A Review of P-Glycoprotein Function and Regulation in Fish
Abstract
1. Introduction
2. P-Glycoprotein Genes in Fish Species
3. P-Glycoprotein Tissue Expression and Function
| Species | Tissue | References |
|---|---|---|
| Anoplarchus insignis (Slender cockscomb blenny) | Liver | [64] |
| Anoplarchus purpurescens (High cockscomb blenny) | Liver | [27] |
| Barbus barbus (Barbel) | Liver | [47] |
| Carassius auratus gibelio (Silver Prussian carp) | Liver | [47] |
| Chelon labrosus (Thicklip grey mullet) | Liver, brain | [65] |
| Chondrostoma nasus (Sneep) | Liver | [47] |
| Cyprinodon variegatus (Sheepshead minnow) | Liver, kidney, intestine, exocrine pancreas | [66] |
| Cyprinus carpio (Common carp) | Liver | [47] |
| Danio rerio (Zebrafish) | Liver, kidney, intestine, ovary, testis, gill, skin, brain, brain vasculature, eye, muscle, heart, embryo (72 hpf), embryonic ionocytes | [52,67,68,69] |
| Fundulus heteroclitus (Killifish, mummichog) | Liver, distal intestine, brain capillaries | [70,71,72] |
| Gambusia affinis (Western mosquitofish) | Liver | [46] |
| Gobiocypris rarus (Chinese rare minnow) | Liver, kidney, intestine, skin, brain, spleen | [51] |
| Hatcheria macraei (Patagonian catfish) | Liver, gill | [45] |
| Ictalurus punctatus (Channel catfish) | Liver, intestine | [73] |
| Jenynsia multidentata (Killifish, one-sided livebearer) | Liver, gill, brain | [74] |
| Lepomis macrochirus (Bluegill sunfish) | Liver | [46] |
| Limanda limanda (Common dab) | Liver | [37] |
| Oncorhynchus mykiss (Rainbow trout) | Liver, kidney, intestine, gonad, gill, skin, brain, larval abdominal viscera, larval skin, larval head, larval muscle, larval yolk sac | [17,19,41,45,54,75,76,77,78] |
| Oreochromis niloticus (Nile tilapia) | Liver, intestine, zygote, embryo, larva | [79,80] |
| Poecilia reticulata (Guppy) | Liver, kidney, intestine, gill, muscle, ovary, esophagus, pancreas, branchial blood vessels, adrenal cortical tissue, gas gland | [49,81] |
| Salmo trutta (Brown trout) | Liver | [45] |
| Scophthalmus maximus (Turbot) | Liver, kidney, intestine, gill, brain, muscle, esophagus, heart | [50] |
| Squalius cephalus (European chub) | Liver | [47] |
| Thunnus albacares (Yellowfin tuna) | Liver, gill, brain, gonad | [43] |
| Trematomus bernacchii (Emerald rockcod) | Liver | [48] |
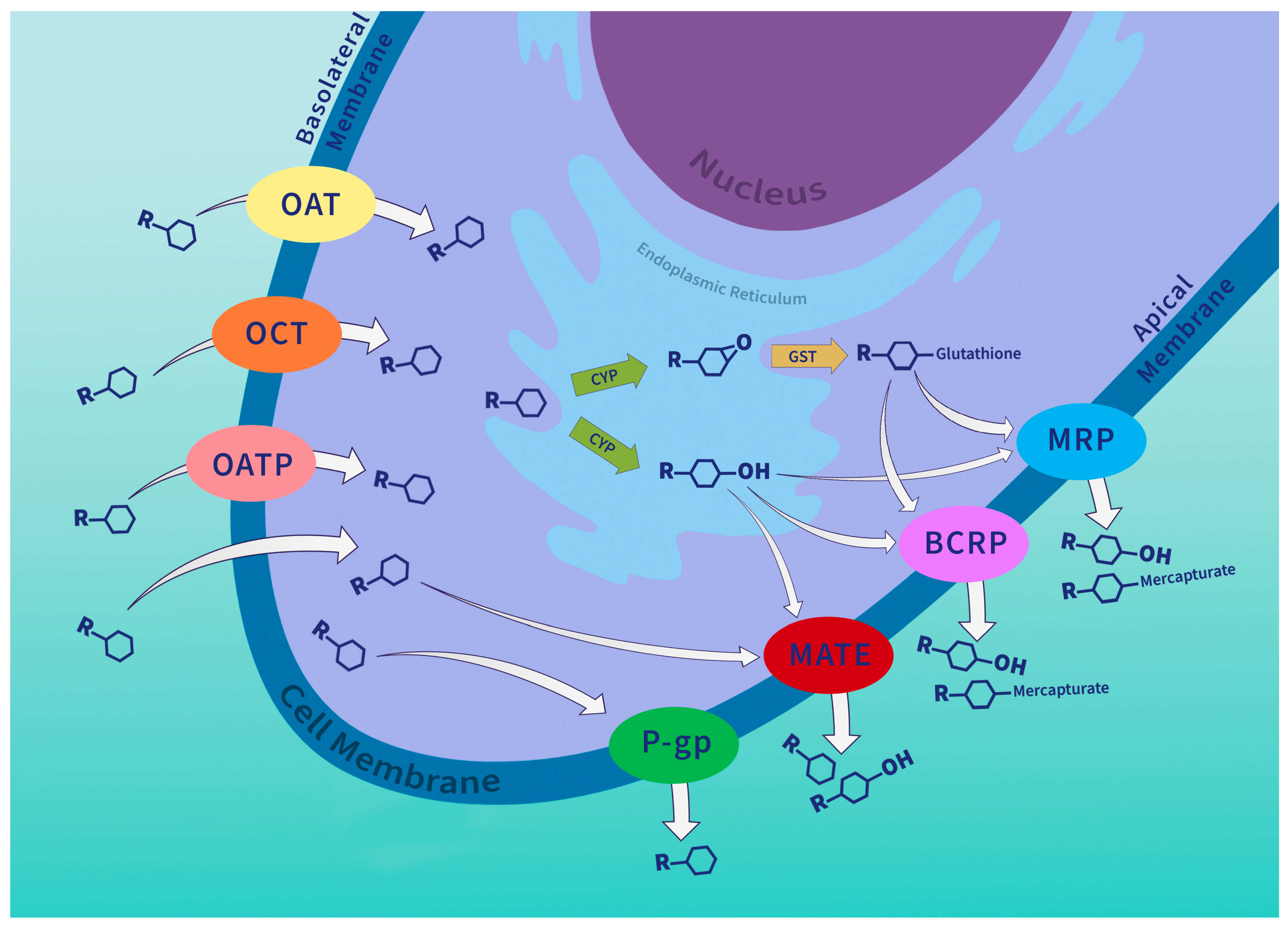
4. P-Glycoprotein in the Chemical Defense System
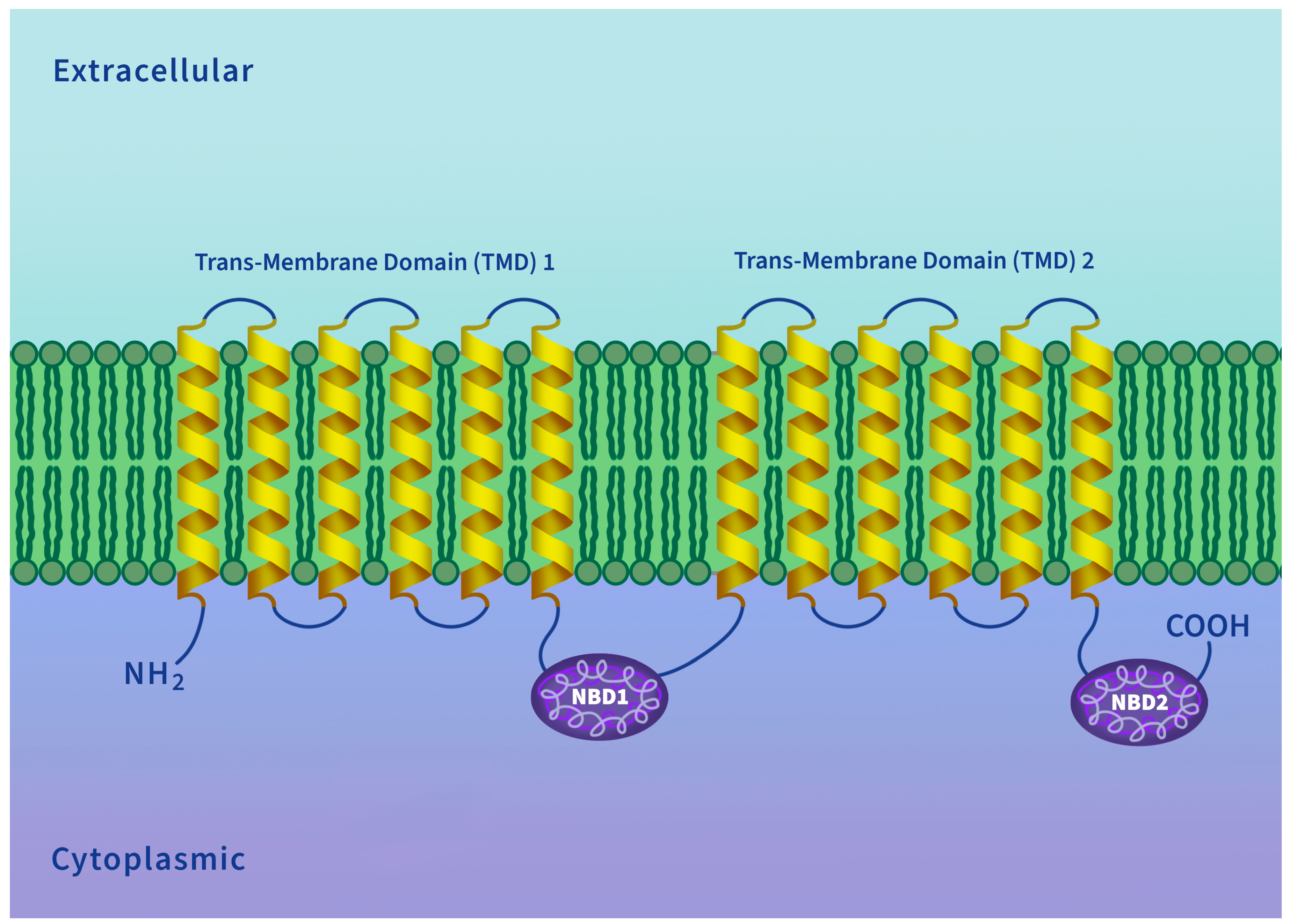
5. P-Glycoprotein Structure and Transport Mechanism
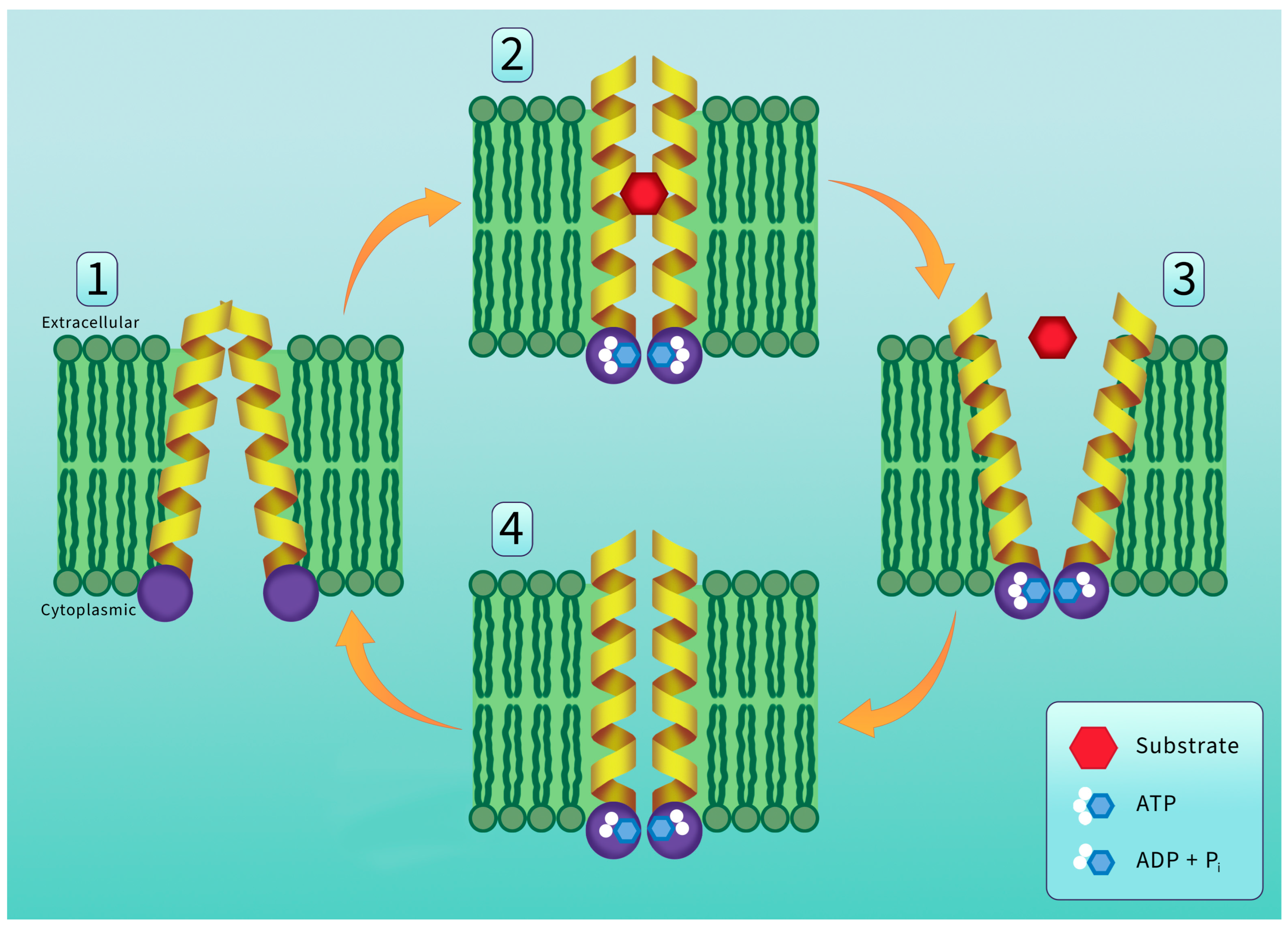
6. P-Glycoprotein Substrates
7. The Regulation of P-Glycoprotein Expression
8. Modulators of P-Glycoprotein Expression
| Expression Inhibitor | Species | Tissue | References |
|---|---|---|---|
| Hormone | |||
| Pregnenolone 16α-carbonitrile (Synthetic steroid) | Danio rerio | Liver | [104] |
| Phytochemical | |||
| Genistein | Ictalurus punctatus | Kidney | [142] |
| Carbon Allotrope | |||
| Fullerene (nC60) | Cyprinus carpio | Liver | [123] |
| Industrial Chemicals | |||
| Metal mine tailings (Mn, Cd, As, Cu, Cr) | Astyanax lacustris | Liver | [159] |
| Pesticides | |||
| Carbendazim | Jenynsia multidentate | Liver | [150] |
| Emamectin benzoate | Oncorhynchus mykiss | Intestine | [152] |
| Malathion (7-day exposure) | Danio rerio | Brain | [94] |
| Triclosan | Xiphophorus helleri (females) | Liver | [153] |
| Analgesic Pharmaceutical | |||
| Morphine | Danio rerio | Embryo | [116] |
9. Inhibitors of P-Glycoprotein Transport Activity
10. The Energetic Costs of P-Glycoprotein Activity
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nigam, S.K. What Do Drug Transporters Really Do? Nat. Rev. Drug Discov. 2015, 14, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jamshidi, N.; Eraly, S.A.; Liu, H.C.; Bush, K.T.; Palsson, B.O.; Nigam, S.K. Multispecific Drug Transporter Slc22a8 (Oat3) Regulates Multiple Metabolic and Signaling Pathways. Drug Metab. Dispos. 2013, 41, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Nagle, M.A.; Kouznetsova, V.L.; Tsigelny, I.F.; Nigam, S.K. Untargeted Metabolomics Identifies Enterobiome Metabolites and Putative Uremic Toxins as Substrates of Organic Anion Transporter 1 (Oat1). J. Proteome Res. 2011, 10, 2842–2851. [Google Scholar] [CrossRef] [PubMed]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of Volatile Organic Compounds from Petunia Flowers Is Facilitated by an ABC Transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Iversen, D.B.; Andersen, N.E.; Dalgård Dunvald, A.-C.; Pottegård, A.; Stage, T.B. Drug Metabolism and Drug Transport of the 100 Most Prescribed Oral Drugs. Basic Clin. Pharmacol. Toxicol. 2022, 131, 311–324. [Google Scholar] [CrossRef]
- Epel, D.; Luckenbach, T.; Stevenson, C.N.; Macmanus-Spencer, L.A.; Hamdoun, A.; Smital, T. Efflux Transporters: Newly Appreciated Roles in Protection against Pollutants. Environ. Sci. Technol. 2008, 42, 3914–3920. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, J.; Reis-Henriques, M.A. ABC Transporters in Fish Species: A Review. Front. Physiol. 2014, 5, 266. [Google Scholar] [CrossRef]
- Kroll, T.; Prescher, M.; Smits, S.H.J.; Schmitt, L. Structure and Function of Hepatobiliary ATP Binding Cassette Transporters. Chem. Rev. 2021, 121, 5240–5288. [Google Scholar] [CrossRef]
- Pierman, B.; Boutry, M.; Lefèvre, F. The ABC of ABC Transporters. In Advances in Botanical Research; Maurel, C., Ed.; Membrane Transport in Plants; Academic Press: New York, NY, USA, 2018; Volume 87, pp. 1–23. [Google Scholar]
- Rice, A.J.; Park, A.; Pinkett, H.W. Diversity in ABC Transporters: Type I, II and III Importers. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 426–437. [Google Scholar] [CrossRef]
- Bieczynski, F.; Painefilú, J.C.; Venturino, A.; Luquet, C.M. Expression and Function of ABC Proteins in Fish Intestine. Front. Physiol. 2021, 12, 791834. [Google Scholar] [CrossRef]
- Dean, M.; Annilo, T. Evolution of the ATP-Binding Cassette (ABC) Transporter Superfamily in Vertebrates. Annu. Rev. Genom. Hum. Genet. 2005, 6, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Mansur, J.; Schrago, C.G.; Salles, T.S.; Alvarenga, E.S.L.; Vasconcellos, B.M.; Melo, A.C.A.; Moreira, M.F. Phylogenetic Analysis of the ATP-Binding Cassette Proteins Suggests a New ABC Protein Subfamily J in Aedes Aegypti (Diptera: Culicidae). BMC Genom. 2020, 21, 463. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.-P.; Tainer, J.A. Rad50/SMC Proteins and ABC Transporters: Unifying Concepts from High-Resolution Structures. Curr. Opin. Struct. Biol. 2003, 13, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Luckenbach, T.; Fischer, S.; Sturm, A. Current Advances on ABC Drug Transporters in Fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 165, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Vilas-Boas, V.; Carmo, H.; Dinis-Oliveira, R.J.; Carvalho, F.; de Lourdes Bastos, M.; Remião, F. Modulation of P-Glycoprotein Efflux Pump: Induction and Activation as a Therapeutic Strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef] [PubMed]
- Love, R.C.; Osachoff, H.L.; Kennedy, C.J. Short Communication: Tissue-Specific Transcript Expression of P-Glycoprotein Isoforms Abcb1a and Abcb1b in Rainbow Trout (Oncorhynchus mykiss) Following Induction with Clotrimazole. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 252, 110538. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P.C. Multidrug Resistance Proteins: Role of P-Glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in Tissue Defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef]
