New Caledonia, with description of new Hysterothylacium larval types XIII and XIV. Parasitol. Int. 2015, 64, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Cannon, L.R.G. Some larval ascaridoids from south-eastern Queensland marine fishes. Int. J. Parasitol. 1977, 7, 233–243. [Google Scholar] [CrossRef]
- Li, L.; Zhao, W.T.; Guo, Y.N.; Zhang, L.P. Nematode parasites infecting in the starry batfish Halieutaea stellata (Vahl) (Lophiiformes: Ogcocephalidae) from the East and South China Sea. J. Fish Dis. 2016, 39, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Berland, B. Identification of larval nematodes from fish. In Nematode Problems in North Atlantic Fish; Moller, H., Ed.; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1989; pp. 15–22. [Google Scholar]
- Shamsi, S. Morphometric and molecular descriptions of three new species of Hysterothylacium (Nematoda: Raphidascarididae) from Australian marine fish. J. Helminthol. 2017, 91, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniac, F.; Jeanmougin, F.; Higgins, D.G. The Clustal X windows interface:flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Hartwich, G. Keys to genera of the Ascaridoidea. In CIH Keys to the Nematode Parasites of Vertebrates; Anderson, R.C., Willmott, A.G.C.S., Eds.; Commonwealth Agricultural Bureaux: Oxfordshire, UK, 1974; Volume 2, pp. 1–15. [Google Scholar]
- Shamsi, S. The occurrence of Anisakis spp. in Australian waters: Past, present, and future trends. Parasitol. Res. 2021, 120, 3007–3033. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Sprohnle-Barrera, C.; Shafaet Hossen, M. Occurrence of Anisakis spp. (Nematoda: Anisakidae) in a pygmy sperm whale Kogia breviceps (Cetacea: Kogiidae) in Australian waters. Dis. Aquat. Org. 2019, 134, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Nascetti, G. Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host—Parasite co-evolutionary processes. Adv. Parasitol. 2008, 66, 47–148. [Google Scholar]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CABI Publishing: Oxfordshire, UK, 2000. [Google Scholar]
- Gregori, M.; Roura, Á.; Abollo, E.; González, Á.F.; Pascual, S. Anisakis simplex complex (Nematoda: Anisakidae) in zooplankton communities from temperate NE Atlantic waters. J. Nat. Hist. 2015, 49, 755–773. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The role of zooplankton in the transmission of helminth-parasites to fish. Rev. Fish Biol. Fish. 1995, 5, 336–371. [Google Scholar] [CrossRef]
- Smith, J.W.; Wootten, R. Anisakis and anisakiasis. In Advances in Parasitology; Academic Press Inc. (London) Ltd.: London, UK, 1978; Volume 16, pp. 93–163. [Google Scholar]
- Abollo, E.; Gestal, C.; Pascual, S. Anisakis infestation in marine fish and cephalopods from Galician waters: An updated perspective. Parasitol. Res. 2001, 87, 492–499. [Google Scholar] [PubMed]
- Klimpel, S.; Kuhn, T.; Münster, J.; Dörge, D.D.; Klapper, R.; Kochmann, J. Parasites of Marine Fish and Cephalopods: A Practical Guide; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- Nagasawa, K.; Moravec, F. Larval anisakid nematodes from four species of squid (Cephalopoda: Teuthoidea) from the central and western North Pacific Ocean. J. Nat. Hist. 2002, 36, 883–891. [Google Scholar] [CrossRef]
- Desowitz, R.S. Human and experimental anisakiasis in the United States. [Hokkaido Igaku Zasshi] Hokkaido J. Med. Sci. 1986, 61, 358–371. [Google Scholar] [PubMed]
- Shamsi, S.; Barton, D.P. A critical review of anisakidosis cases occurring globally. Parasitol. Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on risk assessment of parasites in fishery products. EFSA J. 2010, 8, 1543. [Google Scholar]
- Najjari, M.; Sadjjadi, S.M.; Khodadadi, H.; Farzaneh, M.R.; Mattiucci, S. Anisakis spp, DNA detection in paraffin-embedded tissue biopsies recovered from patients with gastritis using real-time PCR in Bushehr, Persian Gulf, Iran. Mol. Biochem. Parasitol. 2022, 251, 111494. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.J.; Pert, C.C.; Briers, R.A.; Diele, K.; Rueckert, S. Increasing intensities of Anisakis simplex third-stage larvae (L3) in Atlantic salmon of coastal waters of Scotland. Parasites Vectors 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Lanteri, G.; Passantino, A.; De Stefano, C.; Costa, A.; Gaglio, G.; Macri, F. Experimental susceptibility of gilthead sea bream, Sparus aurata, via challenge with Anisakis pegreffii larvae. Biomed Res. Int. 2013, 2013, 701828. [Google Scholar] [CrossRef]
- Macri, F.; Lanteri, G.; Rapisarda, G.; Costa, A.; Marino, F. Anisakis pegreffii experimental challenge in Dicentrarchus labrax: An endoscopic study. Aquaculture 2012, 338, 297–299. [Google Scholar] [CrossRef]
