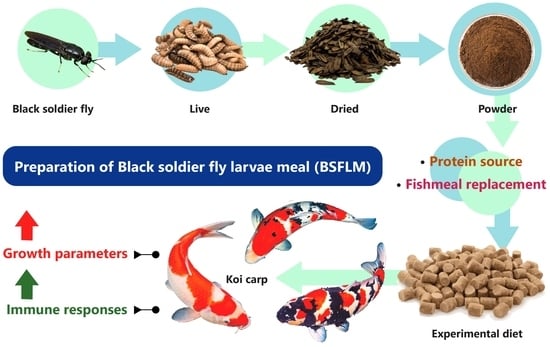
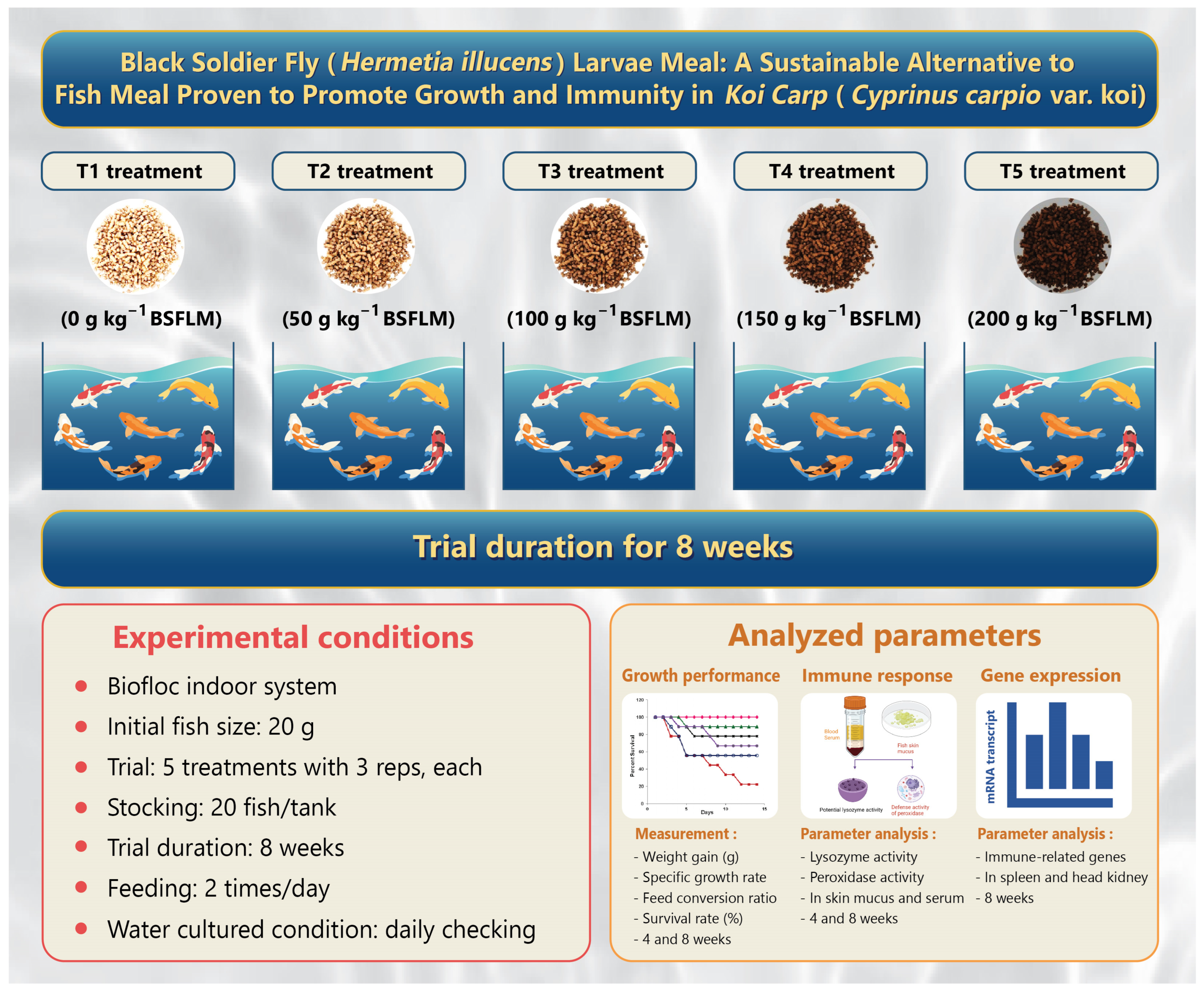
Black Soldier Fly (Hermetia illucens) Larvae Meal: A Sustainable Alternative to Fish Meal Proven to Promote Growth and Immunity in Koi Carp (Cyprinus carpio var. koi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. The Preparation of BSFLM
2.3. Experimental Design
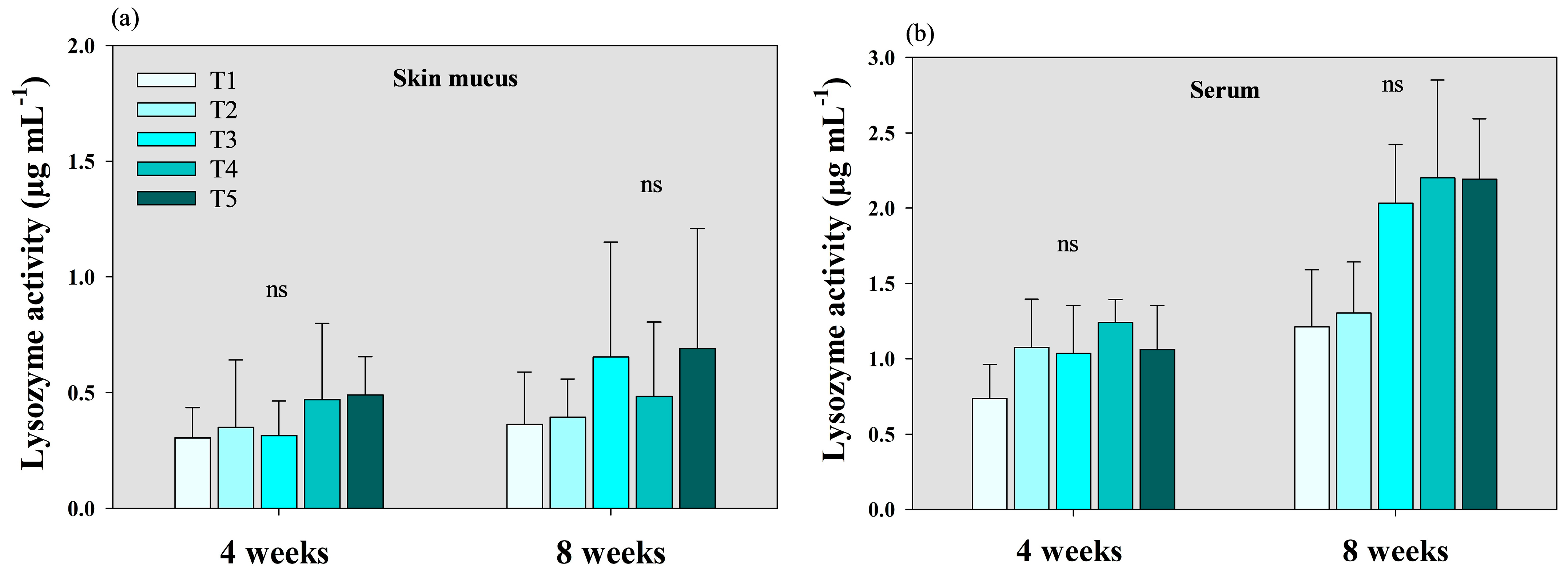
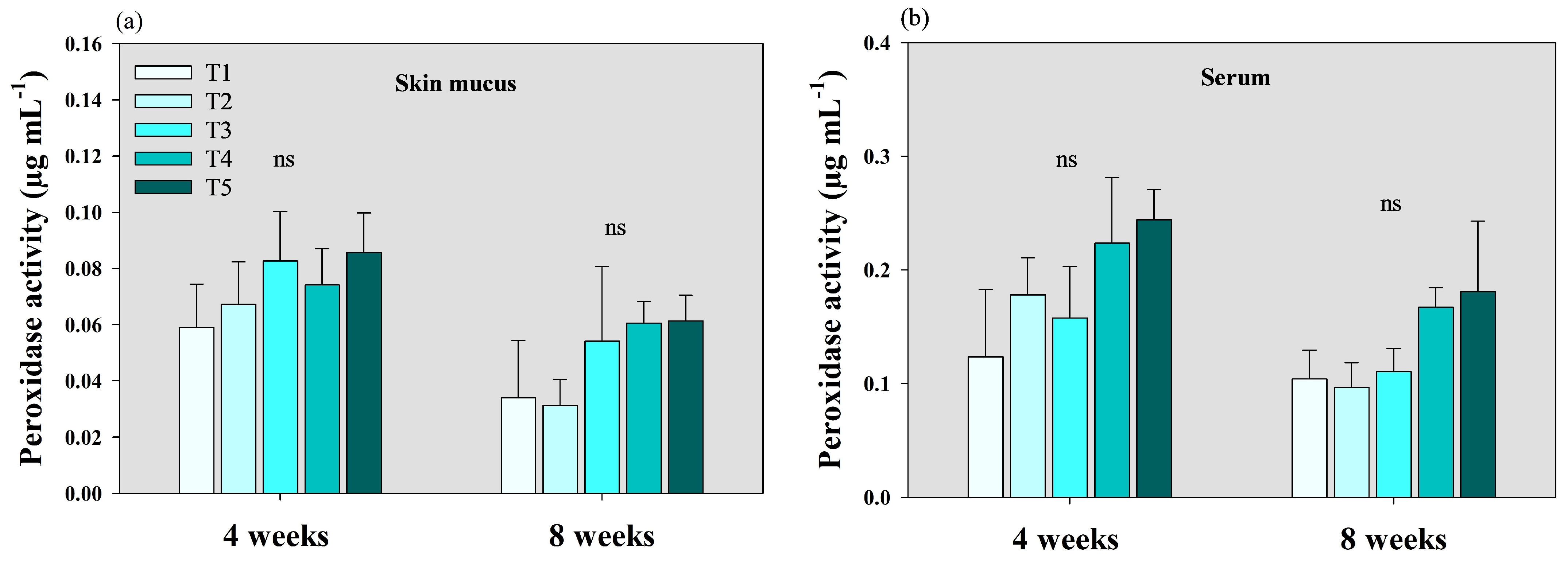
2.4. Lysozyme and Peroxidase Activity Assay
2.5. Growth Performance
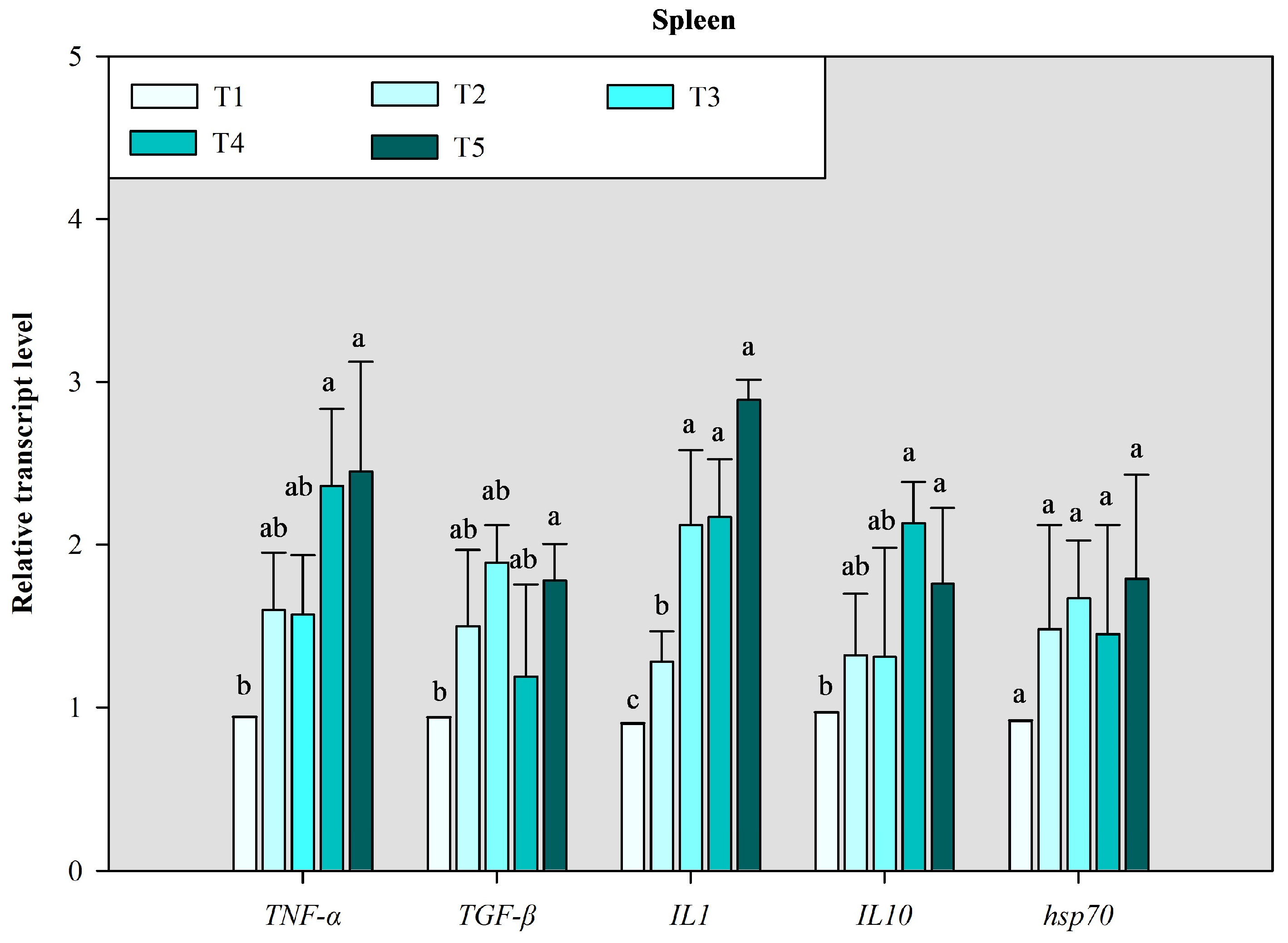
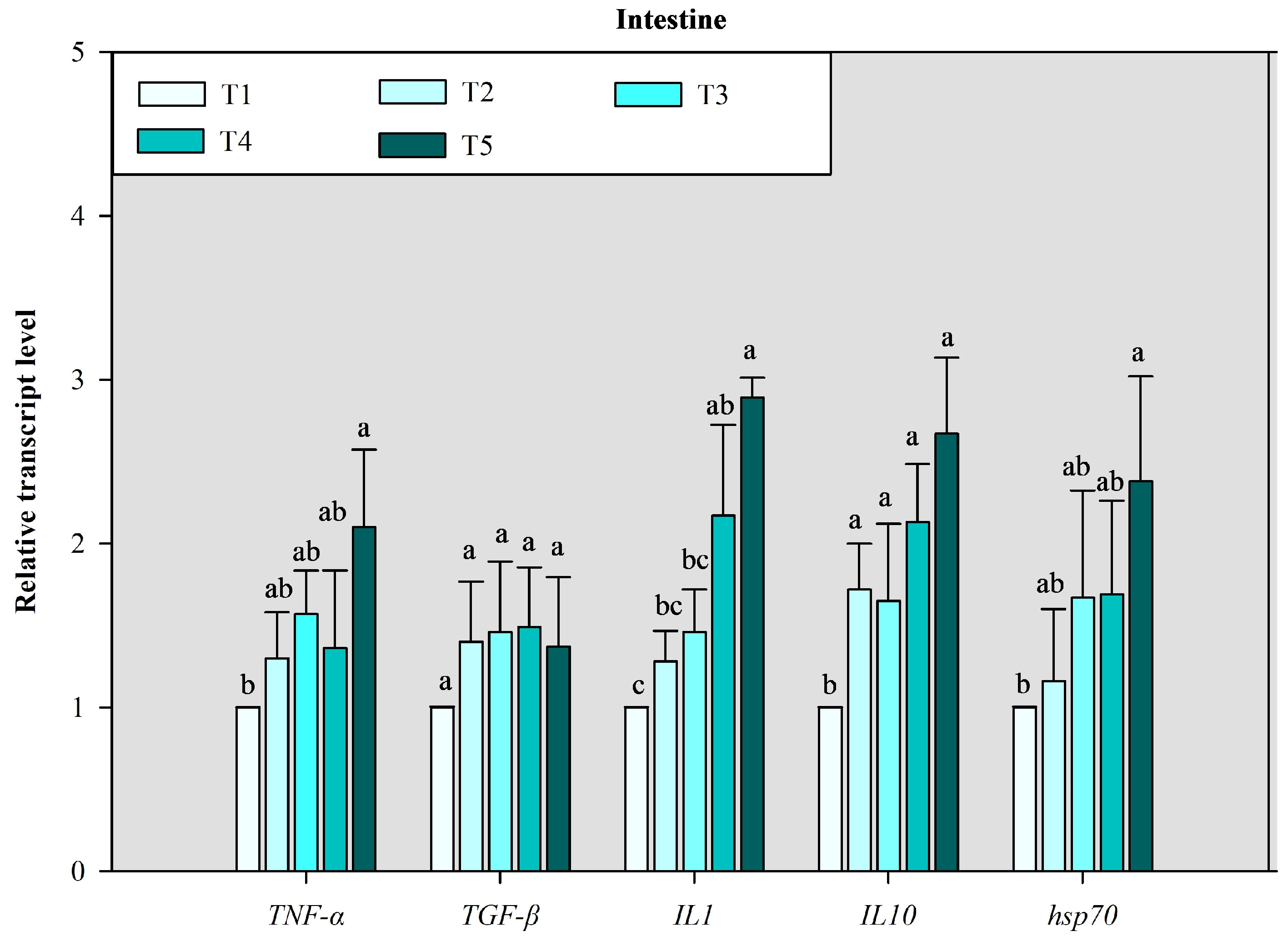
2.6. Quantitative PCR (qPCR) for Gene Expression Analysis
2.7. Statistical Analysis
3. Results
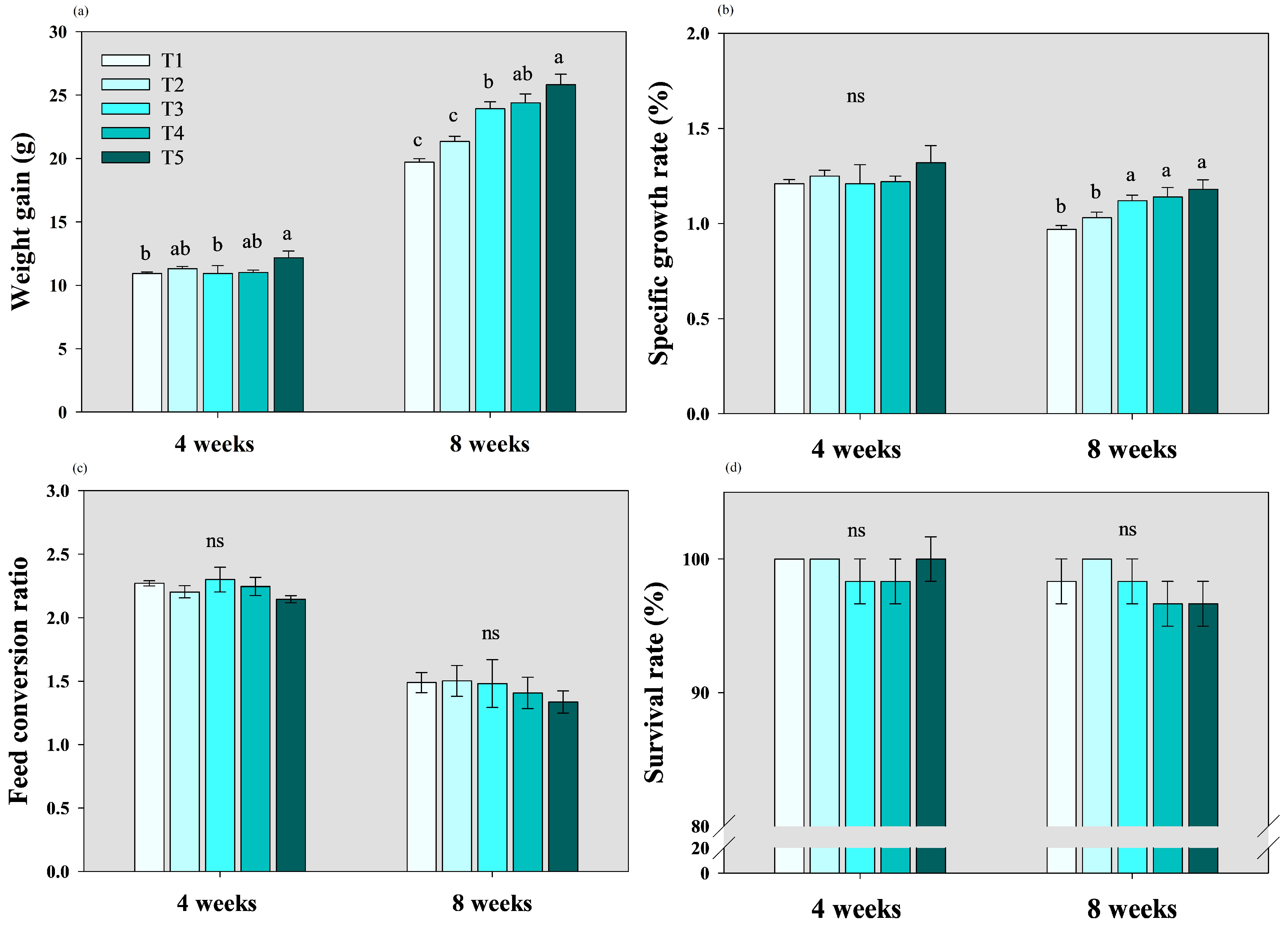
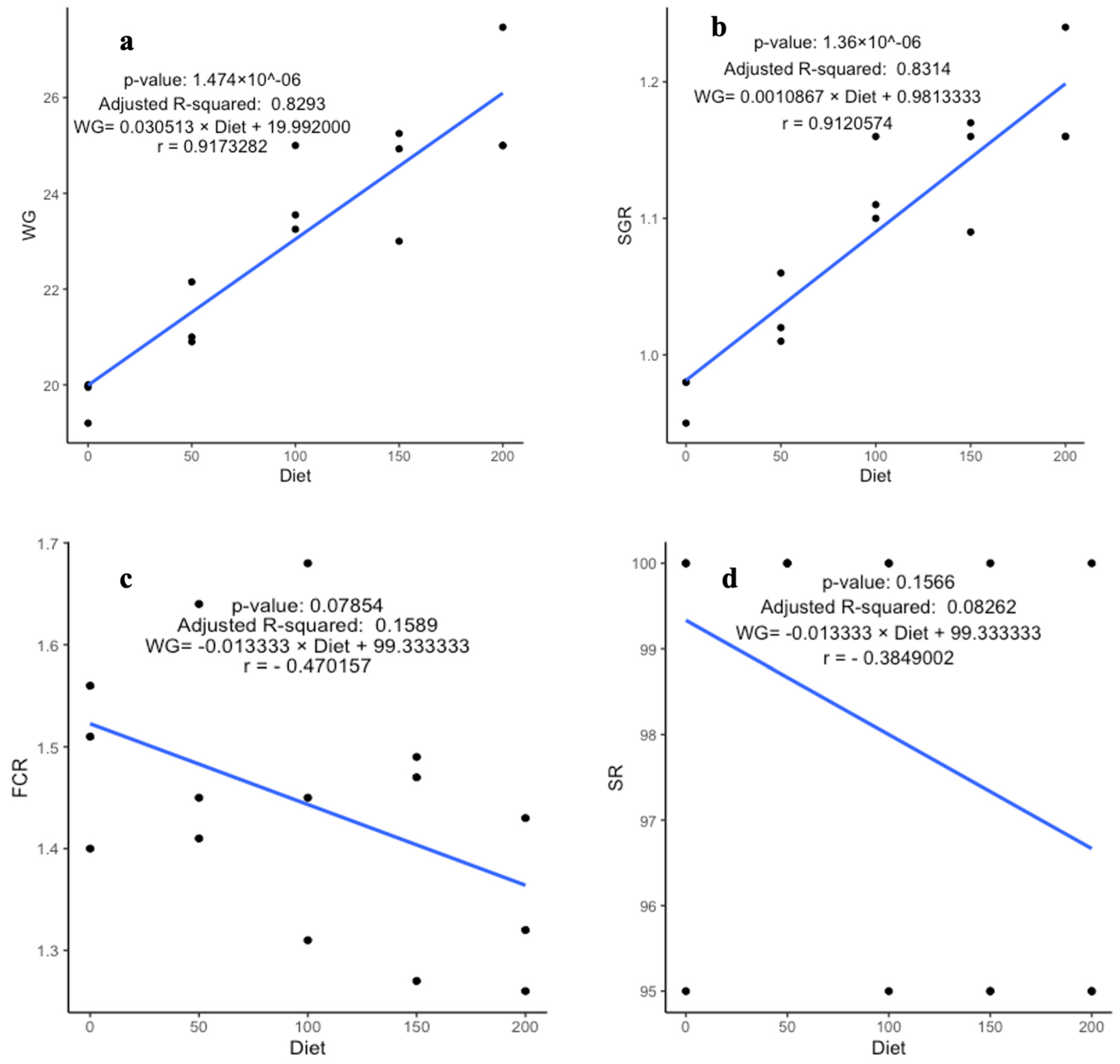
3.1. Growth Parameter Analyses
3.2. Lysozyme and Peroxidase Activity Analysis
3.3. Immune-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saengsitthisak, B.; Punyapornwithaya, V.; Chaisri, W.; Mektrirat, R.; Bernard, J.K.; Pikulkaew, S. The current state of biosecurity and welfare of ornamental fish population in pet fish stores in Chiang Mai Province, Thailand. Vet. Integr. Sci. 2021, 19, 277–294. [Google Scholar] [CrossRef]
- Sicuro, B. The Real Meaning of Ornamental Fish Feeds in Modern Society: The Last Frontier of Pet Nutrition? In Sustainable Aquafeeds; CRC Press: Boca Raton, FL, USA, 2021; pp. 113–120. [Google Scholar]
- Hoseinifar, S.H.; Maradonna, F.; Faheem, M.; Harikrishnan, R.; Devi, G.; Ringø, E.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Carnevali, O. Sustainable Ornamental Fish Aquaculture: The Implication of Microbial Feed Additives. Animals 2023, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J. Fish Disease: Diagnosis and Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Jagoda, S.d.S.; Wijewardana, T.; Arulkanthan, A.; Igarashi, Y.; Tan, E.; Kinoshita, S.; Watabe, S.; Asakawa, S. Characterization and antimicrobial susceptibility of motile aeromonads isolated from freshwater ornamental fish showing signs of septicaemia. Dis. Aquat. Org. 2014, 109, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Parvathi, K.; Bharathi, R.J. Role of Probiotics in Ornamental Fish Farming. Synth. Microb. Res. Chall. Prospect. 2022, 1, 231. [Google Scholar]
- Miles, R.D.; Chapman, F.A. The Benefits of Fish Meal in Aquaculture Diets: FA122/FA122, 5/2006; University of Florida: Gainesville, FL, USA, 2006. [Google Scholar]
