Abstract
This study presents a comprehensive analysis of the length-weight relationship, condition factors, and age of Schizothorax eurystomus in the Shakhimardan River basin in Central Asia, along with a comparative perspective to other Schizothorax species in the region. The study found that S. eurystomus exhibits positive allometric growth, which is consistent with similar patterns observed in this species from the Syr Darya River basin. The two analyzed condition factors showed mean values within the normal range, indicating good feeding and environmental conditions. However, significant disparities between minimum and maximum values of these factors indicated varied growth conditions which may be influenced by anthropogenic factors. Age estimation using opercular bones showed variations in the total length among fish of the same age, and a clear age distribution pattern across different sites. Younger fish predominantly inhabited the shallower, warmer, and lower sections of the river, which is impacted by agricultural water diversion, while older specimens were found in areas with higher discharge and deeper pools. Overall, this research provides valuable insights into the life history traits of S. eurystomus, underlining the need for sustainable fishery management and conservation strategies in the Shakhimardan River basin. The findings also emphasize the importance of considering habitat quality and anthropogenic pressures regarding understanding both fish population dynamics and growth patterns.
Key Contribution:
This study provides the length-weight relationship and two commonly used condition factors for S. eurystomus from the Shakhimardan River basin, as well as age at various lengths using opercular bones with the comparison to other Schizothorax species in Central Asia.
1. Introduction
For over half a century, length-weight relationships have played a crucial role in fish ecological studies [1]. These relationships are instrumental in evaluating population characteristics, such as estimating the size at first maturity [2,3], and calculating condition factors. The condition factor is a vital metric for assessing a fish’s health, indicating that fish with greater weight for a given length are in better condition [4]. This factor, along with the length-weight relationship, is influenced by various environmental conditions, such as food availability [5] and water quality [6,7], as well as biological, temporal, and sampling factors [8]. Sampling factors, for instance, encompass a range of variables such as catchment area, fish community composition, fish weights as measured in each study, time of collection, and potential errors by personnel or instruments. High values of condition factors indicate that the fish is in a good condition and the environment is suitable for fish growth [9]. Additionally, from a population perspective, factors such as age and sexual maturity at a certain length are equally important, and length-weight relationships may vary for fish from different localities and sexes, or for larval, immature, and mature fish [10]. Therefore, these indices can be used to evaluate the wellbeing of aquatic ecosystems [11].
Accurate estimates of age and growth are key to understanding population dynamics [12]. Knowledge about the age structure within these populations can provide insights into current environmental conditions and aid in making management decisions [13,14]. Otoliths are the most reliable structures for estimating a fish’s age, with opercular bones being equally dependable [15]. One study found that age estimates with otoliths and opercular bones yielded the same results in 93% of the cases [16]. In contrast, the use of annual rings on scales for identifying the age of fish is often met with scrutiny due to their tendency to yield unclear and inconsistent data, irrespective of the fish’s size [15]. Aging through scales has been criticized mainly because it frequently leads to the underestimation of age [17].
Despite the acknowledged importance and extensive application in fisheries science, length-weight relationships, condition factors, and age determinations are still unexplored for numerous species [18]. There remains a significant gap in our understanding of the genus Schizothorax which is prevalent in Asia and includes Schizothorax eurystomus, an important snow trout species native to Central Asia (Figure 1). S. eurystomus is widely distributed at higher elevations in China and Central Asia, encompassing many tributaries of the Syr Darya River, particularly those located in the mountainous regions around the Fergana Valley [19,20].

Figure 1.
Schizothorax eurystomus from the Shakhimardan River (total length = 230 mm; weight = 166 g; age = 5 years).
The scientific literature on this species is sparse, with only a few studies documented [19]. Consequently, this study is dedicated to establishing the length-weight relationship, evaluating two commonly used condition factors, and determining the age at various lengths of S. eurystomus using opercular bones, with results being compared to other Schizothorax species in Central Asia. This research contributes essential insights into the ecological characteristics and growth patterns of a species that has been largely understudied.
2. Materials and Methods
2.1. Study Area
The Shakhimardan River, a left tributary of the Syr Darya River, originates near Shakhimardan village within the Uzbekistan exclave of the same name in Kyrgyzstan. The river starts at the confluence of the Aksu and Koksu Rivers, which have their sources on the northern slopes of the Alai and Turkestan Mountain ranges (Figure 2A). Characterized by a permanent flow with seasonal dynamics, the Shakhimardan River flows northwest, traversing the border between Uzbekistan and Kyrgyzstan. Along its course, it passes through Kyrgyzstan before re-entering Uzbekistan near the village of Vodil (Figure 2B). Downstream from Vodil, the river’s flow is extensively regulated. Numerous weirs divert the river’s water into irrigation channels [21]. As the river approaches Fergana city, it re-emerges as the Margilansay River, receiving sustenance from collector-drainage and underground waters. The Margilansay River’s waters are predominantly allocated for agricultural purposes and, consequently, do not reach the Syr Darya River.
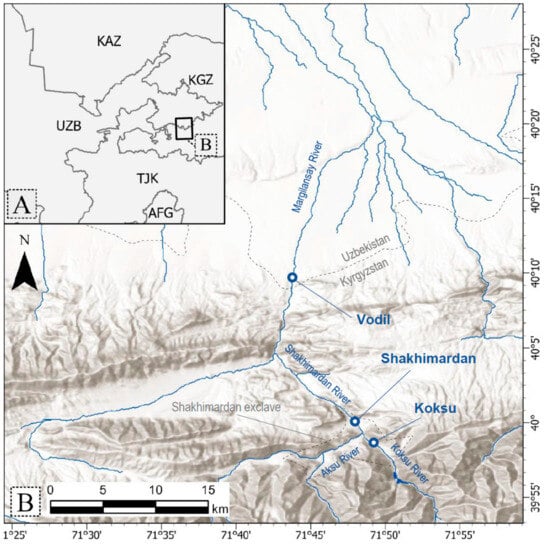
Figure 2.