- Lončar, J.; Popović, M.; Zaja, R.; Smital, T. Gene Expression Analysis of the ABC Efflux Transporters in Rainbow Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 209–215. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ling, V. A Surface Glycoprotein Modulating Drug Permeability in Chinese Hamster Ovary Cell Mutants. Biochim. Biophys. Acta BBA—Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Ling, V. The Molecular Basis of Multidrug Resistance in Cancer: The Early Years of P-Glycoprotein Research. FEBS Lett. 2006, 580, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.; Alpermann, T.; Lauritzen, B.; Van Noorden, C.J.F. Clonal Xenobiotic Resistance during Pollution-Induced Toxic Injury and Hepatocellular Carcinogenesis in Liver of Female Flounder (Platichthys flesus (L.)). Acta Histochem. 2004, 106, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.P.; Cunha, V.; Reis-Henriques, M.A.; Ferreira, M. Histopathological Lesions, P-Glycoprotein and PCNA Expression in Zebrafish (Danio Rerio) Liver after a Single Exposure to Diethylnitrosamine. Environ. Toxicol. Pharmacol. 2014, 38, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Vogelbein, W.K.; Fournie, J.W.; Cooper, P.S.; Van Veld, P.A. Hepatoblastomas in the Mummichog, Fundulus heteroclitus (L.), from a Creosote-Contaminated Environment: A Histologic, Ultrastructural and Immunohistochemical Study. J. Fish Dis. 1999, 22, 419–431. [Google Scholar] [CrossRef]
- Paetzold, C.S.; Ross, N.W.; Richards, R.C.; Jones, M.; Hellou, J.; Bard, S.M. Up-Regulation of Hepatic ABCC2, ABCG2, CYP1A1 and GST in Multixenobiotic-Resistant Killifish (Fundulus heteroclitus) from the Sydney Tar Ponds, Nova Scotia, Canada. Mar. Environ. Res. 2009, 68, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bard, S.M.; Woodin, B.R.; Stegeman, J.J. Expression of P-Glycoprotein and Cytochrome P450 1A in Intertidal Fish (Anoplarchus purpurescens) Exposed to Environmental Contaminants. Aquat. Toxicol. 2002, 60, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kurelec, B.; Pivčević, B. Evidence for a Multixenobiotic Resistance Mechanism in the Mussel Mytilus Galloprovincialis. Aquat. Toxicol. 1991, 19, 291–301. [Google Scholar] [CrossRef]
- Kurelec, B.; Pivčević, B. Distinct Glutathione-Dependent Enzyme Activities and a Verapamil-Sensitive Binding of Xenobiotics in a Fresh-Water Mussel Anodonta Cygnea. Biochem. Biophys. Res. Commun. 1989, 164, 934–940. [Google Scholar] [CrossRef]
- Kurelec, B. The Multixenobiotic Resistance Mechanism in Aquatic Organisms. Crit. Rev. Toxicol. 1992, 22, 23–43. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Rolshausen, G.; Uren Webster, T.M.; Tyler, C.R. Adaptive Capabilities and Fitness Consequences Associated with Pollution Exposure in Fish. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160042. [Google Scholar] [CrossRef]
- Lind, E.E.; Grahn, M. Directional Genetic Selection by Pulp Mill Effluent on Multiple Natural Populations of Three-Spined Stickleback (Gasterosteus aculeatus). Ecotoxicology 2011, 20, 503–512. [Google Scholar] [CrossRef]
- Laporte, M.; Pavey, S.A.; Rougeux, C.; Pierron, F.; Lauzent, M.; Budzinski, H.; Labadie, P.; Geneste, E.; Couture, P.; Baudrimont, M.; et al. RAD Sequencing Reveals Within-Generation Polygenic Selection in Response to Anthropogenic Organic and Metal Contamination in North Atlantic Eels. Mol. Ecol. 2016, 25, 219–237. [Google Scholar] [CrossRef]
- Williams, L.M.; Oleksiak, M.F. Ecologically and Evolutionarily Important SNPs Identified in Natural Populations. Mol. Biol. Evol. 2011, 28, 1817–1826. [Google Scholar] [CrossRef]
- Wirgin, I.; Roy, N.K.; Loftus, M.; Chambers, R.C.; Franks, D.G.; Hahn, M.E. Mechanistic Basis of Resistance to PCBs in Atlantic Tomcod from the Hudson River. Science 2011, 331, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Kurelec, B. Reversion of the Multixenobiotic Resistance Mechanism in Gills of a Marine Mussel Mytilus Galloprovincialis by a Model Inhibitor and Environmental Modulators of P170-Glycoprotein. Aquat. Toxicol. 1995, 33, 93–103. [Google Scholar] [CrossRef]
- Smital, T.; Kurelec, B. The Chemosensitizers of Multixenobiotic Resistance Mechanism in Aquatic Invertebrates: A New Class of Pollutants. Mutat. Res. Mol. Mech. Mutagen. 1998, 399, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Klüver, N.; Burkhardt-Medicke, K.; Pietsch, M.; Schmidt, A.-M.; Wellner, P.; Schirmer, K.; Luckenbach, T. Abcb4 Acts as Multixenobiotic Transporter and Active Barrier against Chemical Uptake in Zebrafish (Danio Rerio) Embryos. BMC Biol. 2013, 11, 69. [Google Scholar] [CrossRef]
- Dean, M.; Moitra, K.; Allikmets, R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Hum. Mutat. 2022, 43, 1162–1182. [Google Scholar] [CrossRef]
- Moitra, K.; Scally, M.; McGee, K.; Lancaster, G.; Gold, B.; Dean, M. Molecular Evolutionary Analysis of ABCB5: The Ancestral Gene Is a Full Transporter with Potentially Deleterious Single Nucleotide Polymorphisms. PLoS ONE 2011, 6, e16318. [Google Scholar] [CrossRef]
- Kropf, C.; Fent, K.; Fischer, S.; Casanova, A.; Segner, H. ABC Transporters in Gills of Rainbow Trout (Oncorhynchus mykiss). J. Exp. Biol. 2020, 223, jeb221069. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Q.; Liu, Z. Genome-Wide Identification, Characterization and Phylogenetic Analysis of 50 Catfish ATP-Binding Cassette (ABC) Transporter Genes. PLoS ONE 2013, 8, e63895. [Google Scholar] [CrossRef]
- Nicklisch, S.C.T.; Pouv, A.K.; Rees, S.D.; McGrath, A.P.; Chang, G.; Hamdoun, A. Transporter-Interfering Chemicals Inhibit P-Glycoprotein of Yellowfin Tuna (Thunnus albacares). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109101. [Google Scholar] [CrossRef]
- Chartrain, M.; Riond, J.; Stennevin, A.; Vandenberghe, I.; Gomes, B.; Lamant, L.; Meyer, N.; Gairin, J.E.; Guilbaud, N.; Annereau, J.P. Melanoma Chemotherapy Leads to the Selection of ABCB5-Expressing Cells. PLoS ONE 2012, 7, e36762. [Google Scholar] [CrossRef]
- Assef, Y.A.; Di Prinzio, C.Y.; Horak, C.N. Differential Activities of the Multixenobiotic Resistance Mechanism in Freshwater Fishes Inhabiting Environments of Patagonia Argentina. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 217, 32–40. [Google Scholar] [CrossRef]
- Damaré, C.L.; Kaddoumi, A.K.; Baer, K.N. Investigation of the Multixenobiotic Resistance Mechanism in the Freshwater Fishes Western Mosquitofish, Gambusia affinis, and Bluegill Sunfish, Lepomis Macrochirus. Bull. Environ. Contam. Toxicol. 2009, 83, 640–643. [Google Scholar] [CrossRef]
- Klobučar, R.; Žaja, R.; Franjević, D.; Brozović, A.; Smital, T. Presence of Ecotoxicologically Relevant Pgp and MRP Transcripts and Proteins in Cyprinid Fish. Arch. Ind. Hyg. Toxicol. 2010, 61, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, S.; Corsi, I.; Luckenbach, T.; Bard, S.M.; Regoli, F.; Focardi, S. Identification of Five Partial ABC Genes in the Liver of the Antarctic Fish Trematomus Bernacchii and Sensitivity of ABCB1 and ABCC2 to Cd Exposure. Environ. Pollut. 2010, 158, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.E.M.; Boulos, J.C.; Machel, K.; Andabili, N.; Marouni, T.; Roth, W.; Efferth, T. Expression of the Stem Cell Marker ABCB5 in Normal and Tumor Tissues. In Vivo 2022, 36, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Tutundjian, R.; Cachot, J.; Leboulenger, F.; Minier, C. Genetic and Immunological Characterisation of a Multixenobiotic Resistance System in the Turbot (Scophthalmus maximus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lv, B.; Zha, J.; Wang, Z. Transcriptional Expression Analysis of ABC Efflux Transporters and Xenobiotic-Metabolizing Enzymes in the Chinese Rare Minnow. Environ. Toxicol. Pharmacol. 2014, 37, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Long, Y.; Sun, R.; Zhou, B.; Lin, L.; Zhong, S.; Cui, Z. Zebrafish Abcb4 Is a Potential Efflux Transporter of Microcystin-LR. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 167, 35–42. [Google Scholar] [CrossRef]