- Dawood, M.A.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Kaiser, F.; Harbach, H.; Schulz, C. Rapeseed proteins as fishmeal alternatives: A review. Rev. Aquac. 2022, 14, 1887–1911. [Google Scholar] [CrossRef]
- Sarker, P.K. Microorganisms in Fish Feeds, Technological Innovations, and Key Strategies for Sustainable Aquaculture. Microorganisms 2023, 11, 439. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Delgado, C.L. Fish to 2020: Supply and Demand in Changing Global Markets; WorldFish: Penang, Malaysia, 2003; Volume 62. [Google Scholar]
- Delgado, C.L.; Wada, N.; Rosegrant, M.W.; Meijer, S.; Ahmed, M. Outlook for Fish to 2020: Meeting Global Demand; International Food Policy Research Institute: Washington, DC, USA, 2003; Volume 15. [Google Scholar]
- Wang, S.; Carter, C.G.; Fitzgibbon, Q.P.; Smith, G.G. Respiratory quotient and the stoichiometric approach to investigating metabolic energy substrate use in aquatic ectotherms. Rev. Aquac. 2021, 13, 1255–1284. [Google Scholar] [CrossRef]
- Alfiko, Y.; Xie, D.; Astuti, R.T.; Wong, J.; Wang, L. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Nesic, K.; Zagon, J. Insects–a promising feed and food protein source? Sci. J. Meat Technol. 2019, 60, 56–67. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Muralisankar, T.; Ganesan, A.R.; Sathishkumar, P.; Revathi, N. Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: A review of past and future needs. Aquaculture 2022, 553, 738095. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.-J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of black soldier fly larvae reared on organic side-streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Amrul, N.F.; Kabir Ahmad, I.; Ahmad Basri, N.E.; Suja, F.; Abdul Jalil, N.A.; Azman, N.A. A review of organic waste treatment using black soldier fly (Hermetia illucens). Sustainability 2022, 14, 4565. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Xu, X.; Ji, H.; Belghit, I.; Sun, J. Black soldier fly larvae as a better lipid source than yellow mealworm or silkworm oils for juvenile mirror carp (Cyprinus carpio var. specularis). Aquaculture 2020, 527, 735453. [Google Scholar] [CrossRef]
