Abstract
Coilia nasus is a typical anadromous migratory fish found in the lower reaches of the Yangtze River. Every year, C. nasus clusters offshore and swims upstream along the Yangtze River into the tributaries and lakes in the middle and lower reaches of the Yangtze River to breed. In this study, female C. nasus were collected as study subjects from the Chongming section of Shanghai, the Taizhou section of Jiangsu, and the Anqing section of Anhui. Their ovaries were used to examine tissue sections and investigate gene expression, including the follicle-stimulating hormone receptor (fshr), the luteinizing hormone receptor (lhr), kisspeptin-1 (kiss1), and forkhead box l2 (foxl2), which are related to reproductive development, while the serum levels of estrogen (including estradiol, E2) and progestins (including 17α,20β-dihydroxy-4-pregenen-3-one, 17α,20β-DHP) were also analyzed. Our results showed that, first, the growth period of the oocytes was small in stage II of ovarian development, in which both E2 and 17α,20β-DHP levels and gene expression were low. Then, in stage III, the growth period of the oocytes became large, and the yolk granules and oil droplets began to appear. Simultaneously, E2 and the expression of kiss1 and foxl2 were significantly elevated. Finally, stage IV was the period of a large amount of accumulation of nutrients in the oocytes, and 17α,20β-DHP levels and the expression of fshr and lhr were significantly elevated. These results enrich the theoretical study of ovarian development in the natural population of C. nasus, supplementing the biological basis of C. nasus reproduction and scientifically supporting the study of C. nasus population ecology and resource conservation.
Keywords:
Coilia nasus; ovarian development; organizational observation; sex steroid hormones; gene expression Key Contribution:
In this study, histological techniques were used to observe the developmental characteristics of C. nasus ovaries, and serum sex steroid hormone contents and ovarian gene expression levels were detected during the development of C. nasus ovaries to provide support for conservation studies of the natural population of C. nasus.
1. Introduction
Ovarian development is mainly regulated by the endocrine system in fish. Estrogens and progestins, including estradiol (E2) and 17α,20β-dihydroxy-4-pregenen-3-one (17α,20β-DHP), play essential roles in the regulation of ovarian maturation and development [1,2]. Studies have shown that E2 is produced in the follicular layer and stimulates the hepatocytes to synthesize vitellogenin through estrogen receptor signaling and then release it into the bloodstream. During the maturation stage of the ovary, E2 causes the synthesis of 17α,20β-DHP in the steroidogenic pathway, which induces the final maturation of the oocyte [3,4]. An oocyte maturates due to 17α,20β-DHP through the stimulation of maturation-promoting factor formation in oocytes [5]. A similar important role is played by genes in the regulation of ovarian development. The follicle-stimulating hormone receptor (fshr) primarily mediates follicle-stimulating hormone (fsh) function in response to the stimulation of ovarian follicle development and ovulation in female animals [6]. A member of the glycoprotein subfamily of the G-protein-coupled receptor superfamily, the luteinizing hormone receptor (lhr) regulates reproduction in animals upon binding to luteinizing hormone (lh) [7]. In female fish, fsh is mainly involved in oocyte differentiation, development, and yolk accumulation, while lh is involved in oocyte maturation and ovulation [8]. Kisspeptin-1 (Kiss1) is a key regulator of the reproductive regulatory cascade, with roles in regulating gonadotropin release and follicular maturation [9]. Kisspeptin is a peptide encoded by kiss1. It stimulates the release of follicle-stimulating hormone and luteinizing hormone [10]. Part of the forkhead transcription factor family, forkhead box l2 (foxl2) is an upstream regulator of the P450 aromatase promoter and is specific to the ovary. The main role of foxl2 is to activate the expression of cyp19a in an effort to regulate estrogen levels and modulate ovarian development [11]. In vertebrates, the foxl2 gene, which encodes a highly conserved amino acid sequence, is the initiating gene for ovarian development [12].
Coilia nasus (Clupeiformes: Engraulidae) is a small–moderate-sized fish [13,14]. Reproductive migratory groups of C. nasus gather at the Yangtze River estuary in spring, and the clusters migrate in an anadromous manner into the tributaries and lakes of the middle and lower reaches of the Yangtze River to finish reproduction [15,16]. During the migration time, the water temperature gradually increases, and the ovaries rapidly develop and mature. The spawning season of C. nasus is from June to October every year under natural conditions [17]. Xu et al. [18] showed that the oocytes in the ovaries of C. nasus at all developmental stages had obvious synchronization and belonged to the type of one-time spawning, and the ovaries could be divided into six developmental stages from I to IV. Recently, more studies have been conducted on the metabolic mechanisms [19], immunity [20,21], energy utilization [22,23,24], and key regulatory pathways in the liver and brain [25] during the reproductive migration of C. nasus, while relatively few studies have been conducted on factors related to ovarian development. In this study, we investigated the changes in the factors related to the reproductive development of C. nasus at the hormone and gene levels. In order to understand the spatial and temporal characteristics of ovarian development and investigate the changes in reproductive development at the hormone and gene levels, female C. nasus from the Chongming section of Shanghai (CM), the Taizhou section of Jiangsu Province (TZ), and the Anqing section of Anhui Province (AQ) were chosen during the C. nasus migratory season in this study, and following further observation of the ovaries at different developmental stages, the levels of serum sex steroid hormones and the expression patterns of genes related to the reproductive development of the ovaries were analyzed. All of these enrich the study of C. nasus reproduction biology and support the conservation of C. nasus germplasm resources.
2. Materials and Methods
2.1. Survey Sample Areas and Methods
During the 2021 C. nasus migration season, three survey sample areas were set up in the CM section (31°29′52″ N, 121°36′36″ E), TZ section (32°12′14″ N, 119°53′40″ E), and AQ section (30°29′11″ N, 116°59′39″ E) (Figure 1). The specifications of the nets used in each segment of the river are shown in Table 1. During the survey period, except for bad weather such as typhoons, C. nasus surveys were carried out daily in the TZ and AQ sections, while in the CM section, they were carried out at the right times according to the tidal conditions and synchronized with the measurement of water temperature data.