- Pons-Bordas, C.; Hazenberg, A.; Hernandez-Gonzalez, A.; Pool, R.V.; Covelo, P.; Sanchez-Hermosin, P.; Lopez, A.; Saavedra, C.; Fraija-Fernandez, N.; Fernandez, M.; et al. Recent increase of ulcerative lesions caused by Anisakis spp. in cetaceans from the north-east Atlantic. J. Helminthol. 2020, 94, e127. [Google Scholar] [CrossRef] [PubMed]
- Hrabar, J.; Bocina, I.; Kurilj, A.G.; Duras, M.; Mladineo, I. Gastric lesions in dolphins stranded along the Eastern Adriatic coast. Dis. Aquat. Org. 2017, 125, 125–139. [Google Scholar] [CrossRef]
- De Benedetto, G.; Giannetto, A.; Riolo, K.; Iaria, C.; Brianti, E.; Gaglio, G. Anisakis pegreffii Larvae in Sphyraena viridensis and Description of Granulomatous Lesions. Animals 2021, 11, 3449. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, T.L.; Overstreet, R.M. Review of Hysterothylacium and Iheringascaris (both previously = Thynnascaris) (Nematoda: Anisakidae) from the northern Gulf of Mexico. Proc. Biol. Soc. Wash. 1981, 93, 1035–1079. [Google Scholar]
- Nadler, S.A.; D’Amelio, S.; Dailey, M.D.; Paggi, L.; Siu, S.; Sakanari, J.A. Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from Northern Pacific marine mammals. J. Parasitol. 2005, 91, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Gasser, R.; Beveridge, I. Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol. Int. 2013, 62, 320–328. [Google Scholar] [CrossRef]
- Klimpel, S.; Rückert, S. Life cycle strategy of Hysterothylacium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol. Res. 2005, 97, 141–149. [Google Scholar] [CrossRef]
- Rokicki, J. The possibility of completing the life cycle of Hysterothylacium aduncum (Rudolphi, 1802) and Contracaecum rudolphii (Hartwich, 1964) (Nematoda) at the waters of Vistula Lagoon. Wiad. Parazytol. 2005, 51, 239–241. [Google Scholar] [PubMed]
- Gonzalez, L. The life cycle of Hysterothylacium aduncum (Nematoda: Anisakidae) in Chilean marine farms. Aquaculture 1998, 162, 173–186. [Google Scholar] [CrossRef]
- Roca-Geronès, X.; Montoliu, I.; Godínez-González, C.; Fisa, R.; Shamsi, S. Morphological and genetic characterization of Hysterothylacium Ward & Magath, 1917 (Nematoda: Raphidascarididae) larvae in horse mackerel, blue whiting and anchovy from Spanish Atlantic and Mediterranean waters. J. Fish Dis. 2018, 41, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Vermeil, C.; Petter, A.; Morin, O.; Le Bodic, M.; Daniel, C.; Guegan, J.; Kerneis, J. Do the eosinophilic granulomas observed in Brittany represent a form of anisakiasis? The larvae of Thynnascaris aduncum do not produce these granulomas experimentally. Bull. Soc. Pathol. Exot. Ses Fil. 1975, 68, 79–83. [Google Scholar]
- Overstreet, R.M.; Meyer, G.W. Hemorrhagic lesions in stomach of rhesus-monkey caused by a piscine ascaridoid nematode. J. Parasitol. 1981, 67, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Nagasawa, K.; Ishikura, H.; Nakagawa, A.; Sato, N.; Kikuchi, K.; Ishikura, H. Female worm Hysterothylacium aduncum excreted from human: A case report. Jpn. J. Parasitol. 1996, 45, 12–23. [Google Scholar]
- Shamsi, S. Recent advances in our knowledge of Australian anisakid nematodes. Int. J. Parasitol. Parasites Wildl. 2014, 3, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S. Seafood-borne parasitic diseases: A “one-health” approach is needed. Fishes 2019, 4, 9. [Google Scholar] [CrossRef]
- Beig, J.; Lane, R.J.; Lane, M.R. Gastric anisakiasis: A rare cause of abdominal pain. Intern. Med. J. 2019, 49, 129. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.M.; Na, B.K.; Kim, T.H.; Park, T.J. Anisakiasis: Report of 15 gastric cases caused by Anisakis Type I larvae and a brief review of Korean anisakiasis cases. Korean J. Parasitol. 2015, 53, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.T.K. Fish Diseases and Disorders. Volume 1: Protozoan and Metazoan Infections; CAB International: Wallingford, UK, 1995. [Google Scholar]
- Wootten, R. The Parasitology of Teleosts. In Fish Pathol; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 292–338. [Google Scholar] [CrossRef]
- Feist, S.W.; Longshaw, M. Histopathology of fish parasite infections—Importance for populations. J. Fish Biol. 2008, 73, 2143–2160. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Cavallero, S.; Zanzani, S.A.; Olivieri, E.; Malandra, R.; Ranghieri, V.; D’Amelio, S.; Manfredi, M.T. Anisakis sp. and Hysterothylacium sp. larvae in anchovies (Engraulis encrasicolus) and chub mackerel (Scomber colias) in the Mediterranean Sea: Molecular identification and risk factors. Food Control 2017, 80, 366–373. [Google Scholar] [CrossRef]
- De Benedetto, G.; Corti, I.; Malandra, R.; Riolo, K.; Giannetto, A.; Gaglio, G. Unusual Localization of Hysterothylacium Incurvum in Xiphias gladius (Linnaeus 1758) Caught in the Atlantic Ocean. Pathogens 2022, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- The European Food Safety Authority. EFSA Evaluates Parasites in Fish. 2010. Available online: https://www.efsa.europa.eu/en/press/news/biohaz100414 (accessed on 15 December 2023).