(A) Location of the study area in Central Asia; (B) detailed map showing the sampling locations Vodil, Shakhimardan, and Koksu.
2.2. Fish Sampling
Our field work, alongside limited available literature, indicate that only two fish species are found in the Shakhimardan River basin upstream of the village of Vodil [20,22]: the stone loach (Triplophysa ferganaensis) and the snow trout (Schizothorax eurystomus Kessler, 1872).
We collected a total of 738 S. eurystomus individuals from three locations along the Shakhimardan and Koksu Rivers: (1) the Koksu River, (2) the Shakhimardan River downstream the confluence of Aksu and Koksu Rivers near the Shakhimardan mosque (hereafter: Shakhimardan), and (3) the Shakhimardan River at Vodil near the Kyrgyzstan border (hereafter: Vodil) (Figure 1). Sampling was conducted qualitatively at six occasions between September 2021 and July 2023 using fish traps and electrofishing gear [20]. The river at Vodil site is characterized by shallow waters which is a result of numerous water distribution facilities upstream. In contrast, the section near the Shakhimardan mosque features deeper pools, and has the highest discharge among the three sites. The Koksu River exhibits water depths intermediate between Vodil and the Shakhimardan site. Fish identification was conducted following the methodologies of Berg [23] and Veselov [24].
Of the total catch, 118 specimens were measured onsite that were anaesthetized, preserved in 10% formalin, and transported to the laboratory for further examination. The remaining 620 specimens were also measured onsite, but were subsequently released back into the river at their capture locations. For each specimen, the total length was recorded to the nearest 1 mm, and their wet weight was recorded to the nearest 0.1 g.
2.3. Laboratory Analyses
For the age determination of the fish, opercular bones were analyzed. The opercular bones extracted from the fish brought to the laboratory (n = 118) underwent a careful preparation process. Initially, they were submerged in boiling water for a few minutes to facilitate the removal of extraneous tissues. Next, broad-tipped forceps and a brush were used to meticulously clean the opercular bones. The clean operculum was then dried to ensure optimal conditions for observation and subsequent interpretation under a microscope and with the naked eye [15,25]. The annual rings, as well as false annual rings, can be clearly seen in well-dried opercular bones when viewed with reflected light against a dark background. The winter period is distinguished by transparent dark zones, and the summer period is distinguished by white zones [25].
2.4. Data Analyses
Following laboratory assessments, we assessed the relationships among various parameters, including total length (TL), wet weight (W), age, and the condition factors of the fish.
The length-weight relation was calculated based on the formula W = aLb, where ‘W’ represents the (wet) weight of fish in grams, ‘L’ is the TL in centimeters, ‘a’ is the scaling constant, and ‘b’ is the allometric coefficient (slope). The values of ‘a’ and ‘b’ were estimated by logarithm-based linear regression, represented as Log(W) = log(a) + b × log(L), following methodologies outlined by Froose [26] and Le Cren [1]. We calculated the 95% confidence limits for ‘a’ and ‘b’, along with the coefficient of determination (r2), using the equations proposed by Sparre and Venema [6].
The ‘b’ value of the length-weight relationship was tested for significant deviation from the expected cube value of 3 using Bailey’s t-test. This test was conducted using the formula t = b − 3/Sb, where ‘b’ is the regression coefficient from the length-weight relationship, and ‘Sb’ is the standard error of ‘b’ [27]. The growth pattern was classified based on the value of ‘b’: isometric growth, which is when small fish have the same form and condition as large specimens for b = 3, positive allometric growth, which is when larger fish have increase in weight more than length for b > 3, and negative allometric growth, which is when larger fish have increase in length more than weight for b < 3 [26].
Following the recommendation by Froese [26], we estimated both Fulton’s condition factor (K) [28] and the relative condition factor (Kn) [1,4] to assess the conditions of the fish.
Fulton’s condition factor (K), also known as the coefficient of condition, was estimated as K = 100 × W/L3, where ‘W’ denotes the (wet) weight of the fish and ‘L’ its TL [28]. While Fulton’s factor is typically used when fish have isometric growth, it remains valuable even when allometric growth is considered more appropriate.
The relative condition factor (Kn) was estimated using the formula Kn = Wo/Wc, where ‘Wo’ is the observed (wet) weight of fish and ‘Wc’ is the calculated (or predicted) (wet) weight, derived using the formula Wc = aLb [1,26]. The Kn values are directly interpreted, with higher values indicating better fish condition. Thereby, Kn serves as an indicator of the environmental conditions impacting the species: a Kn value ≥ 100 suggests good growth conditions, whereas a value < 100 indicates poor growth conditions. We employed the Kruskal-Wallis test to evaluate whether condition factors varied by sampling site or season (March–July vs. September–November). When overall differences were detected, pairwise post-hoc analyses were conducted using Dunn’s test. The significance level (α) was set at 0.05. p-values obtained from Dunn’s test were adjusted for multiple comparisons using the Bonferroni correction.
All other assessments were performed using descriptive statistics, such as mean and standard deviation (SD), and graphical data representation, including histograms and boxplots.