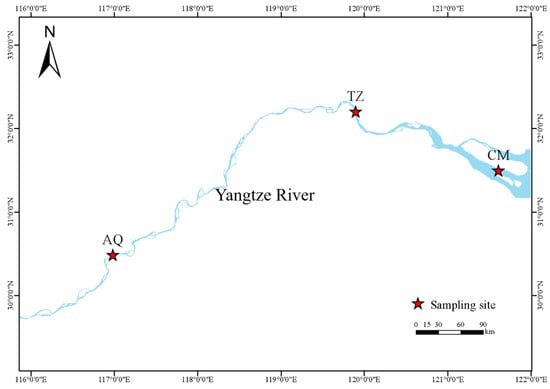
Figure 1.
Sampling site of C. nasus in the Yangtze River. CM, Chongming; TZ, Taizhou; AQ, Anqing.

Table 1.
Type and specifications of the nets used for collection of C. nasus samples.
2.2. Sample Collection and Processing
We placed the captured C. nasus into an insulated box containing crushed ice, placed them on ice to keep them fresh, and then transported the C. nasus to the laboratory for measurement and sampling. It took approximately 3.5–4.5 h from capture to sampling. C. nasus samples were placed on ice trays to measure the body length (accurate to 0.01 mm), body mass (accurate to 0.1 g), and other growth traits. The dissected C. nasus males were used for other experiments, while the females were visually identified according to the shape, size, and color of the dissected ovaries, and the ovaries were weighed (accurate to 0.1 g) [18]. The sample size of stage V ovaries could not be established in this experiment due to limitations in body length control conditions. Therefore, stage II, III, and IV C. nasus females with body lengths of 250–300 mm were selected for this study. One side of the ovary was preserved in 10% paraformaldehyde fixative for sectioning and observation, and the other side of the ovary was put into a freezing tube, preserved in liquid nitrogen, and then transferred to a refrigerator at −80 °C for the determination of gene expression. Meanwhile, the supernatant was collected after the centrifugation of their corresponding blood samples and stored in liquid nitrogen, then transferred to a −80 °C refrigerator for the determination of sex steroid hormones in the serum. A total of 30 C. nasus females in stages II, III, and IV in the AQ section were selected for the characterization of different developmental stages, and a total of 30 female C. nasus in stage IV in the CM, TZ, and AQ sections were selected for spatial characteristic analysis. These fish were all used for histological observation, hormone determination, and gene determination.
2.2.1. Ovarian Tissue Section Preparation
The paraformaldehyde-impregnated ovaries were dehydrated using an automatic dehydrator, and after dehydration, they were prepared in wax blocks by means of xylene transparency and paraffin embedding, after which they were subjected to paraffin sectioning with a slice thickness of 4 μm. Hematoxylin–eosin staining (HE) was performed on paraffin tissue sections, and the sections were sealed with neutral resin. The ovarian condition of development was observed under a microscope (Olympus BX51) and photographed. The following indicators were counted according to the HE section diagrams: the sizes of the oocytes and nuclei in stages II, III, and IV. Additionally, the number of oil droplets and yolk particles in stages III and IV, as well as the ratio of the oil droplet and yolk particle area to the oocyte area, were observed.
2.2.2. Sex Steroid Hormone Measurement
An enzyme-linked immunoassay (ELISA) was used to detect hormone levels in the serum of C. nasus. The basic principle of ELISA is to let the antibody bind to the enzyme complex and then quantitatively detect it by means of color display. A serum E2 and 17α,20β-DHP indicator assay kit was purchased from Nanjing Sembega Biological Company, and all operations were carried out in strict accordance with the instructions of the kit.
2.2.3. Gene Expression Assays
Primer sequences were designed using Primer Premier 5.0 software on the basis of the sequence information of the fshr, lhr, kiss1, and foxl2 genes from the NCBI database (Table 2). The relative expression of the genes involved in the development of the ovaries was established using quantitative real-time PCR. The C. nasus β-actin gene was used as an internal reference, and the multiplicative relationship of the difference in expression between samples was obtained using a 2−(∆∆Ct) mathematical operation between the Ct value of the internal reference gene and the Ct value of the gene to be examined. RNA was extracted using the UNIQ-10 column Trizol total RNA extraction kit; the concentration and purity of the RNA were detected using a multifunctional enzyme labeling instrument; the electrophoresis results of the RNA were detected using an electrophoresis buffer; reverse transcription was carried out on a PCR instrument; and the reaction was carried out in a 10 μL reaction mixture consisting of 5 μL of SybrGreen qPCR Master Mix, 0.2 μL of primer F (10 μm), 0.2 μL of primer R (10 μm), 3.6 μL of ddH2O, and 1 μL of cDNA in a 96-well plate. The reaction conditions were as follows: 94 °C predenaturation for 3 min, amplification for 45 cycles (95 °C denaturation for 15 s, 60 °C annealing for 30 s); the samples were then put into the fluorescence quantitative PCR instrument (LightCycler480 II) for detection.

Table 2.
Primer sequences used in the experiment.
2.3. Data Processing and Analysis
The experimental data were analyzed statistically using SPSS 26.0 software, significant differences were analyzed using one-way ANOVA (p < 0.05 means significant difference), the results were expressed as “mean ± standard deviation”, and the data graphs were created by GraphPad Prism 8.0 software.
Gonadosomatic index (GSI) (%) = weight of ovary (g)/weight of fish body (g) × 100%
3. Results
3.1. Biological Characteristics of C. nasus
The GSIs of stages I–V were 0.79%, 1.24%, 1.80%, 5.58%, and 8.22%, respectively, from March to July. In March, the CM and TZ sections were dominated by stages I and II, accounting for 97.06% and 99.34% of the total, respectively. In April, they were still dominated by stages I and II, accounting for 89.61% and 92.21% of the total, respectively, while the AQ section was dominated by stages II and III, accounting for 49.01% and 45.26% of the total. In May, the CM section was dominated by stage IV, accounting for 46.37% of the total, and stages II and III were dominant in the TZ and AQ sections, accounting for 77.04% and 90.82% of the total, respectively. In June, the CM and TZ sections were mainly dominated by stages IV and V, accounting for 75.79% and 83.87% of the total, respectively. Meanwhile, stages II and III in the AQ section accounted for 91.94% of the total. In July, stages III and IV in the AQ section accounted for 51.31% and 47.38% of the total, respectively (Figure 2). The average water temperature from March to July showed an increasing trend, being 15.47 °C, 17.08 °C, 21.87 °C, 24.63 °C, and 27.69 °C, respectively.