- Romersi, R.F.; Nicklisch, S.C.T. Interactions of Environmental Chemicals and Natural Products with ABC and SLC Transporters in the Digestive System of Aquatic Organisms. Front. Physiol. 2022, 12, 2252. [Google Scholar] [CrossRef]
- Sturm, A.; Ziemann, C.; Hirsch-Ernst, K.I.; Segner, H. Expression and Functional Activity of P-Glycoprotein in Cultured Hepatocytes from Oncorhynchus mykiss. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 281, R1119–R1126. [Google Scholar] [CrossRef]
- Bains, O.S.; Kennedy, C.J. Alterations in Respiration Rate of Isolated Rainbow Trout Hepatocytes Exposed to the P-Glycoprotein Substrate Rhodamine 123. Toxicology 2005, 214, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.L.; Bains, O.S.; Lee, D.S.H.; Kennedy, C.J. Functional and Energetic Characterization of P-Gp-Mediated Doxorubicin Transport in Rainbow Trout (Oncorhynchus mykiss) Hepatocytes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.U.; Kennedy, C.J. Effects of the Chemosensitizer Verapamil on P-Glycoprotein Substrate Efflux in Rainbow Trout Hepatocytes. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2024, 275, 109763. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Daunomycin Secretion by Killfish Renal Proximal Tubules. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 269, R370–R379. [Google Scholar] [CrossRef] [PubMed]
- Schramm, U.; Fricker, G.; Wenger, R.; Miller, D.S. P-Glycoprotein-Mediated Secretion of a Fluorescent Cyclosporin Analogue by Teleost Renal Proximal Tubules. Am. J. Physiol.-Ren. Physiol. 1995, 268, F46–F52. [Google Scholar] [CrossRef]
- Miller, D.S. Aquatic Models for the Study of Renal Transport Function and Pollutant Toxicity. Environ. Health Perspect. 1987, 71, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Fricker, G.; Drewe, J. P-Glycoprotein-Mediated Transport of a Fluorescent Rapamycin Derivative in Renal Proximal Tubule. J. Pharmacol. Exp. Ther. 1997, 282, 440–444. [Google Scholar]
- Fricker, G.; Gutmann, H.; Droulle, A.; Drewe, J.; Miller, D.S. Epithelial Transport of Anthelmintic Ivermectin in a Novel Model of Isolated Proximal Kidney Tubules. Pharm. Res. 1999, 16, 1570–1575. [Google Scholar] [CrossRef]
- Miller, D.S. Sphingolipid Signaling Reduces Basal P-Glycoprotein Activity in Renal Proximal Tubule. J. Pharmacol. Exp. Ther. 2014, 348, 459–464. [Google Scholar] [CrossRef]
- Bard, S.M. Multixenobiotic Resistance as a Cellular Defense Mechanism in Aquatic Organisms. Aquat. Toxicol. 2000, 48, 357–389. [Google Scholar] [CrossRef]
- Diaz de Cerio, O.; Bilbao, E.; Cajaraville, M.P.; Cancio, I. Regulation of Xenobiotic Transporter Genes in Liver and Brain of Juvenile Thicklip Grey Mullets (Chelon labrosus) after Exposure to Prestige-like Fuel Oil and to Perfluorooctane Sulfonate. Gene 2012, 498, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, M.J.; Courtney, L.A.; Benson, W.H. Comparison of Three Histological Fixatives on the Immunoreactivity of Mammalian P-Glycoprotein Antibodies in the Sheepshead Minnow, Cyprinodon Variegatus. J. Exp. Zool. 1998, 281, 251–259. [Google Scholar] [CrossRef]
- Bieczynski, F.; Burkhardt-Medicke, K.; Luquet, C.M.; Scholz, S.; Luckenbach, T. Chemical Effects on Dye Efflux Activity in Live Zebrafish Embryos and on Zebrafish Abcb4 ATPase Activity. FEBS Lett. 2021, 595, 828–843. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.E.; Espinoza, J.A.; Leerberg, D.M.; Yelon, D.; Hamdoun, A. Xenobiotic Transporter Activity in Zebrafish Embryo Ionocytes. Aquat. Toxicol. 2019, 212, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Robinson, A.N.; Ali-Rahmani, F.; Huff, L.M.; Lusvarghi, S.; Vahedi, S.; Hotz, J.M.; Warner, A.C.; Butcher, D.; Matta, J.; et al. Characterization and Tissue Localization of Zebrafish Homologs of the Human ABCB1 Multidrug Transporter. Sci. Rep. 2021, 11, 24150. [Google Scholar] [CrossRef] [PubMed]
- Bard, S.M.; Bello, S.M.; Hahn, M.E.; Stegeman, J.J. Expression of P-Glycoprotein in Killifish (Fundulus heteroclitus) Exposed to Environmental Xenobiotics. Aquat. Toxicol. 2002, 59, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Graeff, C.; Droulle, L.; Fricker, S.; Fricker, G. Xenobiotic Efflux Pumps in Isolated Fish Brain Capillaries. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 282, R191–R198. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.S.; Vogelbein, W.K.; Van Veld, P.A. Altered Expression of the Xenobiotic Transporter P-Glycoprotein in Liver and Liver Tumours of Mummichog Fundulus heteroclitus from a Creosote-Contaminated Environment. Biomarkers 1999, 4, 48–58. [Google Scholar] [CrossRef]
- Doi, A.M.; Holmes, E.; Kleinow, K.M. P-Glycoprotein in the Catfish Intestine: Inducibility by Xenobiotics and Functional Properties. Aquat. Toxicol. 2001, 55, 157–170. [Google Scholar] [CrossRef]
- Amé, M.V.; Baroni, M.V.; Galanti, L.N.; Bocco, J.L.; Wunderlin, D.A. Effects of Microcystin–LR on the Expression of P-Glycoprotein in Jenynsia Multidentata. Chemosphere 2009, 74, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Kropf, C.; Segner, H.; Fent, K. ABC Transporters and Xenobiotic Defense Systems in Early Life Stages of Rainbow Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 185–186, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Shúilleabháin, S.N.; Davoren, M.; Mothersill, C.; Sheehan, D.; Hartl, M.G.J.; Kilemade, M.; O’Brien, N.M.; O’Halloran, J.; Van Pelt, F.N.A.M.; Lyng, F.M. Identification of a Multixenobiotic Resistance Mechanism in Primary Cultured Epidermal Cells from Oncorhynchus mykiss and the Effects of Environmental Complex Mixtures on Its Activity. Aquat. Toxicol. 2005, 73, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Cravedi, J.P.; Segner, H. Prochloraz and Nonylphenol Diethoxylate Inhibit an Mdr1-like Activity in Vitro, but Do Not Alter Hepatic Levels of P-Glycoprotein in Trout Exposed In Vivo. Aquat. Toxicol. 2001, 53, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Zaja, R.; Munić, V.; Klobučar, R.S.; Ambriović-Ristov, A.; Smital, T. Cloning and Molecular Characterization of Apical Efflux Transporters (ABCB1, ABCB11 and ABCC2) in Rainbow Trout (Oncorhynchus mykiss) Hepatocytes. Aquat. Toxicol. 2008, 90, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Reis-Henriques, M.A.; Castro, L.F.C.; Ferreira, M. ABC Transporters, CYP1A and GSTα Gene Transcription Patterns in Developing Stages of the Nile Tilapia (Oreochromis niloticus). Gene 2012, 506, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Reis-Henriques, M.A.; Castro, L.F.C.; Ferreira, M. Gene Expression Analysis of ABC Efflux Transporters, CYP1A and GSTα in Nile Tilapia after Exposure to Benzo(a)Pyrene. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 469–482. [Google Scholar] [CrossRef]
- Hemmer, M.J.; Courtney, L.A.; Ortego, L.S. Immunohistochemical Detection of P-Glycoprotein in Teleost Tissues Using Mammalian Polyclonal and Monoclonal Antibodies. J. Exp. Zool. 1995, 272, 69–77. [Google Scholar] [CrossRef]
- Azevedo, V.C.; Kennedy, C.J. P-Glycoprotein Inhibition Affects Ivermectin-Induced Behavioural Alterations in Fed and Fasted Zebrafish (Danio Rerio). Fish Physiol. Biochem. 2022, 48, 1267–1283. [Google Scholar] [CrossRef]