3. Results
3.1. Length-Weight Relationship
The caught specimens had a TL from 61 to 415 mm (Figure 3a), with a weight from 2 to 787 g. The length-weight relationship exhibited a high coefficient of determination (r2 = 0.98; Figure 3b). The a-value was 0.007 and the b-value 3.126. The 95% Cl for the b-value ranged from 3.098 to 3.154. The results of Bailey’s t-test confirmed that the b-value was statistically significant, indicating a pattern of positive allometric growth.
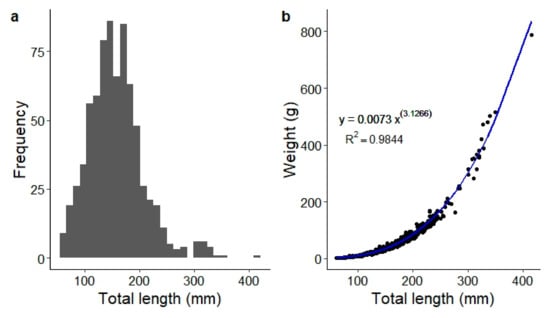
Figure 3.
(a) Length frequency diagram and (b) length-weight relationship of Schizothorax eurystomus in the Shakhimardan River basin (n = 738). The black points represent the observed data points, the blue line depicts the best-fit curve as indicated by the provided equation.
Specimens caught at Vodil had the shortest mean TL (123 mm ± 35.2 SD) of all the sampling sites, followed by the Shakhimardan (mean = 169 mm ± 50.4 SD) and Koksu River (mean = 192 mm ± 41 SD). The shortest fish caught in Koksu River measured 115 mm TL, which was much larger than the smallest specimen from Vodil (62 mm TL) or Shakhimardan (61 mm TL). The largest specimen, with a length of 415 mm, was captured at the Shakhimardan site. The fish size at Vodil did not exceed 212 mm TL.
3.2. Condition Factors
In our analysis, we estimated two distinct types of condition factors for S. eurystomus: Fulton’s condition factor (K) and the relative condition factor (Kn).
For Fulton’s condition factor (K), the mean value was 1.03 ± 0.13 SD. Values ranged from 0.61 to 1.63. Similarly, the relative condition factor (Kn) had a mean value of 101.00 ± 12.60 SD, spanning from 65.50 to 171.00. The frequency distribution for Fulton’s condition factor indicated that 15% of the fish had K values <0.9, 55% had K values ranging between 0.9 and 1.1, and 30% had K values >1.1. Meanwhile, the distribution for the relative condition factor showed that 19% of the fish exhibited Kn values <90, 61% had Kn values between 91 and 110, and 20% had Kn values >110.
Both conditions factors differed according to sampling location (K: χ2 = 46.01, df = 2, p < 0.001; Kn: χ2 = 26.30, df = 2, p < 0.001). Regarding Fulton’s condition factor, pairwise tests indicated that all three sites differed from each other (p < 0.002, respectively). For the relative condition factor, fish from Shakhimardan had a higher condition than from Koksu (p < 0.001) and Vodil (p < 0.001), but the latter sites did not differ from each other (p = 0.112) (Figure 4).
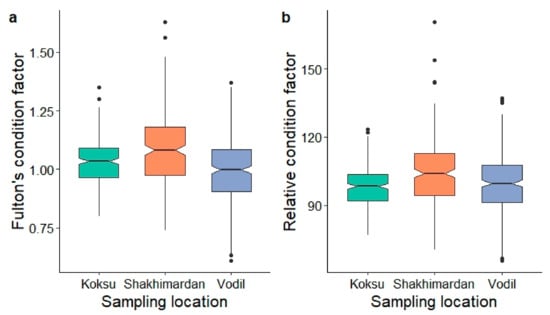
Figure 4.
(a) Fulton’s condition factor (K) and the (b) relative condition factor (Kn) for S. eurystomus specimens (n = 738) from three sampling locations in the Shakhimardan River basin (Koksu, Shakhimardan, Vodil). The boxplots show the median, the bottom and top of the boxes the lower (Q1) and the upper quartile (Q3), the lengths of the whiskers represent the data within 1.5 of the interquartile range, and points are considered outliers. The notches indicate the 95% confidence interval around the median.
Seasonal variation did not significantly influence the condition factor K (χ2 = 0.39, df = 1, p = 0.531); however, it significantly impacted Kn (χ2 = 11.29, df = 1, p < 0.001), with values being marginally higher from March to July when compared to September to November.
When assessing the combined effects of sampling site and season, significant overall differences were observed for both K (χ2 = 51.03, df = 5, p < 0.001) and Kn (χ2 = 33.36, df = 5, p < 0.001). The disparities in K were primarily due to the samples from Vodil in spring/summer, which displayed lower values than those from Koksu in both spring/summer (p = 0.037) and fall/winter (p = 0.035), as well as when compared to Shakhimardan during spring/summer (p < 0.001). Furthermore, K values were higher in Shakhimardan during spring/summer relative to Koksu in fall/winter (p = 0.010). Vodil’s spring/summer samples exhibited lower values than those from Shakhimardan in the same season (p < 0.001), and Kn was lower in Koksu fall/winter samples compared to Shakhimardan in spring/summer (p < 0.001). Pairwise comparisons not mentioned here did not show significant differences.
Table 1 presents a comparative analysis of the length-weight relationship condition factor findings from the present study with those of similar research conducted on various Schizothorax species in Central Asia.

Table 1.
Comparative overview of condition factor findings Schizothorax species in Central Asia.
