Abstract
Environmentally cued hatching is prevalent, diverse, and crucial to many animals’ survival. Fish embryos use a variety of chemical cues to initiate hatching to avoid potential predators, yet the function of chemical cues released from the predatory consumption of heterospecific prey is largely unknown. Zebra cichlids (Metriaclima estherae) are ferocious predators that can feed on medaka (Oryzias latipes), though it is impossible for this to occur in their natural habitat. Zebrafish (Danio rerio) embryos have been employed as experimental subjects due to their sensitivity to a variety of chemical signals. In this study, zebrafish embryos were subjected to three types of chemical signals: predator cues (PCs, released from cichlids), heterospecific cues (HCs, released from medaka), and heterospecific dietary cues (HDCs, released from cichlids that have ingested medaka). As a result, the hatching times of zebrafish embryos were accelerated by 6.8% and 12.6% by PCs and HDCs, respectively. PCs and HDCs cause significantly reduced morphology in zebrafish embryos, including regarding total length, eye length, dorsal fin length, trunk height, caudal fin height, and body cavity, and increase yolk sac height. The PCs and HDCs diminished the larvae’s motion at 120 and 144 h post fertilization (hpf), which could be attributed to non-developmental embryogenesis. Overall, the impacts of HDCs on embryonic hatching, developmental morphology, and locomotor were more pronounced in comparison with PCs. Our findings demonstrate that predators’ dietary cues, even those released after predation on heterospecific prey, can modify embryogenesis, highlighting the critical functions of chemical signals in predation risk assessment using embryos.
Key Contribution:
Zebrafish embryos exhibit responses to chemical signals released by predators, including those released by predators after consuming heterospecific prey.
1. Introduction
Many animals begin their lives enclosed within eggs, protected by a covering such as a capsule or shell. During the early and crucial phases, they develop within a maternal structure. Predation is a significant selective force and psychological stressor that has substantial effects on prey behavior, physiology, and population dynamics [1,2]. Animals have evolved mechanisms for recognizing predation risk and altering their behavior to mitigate their exposure to predators through natural selection [1]. Though previous research on predator–prey interaction has primarily examined adult individuals, recent studies have demonstrated that embryos also respond to predation [3]. Embryos can modify their morphological and behavioral characteristics by responding to external cues prior to hatching [4,5]. Therefore, embryos should not be viewed as “works-in-progress” following a pre-programmed developmental pathway but rather as viable organisms with plasticity that can adapt to dynamic contexts. Embryos are vulnerable to some egg predators due to their immobility. Embryos have certain advantages when encountering larval predators. Accordingly, it has been demonstrated that embryos possess the ability to assess predation risk or receive information from parental care to aid in their assessment [6]. Additionally, hatching is an ecological transition in life history that can be interpreted as a niche shift during the ontogenetic process.
Predator–prey interactions involve the release of various chemical signals by predators and prey. Chemical cues released as by-products serve as reliable sources of public information for guiding anti-predator behavioral decision making [7]. These chemical signals can be classified into three main categories: predator cues (kairomones), alarm cues from damaged prey or undamaged prey (alarm pheromones and disturbance cues), and signals emitted after prey have been digested (dietary cues). Each type of signal elicits distinct responses in prey embryos, which ultimately promote individual fitness and favor population survival. Embryos of some taxa, such as Port Jackson sharks (Heterodontus portusjacksoni) and rainbow trout (Melanotaenia duboulayi), possess an inherent ability to detect and differentiate odors released by predators [8,9]. Embryos of fathead minnows (Pimephales promelas) and amphibians develop the ability to assess predation risk by being exposed to the odor and chemical signals of predators [10,11,12]. Damselfish like the cinnamon clownfish (Amphiprion melanopus) learn about signals from damaged conspecifics [13]. Embryos of convict cichlids (Amatitlania siquia) and wood frogs (Rana sylvatica) can learn to respond to chemical cues from unfamiliar predators [14,15]. Of note, zebrafish embryos have demonstrated the capacity to hatch early in response to chemical signals from crushed conspecific embryos as a predator avoidance mechanism [16]. Although early hatching favors egg escape from predators in development, premature hatching increases the susceptibility of prey to predation during the larval stage. It remains unclear whether the early hatching response is only elicited by cues from crushed embryos or whether embryos also respond to more general cues.
Prey animals may exhibit varying responses to chemical stimuli from predators depending on the predator’s recent diet. Predator excretions consist of metabolites and undigested components of their prey. Prey may exhibit heightened antipredator behavior in response to predators that have consumed conspecifics or closely related species [7]. Numerous studies have been conducted on aquatic animals, such as fish [17] and tadpoles [18], clearly demonstrating their behavior in displaying this phenomenon. Additionally, the dietary cues of predators consuming tadpoles influence the timing of conspecific prey metamorphosis [19]. However, the role of dietary cues from predators consuming heterospecific prey (HDC) in accelerating hatching and influencing the morphological and behavioral responses remains uncertain.