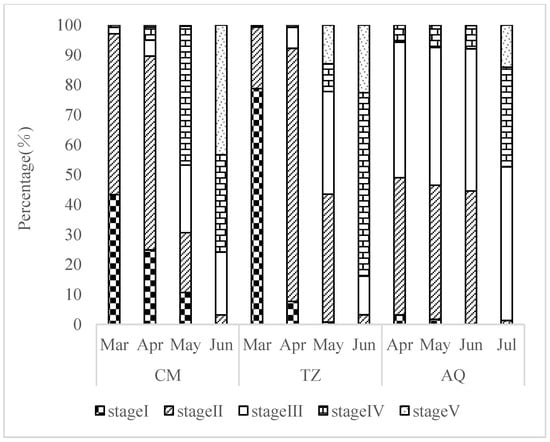
Figure 2.
Spatio-temporal characteristics of ovarian development period in C. nasus breeding population.
3.2. Morphologic and Histologic Sections of the Ovary
The phases of the ovary are differentiated on the basis of the morphological and physiological characteristics of the oocytes during ovarian development, as well as the histological composition of the ovary itself [26], defined by the temporal phases of the germ cells that occupy more than half of the area in the ovarian tissue section or that are present in the highest proportions [18].
3.2.1. Developmental Stage Characteristics of Ovarian Tissue Sections
The ovaries in stage II were finely columnar, with inconspicuous blood vessels in the ovarian membrane and a slightly transparent light flesh red to flesh yellow appearance, with no oocytes visible to the naked eye. Histological observation showed that the oocytes were closely arranged, and in the small growth phase of the primary oocytes, dominated by the cells of the second temporal phase, the oocytes of the second temporal phase were round, oval, or irregularly polygonal; the cells had a long diameter of 78.90–170.20 μm and a short diameter of 40.50–120.30 μm; the cell nucleus had a diameter of 30.00–70.10 μm; the cytoplasm was strongly alkaline and stained dark purple; the chromatin in the nucleus was finely linear; and the cytosol was surrounded by a single layer of a follicular membrane (Figure 3(A1–A3)).
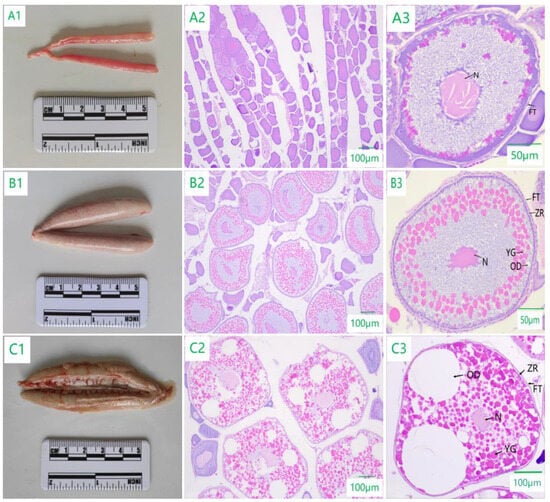
Figure 3.
Morphological and histological characteristics of ovarian development of C. nasus. (A1,B1,C1) are the morphology of ovaries in stages II, III, and IV. (A2,B2,C2) are the tissue section observation results of ovaries in stages II, III, and IV. (A3,B3,C3) are oocytes in phases 2, 3, and 4. N: Nucleus; FT: Follicular membrane; YG: Yolk granule; OD: Oil droplet; ZR: Zona radiata.
The ovaries in stage III were enlarged in the middle and smaller at both ends with blood vessels distributed on the ovarian membrane and had a flesh-colored to light greenish appearance, and the oocytes were macroscopical. Histological observation showed that the cells were in the large growth phase of primary oocytes, dominated by the cells of the third temporal phase, with a cell diameter of 168.43–338.69 μm and a nuclear diameter of 60.62–110.50 μm. The cell volume increased, and oil droplets of different sizes appeared in the form of 10–43 droplets, which accounted for 2.05–5.44% of the area of the oocyte; the yolk began to be deposited in the form of small granules, leading to a total of 130–305 granules, accounting for 21.39–53.68% of the area of the oocyte, and radial bands appeared in the periphery (Figure 3(B1–B3)).
The ovaries in stage IV were enlarged in size, with enlarged oocytes visible to the naked eye and greenish to grayish in appearance with a thin and hyaline ovarian membrane, and they were densely vascularized. Histological observation showed that yolk granules and oil droplets filled the cells, mainly the cells in the fourth temporal phase, with a cell diameter of 278.45–525.33 μm and a nuclear diameter of 80.00–140.58 μm. As the yolk granules accumulated, they accounted for more than 40.20% of the cell volume, and the number of oil droplets increased, their size became larger, and they began to merge to form large oil droplets, which accounted for about 50.50% of the cell volume. At the later stage, they filled the cell, the nucleus gradually became invisible, and the nucleolus was distributed around the nuclear membrane (Figure 3(C1–C3)).
3.2.2. Spatial Characteristics of Ovarian Tissue Sections
The yolk material of C. nasus mainly consisted of yolk granules and oil droplets. The oocytes of stage IV in the TZ section (342.41 ± 56.43 μm) were significantly smaller than those in the CM section (469.63 ± 49.02 μm) and AQ section (413.15 ± 53.40 μm), and oil droplets accounted for a larger proportion than yolk granules in the oocytes of the CM section and the AQ section, while yolk granules dominated in the TZ section (Figure 4).
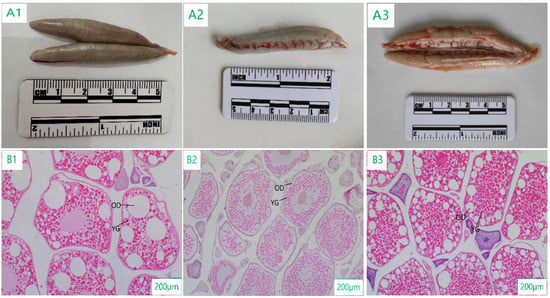
Figure 4.