Notably, S. fedtschenkoi from the Zarafshan River exhibited a b-value slightly less than three [29], and the K value for S. intermedius from Tokyrauyn River was recorded at around 1.5, which is about 30% higher than the value observed in our study. However, it is important to mention that the latter study [30] did not provide other fish parameters or sample size details. Additionally, our literature review did not uncover any other studies addressing length-weight relationships and condition factors for Schizothorax species in Central Asia.
3.3. Age
The age analysis using operculum bones in this study revealed considerable variations in total length among fish of the same age (Figure 5).
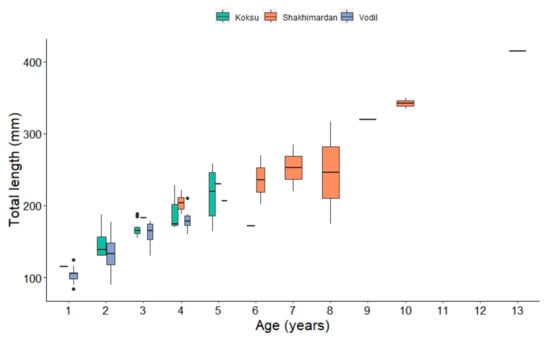
Figure 5.
Length variability by age of S. eurystomus (n = 118) in the Shakhimardan River basin, separated by sampling location.
The operculum bone analysis showed distinct patterns in the age distribution of fish across different sites. Notably, of the fish aged one to two years (n = 38), the majority were captured at Vodil, with only five exceptions from Koksu River. Furthermore, fish aged six years and older were predominantly found at the Shakhimardan site (n = 10), except for a single six-year-old specimen from Koksu River. The mean ages at the respective sites were 2.4 years at Vodil, 3.5 years at Koksu River, and 6.4 years at the Shakhimardan site.
Interestingly, a significant overlap in body lengths across different ages was observed (Figure 5). For instance, an eight-year-old specimen from Shakhimardan location measured 175 mm, which was shorter than the largest two-year-old fish, measuring 188 mm in body length, caught in Koksu River. This finding highlights the variability in growth rates and physical development among individuals of the same species.
The age distribution differed across the three sampling locations (Figure 6). The majority of the younger fish, particularly those under the age of two years, were predominantly captured in the Vodil area. In contrast, all fish aged over seven years were exclusively found at the Shakhimardan site.
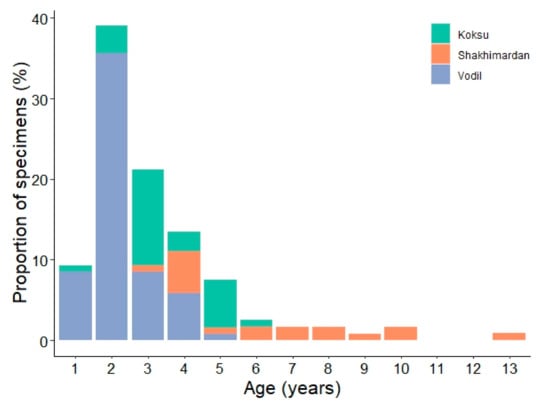
Figure 6.
Age-frequency composition of S. eurystomus specimens (n = 118) in the Shakhimardan River basin, separated by sampling location.
In terms of specific age groups, 9.3% of the fish sampled were one year old. A significant portion, amounting to 60.2%, were within the two to three years age range. Fish aged between four to five years constituted 21.2% of the catch, and those aged six years and above accounted for 8.5%.
The comparative analysis revealed that limited research has been conducted on the age estimation of Schizothorax fish species in Central Asia. Among the published studies, two focused on the age-length relationships of S. argentatus and S. intermedius, measuring total length (Table 2), while two other studies investigated S. intermedius and S. pseudaksaiensis, using standard length measurements (Table 3). Therefore, it is not feasible to directly compare the growth patterns of the same Schizothorax species across different rivers, but only across species. Additionally, a key distinction in age determination techniques exists between our study and previous research. While other studies relied on counting annual rings on scales [23,30,31,32], our study employed operculum bones, offering a different approach to age assessment.

Table 2.
Comparison of mean total length (mm; rounded to the nearest whole number) by age for different Schizothorax species in Central Asia.

Table 3.
Comparison of mean standard length (mm; rounded to the nearest whole number) by age for different Schizothorax species in Central Asia.
Our results illustrated that S. eurystomus exhibits similar total lengths to other Schizothorax species during their first year. However, a notably slower growth rate was observed from ages two to ten (Table 2). In comparison, S. argentatus in the Tokyrauyn River demonstrated a 15% higher growth rate across all ages, with the exception of the first year. S. intermedius in the Kafernigan River ages seven and above were around twice as large as S. eurystomus and S. argentatus. Notably, no data were available for fish aged beyond 11 years, except for one specimen of S. eurystomus in the Shakhimardan River basin (Table 2).
A similar pattern could be detected for standard length assessments on total lengths. As detailed in Table 3, S. eurystomus demonstrated a standard length comparable to that of the S. intermedius in the Chirchik River during their first year. However, from ages two to seven, S. eurystomus exhibited a mean growth rate that was around 25% slower than that of S. intermedius. Additionally, S. pseudaksaiensis displayed a notably faster growth, with a standard length about 50% greater than that of S. eurystomus at ages five to ten (Table 3).
4. Discussion
This study analyzed the length-weight relationship, condition factor, and age of S. eurystomus in the Shakhimardan River basin, Uzbekistan, with comparisons drawn to other Schizothorax species from Central Asia.