- Fisher, H.; Collins, S.; Hanson, C.; Mason, B.; Colombo, S.; Anderson, D. Black soldier fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar). Aquaculture 2020, 521, 734978. [Google Scholar] [CrossRef]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Belghit, I.; Lock, E.-J.; Krogdahl, Å. Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 2020, 520, 734967. [Google Scholar] [CrossRef]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C. First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black soldier fly full-fat larvae meal as an alternative to fish meal and fish oil in Siberian sturgeon nutrition: The effects on physical properties of the feed, animal growth performance, and feed acceptance and utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Ghetas, H.A.; Khallaf, M.A. Black soldier fly (Hermetia illucens) larvae meal in diets of European seabass: Effects on antioxidative capacity, non-specific immunity, transcriptomic responses, and resistance to the challenge with Vibrio alginolyticus. Fish Shellfish. Immunol. 2021, 111, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Maranga, B.; Kagali, R.; Mbogo, K.; Orina, P.; Munguti, J.; Ogello, E. Growth Performance of African Catfish (Clarias Gariepinus) Fed on Diets Containing Black Soldier Fly (Hermetia Illucens) Larvae Under Aquaponic System. Aquac. Stud. 2022, 23, AQUAST910. [Google Scholar] [CrossRef]
- Limbu, S.M.; Shoko, A.P.; Ulotu, E.E.; Luvanga, S.A.; Munyi, F.M.; John, J.O.; Opiyo, M.A. Black soldier fly (Hermetia illucens, L.) larvae meal improves growth performance, feed efficiency and economic returns of Nile tilapia (Oreochromis niloticus, L.) fry. Aquac. Fish Fish. 2022, 2, 167–178. [Google Scholar] [CrossRef]
- Caimi, C.; Biasato, I.; Chemello, G.; Oddon, S.B.; Lussiana, C.; Malfatto, V.M.; Capucchio, M.T.; Colombino, E.; Schiavone, A.; Gai, F. Dietary inclusion of a partially defatted black soldier fly (Hermetia illucens) larva meal in low fishmeal-based diets for rainbow trout (Oncorhynchus Mykiss). J. Anim. Sci. Biotechnol. 2021, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Ratti, S.; Zarantoniello, M.; Chemello, G.; Giammarino, M.; Palermo, F.A.; Cocci, P.; Mosconi, G.; Tignani, M.V.; Pascon, G.; Cardinaletti, G. Spirulina-enriched Substrate to Rear Black Soldier Fly (Hermetia illucens) Prepupae as Alternative Aquafeed Ingredient for Rainbow Trout (Oncorhynchus mykiss) Diets: Possible Effects on Zootechnical Performances, Gut and Liver Health Status, and Fillet Quality. Animals 2023, 13, 173. [Google Scholar] [PubMed]