In the present study, we tested zebrafish (Danio rerio, native to India) embryos with the odor of a cichlid (Metriaclima estherae, native to Africa), the odor of medaka (Oryzias latipes, native to Japan), and the odor of an African cichlid fed on a diet of medaka. Cichlids and medaka belong to a diverse clade of teleosts called percomorphs and have a relatively close relationship (~100 mya) [20]. About 240 million years ago, they shared a common ancestor with Ostariophysi, which includes zebrafish and Mexican cavefish (Astyanax mexicanus) [20]. Given the evolutionary distance between medaka, cichlids, and zebrafish [21], it is evident that these teleost species are ideal for studying and comparing responses elicited by heterospecific chemical cues. While it is impossible for zebra cichlids to coexist with medaka in their natural habitats [22,23], our laboratory observations have revealed that the zebra cichlid, an aggressive species, does predate on medaka. Zebrafish embryos were selected as the exposed species for our study based on their capacity to sense diverse chemical signals [16,24] and behavioral and physiological plasticity in response to various chemical stimuli relevant to predation [16,25]. Zebrafish embryos were exposed to various chemical cues, including heterospecific cues (HCs), predator cues (PCs), and heterospecific dietary cues (HDCs). The hatching times were documented to assess the process of development. Moreover, the morphology and locomotion of larvae were compared to those of a control group to examine the potential outcomes of developmental alterations. This study advances our understanding of the specific signals transmitted using chemicals at varying levels of risk in chemical communication.
2. Materials and Methods
2.1. Animals, Housing, and Maintenance
Zebrafish (five months of age) were purchased from an aquatic culturing center (Qingfeng Aquaculture, Shanghai, China) and subsequently transported to a recirculating flow-through system at the Yantai Institute of Coastal Zone Research. The population was sustained within an enclosed system comprising only one species. The fish were kept at a temperature of 28.5 °C and subjected to a 12 h light–dark cycle. Zebrafish were fed live brine shrimp twice daily. After 10 days of habituation, we relocated the breeding group consisting of one male and two females to a 3 L tank. PVC dividers and tiles in the tank protected the embryos from predators. Adults were placed in the tank at 8 p.m. the night before. The barrier was removed at sunrise the next morning, allowing adults to chase and spawn. Once laid, the tiles were checked for embryos and removed from the tank. Following spawning, embryos were gathered and inspected using an Olympus CKX53 inverted microscope (Olympus, Tokyo, Japan). All embryos in these studies were produced via single breeding between two males and one female. The embryos were subsequently placed in 12-well plates for 6-day post-fertilization (dpf) exposure experiments. Red zebra cichlids (Metriaclima estherae) were purchased from a pet store (Aiyu, Shanghai, China). According to well-established cichlid cultivation protocols [26], the cichlids were housed in 30 L tanks and maintained. Briefly, the water temperature was maintained within the range of 28 ± 3 °C. Before the experiment, the cichlids were fed fish flakes (BESSN, Beijing, China) twice a day. This study used Japanese Medaka (Oryzias latipes) obtained from an experimental fish center (Experimental zebrafish resource center, Weifang, China) and kept in 30 L tanks. The fish were maintained at a temperature of 28.5 °C with a 12 h light–dark cycle according to established protocol [23].
2.2. Preparation of Chemical Stimulus
The medium (a sea salt solution with a conductivity of 500–550 µs/cm) was included in the experiment as a control (C) (Figure 1a). Ten medaka (10 fish) were placed in a 3 L tank for 24 h to collect conditioned water as heterospecific cues (Figure 1b). The cichlids fed flakes were subjected a 12 h isolation and fasting period before being transferred to a new 3 L of water to acquire PCs (Figure 1c). The cichlids that were fed medaka for 10 days were quarantined for 24 h in a 3 L water tank to obtain heterospecific dietary cues (Figure 1d).
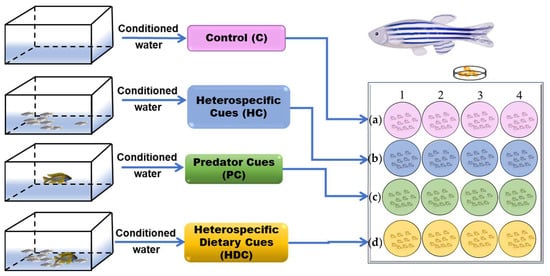
Figure 1.