The target species displayed positive allometric growth, as evidenced by a b-value >3, aligning with the range outlined by Le Cren [1]. This growth pattern, where fish gain weight disproportionately to length increase, was also noted in the Syr Darya River basin population of S. eurystomus [19]. In contrast, a study on the same species in China’s Tarim River reported a significantly higher b-value of 3.38 [33], suggesting variability in growth patterns across different populations, or even within the same population over time [5,6,7,8].
While numerous factors such as sex, gonadal maturity, reproductive cycles, food availability, and environmental conditions can influence fish growth [34], these were not explicitly considered in the current study. In this research, fish specimens were collected over a lengthy period of almost two years, encompassing both spring/summer and fall/winter seasons.
The mean value of Fulton’s condition factor (K) for S. eurystomus was found to be within the normal ranges, as suggested by Soni and Ujjania [34], who posited that a K value of ≥1 is indicative of adequate feeding and favorable environmental conditions. Additionally, we observed a mean relative condition factor (Kn) values close to 100, with values in spring and summer being slightly higher than in fall and winter, which also suggests good growth conditions for the species. Nonetheless, the marked variation between the minimum and maximum values, as highlighted in Figure 4, suggests that a substantial number of specimens are living in less-than-ideal growth conditions. Lower Kn values, for example, may signal poor food availability or high predator density, whereas higher values could indicate high food abundance or lower predator density [35,36]. In contrast, Kn values ≥ 100 typically occur when fish have access to optimal feeding and habitat conditions [37]. Thus, variations in condition factors provide insights into the disparities in various factors [1], including the species’ life history and climatic conditions like temperature, as well as environmental factors such as competition for food and habitat quality, and large-scale anthropogenic interventions in the natural environment [38]. Our field observations revealed notable differences in the river sections sampled. Some areas were characterized by rich and diverse habitats, while others had undergone artificial modifications to their beds and banks. Consequently, the observed variations in condition factor values might be attributed to these river habitat conditions, especially during low water periods. Particularly, fish from the Shakhimardan site exhibited higher condition factors than the other sites, and this was partially seen when including the factor of season. It is worth mentioning that the condition factor does not indicate if fish can successfully complete their life cycle. Moreover, it may well be that the fish are not able to access (optimal) spawning habitats. However, due to the abundance of food they are in good condition. Other sampling techniques in combination with telemetry techniques can provide insights on that [39].
It is recognized that opercular bones provide age estimations closely comparable to those obtained from otoliths [15,16]. This method has been well-documented for various fish species, and is generally considered more accurate than other techniques involving scales, vertebrae, spines, or other hard parts of fish [15]. For instance, age estimates from otoliths in S. curvifrons were found to be in close agreement with those derived from opercular bones [40]. Additionally, opercular bones have been identified as advantageous for age estimation in species such as Esox lucius [41] and Cyprinus carpio [42].
However, there are instances where opercular bones are considered less reliable in comparison to other structures. For example, they were found to be less effective than otoliths and vertebrae in S. o’connori [43], and less accurate than scales and otoliths in Labeo rohita and Channa marulius [44]. In the case of Mastacembelus mastacembelus, age estimation from opercular bones was deemed unreliable due to the loss of primary annuli in the early stages of life [45].
We also tried to estimate age based on scales, i.e., annual rings, collected from the area of the body above the lateral line under the dorsal fin [31,40]. The rings on scales were often indistinct, not obvious, and unclear, regardless of fish size (Figure 7). As well, they were prone to damage during preparation. Therefore, these data have not been presented. Nonetheless, scales have traditionally been the most popular method for aging most cyprinids, owing to the ease of collection and preparation, and also the fact that one can take scales without damaging the fish [46,47]. However, the use of scales has faced criticism, mainly due to the frequent underestimation of age [17]. This inaccuracy in age estimation from scales can often be attributed to the reabsorption of annuli and the formation of false rings due to stress or food scarcity, as well as the diminished growth of scales as fish age, leading to less distinct annuli [15,17,40].
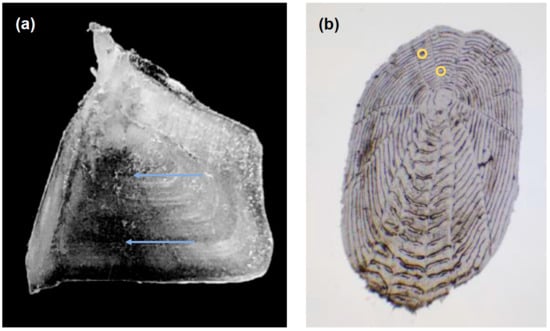
Figure 7.
(a) Opercular bone and (b) scale of a 2-year-old Schizothorax eurystomus from the Shakhimardan River. The blue arrows show annuli on operculum and the yellow dots represent possible annual rings on the scale (Photos: E. Karimov).
A notable pattern in age distribution was observed: most fish aged between one and four years were found in the Vodil area, while older specimens were predominantly caught in the Shakhimardan and Koksu sites. It is important to highlight that specimen older than six years were exclusively found at the Shakhimardan site. This distribution may be linked to environmental factors. The lower section of the Shakhimardan River, characterized by shallower waters due to extensive agricultural water diversion, has lower flow velocities and warmer water temperatures. These factors potentially make it more conducive for younger fish populations. In contrast, the Shakhimardan site, with its higher water flows and deeper pools, likely provides a more suitable habitat for older and larger specimens. Another contributing factor could be the high flow periods and mudflows during spring and summer, caused by snowmelt runoffs. These events may result in the downstream displacement of younger fish [48], explaining their prevalence in the lower river sections. Additionally, the presence of water distribution facilities and dams without fish passages on the Shakhimardan River between the sampling sites likely hinder sub-adult and adult specimens from returning upstream. Consequently, while fish survive in certain areas, their inability to recolonize upstream river reaches has become evident [49]. The snow trout is significant for recreational fishing in the Shakhimardan exclave. However, the local community reported a noticeable decrease in fish abundance in the river over time, also highlighting frequent instances of illegal fishing and overfishing, especially during the spawning season. Such practices could significantly contribute to the declining population of larger specimens in the Shakhimardan River basin.