- Ahmad, I.; Babitha Rani, A.; Verma, A.; Maqsood, M. Biofloc technology: An emerging avenue in aquatic animal healthcare and nutrition. Aquac. Int. 2017, 25, 1215–1226. [Google Scholar] [CrossRef]
- Daniel, N.; Nageswari, P. Exogenous probiotics on biofloc based aquaculture: A review. Curr. Agric. Res. J. 2017, 5, 88. [Google Scholar] [CrossRef]
- Linh, N.V.; Nguyen, D.V.; Khongdee, N.; Wannavijit, S.; Outama, P.; Le Xuan, C.; Mahatheeranont, S.; Sookwong, P.; Le, T.D.; Hoseinifar, S.H.; et al. Influence of black rice (Oryza sativa L.) bran derived anthocyanin-extract on growth rate, immunological response, and immune-antioxidant gene expression in Nile tilapia (Oreochromis niloticus) cultivated in a biofloc system. Fish Shellfish. Immunol. 2022, 128, 604–611. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Chemists International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Outama, P.; Le Xuan, C.; Wannavijit, S.; Lumsangkul, C.; Linh, N.V.; Montha, N.; Tongsiri, S.; Chitmanat, C.; Van Doan, H. Modulation of growth, immune response, and immune-antioxidant related gene expression of Nile tilapia (Oreochromis niloticus) reared under biofloc system using mango peel powder. Fish Shellfish. Immunol. 2022, 131, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Keereelang, J.; Mangumphan, K.; Chitmanat, C.; Tongsiri, S.; Vu Linh, N.; Van Doan, H. Dietary effect of Lactobacillus plantarum (TISTR 912) on digestive enzyme activity, growth performance, immune response, and disease resistance of black sharkminnow (Labeo chrysophekadion) against Aeromonas hydrophila infection. Aquac. Rep. 2022, 27, 101409. [Google Scholar] [CrossRef]
- Tschirner, M.; Kloas, W. Increasing the sustainability of aquaculture systems: Insects as alternative protein source for fish diets. GAIA-Ecol. Perspect. Sci. Soc. 2017, 26, 332–340. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.; Teletchea, F.; Tomasso, J.R., Jr. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Gasco, L.; Biasato, I.; Enes, P.; Gai, F. Potential and challenges for the use of insects as feed for aquaculture. Mass Prod. Benef. Org. 2023, 2023, 465–492. [Google Scholar]
- Hossain, M.S.; Small, B.C.; Hardy, R. Insect lipid in fish nutrition: Recent knowledge and future application in aquaculture. Rev. Aquac. 2023, 15, 1664–1685. [Google Scholar] [CrossRef]