Schematic diagram of the experimental design. Embryos were exposed to control (a), heterospecific cues (b), predator cues (c), and heterospecific dietary cues (d) from day 1 until day 6. Embryonic development was observed at 24 h intervals. The behavior of embryos subjected to four different rearing conditions was evaluated at 5 and 6 dpf.
2.3. Experimental Design
Zebrafish embryos were allocated randomly into 16 groups, with each group comprising 10 embryos. Every four groups were subsequently placed into 6-well plates with 5 mL of chemical cues. Each plate was assigned to a chemical cue, namely, C, HC, PC, and HDC. Hence, each treatment involved four sets of replicates, with daily renewal of the exposure-conditioned water during the entire incubation period (6 days). The bath temperatures in the test chambers were maintained at a constant level of 28 ± 0.1 °C throughout the experiment.
2.3.1. Experiment 1—Hatching Time
Hatching time was assessed from 41 hpf to the point when all eggs hatched (56 hpf). Zebrafish embryos typically incubate for 48 to 96 hpf [27]. Embryos were observed and imaged with a microscope every 30 min and then placed back in the incubator.
2.3.2. Experiment 2—Morphological Assessment
The two or three embryos that hatched randomly in each well were recorded, resulting in a total of ten embryos from four wells [16]. The morphological measurements of newly hatched zebrafish larvae were recorded using an Olympus CKX53 inverted microscope. In accordance with a previous report [16], a total of 13 matrices were measured, including total length, head depth, eye length, eye height, yolk sac height, body cavity length, total trunk height, trunk height, dorsal fin length, dorsal fin height, caudal fin length, caudal fin height, and anal fin length (Figure 2).
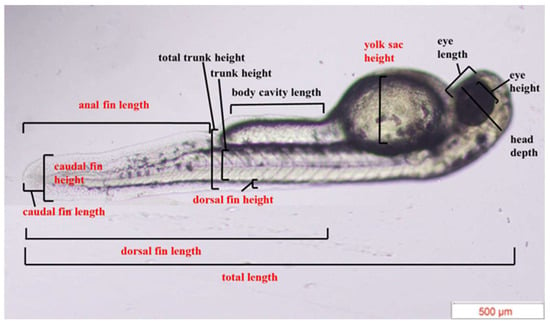
Figure 2.
Morphological measurements of newly hatched zebrafish larvae [16].
2.3.3. Experiment 3—Locomotor Behavior Assay
The behavioral activities of zebrafish larvae were examined using Danio Vision (Noldus, The Netherlands) at the 5-day and 6-day points. This study employed a protocol modified from Padilla et al. [28] to investigate the impact of various chemical cues on the locomotor behavior of zebrafish larvae. Zebrafish larvae were acclimated in a 96-well plate for 10 min prior to starting the experiment. The duration of the observation was 10 min without additional stimulation. The movement speed (mm/s) and movement duration (s) of the hatchlings were recorded. The behavioral phenotypes of 10 hatchlings were recorded for each treatment. Behavioral data analysis was performed for each larva using movement analysis, as previously reported [29,30].
2.4. Statistical Analysis
All data underwent normality testing, and each group was compared. A one-way ANOVA was performed followed by Bonferroni multiple comparisons if the data showed a normal distribution and homogeneity of variance. Nonparametric tests (Kruskal–Wallis test) followed by Dunn–Bonferroni test were used if the data were not normally distributed or were normally distributed but there was heterogeneity of variance. In zebrafish larvae locomotion analysis, t-tests were used for comparison of the movement speed and cumulative duration. The 5% limit for alpha, known as the significance level of a study, was set for a single comparison between groups.
3. Results
3.1. Heterospecific Cues (HDC) and Predator Cues (PC) Accelerate Hatching Time
The fish in the control group hatched within a time frame of 45 to 60 hpf (Figure 3). The hatching times in the PC group (mean 46.6 hpf) and HDC group (mean 43.7 hpf) were both accelerated compared to those of the control group (mean 50.0 hpf) by 6.8% (Kruskal–Wallis p < 0.001) and 12.6% (Kruskal–Wallis p < 0.001), respectively. There was a significant difference between the PC group and the HDC group (Kruskal–Wallis p = 0.013). However, no significant differences were observed in the HC group compared to the control (Kruskal–Wallis p = 1) (Figure 3).