- Bard, S.M.; Gadbois, S. Assessing Neuroprotective P-Glycoprotein Activity at the Blood-Brain Barrier in Killifish (Fundulus heteroclitus) Using Behavioural Profiles. Mar. Environ. Res. 2007, 64, 679–682. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Tierney, K.B.; Mittelstadt, M. Inhibition of P-Glycoprotein in the Blood–Brain Barrier Alters Avermectin Neurotoxicity and Swimming Performance in Rainbow Trout. Aquat. Toxicol. 2014, 146, 176–185. [Google Scholar] [CrossRef]
- Azevedo, V.C.; Johnston, C.U.; Kennedy, C.J. Ivermectin Toxicokinetics in Rainbow Trout (Oncorhynchus mykiss) Following P-Glycoprotein Inhibition. Arch. Environ. Contam. Toxicol. 2024; manuscrript submitted. [Google Scholar] [CrossRef]
- Cserr, H.F.; Bundgaard, M. Blood-Brain Interfaces in Vertebrates: A Comparative Approach. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 246, R277–R288. [Google Scholar] [CrossRef]
- Kajikawa, T.; Mishima, H.; Mishima, H.; Murakami, T.; Takano, M. Role of P-Glycoprotein in Distribution of Rhodamine 123 into Aqueous Humor in Rabbits. Curr. Eye Res. 1999, 18, 240–246. [Google Scholar] [CrossRef]
- Chen, L. Visual System: An Understudied Target of Aquatic Toxicology. Aquat. Toxicol. 2020, 225, 105542. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, D.D.; de Lima, L.F.; Mbemya, G.T.; Maside, C.M.; Miranda, A.M.; Tavares, K.C.S.; Alves, B.G.; Faustino, L.R.; Smitz, J.; de Figueiredo, J.R.; et al. ATP-Binding Cassette (ABC) Transporters in Caprine Preantral Follicles: Gene and Protein Expression. Cell Tissue Res. 2018, 372, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cheng, C.Y.; Mruk, D.D. Drug Transporter, P-Glycoprotein (MDR1), Is an Integrated Component of the Mammalian Blood–Testis Barrier. Int. J. Biochem. Cell Biol. 2009, 41, 2578–2587. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Nie, X.; Liu, Y.; Wang, C.; Liu, S. Response of PXR Signaling Pathway to Simvastatin Exposure in Mosquitofish (Gambusia affinis) and Its Histological Changes. Ecotoxicol. Environ. Saf. 2018, 154, 228–236. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Danio Rerio Embryos on Prozac—Effects on the Detoxification Mechanism and Embryo Development. Aquat. Toxicol. 2016, 178, 182–189. [Google Scholar] [CrossRef]
- Karmakar, S.; Sen Gupta, P.; Bhattacharya, S.; Sarkar, A.; Rahaman, A.; Mandal, D.P.; Bhattacharjee, S. Vitamin B12 Alleviates Malathion-Induced Toxicity in Zebra Fish by Regulating Cytochrome P450 and PgP Expressions. Toxicol. Mech. Methods 2022, 33, 364–377. [Google Scholar] [CrossRef]
- Meinan, X.; Yimeng, W.; Chao, W.; Tianli, T.; Li, J.; Peng, Y.; Xiangping, N. Response of the Sirtuin/PXR Signaling Pathway in Mugilogobius Chulae Exposed to Environmentally Relevant Concentration Paracetamol. Aquat. Toxicol. 2022, 249, 106222. [Google Scholar] [CrossRef]
- Vannuccini, M.L.; Grassi, G.; Leaver, M.J.; Corsi, I. Combination Effects of Nano-TiO2 and 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) on Biotransformation Gene Expression in the Liver of European Sea Bass Dicentrarchus Labrax. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 176–177, 71–78. [Google Scholar] [CrossRef]
- Bolten, J.S.; Pratsinis, A.; Alter, C.L.; Fricker, G.; Huwyler, J. Zebrafish (Danio Rerio) Larva as an in Vivo Vertebrate Model to Study Renal Function. Am. J. Physiol.-Ren. Physiol. 2022, 322, F280–F294. [Google Scholar] [CrossRef]
- Muzzio, A.M.; Noyes, P.D.; Stapleton, H.M.; Lema, S.C. Tissue Distribution and Thyroid Hormone Effects on mRNA Abundance for Membrane Transporters Mct8, Mct10, and Organic Anion-Transporting Polypeptides (Oatps) in a Teleost Fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 167, 77–89. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Jaini, R.; Morgan, J.A.; Dudareva, N. Rethinking How Volatiles Are Released from Plant Cells. Trends Plant Sci. 2015, 20, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Mihaljević, I.; Popović, M.; Žaja, R.; Maraković, N.; Šinko, G.; Smital, T. Interaction between the Zebrafish (Danio Rerio) Organic Cation Transporter 1 (Oct1) and Endo- and Xenobiotics. Aquat. Toxicol. 2017, 187, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Zaja, R.; Fent, K.; Smital, T. Interaction of Environmental Contaminants with Zebrafish Organic Anion Transporting Polypeptide, Oatp1d1 (Slco1d1). Toxicol. Appl. Pharmacol. 2014, 280, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Willi, R.A.; Fent, K. Interaction of Environmental Steroids with Organic Anion Transporting Polypeptide (Oatp1d1) in Zebrafish (Danio Rerio). Environ. Toxicol. Chem. 2018, 37, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Bresolin, T.; de Freitas Rebelo, M.; Celso Dias Bainy, A. Expression of PXR, CYP3A and MDR1 Genes in Liver of Zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.S.; Kennedy, C.J. Regulation of Hepatic Abcb4 and Cyp3a65 Gene Expression and Multidrug/Multixenobiotic Resistance (MDR/MXR) Functional Activity in the Model Teleost, Danio Rerio (Zebrafish). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 200, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Reschly, E.J.; Bainy, A.C.D.; Mattos, J.J.; Hagey, L.R.; Bahary, N.; Mada, S.R.; Ou, J.; Venkataramanan, R.; Krasowski, M.D. Functional Evolution of the Vitamin D and Pregnane X Receptors. BMC Evol. Biol. 2007, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- De Anna, J.S.; Darraz, L.A.; Painefilú, J.C.; Cárcamo, J.G.; Moura-Alves, P.; Venturino, A.; Luquet, C.M. The Insecticide Chlorpyrifos Modifies the Expression of Genes Involved in the PXR and AhR Pathways in the Rainbow Trout, Oncorhynchus mykiss. Pestic. Biochem. Physiol. 2021, 178, 104920. [Google Scholar] [CrossRef]
- Mealey, K.L.; Owens, J.G.; Freeman, E. Canine and Feline P-Glycoprotein Deficiency: What We Know and Where We Need to Go. J. Vet. Pharmacol. Ther. 2023, 46, 1–16. [Google Scholar] [CrossRef]
- Borst, P.; Schinkel, A.H. P-Glycoprotein ABCB1: A Major Player in Drug Handling by Mammals. J. Clin. Investig. 2013, 123, 4131–4133. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Nosol, K.; Romane, K.; Irobalieva, R.N.; Alam, A.; Kowal, J.; Fujita, N.; Locher, K.P. Cryo-EM Structures Reveal Distinct Mechanisms of Inhibition of the Human Multidrug Transporter ABCB1. Proc. Natl. Acad. Sci. USA 2020, 117, 26245–26253. [Google Scholar] [CrossRef]
- Löscher, W.; Gericke, B. Novel Intrinsic Mechanisms of Active Drug Extrusion at the Blood-Brain Barrier: Potential Targets for Enhancing Drug Delivery to the Brain? Pharmaceutics 2020, 12, 966. [Google Scholar] [CrossRef]
- Srikant, S.; Gaudet, R. Mechanics and Pharmacology of Substrate Selection and Transport by Eukaryotic ABC Exporters. Nat. Struct. Mol. Biol. 2019, 26, 792–801. [Google Scholar] [CrossRef]
- Zaja, R.; Lončar, J.; Popovic, M.; Smital, T. First Characterization of Fish P-Glycoprotein (Abcb1) Substrate Specificity Using Determinations of Its ATPase Activity and Calcein-AM Assay with PLHC-1/Dox Cell Line. Aquat. Toxicol. 2011, 103, 53–62. [Google Scholar] [CrossRef]
- Johnston, C.U.; Kennedy, C.J. Potency and Mechanism of P-Glycoprotein Chemosensitizers in Rainbow Trout (Oncorhynchus mykiss) Hepatocytes. Department of Biological Sciences, Simon Fraser University, Burnaby, BC, Canada. 2024; manuscrript submitted. [Google Scholar]
- Gutmann, H.; Miller, D.S.; Droulle, A.; Drewe, J.; Fahr, A.; Fricker, G. P-Glycoprotein- and Mrp2-Mediated Octreotide Transport in Renal Proximal Tubule: Octreotide Transport. Br. J. Pharmacol. 2000, 129, 251–256. [Google Scholar] [CrossRef]