A comparison of the age-length relationships determined in our study with those reported in other studies reveals that S. eurystomus in the Shakhimardan River basin exhibited the slowest growth rate among the Schizothorax species in Central Asia. For example, the growth rate of S. eurystomus in our study was nearly half of that observed for S. intermedius from the Kafernigan River [32]. Notably, the Kafernigan River is characterized by a longer free-flowing section and a higher mean annual discharge of 156 m3/s, compared to a mere 9.7 m3/s in the Shakhimardan River [21,50]. This disparity suggests that a longer river continuum and higher mean flows, offering more diverse habitats and food resources, likely facilitates enhanced fish growth.
5. Conclusions
This study on Schizothorax eurystomus within the Shakhimardan River basin has significantly advanced our understanding of the species’ growth, condition, and age patterns. The observed positive allometric growth pattern, as well as the condition factors’ values, underscore the species’ effective adaptation to its natural habitat. The age analysis, facilitated using opercular bones, has revealed a distinct spatial segregation of age groups within the river system, with younger specimens primarily located in the lower river sections that are affected by human activities. This pattern suggests that habitat fragmentation and anthropogenic pressures, such as water abstraction, could pose substantial threats to the snow trout population. In summary, the findings from this study provide a deeper understanding of the ecology of S. eurystomus and offer valuable insights for fishery research, management, and conservation efforts.
Author Contributions
Conceptualization, E.K., M.S. and D.S.H.; methodology, E.K., B.Z., B.K., M.S. and D.S.H.; validation, E.K., M.S. and D.S.H.; formal analysis, E.K.; investigation, E.K., B.Z., J.C., P.V., B.K., O.O., M.S. and D.S.H.; resources, B.K.; data curation, E.K.; writing—original draft preparation, E.K., M.S. and D.S.H.; writing—review and editing, E.K., B.Z., J.C., P.V., B.K., O.O., M.S. and D.S.H.; visualization, E.K., P.V., M.S. and D.S.H.; supervision, M.S. and D.S.H.; project administration, B.K.; funding acquisition, E.K. and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 101022905.
Institutional Review Board Statement
State Committee for Ecology and Environmental Protection of the Republic of Uzbekistan, Approval Code: 03-02/1-631, 02-02/1-1416, and 02-02/1-1465, Approval Date: 29 March 2022, 20 September 2022, and 27 September 2022; Ministry of Natural Resources of the Republic of Uzbekistan, Approval Code: 04-04/2-1567, Approval Date: 2 June 2023.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Thanks to Jan De Keyser and Justine Carey for helping with some of the figures. Finally, we express our gratitude to three reviewers who helped improve the quality of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Le Cren, E.D. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca Fluviatilis). J. Anim. Ecol. 1951, 20, 201. [Google Scholar] [CrossRef]
- Soares, B.E.; Barros, T.F.; Hashiguti, D.T.; Pereira, D.C.; Ferreira, K.C.F.; Caramaschi, É.P. Traditional Approaches to Estimate Length at First Maturity (L50) Retrieve Better Results than Alternative Ones in a Neotropical Heptapterid. J. Fish Biol. 2020, 97, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Hashiguti, D.T.; Soares, B.E.; Wilson, K.L.; Oliveira-Raiol, R.D.; de Assis Montag, L.F. Comparing Three Methods to Estimate the Average Size at First Maturity: A Case Study on a Curimatid Exhibiting Polyphasic Growth. Ecol. Freshw. Fish 2019, 28, 266–273. [Google Scholar] [CrossRef]
- Bagenal, T.B.; Tesch, F.W. Age and Growth. In Methods for Assessment of Fish Production in Fresh Waters; Bagenal, T., Ed.; Blackwell Science Publications: Oxford, UK, 1978; pp. 101–136. [Google Scholar]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations. Bull. Fish. Research. Board Can. 1975, 191, 207–211. [Google Scholar]
- Sparre, P.; Venema, C.S. Introduction to Tropical Fish Stock Assessment; Part 1:Manual; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1998; Volume 306. [Google Scholar]
- Mommsen, T.P. Growth and Metabolism. In The Physiology of Fishes; CRC Press: New York, NY, USA, 1998; pp. 65–98. [Google Scholar]
- Mehanna, S.F.; Farouk, A.E. Length-Weight Relationship of 60 Fish Species From the Eastern Mediterranean Sea, Egypt (GFCM-GSA 26). Front. Mar. Sci. 2021, 8, 625422. [Google Scholar] [CrossRef]