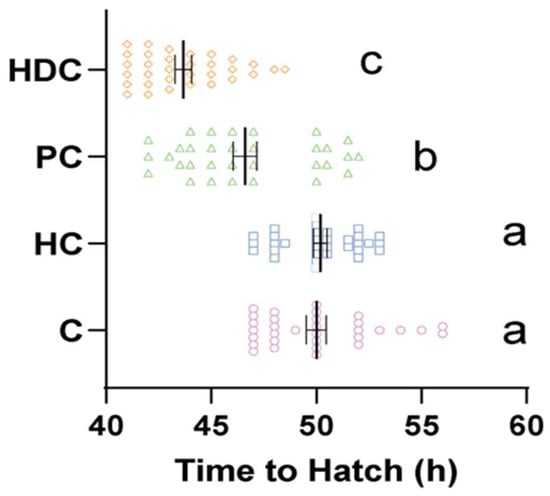
Figure 3.
Hatching time of zebrafish embryos in response to various stimuli: control (C), heterospecific cues (HCs), predator cues (PCs), and heterospecific dietary cues (HDCs). n = 32. The mean ± standard deviation of the mean (SEM) was utilized to represent the data. Letters denote significant pairwise differences between the treatments (p < 0.05).
3.2. Heterospecific Dietary Cues (HDCs) and Predator Cues (PCs) Altered Developmental Morphology
Representative developmental images at 45.0 hpf are shown in Figure 4a–c, suggesting that the embryo treated with HDC and PC exhibited had accelerated development compared to the control group.
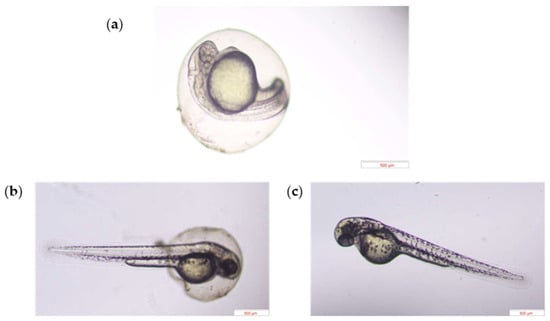
Figure 4.
The representative morphology of zebrafish embryos after exposure to C (a), PC (b), and HDC (c) at 45.0 hpf.
In accordance with a previous report [16], we measured a total of 13 morphological indexes immediately after hatching (Figure 2). No significant changes were observed among treatments for six indexes, namely, head depth, eye height, dorsal fin height, total trunk height, anal fin length, and caudal fin length (Figure A1). Significant changes were observed in seven indexes across the treatments, namely, total length, eye length, yolk height, body cavity length, dorsal fin length, trunk height, and caudal fin height (Figure 5a–g). There were no significant variations observed between the HC treatment group and the C treatment groups in terms of each morphological index. The larvae in the HDC treatment group exhibited an apparently decreased body total length, eye length, dorsal fin length, trunk height, caudal fin height, and body cavity length and an increased yolk sac height in comparison with the control. Both the PC and HDC treatment groups exhibited dysplasia in terms of morphological development in comparison to the control and HC treatment groups.
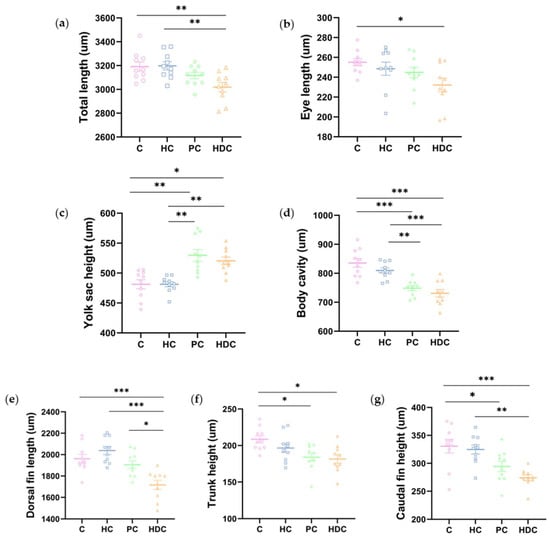
Figure 5.
Morphological measurements of newly hatched zebrafish larvae: (a) total length; (b) eye length; (c) yolk sac height; (d) body cavity length; (e) dorsal fin length; (f) trunk height; and (g) caudal fin height. The mean ± standard deviation of the mean (SEM) was utilized to represent the data. n = 10. p < 0.05 is indicated by *, p < 0.01 is indicated by **, and p < 0.001 is indicated by ***.