- Wang, S.; Carter, C.G.; Fitzgibbon, Q.P.; Codabaccus, B.M.; Smith, G.G. Effect of dietary protein on energy metabolism including protein synthesis in the spiny lobster Sagmariasus verreauxi. Sci. Rep. 2021, 11, 11814. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. The relationship between nutrition and the immune system. Front. Nutr. 2022, 9, 1082500. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional modulation of immune function: Analysis of evidence, mechanisms, and clinical relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of fish meal by Black soldier fly (Hermetia illucens) larvae meal: Effects on growth, haematology, and skin mucus immunity of Nile Tilapia, Oreochromis niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef]
- Carral, J.M.; Sáez-Royuela, M. Replacement of Dietary Fishmeal by Black Soldier Fly Larvae (Hermetia illucens) Meal in Practical Diets for Juvenile Tench (Tinca tinca). Fishes 2022, 7, 390. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Truzzi, C.; Giorgini, E.; Marcellucci, C.; Vargas-Abúndez, J.A.; Zimbelli, A.; Annibaldi, A.; Parisi, G.; Tulli, F. A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019, 9, 8598. [Google Scholar] [CrossRef]
- English, G.; Wanger, G.; Colombo, S.M. A review of advancements in black soldier fly (Hermetia illucens) production for dietary inclusion in salmonid feeds. J. Agric. Food. Res. 2021, 5, 100164. [Google Scholar] [CrossRef]
- Yamamoto, F.Y.; Suehs, B.A.; Ellis, M.; Bowles, P.R.; Older, C.E.; Hume, M.E.; Bake, G.G.; Cammack, J.A.; Tomberlin, J.K.; Gatlin III, D.M. Dietary fishmeal replacement by black soldier fly larvae meals affected red drum (Sciaenops ocellatus) production performance and intestinal microbiota depending on what feed substrate the insect larvae were offered. Anim. Feed Sci. Technol. 2022, 283, 115179. [Google Scholar] [CrossRef]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Bruni, L.; Pastorelli, R.; Viti, C.; Gasco, L.; Parisi, G. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 2018, 487, 56–63. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Khosravi, S.; Mauliasari, I.R.; Lee, S.-M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for rainbow trout (Oncorhynchus mykiss) fry. Fish. Aquat. Sci. 2020, 23, 12. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Chatzifotis, S.; Piccolo, G. Does dietary insect meal affect the fish immune system? The case of mealworm, Tenebrio molitor on European sea bass, Dicentrarchus labrax. Dev. Comp. Immunol. 2018, 81, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Ye, J.; Zhang, Y.; Yang, X.; Wu, C.; Shao, X.; Liu, P. The influence of maggot meal and l-carnitine on growth, immunity, antioxidant indices and disease resistance of black carp (Mylopharyngodon piceus). J. Chin. Cereals Oils Assoc. 2013, 28, 80–86. [Google Scholar]
- Hou, L.; Shi, Y.; Zhai, P.; Le, G. Antibacterial activity and in vitro anti-tumor activity of the extract of the larvae of the housefly (Musca domestica). J. Ethnopharmacol. 2007, 111, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, G.; Huang, Y.; Sun, Y.; He, F.; Zhao, H.; Li, N. Effects of substitution of fish meal with black soldier fly (Hermetia illucens) larvae meal, in yellow catfish (Pelteobagrus fulvidraco) diets. Isr. J. Aquac. Bamidgeh 2017, 69, 9. [Google Scholar]
- Schuhmann, B.; Seitz, V.; Vilcinskas, A.; Podsiadlowski, L. Cloning and expression of gallerimycin, an antifungal peptide expressed in immune response of greater wax moth larvae, Galleria mellonella. Arch. Insect Biochem. Physiol. 2003, 53, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Lee, J.-Y.; Hartl, D.; Elias, J.A. Chitin regulation of immune responses: An old molecule with new roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish. Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef]
- Kumar, S.; Choubey, A.K.; Srivastava, P.K. The effects of dietary immunostimulants on the innate immune response of Indian major carp: A review. Fish Shellfish. Immunol. 2022, 123, 36–49. [Google Scholar] [CrossRef]
- Roberts, R.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef]
- Tang, T.; Wu, C.; Li, J.; Ren, G.; Huang, D.; Liu, F. Stress-induced HSP70 from Musca domestica plays a functionally significant role in the immune system. J. Insect Physiol. 2012, 58, 1226–1234. [Google Scholar] [CrossRef]
- Wali, A.; Balkhi, M. Heat shock proteins, importance and expression in fishes. Eur. J. Biotechnol. Biosci. 2016, 4, 29–35. [Google Scholar]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Fish Shellfish. Immunol. 2022, 120, 497–506. [Google Scholar] [CrossRef]