Morphological and histological characteristics in stage IV of ovarian development of C. nasus at different sampling points. (A1): ovarian morphological characteristics at CM; (A2): ovarian morphological characteristics at TZ; (A3): ovarian morphological characteristics at AQ; (B1): ovarian histological characteristics at CM; (B2): ovarian histological characteristics at TZ; (B3): ovarian histological characteristics at AQ; YG: Yolk granule; OD: Oil droplet.
3.3. Sex Steroid Hormone Levels in Serum
3.3.1. Characterization of the Developmental Phase of Sex Steroid Hormones
The levels of sex steroid hormones in C. nasus changed significantly throughout the process of ovarian development. The E2 level in the serum was significantly higher in stage III (46.22 ± 4.32 ng/L) than in stage II (38.12 ± 4.03 ng/L) and was significantly lower in stage IV (33.91 ± 4.72 ng/L). The 17α,20β-DHP level was significantly higher in stage IV (31.79 ± 4.11 ng/L) than in stage II (24.48 ± 1.25 ng/L) and stage III (23.27 ± 1.41 ng/L) (Figure 5).
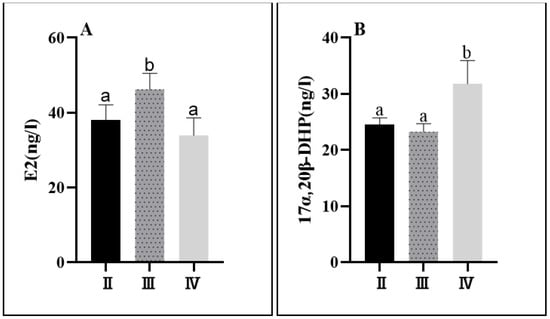
Figure 5.
Serum sex steroid levels at different developmental stages of C. nasus. E2 level (A); 17α,20β-DHP level (B). Significant differences are presented with different superscript letters (p < 0.05).
3.3.2. Spatial Characterization of Sex Steroid Hormones
Both estrogen and progesterone showed significant differences during the anadromous migration of C. nasus. The E2 level was significantly higher in the TZ section than in the CM section and the AQ section during stage IV, and the 17α,20β-DHP level was significantly lower in the TZ section than in the CM section and the AQ section. (Figure 6).
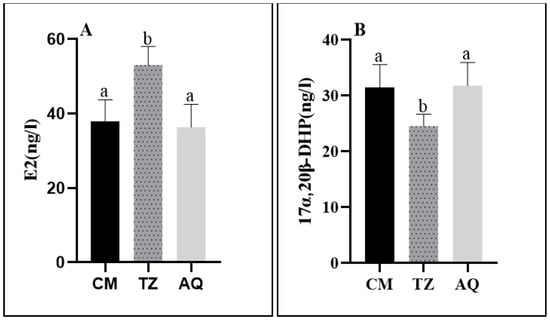
Figure 6.
Serum sex steroid levels at different sampling points of C. nasus. E2 level (A); 17α,20β-DHP level (B). Significant differences are presented with different superscript letters (p < 0.05).
3.4. Expression Patterns of Ovarian Development-Related Genes
3.4.1. Characterization of Developmental Stages of Gene Expression
The expression of the fshr, lhr, kiss1, and foxl2 genes changed significantly during the development of the ovaries in C. nasus. There was no significant change in the expression of fshr and lhr in stages II and III, and it was significantly higher in stage IV. The expression of the kiss1 gene was significantly higher in stage III than in stages II and IV. The expression of foxl2 showed an increasing change with the maturation of the ovary, with the lowest expression level in stage II and a significant increase in its expression in stages III and IV (Figure 7).
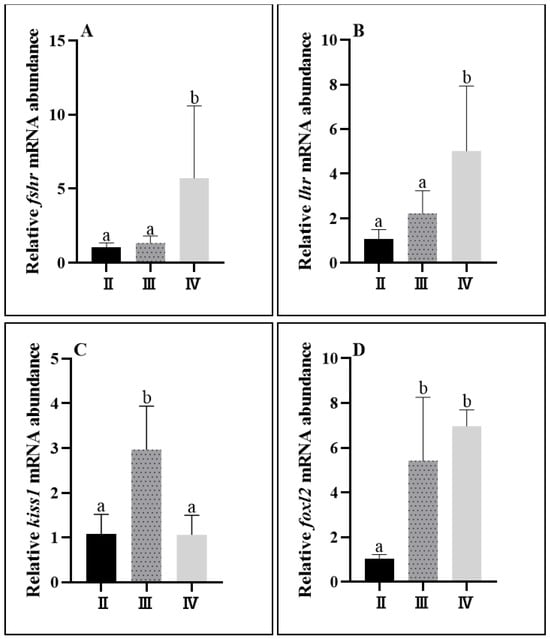
Figure 7.
Ovarian gene expression levels at different developmental stages of C. nasus. Fshr mRNA level (A); lhr mRNA level (B); kiss1 mRNA level (C); foxl2 mRNA level (D). Significant differences are presented with different superscript letters (p < 0.05).
3.4.2. Spatial Characterization of Gene Expression
The expression of fshr was significantly higher in the TZ section than in the CM and AQ sections, and the expression of lhr was significantly higher in the TZ section than in the CM section. There was no significant difference in the expression of kiss1 and foxl2 (Figure 8).
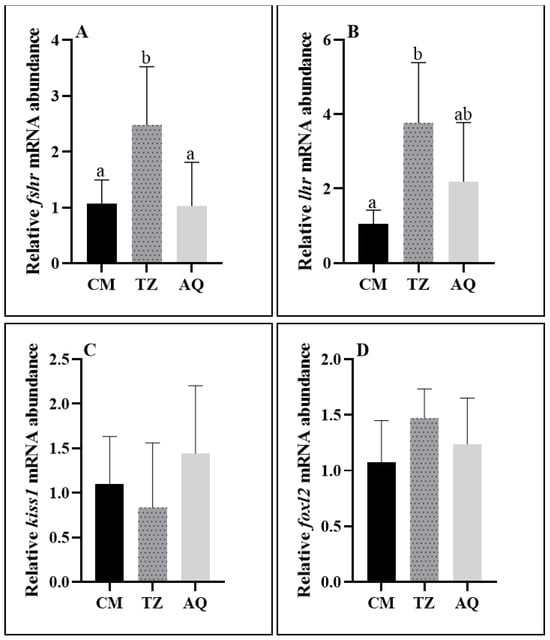
Figure 8.