3.3. Heterospecific Dietary Cues (HDCs) and Predator Cues (PCs) Attenuate the Locomotor Ability of Zebrafish Larvae
PC and HDC exposure significantly inhibited the locomotor behavior of zebrafish larvae at 120 and 144 hpf, respectively (Figure 6). At 120 hpf, the duration of locomotion decreased by 42.3% in the PC treatment group (ANOVA F3,36 = 10.816, p < 0.001) and by 61.9% in the HDC exposure groups (ANOVA F3,36 = 10.816, p < 0.001) (Figure 6a). At 144 hpf, the duration of locomotion decreased by 32.6% in the PC treatment group (ANOVA F3,36 = 17.257, p = 0.004) and by 46.5% in the HDC exposure groups (ANOVA F3,36 = 17.257, p < 0.001) (Figure 6a). However, there were no significant effects observed following HC exposure at the two observation timepoints (Figure 6a). A notable reduction in velocity, namely, 41.8% (ANOVA F3,36 = 10.837, p = 0.003) and 52.5% (ANOVA F3,36 = 10.837, p < 0.001), was observed in the fish exposed to PC and HDC treatments at 120 hpf, respectively (Figure 6b). Similarly, PC and HDC treatments reduced the velocity of zebrafish larvae by 28.7% (ANOVA F3,36 = 13.530, p = 0.012) and 39.4% (ANOVA F3,36 = 13.530, p < 0.001) at 144 hpf, respectively (Figure 6b). Larvae exposed to the HC treatment did not show any significant differences in velocity compared with the control (ANOVA p > 0.05) (Figure 6b). The PC and HDC treatments exhibited a higher degree of static status (Figure 6c,d).
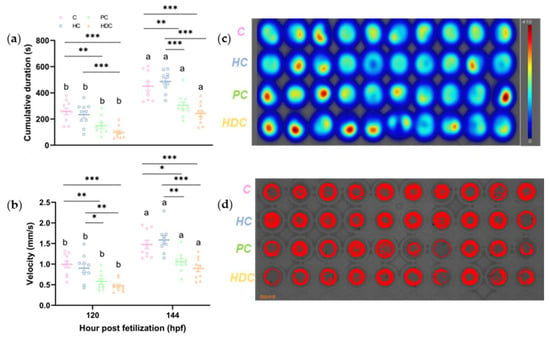
Figure 6.
Effects of heterospecific cues (HCs), predator cues (PCs), heterospecific dietary cues (HDCs), and control (C) on swimming performance: (a) cumulative movement duration and (b) velocity of zebrafish larvae at 120 and 144 hpf. Results are expressed as means ± SEM (n = 10). p < 0.05 is indicated by *, p < 0.01 is indicated by **, and p < 0.001 is indicated by ***. Letters denote significant pairwise differences of each chemical treatment between 120 and 144 hpf (p < 0.05). A typical thermal diagram (c) and trajectory diagram (d) of zebrafish larvae at 144 hpf are shown.
4. Discussion
Our results indicated that the chemical stimuli employed in this experiment were maintained at appropriate concentrations, ensuring the well-being of the zebrafish embryos, as the hatch rate in all the treatments approached almost 100% at 56 hpf. Unlike certain toxins and pollutants [31], these chemical stimuli did not induce any malformations. Although hatchability and embryo morphogenesis were normal, the hatching process demonstrated significant alterations in response to different stimuli.
The predator cues showed an approximate 6.8% acceleration in hatch time compared to the control, suggesting that zebrafish embryos have an innate response to predator cues, which has also been observed in amphibians [6]. Moreover, the chemical cues released by predators who consumed medaka accelerated the hatching time of the zebrafish embryos by 12.6%, indicating that the dietary cues could be “public information” that can inform species with distant correlations in phylogeny. The results suggested that zebrafish possess very general sensitivity to cues that indicate predation. However, HCs had no significant effect on the hatching time of the zebrafish embryos. The zebra cichlid is a piscivorous fish [32], while the medaka is an omnivorous fish and may ignore zebrafish embryos due to the mismatched particle size and gape [33]. We speculated that there is something in the body odor of a cichlid that is absent in the body odor of medaka that triggers the early hatching response. Characterization of the difference in molecular identity between medaka and cichlid chemical signals is needed to reveal the mechanism underlying the hatching response.
Our findings revealed that dietary cues significantly accelerated hatching when compared to predator cues, implying that damaged medaka or medaka products ingested by cichlids are indicative of a higher predation risk. Most embryonic learning research has used chemical stimuli consisting of a predator cue and an alarm substance (extracted from damaged conspecific skin) [10,11,12]. As a result, the chemical mixture that indicates immediate predation is more effective than the predator cue or alarm ingredient alone. Prey may respond to residual chemical alarm cues from ingested prey in a predator’s feces, limiting predator generalization because alarm cue identification must be learned and is species specific. These studies provide crucial insights into the link between predator recognition and diet. In our experiments, we utilized a diet that does not contain a chemical alarm cue (produced when a conspecific is injured) and a cichlid that is geographically distant and not often considered a predator. The diet of a nonpredator caused the embryos to sense a higher predation risk, demonstrating the importance of dietary components in predator recognition while excluding the potential impacts of chemical alarm cues and the predator.