- Ndimele, P.E. Re-Validation of Biochemophysical Variables as Metrics for Assessing the Ecological Integrity of an Aquatic Ecosystem (Badagry Lagoon, Lagos, Nigeria). Appl. Water Sci. 2023, 13, 164. [Google Scholar] [CrossRef]
- Kuriakose, S. Estimation of Length Weight Relationship in Fishes. In Summer School on Advanced Methods for Fish Stock Assessment and Fisheries Management; CMFRI: Kochi, India, 2017; pp. 215–220. [Google Scholar]
- Afamdi, A. Condition Factor of Four Cichlid Species of a Man-Made Lake in Imo State, Southeastern Nigeria. Turk. J. Fish. Aquat. Sci. 2005, 5, 43–47. [Google Scholar]
- Campana, S.E.; Thorrold, S.R. Otoliths, Increments, and Elements: Keys to a Comprehensive Understanding of Fish Populations? Can. J. Fish. Aquat. Sci. 2011, 58, 30–38. [Google Scholar] [CrossRef]
- Mayne, B.; Espinoza, T.; Roberts, D.; Butler, G.L.; Brooks, S.; Korbie, D.; Jarman, S. Nonlethal Age Estimation of Three Threatened Fish Species Using DNA Methylation: Australian Lungfish, Murray Cod and Mary River Cod. Mol. Ecol. Resour. 2021, 21, 2324–2332. [Google Scholar] [CrossRef]
- Unfer, G.; Pinter, K. Fisheries Management of Stream-Resident Brown Trout Populations—Possibilities and Restrictions In Brown Trout: Life History, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 649–665. ISBN 9781119268352. [Google Scholar] [CrossRef]
- Khan, S.; Afzal Khan, M.; Miyan, K. Precision of Age Determination from Otoliths, Opercular Bones, Scales and Vertebrae in the Threatened Freshwater Snakehead, Channa Punctata (Bloch, 1793). J. Appl. Ichthyol. 2013, 29, 757–761. [Google Scholar] [CrossRef]
- Wright, R.M.; Giles, N. The Population Biology of Tench, Tinea Tinea (L.), in Two Gravel Pit Lakes. J. Fish. Biol. 1991, 38, 17–28. [Google Scholar] [CrossRef]
- Beamish, R.J.; MacFarlane, G.A. Current Trends in Age Determination Methodology. In Age and Growth of Fish; Summerfelt, R.C., Hall, G.H., Eds.; Iowa State University Press: Ames, IA, USA, 1987; pp. 15–42. [Google Scholar]
- Kulbicki, M.; Guillemot, N.; Amand, M. A General Approach to Length-Weight Relationships for New Caledonian Lagoon Fishes. Cybium 2005, 29, 235–252. [Google Scholar]
- Sheraliev, B.; Komilova, D.; Kayumova, Y. Length-Weight Relationship and Relative Condition Factor of Schizothorax Eurystomus Kessler, 1872 from Fergana Valley. J. Entomol. Zool. Stud. 2019, 7, 409–412. [Google Scholar]
- Karimov, E.B.; Karimov, B.K.; Schletterer, M.; Hayes, D.S. Ichthyological Research in the Koksu River, Uzbekistan to Identify Key Fish Species in the Context of Small Hydropower Development. Exp. Biol. 2023, 96, 133–141. [Google Scholar] [CrossRef]
- Mirzaev, N.N. Materialyi k Izucheniyu Problem Ekologii, Pitevogo Vodosnabzheniya, Melioratsii, Energosnabzheniya i Mashinnogo Orosheniya v Zone Pilotnyih Kanalov; Tashkent, Uzbekistan. 2006, Volume 1. Available online: http://www.cawater-info.net/library/rus/iwrm/canals_part_1.pdf (accessed on 28 January 2024).
- Sheraliev, B.; Peng, Z. Molecular Diversity of Uzbekistan’s Fishes Assessed with DNA Barcoding. Sci. Rep. 2021, 11, 16894. [Google Scholar] [CrossRef]
- Berg, L.S. Freshwater Fishes of the USSR and Neighboring Countries; Academy of Sciences of the USSR: Moscow, Russia, 1949; Volume 2. [Google Scholar]
- Veselov, E.A. Determinant of Freshwater Fish of Fauna of the USSR; Prosveshcheniye: Moscow, Russia, 1977. [Google Scholar]
- Le Cren, E.D. The Determination of the Age and Growth of the Perch (Perca Fluviatilis) from the Opercular Bone. J. Anim. Ecol. 1947, 16, 188. [Google Scholar] [CrossRef]
- Froese, R. Cube Law, Condition Factor and Weight–Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; Oxford and IBH Publishing Company: New Delhi, India, 1967. [Google Scholar]
- Fulton, T.W. The Sovereignty of the Sea: An Historical Account of the Claims of England to the Dominion of the British Seas, and of the Evolution of the Territorial Waters; W. Blackwood: London, UK, 1911. [Google Scholar]
- Sheraliev, B.; Kayumova, Y.; Allayarov, S.; Rozimov, A.; Komilova, D.; Urmonova, D.; Peng, Z. Length–Weight Relations of 14 Endemic and Indigenous Freshwater Fish Species (Actinopterygii) from the Aral Sea Basin, Uzbekistan. Acta Ichthyol. Piscat. 2022, 52, 239–243. [Google Scholar] [CrossRef]
- Amirbekova, F.; Isbekov, K.B.; Assylbekova, S.Z.; Sharipova, O.A.; Adyrbekova, K.; Bulavina, N. Biological Characteristics of a Rare and Vulnerable Species (SCHIZOTHORAX ARGENTATUS (Kessler, 1874)) of TOKYRAUYN RIVER and Approbation of Its Artificial Reproduction. Agriculture 2022, 12, 1121. [Google Scholar] [CrossRef]