- Roden, C. The New Book of Middle Eastern Food; Knopf: New York, NY, USA, 2008. [Google Scholar]
- Bargain, O. Globalization and cultural spillover in trade: Evidence from the Japanese food culture. Rev. World Econ. 2024, 160, 55–73. [Google Scholar] [CrossRef]
- Tian, C.; Luan, W.; Wang, H. Exotic Food, Food Environment, and Geographical Patterns: Big Data Analytics From Japanese Cuisine in China. Front. Earth Sci. 2022, 10, 944927. [Google Scholar] [CrossRef]
- Savvaidis, I.N.; Al Katheeri, A.; Lim, S.-H.E.; Lai, K.-S.; Abushelaibi, A. Traditional foods, food safety practices, and food culture in the Middle East. In Food Safety in the Middle East; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–31. [Google Scholar]
- Ahmed, N. Tourism Impact on the Cultural Heritage of Countries in the Middle East. J. Appl. Geogr. Stud. 2023, 3, 41–53. [Google Scholar] [CrossRef]
- Derek, M. Ethnic Cuisine in Urban Space. Gastron. Urban Space Chang. Chall. Geogr. Perspect. 2020, 225–237. [Google Scholar] [CrossRef]
- Shamsi, S.; Ghadam, M.; Suthar, J.; Ebrahimzadeh Mousavi, H.; Soltani, M.; Mirzargar, S. Occurrence of ascaridoid nematodes in selected edible fish from the Persian Gulf and description of Hysterothylacium larval type XV and Hysterothylacium persicum n. sp. (Nematoda: Raphidascarididae). Int. J. Food Microbiol. 2016, 236, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Naderi Beni, A.; Marriner, N.; Sharifi, A.; Azizpour, J.; Kabiri, K.; Djamali, M.; Kirman, A. Climate change: A driver of future conflicts in the Persian Gulf Region? Heliyon 2021, 7, e06288. [Google Scholar] [CrossRef] [PubMed]
- Le Quesne, W. Too Hot to Handle? Adapting to the Impacts of Marine Climate Change in the Gulf. Marine Science Gove.UK. 2022. Available online: https://marinescience.blog.gov.uk/2022/11/15/too-hot-to-handle-adapting-to-the-impacts-of-marine-climate-change-in-the-gulf/ (accessed on 4 April 2024).
- Højgaard, D.P. Impact of temperature, salinity and light on hatching of eggs of Anisakis simplex (Nematoda, Anisakidae), isolated by a new method, and some remarks on survival of larvae. Sarsia 1998, 83, 21–28. [Google Scholar] [CrossRef]
- Habibi, F.; Shamsi, S. Preliminary report of occurrence of Corynosoma spp. (Acanthocephala: Polymorphidae) in Southern Caspian sprat (Clupeonella grimmi). Parasitol. Res. 2018, 17, 3327–3331. [Google Scholar] [CrossRef]
- Zuo, S.; Kania, P.W.; Mehrdana, F.; Marana, M.H.; Buchmann, K. Contracaecum osculatum and other anisakid nematodes in grey seals and cod in the Baltic Sea: Molecular and ecological links. J. Helminthol. 2017, 92, 81–89. [Google Scholar] [CrossRef]
- Zuo, S.; Huwer, B.; Bahlool, Q.; Al-Jubury, A.; Christensen, N.D.; Korbut, R.; Kania, P.; Buchmann, K. Host size-dependent anisakid infection in Baltic cod Gadus morhua associated with differential food preferences. Dis. Aquat. Org. 2016, 120, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Pironi, F.; Shinn, A.P.; Manera, M.; Giari, L. Histopathology and ultrastructure of Platichthys flesus naturally infected with Anisakis simplex sl larvae (Nematoda: Anisakidae). J. Parasitol. 2007, 93, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).