Zebrafish exhibit a comparatively lower-amplitude regulation of hatching times in response to HDCs, while red-eyed treefrogs (Agalychnis callidryas) can accelerate hatching by 30% in response to predators in the wild [34,35]. Chemical cues signal embryonic development in zebrafish embryos, while amphibian arboreal embryos are more susceptible to mechanical signals, such as vibrations [36]. When animals hatch from their egg capsules and venture into the external environment, they transition from one set of potential dangers and advantages to another. Therefore, the time of hatching is crucial to protecting eggs from possible damage. Species with innate predator recognition have a selective advantage, particularly when encountering a predator at their early development stage [1,37]. Furthermore, the hatching times accelerated under the stimulation of predator and heterospecific dietary cues, respectively, suggesting that the hatching time of zebrafish embryos could be more responsive than morphological alteration in embryogenesis.
Our results demonstrated the morphological disparity induced by various stimuli, suggesting that embryos exhibit a lag in development induced by PCs and HDCs. Early hatching can present a trade-off as it offers the advantage of allowing embryos to evade immediate predation. Nevertheless, this trade-off results in hatching at a less advanced stage, which subsequently decreases the hatchlings’ capacity to defend themselves against potential predators [16]. Notably, the HDCs had a significant effect on dorsal fin length and caudal fin height in larvae, indicating that these heterospecific dietary cues play a role in inducing morphological changes, which may have a close correlation with locomotion. Morphological development may also be highly predator-specific [38]. Taken together, the insufficiency of morphological development may result from premature hatching, which may mitigate the locomotor activity of hatchlings [6].
Consequently, the behavioral response of larvae exhibited attenuation in the treatments with PCs and HDCs, indicating plastic responses to risk in embryos have carryover effects on the larval stage and subsequent developmental stages. Embryos display a diverse range of behavioral responses to adapt to changes in their developmental environment during interactions with predators. For instance, the exposure of red-capped plover (Charadrius ruficapillus) embryos to predator calls resulted in a decrease in embryonic vocalizations [39]. Cuttlefish (Sepia pharaonis) embryos exhibit reduced ventilation rates when exposed to visual and chemical cues associated with predators but not in response to non-predatory cues [40]. Embryonic skates (Raja clavata) and bamboo sharks (Chiloscyllium punctatum) exhibit a response to the presence of a predator by ceasing gill movements and curling their tails around their bodies in response to the detection of a weak electrical field due to the absence of the ability to move rapidly to evade predators [41,42]. Moreover, they possess the capability to reduce their movement or activity to eliminate their bioelectric signatures when they perceive the presence of a predator, ultimately allowing them to escape from predators. Predator-cue-induced behavioral mediation is processed by prey sensory systems through neurophysiological alterations [43]. Our findings demonstrated that zebrafish embryos alter locomotor activity at early developmental stages to respond to chemical signals, which could be attributed to incomplete embryonic development.
Taken together, our experiments demonstrated that zebrafish embryos exhibited responses to chemical signals released by a predator, including after it consumed heterospecific prey. The predator and prey used in this experiment are evolutionarily distant from the zebrafish embryo. Zebrafish embryos accelerated their hatching times, altered their morphology, and reduced their movement tendencies, which are assumed to promote their escape from predation. These findings suggest that zebrafish embryos have an inherent capacity to detect and react to predatory signals.
5. Conclusions
Our results demonstrate the morphological disparity induced by various stimuli. Early hatching can present a trade-off, as it offers the advantage of allowing embryos to evade immediate predation. Early hatching leads to morphological changes and incomplete development, which may mitigate the locomotor activity of hatchlings. These findings enhance our knowledge of how organisms adapt to their environments during the early stages of development, highlighting the significance of assessing the risk of predation through chemical signals.
Author Contributions
Conceptualization: K.L. and A.L.; Methodology: A.L., Z.Y., Z.L. and Y.L.; Investigation: A.L., Z.Y., H.Z., J.C., D.W., B.J., H.W., Z.L. and X.L.; Supervision: K.L.; Writing—original draft: A.L. and K.L.; Writing—review and editing: K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32270533), the Yantai Science and Technology Innovation Development Program (Grant No. 2023JCYJ098), and the seed project of Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences (Grant No. YICE351010101). Ke Li appreciates the support provided by the National Overseas High-level Talent Project and expresses gratitude for the Taishan Scholar Program provided by the Shandong Province of China (tsqn20190403), as well as for the support received from the Yantai Municipal City’s Shuangbai Plan (2018020).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences (protocol code 2022R001 and date of 16 November 2021).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
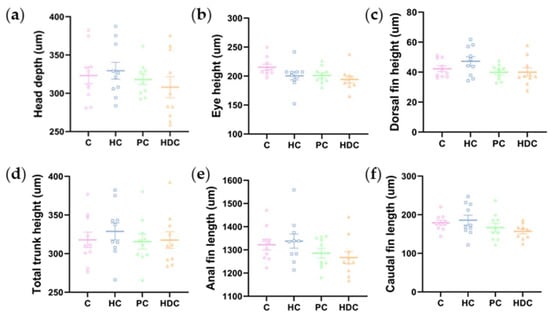
Figure A1.