- Mottaz, H.; Schönenberger, R.; Fischer, S.; Eggen, R.I.L.; Schirmer, K.; Groh, K.J. Dose-Dependent Effects of Morphine on Lipopolysaccharide (LPS)-Induced Inflammation, and Involvement of Multixenobiotic Resistance (MXR) Transporters in LPS Efflux in Teleost Fish. Environ. Pollut. 2017, 221, 105–115. [Google Scholar] [CrossRef]
- Caminada, D.; Zaja, R.; Smital, T.; Fent, K. Human Pharmaceuticals Modulate P-Gp1 (ABCB1) Transport Activity in the Fish Cell Line PLHC-1. Aquat. Toxicol. 2008, 90, 214–222. [Google Scholar] [CrossRef]
- Fischer, S.; Loncar, J.; Zaja, R.; Schnell, S.; Schirmer, K.; Smital, T.; Luckenbach, T. Constitutive mRNA Expression and Protein Activity Levels of Nine ABC Efflux Transporters in Seven Permanent Cell Lines Derived from Different Tissues of Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 2011, 101, 438–446. [Google Scholar] [CrossRef]
- Zaja, R.; Klobučar, R.S.; Smital, T. Detection and Functional Characterization of Pgp1 (ABCB1) and MRP3 (ABCC3) Efflux Transporters in the PLHC-1 Fish Hepatoma Cell Line. Aquat. Toxicol. 2007, 81, 365–376. [Google Scholar] [CrossRef]
- Uchea, C.; Owen, S.F.; Chipman, J.K. Functional Xenobiotic Metabolism and Efflux Transporters in Trout Hepatocyte Spheroid Cultures. Toxicol. Res. 2015, 4, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Smital, T.; Sauerborn, R. Measurement of the Activity of Multixenobiotic Resistance Mechanism in the Common Carp Cyprinus Carpio. Mar. Environ. Res. 2002, 54, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tian, J.; Zhang, F.; Wang, H.; Yin, J. Pxr- and Nrf2- Mediated Induction of ABC Transporters by Heavy Metal Ions in Zebrafish Embryos. Environ. Pollut. 2019, 255, 113329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, X.; Wang, R.; Yuan, J.; Yin, D. Fullerene Inhibits Benzo(a)Pyrene Efflux from Cyprinus Carpio Hepatocytes by Affecting Cell Membrane Fluidity and P-Glycoprotein Expression. Aquat. Toxicol. 2016, 174, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.; Burkhardt-Medicke, K.; Wellner, P.; Santos, M.M.; Moradas-Ferreira, P.; Luckenbach, T.; Ferreira, M. Effects of Pharmaceuticals and Personal Care Products (PPCPs) on Multixenobiotic Resistance (MXR) Related Efflux Transporter Activity in Zebrafish (Danio Rerio) Embryos. Ecotoxicol. Environ. Saf. 2017, 136, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Nornberg, B.F.; Batista, C.R.; Almeida, D.V.; Trindade, G.S.; Marins, L.F. ABCB1 and ABCC4 Efflux Transporters Are Involved in Methyl Parathion Detoxification in ZFL Cells. Toxicol. In Vitro 2015, 29, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Masereeuw, R.; Terlouw, S.A.; Aubel, R.A.M.H.v.; Russel, F.G.M.; Miller, D.S. Endothelin B Receptor-Mediated Regulation of ATP-Driven Drug Secretion in Renal Proximal Tubule. Mol. Pharmacol. 2000, 57, 59–67. [Google Scholar] [PubMed]
- Bourgeois, Z.M.; Comfort, J.; Schultz, M.; Challis, J.K.; Cantin, J.; Ji, X.; Giesy, J.P.; Brinkmann, M. Predicting Hepatic Clearance of Psychotropic Drugs in Isolated Perfused Fish Livers Using a Combination of Two In Vitro Assays. Environ. Sci. Technol. 2022, 56, 15839–15847. [Google Scholar] [CrossRef] [PubMed]
- Stott, L.C.; Schnell, S.; Hogstrand, C.; Owen, S.F.; Bury, N.R. A Primary Fish Gill Cell Culture Model to Assess Pharmaceutical Uptake and Efflux: Evidence for Passive and Facilitated Transport. Aquat. Toxicol. 2015, 159, 127–137. [Google Scholar] [CrossRef]
- Gutmann, H.; Fricker, G.; Drewe, J.; Toeroek, M.; Miller, D.S. Interactions of HIV Protease Inhibitors with ATP-Dependent Drug Export Proteins. Mol. Pharmacol. 1999, 56, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Sussman-Turner, C.; Renfro, J.L. Heat-Shock-Stimulated Transepithelial Daunomycin Secretion by Flounder Renal Proximal Tubule Primary Cultures. Am. J. Physiol.-Ren. Physiol. 1995, 268, F135–F144. [Google Scholar] [CrossRef]
- Zhou, S.-F. Structure, Function and Regulation of P-Glycoprotein and Its Clinical Relevance in Drug Disposition. Xenobiotica 2008, 38, 802–832. [Google Scholar] [CrossRef]
- Yano, K.; Seto, S.; Kamioka, H.; Mizoi, K.; Ogihara, T. Testosterone and Androstenedione Are Endogenous Substrates of P-Glycoprotein. Biochem. Biophys. Res. Commun. 2019, 520, 166–170. [Google Scholar] [CrossRef]
- Abulrob, A.G.; Gumbleton, M. Transport of Phosphatidylcholine in MDR3-Negative Epithelial Cell Lines via Drug-Induced MDR1 P-Glycoprotein. Biochem. Biophys. Res. Commun. 1999, 262, 121–126. [Google Scholar] [CrossRef]
- Wang, E.; Casciano, C.N.; Clement, R.P.; Johnson, W.W. Two Transport Binding Sites of P-Glycoprotein Are Unequal yet Contingent: Initial Rate Kinetic Analysis by ATP Hydrolysis Demonstrates Intersite Dependence. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 2000, 1481, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, C.; Ducharme, J.; Pollack, G.M. Uptake and Efflux of the Peptidic Delta-Opioid Receptor Agonist [D-Penicillamine2,5]-Enkephalin at the Murine Blood–Brain Barrier by In Situ Perfusion. Neurosci. Lett. 2001, 301, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Patiño, R.; Sullivan, C.V. Ovarian Follicle Growth, Maturation, and Ovulation in Teleost Fish. Fish Physiol. Biochem. 2002, 26, 57–70. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yamashita, M. Regulation of Oocyte Maturation in Fish. Dev. Growth Differ. 2008, 50, S195–S219. [Google Scholar] [CrossRef]
- Goss, G.G.; Perry, S.F.; Wood, C.M.; Laurent, P. Mechanisms of Ion and Acid-Base Regulation at the Gills of Freshwater Fish. J. Exp. Zool. 1992, 263, 143–159. [Google Scholar] [CrossRef]
- Frank, N.Y.; Pendse, S.S.; Lapchak, P.H.; Margaryan, A.; Shlain, D.; Doeing, C.; Sayegh, M.H.; Frank, M.H. Regulation of Progenitor Cell Fusion by ABCB5 P-Glycoprotein, a Novel Human ATP-Binding Cassette Transporter*. J. Biol. Chem. 2003, 278, 47156–47165. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in Understanding the Role of P-Gp in Doxorubicin Resistance: Molecular Pathways, Therapeutic Strategies, and Prospects. Drug Discov. Today 2022, 27, 436–455. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.N.Y.; Hoque, M.T.; Bendayan, R. Role of Nuclear Receptors in the Regulation of Drug Transporters in the Brain. Trends Pharmacol. Sci. 2013, 34, 361–372. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Ai, X.; Zhong, L.; Han, G.; Song, J.; Yang, Q.; Dong, J. Effects of 27 Natural Products on Drug Metabolism Genes in Channel Catfish (Ictalurus punctatus) Cell Line. Xenobiotica 2020, 50, 1043–1051. [Google Scholar] [CrossRef]
- Wang, C.; Ku, P.; Nie, X.; Bao, S.; Wang, Z.; Li, K. Effects of Simvastatin on the PXR Signaling Pathway and the Liver Histology in Mugilogobius Abei. Sci. Total Environ. 2019, 651, 399–409. [Google Scholar] [CrossRef]