- Yong-Kang, C.; Chao-Zhong, Z.; Shuang, Z.; Shu-Yan, C.; Xiao-Hui, D.; Qi-Hui, Y.; Hong-Yu, L.; Bei-Ping, T.; Shi-Wei, X. Dietary Black Soldier Fly (Hermetia Illucens) Larvae Meal on Growth Performance, Non-Specific Immunity and Lipid Metabolism of Litopenaeus Vannamei. J. Hydrobiol. 2023, 47, 269–278. [Google Scholar]
- Agulló, E.; Rodríguez, M.S.; Ramos, V.; Albertengo, L. Present and future role of chitin and chitosan in food. Macromol. Biosci. 2003, 3, 521–530. [Google Scholar] [CrossRef]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-López, F.; Teles, M.; MacKenzie, S. The response of fish to immunostimulant diets. Fish Shellfish. Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A review on the involvement of heat shock proteins (extrinsic chaperones) in response to stress conditions in aquatic organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Renteria, P.; Vizcaino, A.; Barroso, F.G. Innovative protein sources in shrimp (Litopenaeus vannamei) feeding. Rev. Aquac. 2020, 12, 186–203. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Rocha, S.D.; Øyås, O.; Lagos, L.; Hansen, J.Ø.; Mydland, L.T.; Øverland, M. Modulation of Atlantic salmon (Salmo salar) gut microbiota composition and predicted metabolic capacity by feeding diets with processed black soldier fly (Hermetia illucens) larvae meals and fractions. Anim. Microbiome 2022, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Foysal, M.J.; Gupta, S.K. A systematic meta-analysis reveals enrichment of Actinobacteria and Firmicutes in the fish gut in response to black soldier fly (Hermetica illucens) meal-based diets. Aquaculture 2022, 549, 737760. [Google Scholar] [CrossRef]
| Ingredients | BSFLM Levels (g/kg Diet) | ||||
|---|---|---|---|---|---|
| Control (T1) | T2 | T3 | T4 | T5 | |
| Fish meal | 200 | 150 | 100 | 50 | 0 |
| Soybean meal | 400 | 400 | 400 | 400 | 400 |
| Corn meal | 100 | 100 | 100 | 100 | 100 |
| Rice bran | 200 | 200 | 200 | 200 | 200 |
| Wheat flour | 80 | 80 | 80 | 80 | 80 |
| Binder | 5 | 5 | 5 | 5 | 5 |
| Soybean oil | 3 | 3 | 3 | 3 | 3 |
| BSFLM 1 | 0 | 50 | 100 | 150 | 200 |
| Vitamin C 98% | 2 | 2 | 2 | 2 | 2 |
| Premix 2 | 10 | 10 | 10 | 10 | 10 |
| Proximate chemical composition | |||||
| Dry matter | 93.64 | 93.95 | 93.81 | 94.01 | 94.09 |
| Ash | 11.09 | 11.40 | 11.49 | 11.45 | 11.32 |
| Fiber | 9.73 | 9.24 | 9.39 | 9.92 | 9.30 |
| Crude protein | 35.56 | 36.00 | 35.94 | 35.81 | 35.50 |