- Abdujalilov, M.; Rakhmatova, G.; Alimova, A.; Yuldashov, M. To the Biology of the Snow Trout in the Upper Reach of the Chirchik River. Eur. J. Interdiscip. Res. Dev. 2023, 20, 18–22. [Google Scholar]
- Rasulov, A.H.; Musofirov, H.K. Bioekologicheskie Osobennosti Obyiknovennoy Marinki (Schizothorax Intermedius Ms Clelland) r. Kafirnigan. Bull. Tajik SSR Acad. Sciences. Sect. Biol. Sci. 2018, 3, 29–34. [Google Scholar]
- Huo, T.B.; Jiang, Z.F.; Karjan, A.; Wang, Z.C.; Tang, F.J.; Yu, H.X. Length-Weight Relationships of 16 Fish Species from the Tarim River, China. J. Appl. Ichthyol. 2012, 28, 152–153. [Google Scholar] [CrossRef]
- Soni, N.; Ujjania, N.C. Length-Weight Relationship and Condition Factor of Indian Major Carps of Vallabhsagar Reservoir, Gujarat, India. Indian J. Fish. 2017, 64, 186–189. [Google Scholar] [CrossRef]
- Anderson, R.O.; Neumann, R.M. Length-Weight and Associated Structural Indices. In Fisheries Techniques; Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 447–482. [Google Scholar]
- Muchlisin, Z.A.; Musman, M.; Siti Azizah, M.N. Length-Weight Relationships and Condition Factors of Two Threatened Fishes, Rasbora Tawarensis and Poropuntius Tawarensis, Endemic to Lake Laut Tawar, Aceh Province, Indonesia. J. Appl. Ichthyol. 2010, 26, 949–953. [Google Scholar] [CrossRef]
- Jisr, N.; Younes, G.; Sukhn, C.; El-Dakdouki, M.H. Length-Weight Relationships and Relative Condition Factor of Fish Inhabiting the Marine Area of the Eastern Mediterranean City, Tripoli-Lebanon. Egypt. J. Aquat. Res. 2018, 44, 299–305. [Google Scholar] [CrossRef]
- Ragheb, E. Length-Weight Relationship and Well-Being Factors of 33 Fish Species Caught by Gillnets from the Egyptian Mediterranean Waters off Alexandria. Egypt. J. Aquat. Res. 2023, 49, 361–367. [Google Scholar] [CrossRef]
- Verhelst, P.; Brys, R.; Cooke, S.J.; Pauwels, I.; Rohtla, M.; Reubens, J. Enhancing Our Understanding of Fish Movement Ecology through Interdisciplinary and Cross-Boundary Research. Rev. Fish. Biol. Fish. 2022, 33, 111–135. [Google Scholar] [CrossRef]
- Sabah; Khan, M.A. Precise Age Estimation and Growth of Three Schizothoracinae Fishes from Kashmir Valley. Zool. Ecol. 2014, 24, 16–25. [Google Scholar] [CrossRef]
- Frost, W.E.; Kipling, C. The Determination of the Age and Growth of Pike (Esox lucius L.) from Scales and Opercular Bones. J. Du Cons. Int. Explor. Mer 1959, 24, 314–341. [Google Scholar] [CrossRef]
- McConell, W.J. The Opercular Bone As An Indicator of Age and Growth of the Carp Cyprinus carpio Linnaeus. Master’s Thesis, Utah State University, Logan, UT, USA, 1951. [Google Scholar] [CrossRef]
- Ma, B.; Xie, C.; Huo, B.; Yang, X.; Li, P. Age Validation, and Comparison of Otolith, Vertebra and Opercular Bone for Estimating Age of Schizothorax o’connori in the Yarlung Tsangpo River, Tibet. Environ. Biol. Fishes 2011, 90, 159–169. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S. Comparison of Age Estimates from Scale, Opercular Bone, Otolith, Vertebrae and Dorsal Fin Ray in Labeo Rohita (Hamilton), Catla Catla (Hamilton) and Channa Marulius (Hamilton). Fish. Res. 2009, 100, 255–259. [Google Scholar] [CrossRef]
- Gümüş, A.; Şahinöz, E.; Doǧu, Z.; Polat, N. Age and Growth of the Mesopotamian Spiny Eel, Mastacembelus Mastacembelus (Banks & Solender, 1794), from Southeastern Anatolia. Turk. J. Zool. 2010, 34, 399–407. [Google Scholar] [CrossRef]
- Kamilov, B.G. Age and Growth of the Silver Carp (Hypophthalmichthys Molitrix Val.) in Tudakul reservoir, Uzbekistan. Croat. J. Fish. 2014, 72, 12–16. [Google Scholar] [CrossRef][Green Version]
- Devries, D.R.; Frie, R.V. Determination of Age and Growth. In Fisheries Techniques; Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 483–512. [Google Scholar]
- Pavlov, D.S.; Kirillova, E.A.; Kirillov, P.I.; Nezdoliy, V.K. Pokatnaya Migratsiya, Povedenie i Raspredelenie Molodi Ryib v Nizovyah Reki Ozernoy (Yugo-Zapadnaya Kamchatka). Proc. Russ. Acad. Sci. Biol. Ser. 2015, 2015, 52–62. [Google Scholar] [CrossRef]
- Stoffers, T.; Buijse, A.D.; Verreth, J.A.J.; Nagelkerke, L.A.J. Environmental Requirements and Heterogeneity of Rheophilic Fish Nursery Habitats in European Lowland Rivers: Current Insights and Future Challenges. Fish Fish. 2022, 23, 162–182. [Google Scholar] [CrossRef]
- Prohorov, A.M. (Ed.) The Great Soviet Encyclopedia, 3rd ed.; Sovetskaya Entsiklopediya: Moscow, Russia, 1978. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).