- Wu, B.; Li, H.-X.; Lian, J.; Guo, Y.-J.; Tang, Y.-H.; Chang, Z.-J.; Hu, L.-F.; Zhao, G.-J.; Hong, G.-L.; Lu, Z.-Q. Nrf2 Overexpression Protects against Paraquat-induced A549 Cell Injury Primarily by Upregulating P-glycoprotein and Reducing Intracellular Paraquat Accumulation. Exp. Ther. Med. 2019, 17, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Baumgarner, B.L.; Bharadwaj, A.S.; Inerowicz, D.; Goodman, A.S.; Brown, P.B. Proteomic Analysis of Rainbow Trout (Oncorhynchus mykiss) Intestinal Epithelia: Physiological Acclimation to Short-Term Starvation. Comp. Biochem. Physiol. Part D Genomics Proteomics 2013, 8, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wassmur, B.; Gräns, J.; Kling, P.; Celander, M.C. Interactions of Pharmaceuticals and Other Xenobiotics on Hepatic Pregnane X Receptor and Cytochrome P450 3A Signaling Pathway in Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 2010, 100, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.M.; Garcia, M.L.; Ventura-Lima, J.; González, M.; Ballesteros, M.L.; Miglioranza, K.S.B.; Amé, M.V.; Wunderlin, D.A. Antioxidant, Phase II and III Responses Induced by Lipoic Acid in the Fish Jenynsia Multidentata (Anablapidae) and Its Influence on Endolsulfan Accumulation and Toxicity. Pestic. Biochem. Physiol. 2014, 108, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Zaja, R.; Loncar, J.; Smital, T.; Focardi, S.; Corsi, I. Interaction of ABC Transport Proteins with Toxic Metals at the Level of Gene and Transport Activity in the PLHC-1 Fish Cell Line. Chem. Biol. Interact. 2012, 198, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Mariottini, M.; Vannuccini, M.L.; Trisciani, A.; Marchi, D.; Corsi, I. Induction of CYP1A and ABC Transporters in European Sea Bass (Dicentrarchus Labrax) upon 2,3,7,8-TCDD Waterborne Exposure. Mar. Environ. Res. 2014, 99, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Götte, J.Y.; Carrizo, J.C.; Panzeri, A.M.; Amé, M.V.; Menone, M.L. Sublethal Effects of Carbendazim in Jenynsia Multidentata Detected by a Battery of Molecular, Biochemical and Genetic Biomarkers. Ecotoxicol. Environ. Saf. 2020, 205, 111157. [Google Scholar] [CrossRef]
- Bonansea, R.I.; Marino, D.J.G.; Bertrand, L.; Wunderlin, D.A.; Amé, M.V. Tissue-Specific Bioconcentration and Biotransformation of Cypermethrin and Chlorpyrifos in a Native Fish (Jenynsia Multidentata) Exposed to These Insecticides Singly and in Mixtures. Environ. Toxicol. Chem. 2017, 36, 1764–1774. [Google Scholar] [CrossRef]
- Cárcamo, J.G.; Aguilar, M.N.; Barrientos, C.A.; Carreño, C.F.; Quezada, C.A.; Bustos, C.; Manríquez, R.A.; Avendaño-Herrera, R.; Yañez, A.J. Effect of Emamectin Benzoate on Transcriptional Expression of Cytochromes P450 and the Multidrug Transporters (Pgp and MRP1) in Rainbow Trout (Oncorhynchus mykiss) and the Sea Lice Caligus Rogercresseyi. Aquaculture 2011, 321, 207–215. [Google Scholar] [CrossRef]
- Liang, X.; Nie, X.; Ying, G.; An, T.; Li, K. Assessment of Toxic Effects of Triclosan on the Swordtail Fish (Xiphophorus helleri) by a Multi-Biomarker Approach. Chemosphere 2013, 90, 1281–1288. [Google Scholar] [CrossRef]
- Ren, T.; Fu, G.-H.; Liu, T.-F.; Hu, K.; Li, H.-R.; Fang, W.-H.; Yang, X.-L. Toxicity and Accumulation of Zinc Pyrithione in the Liver and Kidneys of Carassius Auratus Gibelio: Association with P-Glycoprotein Expression. Fish Physiol. Biochem. 2017, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Nie, X.; Liu, Y.; Wang, C.; Li, W.; Liu, S. Diclofenac Exposure Alter the Expression of PXR and Its Downstream Target Genes in Mosquito Fish (Gambusia affinis). Sci. Total Environ. 2018, 616–617, 583–593. [Google Scholar] [CrossRef]
- Liang, X.; Wang, L.; Ou, R.; Nie, X.; Yang, Y.; Wang, F.; Li, K. Effects of Norfloxacin on Hepatic Genes Expression of P450 Isoforms (CYP1A and CYP3A), GST and P-Glycoprotein (P-Gp) in Swordtail Fish (Xiphophorus helleri). Ecotoxicology 2015, 24, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Weltje, L.; Simpson, P.; Gross, M.; Crane, M.; Wheeler, J.R. Comparative Acute and Chronic Sensitivity of Fish and Amphibians: A Critical Review of Data. Environ. Toxicol. Chem. 2013, 32, 984–994. [Google Scholar] [CrossRef]
- Wassmur, B.; Gräns, J.; Norström, E.; Wallin, M.; Celander, M.C. Interactions of Pharmaceuticals and Other Xenobiotics on Key Detoxification Mechanisms and Cytoskeleton in Poeciliopsis Lucida Hepatocellular Carcinoma, PLHC-1 Cell Line. Toxicol. In Vitro 2013, 27, 111–120. [Google Scholar] [CrossRef]
- Macêdo, A.K.S.; Santos, K.P.E.d.; Brighenti, L.S.; Windmöller, C.C.; Barbosa, F.A.R.; Ribeiro, R.I.M.d.A.; Santos, H.B.d.; Thomé, R.G. Histological and Molecular Changes in Gill and Liver of Fish (Astyanax Lacustris Lütken, 1875) Exposed to Water from the Doce Basin after the Rupture of a Mining Tailings Dam in Mariana, MG, Brazil. Sci. Total Environ. 2020, 735, 139505. [Google Scholar] [CrossRef]
- Kurth, D.; Brack, W.; Luckenbach, T. Is Chemosensitisation by Environmental Pollutants Ecotoxicologically Relevant? Aquat. Toxicol. 2015, 167, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Pavlichenko, V.; Burkhardt-Medicke, K.; Soares, A.M.V.M.; Altenburger, R.; Barata, C.; Luckenbach, T. Use of a Combined Effect Model Approach for Discriminating between ABCB1- and ABCC1-Type Efflux Activities in Native Bivalve Gill Tissue. Toxicol. Appl. Pharmacol. 2016, 297, 56–67. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Riemer, S.; Kurelec, B.; Smodlaka, N.; Puskaric, S.; Jagic, B.; Müller-Niklas, G.; Queric, N.V. Chemosensitizers of the Multixenobiotic Resistance in Amorphous Aggregates (Marine Snow): Etiology of Mass Killing on the Benthos in the Northern Adriatic? Environ. Toxicol. Pharmacol. 1998, 6, 229–238. [Google Scholar] [CrossRef]
- Smital, T.; Luckenbach, T.; Sauerborn, R.; Hamdoun, A.M.; Vega, R.L.; Epel, D. Emerging Contaminants—Pesticides, PPCPs, Microbial Degradation Products and Natural Substances as Inhibitors of Multixenobiotic Defense in Aquatic Organisms. Mutat. Res. Mol. Mech. Mutagen. 2004, 552, 101–117. [Google Scholar] [CrossRef]
- Wu, B.; Torres-Duarte, C.; Cole, B.J.; Cherr, G.N. Copper Oxide and Zinc Oxide Nanomaterials Act as Inhibitors of Multidrug Resistance Transport in Sea Urchin Embryos: Their Role as Chemosensitizers. Environ. Sci. Technol. 2015, 49, 5760–5770. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Pang, K.S.; Isoherranen, N.; Zhao, P. Dealing with the Complex Drug–Drug Interactions: Towards Mechanistic Models. Biopharm. Drug Dispos. 2015, 36, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Bieczynski, F.; De Anna, J.S.; Pirez, M.; Brena, B.M.; Villanueva, S.S.M.; Luquet, C.M. Cellular Transport of Microcystin-LR in Rainbow Trout (Oncorhynchus mykiss) across the Intestinal Wall: Possible Involvement of Multidrug Resistance-Associated Proteins. Aquat. Toxicol. 2014, 154, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.A.; Saunders, D.M.V.; Al-Mousa, A.; Alcorn, J.; Pereira, A.S.; Martin, J.W.; Giesy, J.P.; Wiseman, S.B. Inhibition of ABC Transport Proteins by Oil Sands Process Affected Water. Aquat. Toxicol. 2016, 170, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Zaja, R.; Terzić, S.; Senta, I.; Lončar, J.; Popović, M.; Ahel, M.; Smital, T. Identification of P-Glycoprotein Inhibitors in Contaminated Freshwater Sediments. Environ. Sci. Technol. 2013, 47, 4813–4821. [Google Scholar] [CrossRef]
- Tan, X.; Yim, S.-Y.; Uppu, P.; Kleinow, K.M. Enhanced Bioaccumulation of Dietary Contaminants in Catfish with Exposure to the Waterborne Surfactant Linear Alkylbenzene Sulfonate. Aquat. Toxicol. 2010, 99, 300–308. [Google Scholar] [CrossRef]
- Keiter, S.; Burkhardt-Medicke, K.; Wellner, P.; Kais, B.; Färber, H.; Skutlarek, D.; Engwall, M.; Braunbeck, T.; Keiter, S.H.; Luckenbach, T. Does Perfluorooctane Sulfonate (PFOS) Act as Chemosensitizer in Zebrafish Embryos? Sci. Total Environ. 2016, 548–549, 317–324. [Google Scholar] [CrossRef]
- Zaja, R.; Caminada, D.; Lončar, J.; Fent, K.; Smital, T. Development and Characterization of P-Glycoprotein 1 (Pgp1, ABCB1)-Mediated Doxorubicin-Resistant PLHC-1 Hepatoma Fish Cell Line. Toxicol. Appl. Pharmacol. 2008, 227, 207–218. [Google Scholar] [CrossRef]