Ovarian gene expression at different sampling points of C. nasus. Fshr mRNA level (A); lhr mRNA level (B); kiss1 mRNA level (C); foxl2 mRNA level (D). Significant differences are presented with different superscript letters (p < 0.05).
4. Discussion
4.1. Characterization of Ovarian Development in C. nasus
As a typical river–sea migratory fish, the anadromous migration of C. nasus is a seasonal reproductive activity [19]. C. nasus mostly lives in coastal and nearby waters and aggregates in the offshore waters of the Yangtze River estuary during spawning migration [15,23]. The increase in water temperature induces the pre-spawning migratory behavior of C. nasus and promotes its gonadal development and maturation [27,28]. The study that analyzed C. nasus in April–July 2018 in the AQ section showed that the main developmental stage of the ovaries was stage III, and in June, the ovaries began to develop to stage V [29]. He et al. [30] investigated and studied C. nasus in the AQ section from April to August 2005 and found that the gonadosomatic index of ovaries in stages II and III in April was 1.36% on average, while in June, the gonadosomatic index of ovaries developing to stages IV and V reached 6.66%. In this study, in the early stage of migration from March to April, the ovarian development of C. nasus was dominated by stages I and III, and the proportion of stages III and IV increased in May. The number of C. nasus individuals in stages IV and V increased in June and July. Overall, the proportion of ovarian-mature individuals and the coefficient of ovarian maturity increased with the passage of time and the increase in water temperature in all water segments. This was consistent with the results of historical studies. The yolk, as an energy-supplying substance for ovarian development in fish, has also attracted much attention in terms of changes in its content. The observation of tissue sections showed that the ovaries in stage II were dominated by the second time-phase oocytes, which were smaller, and no yolk material was produced, which was consistent with the histological characteristics in stage II of Rhinobio ventralis [31]. In addition, the ovaries in stage III were dominated by oocytes in the third temporal phase, which were enlarged about onefold and began to show nutrients such as yolk granules and oil droplets, which was consistent with the findings for Girella leonina [32] and Xenocypris microlepis [33]. Moreover, the ovaries in stage IV were dominated by oocytes in the fourth temporal phase, which were almost full of nutrients such as yolk granules and oil droplets, with yolk granules becoming fuller and the area of oil droplets fusing and becoming larger. Similarly, a large number of oil droplets were found to be synthesized into oil globules in stage IV of Girella leonina [32]. The results of ovarian development and the ovarian gonadosomatic index in the present study are consistent with those of previous studies on C. nasus [17,34], reflecting the typical ecological characteristics of C. nasus in fattening and long-distance reproductive migration in different waters.
In this study, the observation of ovarian tissue sections belonging to stage IV in the CM, TZ, and AQ sections revealed no significant differences in oocyte size. However, the proportion of yolk material in the composition of oocytes was different. Concretely, the ovarian tissue sections of the CM and AQ sections showed that oil droplets occupied about half of the cell area, and the proportion of yolk granules was slightly smaller than the proportion of oil droplets, which was consistent with the results of stage IV ovarian tissue from a pond culture of C. nasus, Parabramis pekinensis, and Hippocampus erectus [18,35,36]. By contrast, ovarian tissue sections from the TZ section showed that yolk granules accounted for a larger proportion of oocytes and fewer oil droplets. This suggests that the maturity level of C. nasus collected in the TZ section was lower than that in the CM and AQ sections. Further development and maturation of C. nasus collected in the TZ section were needed, probably because the target spawning grounds had not been reached yet, and it was necessary to continue migrating upstream to complete the spawning process [18,37].
4.2. Characterization of Changes in Sex Steroid Hormones in Serum
Sex steroid hormone levels can reflect the level of ovarian development in fish, among which E2 and 17α,20β-DHP are the main endogenous sex steroid hormones in fish and play an important role in regulating the ovarian development process.
E2 was at a low level in stage II, consistent with the results of Oreochromis niloticus [38], Epinephelus fuscoguttatus [39], and Schizothorax labrosus [40]. Upon ovarian development to stage III, gonadotropins stimulate follicular and granulosa cells to co-synthesize E2, which acts through nuclear estrogen receptor signaling to induce the hepatic synthesis of yolk proteins and thus promotes ovarian vitellogenesis and accumulation [39], exhibiting a significant stage III elevation. Existing studies have shown that high concentrations of E2 inhibit oocyte development and ovulation [41] and that there is a significant decrease in E2 concentration when the C. nasus ovary enters stage IV in order to ensure the normal process of oogenesis, which may be closely related to the high expression of maturation-inducing hormones (MIHs, e.g., 17α,20β-DHP), which induce the final maturation of the oocyte [3]. The findings in fish such as Danio rerio [42], Epinephelus fasciatus [43], and Oncorhynchus keta [44] were the same as those of C. nasus in the present study, in which 17α,20β-DHP was significantly elevated in stage IV. These factors also have a role in inducing ovarian cell maturation and the final maturation of the ovarian hormones. Sex steroid hormones are involved in the regulation of ovarian maturation and play a role in the completion of reproductive migration. It is worth noting that the levels of sex steroid hormones in the C. nasus were much lower than those in other fish such as Oncorhynchus nerka [45]; the E2 level in migrating river was on average 10.9 ng/mL, declining to 2.1 ng/mL in spawning streams, whereas the 17α,20β-DHP level was 0.01 ng/mL in the river and rose to 190 ng/mL in the spawning streams. As for the captive female Clupea pallasii [46], E2 levels in the serum could exhibit a peak concentration at 15.8 ng/mL, and 17α,20β-DHP levels could exhibit a peak concentration at 2.4 ng/mL.