Morphological measurements of newly hatched zebrafish larvae: (a) head depth (ANOVA F3,36 = 0.683, p = 0.568); (b) eye height (ANOVA F3,36 = 2.520, p = 0.073); (c) dorsal fin height (ANOVA F3,36 = 1.981, p = 0.134); (d) total trunk height (ANOVA F3,36 = 0.336, p = 0.799); (e) anal fin length (ANOVA F3,36 = 1.629, p = 0.200); and (f) caudal fin length (ANOVA F3,36 = 1.819, p = 0.161).
References
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Zanette, L.Y.; Clinchy, M. Ecology and neurobiology of fear in free-living wildlife. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 297–318. [Google Scholar] [CrossRef]
- Du, W.G.; Shine, R. The behavioural and physiological ecology of embryos: Responding to the challenges of life inside an egg. Biol. Rev. 2022, 97, 1272–1286. [Google Scholar] [CrossRef]
- Hamdoun, A.; Epel, D. Embryo stability and vulnerability in an always changing world. Proc. Natl. Acad. Sci. USA 2007, 104, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Ituarte, R.B.; Vázquez, M.G.; Bas, C.C. Chemically induced plasticity in early life history of Palaemon argentinus: Are chemical alarm cues conserved within palaemonid shrimps? J. Exp. Biol. 2019, 222, jeb199984. [Google Scholar]
- Warkentin, K.M. Environmentally cued hatching across taxa: Embryos respond to risk and opportunity. Integr. Comp. Biol. 2011, 51, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Wisenden, B.D. Chemical cues that indicate risk of predation. In Fish Pheromones Related Cues; Sorensen, P.W., Wisenden, B.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 131–148. [Google Scholar]
- Gervais, C.R.; Nay, T.; Brown, C. Friend or foe? Development of odour detection, differentiation and antipredator response in an embryonic elasmobranch. Mar. Freshw. Res. 2021, 72, 942–949. [Google Scholar] [CrossRef]
- Oulton, L.J.; Haviland, V.; Brown, C. Predator recognition in rainbowfish, Melanotaenia duboulayi, embryos. PLoS ONE 2013, 8, e76061. [Google Scholar] [CrossRef]
- Crowder, C.; Ward, J. Embryonic antipredator defenses and behavioral carryover effects in the fathead minnow (Pimephales promelas). Behav. Ecol. Sociobiol. 2022, 76, 27. [Google Scholar] [CrossRef]
- Horn, M.E.; Chivers, D.P. Preschool for small frys: Threat-sensitive learning of predators by embryonic fathead minnows. Anim. Behav. 2021, 178, 49–55. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Chivers, D.P. The ghost of predation future: Threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behav. Ecol. Sociobiol. 2010, 64, 549–555. [Google Scholar] [CrossRef]
- Atherton, J.A.; McCormick, M.I. Active in the sac: Damselfish embryos use innate recognition of odours to learn predation risk before hatching. Anim. Behav. 2015, 103, 1–6. [Google Scholar] [CrossRef]
- Mathis, A.; Ferrari, M.C.; Windel, N.; Messier, F.; Chivers, D.P. Learning by embryos and the ghost of predation future. Proc. R. Soc. B 2008, 275, 2603–2607. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.B.; Alemadi, S.D.; Wisenden, B.D. Learned recognition of novel predator odour by convict cichlid embryos. Behav. Ecol. Sociobiol. 2013, 67, 1269–1273. [Google Scholar] [CrossRef]
- Wisenden, B.D.; Paulson, D.C.; Orr, M. Zebrafish embryos hatch early in response to chemical and mechanical indicators of predation risk, resulting in underdeveloped swimming ability of hatchling larvae. Open Biol. 2022, 11, bio059229. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Smith, R.J.F. Chemical labeling of northern pike (Esox lucius) by the alarm pheromone of fathead minnows (Pimephales promelas). J. Chem. Ecol. 1993, 19, 1967–1979. [Google Scholar] [CrossRef]
- Laurila, A.; Kujasalo, J.; Ranta, E. Different antipredator behaviour in two anuran tadpoles: Effects of predator diet. Behav. Ecol. Sociobiol. 1997, 40, 329–336. [Google Scholar] [CrossRef]
- Laurila, A.; Kujasalo, J.; Ranta, E. Predator-induced changes in life history in two anuran tadpoles: Effects of predator diet. Oikos 1998, 83, 307–317. [Google Scholar] [CrossRef]