- Tutundjian, R.; Minier, C.; Le Foll, F.; Leboulenger, F. Rhodamine Exclusion Activity in Primary Cultured Turbot (Scophthalmus maximus) Hepatocytes. Mar. Environ. Res. 2002, 54, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Doppenschmitt, S.; Spahn-Langguth, H.; Regårdh, C.G.; Langguth, P. Role of P-glycoprotein-mediated Secretion in Absorptive Drug Permeabiity: An Approach Using Passive Membrane Permeability and Affinity to P-glycoprotein††Dedicated to Prof. B. C. Lippold on the Occasion of His 60th Birthday. J. Pharm. Sci. 1999, 88, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- von Richter, O.; Glavinas, H.; Krajcsi, P.; Liehner, S.; Siewert, B.; Zech, K. A Novel Screening Strategy to Identify ABCB1 Substrates and Inhibitors. Naunyn. Schmiedebergs Arch. Pharmacol. 2009, 379, 11–26. [Google Scholar] [CrossRef]
- Chu, X.; Bleasby, K.; Evers, R. Species Differences in Drug Transporters and Implications for Translating Preclinical Findings to Humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.J. P-Glycoprotein Induction and Its Energetic Costs in Rainbow Trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2021, 47, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Gourley, M.E.; Kennedy, C.J. Energy Allocations to Xenobiotic Transport and Biotransformation Reactions in Rainbow Trout (Oncorhynchus mykiss) during Energy Intake Restriction. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K.; Bush, K.T.; Bhatnagar, V.; Poloyac, S.M.; Momper, J.D. The Systems Biology of Drug Metabolizing Enzymes and Transporters: Relevance to Quantitative Systems Pharmacology. Clin. Pharmacol. Ther. 2020, 108, 40–53. [Google Scholar] [CrossRef] [PubMed]
| Species | Abcb1 | Abcb4 | Abcb5 | References |
|---|---|---|---|---|
| Danio rerio (Zebrafish) | − | + | + | [38] |
| Gadus morhua (Atlantic cod) | − | + | − | [15,38] |
| Gasterosteus aculeatus (Three-spined stickleback) | − | + | − | [15,38] |
| Ictalurus punctatus (Channel catfish) | − | + | + | [15,42] |
| Latimeria chalumnae (African coelacanth) | − | + | + | [38,41] |
| Oncorhynchus mykiss (Rainbow trout) | − | + | + | [41] |
| Oreochromis niloticus (Nile tilapia) | − | + | − | [41] |
| Oryzias latipes (Medaka) | − | + | − | [15,38] |
| Takifugu rubripes (Japanese pufferfish) | + | + | − | [15,38] |
| Tetraodon nigroviridis (Green spotted pufferfish) | + | + | − | [38] |
| Substrate | Species | References |
|---|---|---|
| Hormones | ||
| 19-methyl testosterone | Poeciliopsis lucida | [113] |
| Cortisol | Oncorhynchus mykiss | [114] |
| Octreotide (synthetic somatostatin) | Fundulus heteroclitus | [115] |
| Bacterial Toxins | ||
| Lipopolysaccharides | Danio rerio | [116] |
| Microcystin-LR | Danio rerio | [52] |
| Fluorescent Dyes | ||
| BCECF-AM | Danio rerio | [68] |
| Calcein-AM | Danio rerio, Oncorhynchus mykiss, Poeciliopsis lucida | [38,68,78,117,118,119] |
| DiOC6 | Danio rerio | [68] |
| Rhodamine 123 | Oncorhynchus mykiss, Poeciliopsis lucida | [54,78,113,114,117,119,120] |
| Rhodamine B | Hatcheria macraei, Salmo trutta, Oncorhynchus mykiss, Oncorhynchus tshawytscha, Danio rerio, Cyprinus carpio | [38,45,67,121] |
| Metal Compounds | ||
| Arsenic trioxide (As2O3) | Poeciliopsis lucida | [113] |
| Cadmium chloride (CdCl2) | Danio rerio | [122] |
| Silver nitrate (AgNO3) | Danio rerio | [122] |
| Polycyclic Aromatic Hydrocarbons | ||
| 2-aminoanthracene | Leuciscus idus melanotus | [30] |
| Benzo[a]pyrene | Cyprinus carpio | [123] |
| Phenanthrene | Danio rerio | [38] |
| Fragrance Ingredients | ||
| α-amylcinnamaldehyde | Danio rerio | [124] |
| α-hexylcinnamaldehyde | Danio rerio | [124] |
| Galaxolide | Danio rerio | [38] |
| Isoeugenol | Danio rerio | [124] |
| Musk xylene | Danio rerio | [124] |
| Nerol | Danio rerio | [124] |
| Tonalide | Danio rerio | [38] |
| Pesticides | ||
| Chlorpyrifos | Poeciliopsis lucida | [113] |
| Diazinon | Poeciliopsis lucida | [113] |
| Emamectin benzoate | Oncorhynchus mykiss | [84] |
| Methyl parathion | Danio rerio | [125] |
| Phosalone | Poeciliopsis lucida | [113] |
| Hydrophobic Peptide | ||
| Reversin 205 | Poeciliopsis lucida | [113] |
| Immunosuppressants | ||
| Cyclosporin A | Fundulus heteroclitus, Squalus acanthias | [71,97,126] |
| Rapamycin | Fundulus heteroclitus | [61] |
| Psychoactive Pharmaceuticals | ||
| Bupropion | Oncorhynchus mykiss | [127] |
| Citalopram | Oncorhynchus mykiss | [127] |
| Clozapine | Oncorhynchus mykiss | [127] |
| Fluoxetine | Danio rerio | [124] |
| Venlafaxine | Oncorhynchus mykiss | [127] |
| Analgesic Pharmaceuticals | ||
| Colchicine | Poeciliopsis lucida | [113] |
| Diclofenac | Danio rerio | [124] |
| Antiarrhythmics | ||
| Acebutolol | Poeciliopsis lucida | [117] |
| Diltiazem | Poeciliopsis lucida | [113] |
| Nicardipine | Poeciliopsis lucida | [113] |
| Prazosin | Poeciliopsis lucida | [113] |
| Propanolol | Oncorhynchus mykiss, Poeciliopsis lucida | [113,128] |
| Quinidine | Oncorhynchus mykiss, Poeciliopsis lucida | [113,114] |
| Verapamil | Danio rerio, Poeciliopsis lucida, Fundulus heteroclitus, Squalus acanthias, Oncorhynchus mykiss | [38,71,78,113] |
| Antiretroviral | ||
| Saquinavir | Fundulus heteroclitus | [129] |
| Lipid-Lowering Pharmaceuticals | ||
| Atorvastatin | Poeciliopsis lucida | [117] |
| Pravastatin | Poeciliopsis lucida | [113] |
| Simvastatin | Danio rerio | [124] |
| Antiparasitic | ||
| Ivermectin | Danio rerio, Oncorhynchus mykiss, Fundulus heteroclitus | [62,82,83,84] |
| Phosphodiesterase Inhibitor | ||
| Sildenafil | Poeciliopsis lucida | [113] |
| Anticancer Pharmaceuticals | ||
| AT9283 | Danio rerio | [69] |
| Bisantrene | Danio rerio | [69] |
| Daunomycin (Daunorubicin) | Fundulus heteroclitus, Pleuronectes americanus | [58,130] |
| Doxorubicin | Oncorhynchus mykiss, Poeciliopsis lucida, Danio rerio | [54,56,69,117] |
| Etoposide | Danio rerio | [69] |
| KW-2478 | Danio rerio | [69] |
| Mitoxantrone | Danio rerio | [69] |
| Paclitaxel | Danio rerio | [69] |
| Romidepsin | Danio rerio | [69] |
| Sepantronium bromide (YM-155) | Danio rerio | [69] |
| Tanespimycin (17-AAG) | Danio rerio | [69] |
| Tozasertib (VX-680) | Danio rerio | [69] |
| Vinblastine | Danio rerio, Ictalurus punctatus | [38,69,73] |
| Vincristine | Danio rerio | [38] |
| Vinorelbine | Oncorhynchus mykiss | [57] |
| Expression Inducer | Species | Tissue | References |
|---|---|---|---|
| Bile Acids and Salts | |||
| 5α-cyprinol 27-sulfate | Danio rerio | Liver | [105] |
| Lithocholic acid | Oncorhynchus mykiss | Liver | [146] |
| Enzyme Cofactor | |||
| Lipoic acid | Jenynsia multidentata | Liver | [147] |
| Hormone | |||
| Pregnenolone 16α-carbonitrile (Synthetic steroid) | Oncorhynchus mykiss | Liver | [146] |
| Vitamin | |||
| Vitamin B12 | Danio rerio | Liver | [94] |
| Cyanotoxin | |||
| Microcystin-LR | Danio rerio | Embryo | [52] |
| Microcystin-LR | Jenynsia multidentata | Liver, gill, brain | [74] |
| Metal Compounds | |||
| Arsenic trioxide (As2O3) | Poeciliopsis lucida | Liver | [148] |
| Cadmium chloride (CdCl2) | Trematomus bernacchii | Liver | [48] |
| Cadmium chloride (CdCl2) | Danio rerio | Embryo | [122] |
| Silver nitrate (AgNO3) | Danio rerio | Embryo | [122] |
| Phytochemicals | |||
| Glycyrrhizic acid | Ictalurus punctatus | Kidney | [142] |
| Quercetin | Ictalurus punctatus | Kidney | [142] |
| Schisandrin A | Ictalurus punctatus | Kidney | [142] |
| Schisandrin B | Ictalurus punctatus | Kidney | [142] |
| Schisandrol A | Ictalurus punctatus | Kidney | [142] |
| schisandrol B | Ictalurus punctatus | Kidney | [142] |
| Industrial Chemicals | |||
| 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) | Dicentrarchus labrax | Liver | [149] |