Regarding different river sections, the E2 level in the TZ section was significantly higher than that in the CM and AQ sections, while the 17α,20β-DHP level was significantly lower in the TZ section than in the CM and AQ sections, which indicated that the level of maturation of C. nasus in the TZ section was lower than that in the CM and AQ sections. He, W. [30] and Dai, P. [47] considered that C. nasus migrates at the same speed and that the developmental speed of C. nasus with a similar body length is also similar, so the target spawning sites of C. nasus differ from each other due to the different starting points of C. nasus migration. In this study, the stage IV C. nasus subjects migrating to the CM section were more mature; it was hypothesized that they might have come from waters further away from the entrance of the Yangtze River, and their target spawning grounds might have been located near the CM section [28]. Similarly, the stage IV ovaries of C. nasus migrating to the AQ section were also more mature, suggesting that this population may have come from waters closer to the entrance of the Yangtze River and that the target spawning grounds may be in the AQ section and its neighboring waters after the same distance of migration [29]. The stage IV ovaries of C. nasus in the TZ section were less mature, and it was hypothesized that there might not be any suitable target spawning grounds in the TZ section and its neighboring waters. As a result, the TZ section mainly functions as a migration corridor during migration [48].
4.3. Characterization of Gene Expression Related to Ovarian Development
The fshr, lhr, kiss1, and foxl2 genes regulate the process of ovarian development and maturation by participating in physiological activities, such as the synthesis of sex steroid hormones and the accumulation of yolk material in oocytes. In female fish, fshr is mainly expressed in sphingocytes and granulosa cells, and lhr is mainly expressed in granulosa cells [49]. The traditional theory of animal reproduction suggests that the target organs of gonadotropin receptors (fshr and lhr) are the gonads, which indirectly affect the development and function of the reproductive organs through the regulation of gonadotropin secretion [50]. In Oncorhynchus mykiss [51] and Ctenopharyngodon idella [52], the expression of fshr mRNA and lhr mRNA gradually increased with oocyte development. Similarly, in this study, fshr promoted yolk production and accumulation [53], while lhr was highly expressed in stage IV of C. nasus, which could promote the secretion of 17α,20β-DHP from the ovary. Thus, lhr and 17α,20β-DHP were the main factors regulating reproduction during oocyte maturation. Kiss1 was significantly higher in stage III than in stages II and IV, and it mainly promoted yolk formation. The expression of foxl2 was significantly higher in stages III and IV than in stage II. Foxl2 indirectly regulates the synthesis of E2 through the regulation of the transcription gene cyp19 and may play a promotional role in yolk production and deposition [54].
Gene expression levels also varied in C. nasus in different river sections. Fhsr and lhr expression levels were both higher in the TZ section. When migrating from the CM section, where fresh and sea water meet, to the TZ section, which is freshwater, salinity changes occur, while the expression of fhsr and lhr increases, indicating a significant increase in the synthesis of sex steroid hormone-related precursors [55], resulting in the elevated expression of fhsr and lhr. This also suggests that these genes respond to the effects of osmotic pressure on the gonads by regulating the synthesis of regulatory steroid hormones.
5. Conclusions
In this study, we analyzed the characteristics of ovarian reproductive development in C. nasus at the histological, hormonal, and genetic levels. As the Coilia nasus sinensis matures, its ovaries gradually become plump. The oocytes increased in size and yolk material during the development and maturation of C. nasus. The related hormones’ (E2 and 17α,20β-DHP) and genes’ (fshr, lhr, kiss1, and foxl2) expression levels varied at different stages of ovarian development in C. nasus. They jointly regulate the ovaries, enabling the mature development of the Coilia nasus, which is reflected in the morphology and histology of the ovaries. The results of this study contribute to a better understanding of the reproductive biology of the natural population of C. nasus in the Yangtze River.
Author Contributions
Conceptualization, Y.Y. and K.L.; Methodology, S.W. and Y.Y.; Software, S.W., Y.D. and F.M.; Validation, Z.H., Y.Y. and K.L.; Formal Analysis, Y.Y.; Investigation, S.W., W.Z. and C.Y.; Resources, Z.H. and K.L.; Data Curation, W.H.; Writing—Original Draft Preparation, S.W.; Writing—Review and Editing, Y.Y., Z.H. and K.L.; Visualization, S.W.; Supervision, Z.H.; Project Administration, K.L.; Funding Acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financed by the Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO. 2023TD11), the Monitoring of Aquatic Biological Resources in the Jiangsu section of the main stream of the Yangtze River (2021-SJ-110-04), the Study on Resources and Fishing Management of Coilia nasus (CJBYZGL2021-24), and the Investigation of Fishery Resources of the Yangtze River Estuary (JC202104).
Institutional Review Board Statement