| Ether extract | 2.14 | 2.03 | 2.12 | 2.58 | 2.72 |
| Nitrogen-free extract | 38.12 | 39.28 | 39.87 | 39.24 | 39.26 |
| Primer | Sequences | Tm (°C) | Size (bp) | Reference |
|---|---|---|---|---|
| β-actin | AGACATCAGGGTGTCATGGTTGGT | 60 | 190 | NM_001279635 |
| CTCAAACATGATCTGTGTCAT | ||||
| TNF-α | GCCATAGGAATCAGAGTAGCG | 59 | 196 | EU047718 |
| GACCAGGCTTTCACTTCAGG | ||||
| TGF-β | CCTGGGCTGGAAGTGGATAC | 59 | 200 | JF957371 |
| GTAAAAGATGGGCAGTGGGTC | ||||
| IL1 | GATGCAAATGCCCTCAAATACA | 60 | 172 | XM_005467348 |
| GGCTCTTGACGTTCCTTTTG | ||||
| IL10 | GGAGGGCTTTCCAGTGAGAC | 60 | 200 | XM_042734270.1 |
| TGTTGCACGTTTTCGTCCAG | ||||
| Hsp70 | GTGTCCATCCTGACCATTGAAGA | 60 | 190 | NM_001279635 |
| CTGACTGATGTCCTTCTTGTGCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linh, N.V.; Wannavijit, S.; Tayyamath, K.; Dinh-Hung, N.; Nititanarapee, T.; Sumon, M.A.A.; Srinual, O.; Permpoonpattana, P.; Doan, H.V.; Brown, C.L. Black Soldier Fly (Hermetia illucens) Larvae Meal: A Sustainable Alternative to Fish Meal Proven to Promote Growth and Immunity in Koi Carp (Cyprinus carpio var. koi). Fishes 2024, 9, 53. https://doi.org/10.3390/fishes9020053
Linh NV, Wannavijit S, Tayyamath K, Dinh-Hung N, Nititanarapee T, Sumon MAA, Srinual O, Permpoonpattana P, Doan HV, Brown CL. Black Soldier Fly (Hermetia illucens) Larvae Meal: A Sustainable Alternative to Fish Meal Proven to Promote Growth and Immunity in Koi Carp (Cyprinus carpio var. koi). Fishes. 2024; 9(2):53. https://doi.org/10.3390/fishes9020053
Chicago/Turabian StyleLinh, Nguyen Vu, Supreya Wannavijit, Khambou Tayyamath, Nguyen Dinh-Hung, Thitikorn Nititanarapee, Md Afsar Ahmed Sumon, Orranee Srinual, Patima Permpoonpattana, Hien Van Doan, and Christopher L. Brown. 2024. "Black Soldier Fly (Hermetia illucens) Larvae Meal: A Sustainable Alternative to Fish Meal Proven to Promote Growth and Immunity in Koi Carp (Cyprinus carpio var. koi)" Fishes 9, no. 2: 53. https://doi.org/10.3390/fishes9020053
APA StyleLinh, N. V., Wannavijit, S., Tayyamath, K., Dinh-Hung, N., Nititanarapee, T., Sumon, M. A. A., Srinual, O., Permpoonpattana, P., Doan, H. V., & Brown, C. L. (2024). Black Soldier Fly (Hermetia illucens) Larvae Meal: A Sustainable Alternative to Fish Meal Proven to Promote Growth and Immunity in Koi Carp (Cyprinus carpio var. koi). Fishes, 9(2), 53. https://doi.org/10.3390/fishes9020053