| Crude oil (Exxon Valdez) | Anoplarchus purpurescens | Liver | [27] |
| Heavy fuel oil (fresh) | Chelon labrosus | Liver | [65] |
| Perfluorooctane sulfonate (PFOS) | Chelon labrosus | Liver | [65] |
| Pesticides | |||
| Carbendazim | Jenynsia multidentata | Gill | [150] |
| Chlorpyrifos | Jenynsia multidentata | Liver | [151] |
| Emamectin benzoate | Oncorhynchus mykiss | Liver | [152] |
| Malathion | Danio rerio | Liver, brain | [94] |
| Rotenone | Ictalurus punctaus | Kidney | [142] |
| Triclosan | Xiphophorus helleri (males) | Liver | [153] |
| Zinc pyrithione | Carassius auratus gibelio | Liver, kidney | [154] |
| Analgesic Pharmaceuticals | |||
| Diclofenac | Gambusia affinis | Liver | [155] |
| Paracetamol (Acetaminophen) | Mugilogobius chulae | Liver | [95] |
| Antibiotic | |||
| Norfloxacin | Xiphophorus Helleri | Liver | [156] |
| Antifungal Pharmaceutical | |||
| Clotrimazole | Oncorhynchus mykiss | Intestine, brain | [17] |
| Lipid-Lowering Pharmaceutical | |||
| Simvastatin | Mugilogobius abei | Liver | [143] |
| Simvastatin | Gambusia affinis | Liver | [92] |
| Inhibitor | Species | References |
|---|---|---|
| Fluorescent Dye | ||
| Hoechst 3334 | Poeciliopsis lucida | [113] |
| Cyanotoxin | ||
| Microcystin-LR | Oncorhynchus mykiss | [166] |
| Metal Compounds | ||
| Arsenic trioxide (As2O3) | Poeciliopsis lucida | [148] |
| Cadmium chloride (CdCl2) | Poeciliopsis lucida | [148] |
| Mercury (II) chloride (HgCl2) | Poeciliopsis lucida | [148] |
| Orthovanadate (VO4) | Poeciliopsis lucida | [113] |
| Potassium dichromate (K2Cr2O7) | Poeciliopsis lucida | [148] |
| Hormone | ||
| Octreotide (Synthetic somatostatin) | Fundulus heteroclitus | [115] |
| Bile Acid | ||
| Taurochenodeoxycholate | Poeciliopsis lucida | [113] |
| Fragrance Ingredients | ||
| Galaxolide | Danio rerio | [38] |
| α-hexylcinnamaldehyde | Danio rerio | [124] |
| Isoeugenol | Danio rerio | [124] |
| Musk ketone | Danio rerio | [124] |
| Musk xylene | Danio rerio | [124] |
| Nerol | Danio rerio | [124] |
| Tonalide | Danio rerio | [38,67] |
| Polycyclic Aromatic Hydrocarbon | ||
| Phenanthrene | Danio rerio | [38] |
| Industrial Chemicals | ||
| Oil-Sands Process-Affected Water (fresh) | Oryzias latipes | [167] |
| Polychlorinated Biphenyls (PCBs) | Thunnus albacares | [43] |
| Surfactants | ||
| Alcohol polyethoxylates | Poeciliopsis lucida | [168] |
| C-12 linear alkylbenzene sulfonate | Ictalurus punctatus | [169] |
| Nonylphenol diethoxylate | Oncorhynchus mykiss | [77] |
| Perfluorooctane sulfonate (PFOS) | Danio rerio | [170] |
| Polypropylene glycols | Poeciliopsis lucida | [168] |
| Flame Retardants | ||
| Polybrominated diphenyl ethers (BDEs) | Thunnus albacares | [43] |
| Pesticides | ||
| Azinphos-methyl | Danio rerio | [67] |
| Diazinon | Poeciliopsis lucida | [113] |
| Dichlorodiphenyldichloroethane (DDD) | Thunnus albacares | [43] |
| Dichlorodiphenyldichloroethylene (DDE) | Thunnus albacares, Poeciliopsis lucida | [43,113] |
| Dichlorodiphenyltrichloroethane (DDT) | Thunnus albacares | [43] |
| Dieldrin | Thunnus albacares | [43] |
| 2,4-dinitrophenol | Fundulus heteroclitus | [59] |
| Emamectin benzoate | Oncorhynchus mykiss | [84] |
| Endosulfan | Poeciliopsis lucida | [113] |
| Endrin | Thunnus albacares | [43] |
| Metazachlor | Danio rerio | [67] |
| Mirex | Thunnus albacares | [43] |
| Phosalone | Poeciliopsis lucida | [113] |
| Prochloraz | Oncorhynchus mykiss | [77] |
| Terbuthylazine | Danio rerio | [67] |
| Hydrophobic Peptide | ||
| Reversin 205 | Oncorhynchus mykiss, Poeciliopsis lucida | [41,113,118,119] |
| Anticoagulant | ||
| Dipyridamole | Danio rerio | [67] |
| Antimalarial | ||
| Quinine | Fundulus heteroclitus | [59] |
| Antiparasitic | ||
| Ivermectin | Danio rerio, Oncorhynchus mykiss, Fundulus heteroclitus | [62,67,84] |
| Leukotriene Antagonist | ||
| MK571 | Danio rerio, Poeciliopsis lucida | [38,100,142] |
| Antihistamine | ||
| Cimetidine | Oncorhynchus mykiss | [128] |
| Antibiotics | ||
| Erythromycin | Danio rerio, Poeciliopsis lucida | [97,113] |
| Troleandomycin | Poeciliopsis lucida | [158] |
| Analgesic Pharmaceutical | ||
| Diclofenac | Danio rerio, Poeciliopsis lucida | [124,158] |
| Antifungal Pharmaceutical | ||
| Clotrimazole | Oncorhynchus mykiss | [75] |
| Anti-Inflammatories | ||
| Indomethacin | Poeciliopsis lucida | [119] |
| Sulfasalazine | Poeciliopsis lucida | [113] |
| Antihypertensives | ||
| Furosemide | Poeciliopsis lucida | [117] |
| Reserpine | Poeciliopsis lucida | [113] |
| Antihyperuricemic | ||
| Probenecid | Poeciliopsis lucida | [119] |
| Phosphodiesterase Inhibitor | ||
| Sildenafil | Poeciliopsis lucida | [113,117] |
| Antiretrovirals | ||
| Ritonavir | Fundulus heteroclitus | [129] |
| Saquinavir | Fundulus heteroclitus | [129] |
| Lipid-Lowering Pharmaceuticals | ||
| Atorvastatin | Poeciliopsis lucida | [113,117] |
| Gemfibrozil | Poeciliopsis lucida | [117] |
| Pravastatin | Poeciliopsis lucida | [117] |
| Simvastatin | Poeciliopsis lucida | [117] |
| Psychoactive Pharmaceuticals | ||
| Carbamazepine | Danio rerio | [67] |
| Fluoxetine | Danio rerio | [124] |
| Sertraline | Danio rerio | [124] |
| Trifluoperazine | Poeciliopsis lucida | [113] |
| Immunosuppressants | ||
| Cyclosporin A | Danio rerio, Oncorhynchus mykiss, Thunnus albacares, Poeciliopsis lucida, Cyprinus carpio, Fundulus heteroclitus, Pleuronectes americanus | [38,41,43,58,61,67,68,77,113,114,115,117,118,119,121,128,130,148,168,171] |
| Cyclosporin G | Fundulus heteroclitus | [59] |
| Rapamycin | Fundulus heteroclitus | [61] |
| Tacrolimus (FK506) | Fundulus heteroclitus | [61] |
| Antiarrhythmics | ||
| Diltiazem | Poeciliopsis lucida | [113] |
| Nicardipine | Poeciliopsis lucida | [113] |
| Prazosin | Poeciliopsis lucida | [113] |
| Propranolol | Poeciliopsis lucida | [113] |
| Quinidine | Oncorhynchus mykiss, Poeciliopsis lucida | [113,114,128] |
| Verapamil | Danio rerio, Oncorhynchus mykiss, Thunnus albacares, Hatcheria macraei, Salmo trutta, Oncorhynchus tshawytscha, Poeciliopsis lucida, Scophthalmus maximus, Cyprinus carpio, Ictalurus punctatus, Fundulus heteroclitus, Pleuronectes americanus | [38,43,45,54,59,61,62,67,69,73,77,78,97,113,114,115,119,121,124,130,172] |
| Anti-Cancer Pharmaceuticals | ||
| Doxorubicin | Oncorhynchus mykiss | [54] |
| Elacridar | Danio rerio | [69] |
| Fluorouracil | Poeciliopsis lucida | [113] |
| Mitoxantrone | Poeciliopsis lucida | [113] |
| Tamoxifen | Poeciliopsis lucida | [113] |
| Tariquidar (XR9576) | Danio rerio, Oncorhynchus mykiss | [56,69] |
| Valspodar (PSC833) | Oncorhynchus mykiss, Thunnus albacares, Danio rerio, Poeciliopsis lucida, Fundulus heteroclitus, Squalus acanthias | [38,43,61,62,68,69,71,113,114,115,118,171] |
| Vinblastine | Danio rerio, Oncorhynchus mykiss, Fundulus heteroclitus, Pleuronectes americanus | [38,54,59,68,77,130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, C.U.; Kennedy, C.J. A Review of P-Glycoprotein Function and Regulation in Fish. Fishes 2024, 9, 51. https://doi.org/10.3390/fishes9020051
Johnston CU, Kennedy CJ. A Review of P-Glycoprotein Function and Regulation in Fish. Fishes. 2024; 9(2):51. https://doi.org/10.3390/fishes9020051
Chicago/Turabian StyleJohnston, Christina U., and Christopher J. Kennedy. 2024. "A Review of P-Glycoprotein Function and Regulation in Fish" Fishes 9, no. 2: 51. https://doi.org/10.3390/fishes9020051
APA StyleJohnston, C. U., & Kennedy, C. J. (2024). A Review of P-Glycoprotein Function and Regulation in Fish. Fishes, 9(2), 51. https://doi.org/10.3390/fishes9020051