The experimental subject, Coilia nasus, died immediately after being exposed to water, so it does not involve any ethical issues. In addition, the collection of coilia nasus has obtained fishing permits from Anhui Province, Jiangsu Province, and Shanghai City, with numbers: No.00002582057, No.ZX-006, and No.ZT-90004, respectively.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sang, H.; Lam, H.; Hy, L.; Ky, P.; Minh, T. Changes in plasma and ovarian steroid hormone level in wild female blue tang fish Paracanthurus hepatus during a reproductive cycle. Animals 2019, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Tenugu, S.; Pranoty, A.; Mamta, S.; Senthilkumaran, B. Development and organisation of gonadal steroidogenesis in bony fishes—A review. Aquac. Fish. 2021, 6, 223–246. [Google Scholar] [CrossRef]
- Moreira, R.; Honji, R.; Melo, R.; Moraes, N.; Amaral, J.; Carvalho, A.; Hilsdorf, A. The involvement of gonadotropins and gonadal steroids in the ovulatory dysfunction of the potamodromous Salminus hilarii (Teleostei: Characidae) in captivity. Fish Physiol. Biochem. 2015, 41, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, T.; Tokumoto, M.; Horiguchi, R.; Ishikawa, K.; Nagahama, Y. Diethylstilbestrol induces fish oocyte maturation. Proc. Natl. Acad. Sci. USA 2004, 101, 3686–3690. [Google Scholar] [CrossRef] [PubMed]
- Brzuska, E.; Socha, M.; Chyb, J.; Sokołowska-Mikołajczyk, M.; Inglot, M. The Effect of [(D-Ala6, Pro9NEt) mGnRH-α+ Metoclopramide] (Ovopel) on Propagation Effectiveness of Two Breeding Lines of Common Carp (Cyprinus carpio L.) and on Luteinizing Hormone and 17α, 20β-Dihydroxyprogesterone Levels in Females during Ovulation Induction. Animals 2023, 13, 1428. [Google Scholar] [PubMed]
- Luo, M. Effects of Heat Stress on the Function and Related Genes Expression in Mice Ovarian Granulosa Cells; Nanjing Agricultural University: Nanjing, China, 2016. [Google Scholar]
- Menon, K.; Munshi, U.; Clouser, C.; Nair, A. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: A perspective. Biol. Reprod. 2004, 70, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, Y.; Rao, J.; Liu, Z. The expression of three gonadotropin subunits in response to 17α-ethynylestadiol in male Pelteobagrus fulvidraco. Acta Hydrobiol. Sin. 2015, 39, 1117–1125. [Google Scholar]
- Nie, Z.; Zhao, N.; Zhao, H.; Fu, Z.; Ma, Z.; Wei, J. Cloning, Expression Analysis and SNP Screening of the kiss1 Gene in Male Schizothorax biddulphi. Genes 2023, 14, 862. [Google Scholar] [CrossRef]
- Kuohung, W.; Kaiser, U. GPR54 and KiSS-1: Role in the regulation of puberty and reproduction. Rev. Endocr. Metab. Disord. 2006, 7, 257–263. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, M.; Jiang, D.; Zhang, J.; Hou, X.; Zhu, C. Induction of Premature Reversal in Plectropomus leopardus by Letrozole. J. Zhanjiang Ocean Univ. 2023, 43, 19–25. [Google Scholar]
- Fan, Z.; Zou, Y.; Liang, D.; Tan, X.; Jiao, S.; Wu, Z.; Li, J.; Zhang, P.; You, F. Roles of forkhead box protein L2 (foxl2) during gonad differentiation and maintenance in a fish, the olive flounder (Paralichthys olivaceus). Reprod. Fertil. Dev. 2019, 31, 1742–1752. [Google Scholar] [CrossRef]
- Zhai, L.; Li, Z.; Hu, Y.; Huangs, C.; Tian, S.; Wan, R.; Pauly, D. Assessment of Coilia mystus and C. nasus in the Yangtze River Estuary, China, using a length-based approach. Fishes 2022, 7, 95. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, P. Migration Biology and Stress Response of Coilia nasus: A Review. Chin. Agric. Sci. Bull. 2023, 39, 130–134. [Google Scholar]
- Yuan, C. Spawning migration of Coilia nasus. Bull. Biol. 1987, 12, 1–3. [Google Scholar]
- He, W.; Li, J.; Jiang, Z. Cytological observations on the gonad of Coilia ectenes in the Yangtze River. J. Shanghai Fish. Univ. 2006, 15, 292–295. [Google Scholar]
- He, W.; Li, J. Histological studies of gonadal development of gonad of Coilia ectenes in the Changjiang River. J. Fish. China 2006, 6, 773–777. [Google Scholar]
- Xu, G.; Wan, J.; Gu, R.; Zhang, C.; Xu, P. Morphological and histological studies on ovary development of Coilia nasus under artificial farming conditions. J. Fish. Sci. China 2011, 18, 537–546. [Google Scholar] [CrossRef]
- Yin, D.; Lin, D.; Ying, C.; Ma, F.; Yang, Y.; Wang, Y.; Tan, J.; Liu, K. Metabolic mechanisms of Coilia nasus in the natural food intake state during migration. Genomics 2020, 112, 3294–3305. [Google Scholar] [CrossRef]
- Gao, J.; Xu, G.; Xu, P. Whole-genome resequencing of three Coilia nasus population reveals genetic variations in genes related to immune, vision, migration, and osmoregulation. BMC Genom. 2021, 22, 878. [Google Scholar] [CrossRef]
- Liu, K.; Yin, D.; Shu, Y.; Dai, P.; Yang, Y.; Wu, H. Transcriptome and metabolome analyses of Coilia nasus in response to Anisakidae parasite infection. Fish Shellfish. Immunol. 2019, 87, 235–242. [Google Scholar] [CrossRef]
- Li, L.; Tang, W.; Zhang, Y. Changes of fatty acid content and its components in different tissues during spawning migration processes of female Coilia nasus in the lower reaches of the Yangtze River. J. Fish. China 2019, 43, 790–800. [Google Scholar]
- Guo, W. Fatty acid Composition and Energy Utilization of Coilia nasus during Spawning Migration in Yangtze River; Shanghai Ocean University: Shanghai, China, 2021. [Google Scholar]
- Shi, Y.; Xu, J.; Yuan, X.; Yang, M.; Zhang, Z.; Xie, Y.; Shui, C. Muscle nutrient composition of Coilia nasus in brackish water andfresh water cultured modes. J. Fish. China 2023, 47, 73–82. [Google Scholar]
- Wang, M.; Xu, P.; Zhu, Z. Regulation of signal transduction in Coilia nasus during migration. Genomics 2020, 112, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X. Study on the development and annual change in the ovary of Pelteobagrus fulvidraco. J. Nat. Sci. Hunan Norm. Univ. 2003, 26, 73–78. [Google Scholar]
- Zhang, S.; Wan, S.; Chen, G.; Gu, S.; Gao, Z. Research progress on artificial breeding technology of Yangtze River Coilia nasus. Sci. Fish Farming 2023, 1–2. [Google Scholar] [CrossRef]
- Xue, X.; Peng, Y.; Fang, D.; Xu, D.; Wang, X.; Ren, K. Preliminary study of Coilia nasus spawning grouds at Sutong section in the lower reaches of the Yangtze River. J. Fish. China 2022, 46, 1377–1388. [Google Scholar]