- Betancur-R, R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Ortí, G. Phylogenetic classification of bony fishes. BMC Evol. Biol. 2017, 17, 162. [Google Scholar] [CrossRef]
- Wittbrodt, J.; Shima, A.; Schartl, M. Medaka—A model organism from the far East. Nat. Rev. Genet. 2002, 3, 53–64. [Google Scholar] [CrossRef]
- Santos, M.E.; Lopes, J.F.; Kratochwil, C.F. East African cichlid fishes. EvoDevo 2023, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Larsen, G.D. Uncommon uses for a common fish. Lab. Anim. 2015, 44, 429. [Google Scholar] [CrossRef] [PubMed]
- Mourabit, S.; Rundle, S.; Spicer, J.; Sloman, K. Alarm substance from adult zebrafish alters early embryonic development in offspring. Biol. Lett. 2010, 6, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, W. Embryonic substances induce alarm response in adult zebrafish (Danio rerio). J. Fish Biol. 2020, 97, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, E.; Páramo-Delgadillo, S. The culture of cichlids of southeastern Mexico. Aquac. Res. 2008, 39, 777–783. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Padilla, S.; Hunter, D.L.; Padnos, B.; Frady, S.; MacPhail, R.C. Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol. 2011, 33, 624–630. [Google Scholar] [CrossRef]
- Murphy, C.A.; Rose, K.A.; del Carmen Alvarez, M.; Fuiman, L.A. Modeling larval fish behavior: Scaling the sublethal effects of methylmercury to population-relevant endpoints. Aquat. Toxicol. 2008, 86, 470–484. [Google Scholar] [CrossRef]
- Ulhaq, M.; Örn, S.; Carlsson, G.; Morrison, D.A.; Norrgren, L. Locomotor behavior in zebrafish (Danio rerio) larvae exposed to perfluoroalkyl acids. Aquat. Toxicol. 2013, 144, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kohda, M. Individual specialized foraging repertoires in the piscivorous cichlid fish Lepidiolamprologus profundicola. Anim. Behav. 1994, 48, 1123–1131. [Google Scholar] [CrossRef]
- Shima, A.; Mitani, H. Medaka as a research organism: Past, present and future. Mech. Dev. 2004, 121, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, K.M. Adaptive plasticity in hatching age: A response to predation risk trade-offs. Proc. Natl. Acad. Sci. USA 1995, 92, 3507–3510. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, K.M. Wasp predation and wasp-induced hatching of red-eyed treefrog eggs. Anim. Behav. 2000, 60, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Serrano-Rojas, S.J.; Warkentin, K.M. Multimodal mechanosensing enables treefrog embryos to escape egg-predators. J. Exp. Biol. 2020, 223, jeb236141. [Google Scholar] [CrossRef]
- Ferrari, M.C.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator–prey interactions in aquatic ecosystems: A review and prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Touchon, J.C.; Wojdak, J.M. Plastic hatching timing by red-eyed treefrog embryos interacts with larval predator identity and sublethal predation to affect prey morphology but not performance. PLoS ONE 2014, 9, e100623. [Google Scholar] [CrossRef]
- Kostoglou, K.N.; van Dongen, W.F.; Bowe, S.J.; Weston, M.A. Shorebird embryos exhibit anti-predator responses. Ibis 2021, 163, 1425–1436. [Google Scholar] [CrossRef]
- Mezrai, N.; Arduini, L.; Dickel, L.; Chiao, C.-C.; Darmaillacq, A.-S. Awareness of danger inside the egg: Evidence of innate and learned predator recognition in cuttlefish embryos. Learn. Behav. 2020, 48, 401–410. [Google Scholar] [CrossRef]
- Kempster, R.M.; Hart, N.S.; Collin, S.P. Survival of the stillest: Predator avoidance in shark embryos. PLoS ONE 2013, 8, e52551. [Google Scholar] [CrossRef]
- Ball, R.E.; Oliver, M.K.; Gill, A.B. Early life sensory ability—Ventilatory responses of thornback ray embryos (Raja clavata) to predator-type electric fields. Dev. Neurobiol. 2016, 76, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, A.; Brandalise, F.; Rubolini, D.; Rossi, P.; Galeotti, P. Fear is the mother of invention: Anuran embryos exposed to predator cues alter life-history traits, post-hatching behaviour and neuronal activity patterns. J. Exp. Biol. 2015, 218, 3919–3930. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).