- Dai, P.; Yan, Y.; Zhu, X.; Tian, J.; Ma, F.; Liu, K. Status of Coilia nasus resources in the National Aquatic Germplasm Resources Conservation Area in the Anqing Section of the Yangtze River. J. Fish. Sci. China 2020, 27, 1267–1276. [Google Scholar]
- He, W.; Li, J. Study on the gonadal development pattern of the Coilia nasus in the Yangtze River. China Fish. 2006, 5, 70–72. [Google Scholar]
- Qu, H.; Liu, Y.; Yang, Y.; Li, S.; Hu, M.; Guan, M.; Lu, X.; Wen, Z.; Guo, W. Histological change in annual development of ovary in gudgeon Rhinogobio ventralis. Fish. Sci. 2015, 34, 32–37. [Google Scholar]
- Zhu, L.; Sun, M.; Zhang, D.; Yuan, J.; Xu, S. Histological study on the ovary development of Girella leonine. J. Appl. Oceanogr. 2018, 37, 255–262. [Google Scholar]
- Liu, M.; Xiong, B.; Lv, G. Histological observation of ovary development in Xenocypris microlepis. Fish. Sci. 2010, 29, 125–130. [Google Scholar]
- Ma, F.; Yang, Y.; Fang, D.; Ying, C.; Xu, P.; Liu, K.; Yin, G. A review on the biological characteristics and resource protection status of Coilia nasus in the Yangtze River basin. J. Aquac. 2022, 46, 1580–1590. [Google Scholar]
- Gu, H.; Feng, Y.; Yang, Z.; Wang, J. Histological observation of gonadal development of white bream Parabramis pekinensis. J. Fish. China 2022, 35, 44–50. [Google Scholar]
- Chen, L.; Qin, G.; Wang, X.; Liu, Y.; Xiao, W.; Lin, Q. Distinct structures of gonads and germ cell development of lined seahorse, Hippocampus erectus. J. Trop. Oceanogr. 2020, 39, 93–102. [Google Scholar]
- Zhao, H. Study on Long-Term Sperm Storage and Sperm Energy Metabolism of Sebastes schlegelii; University of Chinese Acedemy of Science: Beijing, China, 2020. [Google Scholar]
- Li, Y. Study on the Function and Regulatory Mechanism of Transcription Factor Sox3 in Oogenesis of Nile Tilapia (Oreochromis niloticus); Southwest University: Chongqing, China, 2021. [Google Scholar]
- Qiu, Y.; Ding, X.; Li, Z.; Duan, P.; Wang, X.; Li, L.; Wang, L.; Liu, Y.; Ma, W.; Pang, Z.; et al. Comparative analysis of ovarian development and sex steroid hormone levels in hybrid Jinhu grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) and Epinephelus fuscoguttatus. J. Fish. Sci. China 2023, 30, 457–467. [Google Scholar]
- Zhao, H.; Zhao, N.; Li, L.; Qiang, Z.; Nie, Z.; Wei, J. Gonadal development and changes inconcentration of serum sex hormones in Schizothorax irregularis. Fish. Sci. 2023, 42, 233–240. [Google Scholar]
- Liu, C. Effects of Photoperiod on Fecundity, Plasma Hormone Content and Gene Expression Related to Ovarian Development in Zebrafish; Shanghai Ocean University: Shanghai, China, 2022. [Google Scholar]
- Tokumoto, T.; Yamaguchi, T.; Ii, S.; Tokumoto, M. In vivo induction of oocyte maturation and ovulation in zebrafish. PLoS ONE 2011, 6, e25206. [Google Scholar] [CrossRef]
- Xu, W.; Tang, Y.; Zhang, J.; Soyano, K.; Li, B. Annual ovarian development and changes in the concentration of serum sex hormones in blacktip grouper Epinephelus fasciatus. J. Dalian Ocean Univ. 2020, 35, 657–662. [Google Scholar]
- Li, P.; Wang, J.; Lu, W.; Tang, F.; Liu, W. Reproduction related endocrine hormone level and gonadal decelopment of Oncorhynchus ketain pre-growth period. Guizhou Agric. Sci. 2020, 48, 54–59. [Google Scholar]
- Baker, M.; Swanson, P.; Young, G. Injuries from non-retention in gillnet fisheries suppress reproductive maturation in escaped fish. PLoS ONE 2013, 8, e69615. [Google Scholar] [CrossRef] [PubMed]
- Koya, Y.; Soyano, K.; Yamamoto, K.; Obana, H.; Matsubara, T. Oocyte development and serum profiles of vitellogenin and steroid hormone levels in captive female Pacific herring Clupea pallasii during their first maturational cycle. Fish. Sci. 2003, 69, 137–145. [Google Scholar] [CrossRef]
- Dai, P.; Ma, F.; Tian, J.; Wang, Y.; Yang, Y.; Liu, K. Community structure and infection characteristics of nematodes in the Coilia nasus Anqing section of the Yangtze River. Acta Hydrobiol. Sin. 2023, 47, 917–923. [Google Scholar]
- Hu, Y.; Jiang, T.; Liu, H.; Chen, X.; Yang, J. Otolith microchemical “fingerprints” of Coilia nasus from the Taizhou section of Changjiang River in Jiangsu Province. Chin. J. Ecol. 2023, 1–10. Available online: http://kns.cnki.net/kcms/detail/21.1148.Q.20230407.1729.004.Html (accessed on 20 December 2023).
- Levavi-Sivan, B.; Bogerd, J.; Mañanós, E.L.; Gómez, A.; Lareyre, J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Effects of Gonadotropin and Receptor on Oocytes of Micropterus salmoides; Henan Normal University: Xinxiang City, China, 2022. [Google Scholar]
- Sambroni, E.; Gac, L.; Breton, B.; Lareyre, J. Functional specificity of the rainbow trout (Oncorhynchus mykiss) gonadotropin receptors as assayed in a mammalian cell line. J. Endocrinol. 2007, 195, 213–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- She, D.; Chen, D.; Wang, Y.; Zhou, Y.; Liu, Q.; Li, J. Molecular gene cloning and expression of gonadotropin receptors from Ctenopharyngodon idella. Life Sci. Res. 2015, 19, 39–43. [Google Scholar]
- Lee, E.; Chakravarthi, V.; Wolfe, M.; Rumi, M. ERβ regulation of gonadotropin responses during folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef]
- Miao, X. Estradiol-Induced Sexual Reversal and Its Effects on Gonadal Differentiation and Development in Mandarin Fish (Siniperca chuatsi); Southwest University: Chongqing, China, 2023. [Google Scholar]
- Wang, S.; Huang, J.; Liang, L.; Su, B.; Zhang, Y.; Liew, H.J.; Sun, B.; Zhang, L.; Chang, Y. Distinctive metabolite profiles in migrating Amur ide (Leuciscus waleckii) reveal changes in osmotic pressure, gonadal development, and energy allocation strategies. Front. Environ. Sci. 2022, 10, 1–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).