Abstract
The aquaculture industry in Chile, as in the rest of the world, has rapidly grown, becoming a crucial economic sector. However, diseases pose a major threat, causing significant economic losses and environmental impacts. Various antimicrobials, particularly Oxytetracycline and Florfenicol, are used to combat these diseases, which has boosted production and mitigated economic losses. However, excessive antibiotic use has led to pathogen resistance, necessitating higher doses. This overuse can cause side effects in fish, including liver damage and immunosuppression. This study aimed to determine the impact of multiple doses of florfenicol and oxytetracycline on the SHK-11 cell line of Salmo salar by analyzing the expression of genes related to innate immunity and oxidative stress by qRT-PCR in addition to the quantification of immune system proteins via dot blot. The experimental treatments were the following: cells were stimulated with different concentrations of oxytetracycline (0.25, 0.5, and 1.5 µg/mL) and florfenicol (1, 10, and 20 µg/mL) for time kinetics of 0.5, 1, 3, 6, 12, 24, and 48 h. For both cases, controls consisting of cells without antibiotics were included. The expression of the immune system genes was mostly inhibited compared to the control. However, it was observed that TLR-1 and MyD88 present a joint activation pattern at different times and concentrations for both antibiotics. Regarding the expression of CAT and GPx, transcripts were increased in the early stages of stimulation with oxytetracycline and florfenicol, followed by a subsequent decrease in gene expression. This study provides relevant information to understand the effect of antibiotics at the cellular level in one of the most important species for global aquaculture, the Atlantic salmon.
Key Contribution:
The antibiotics are affecting the fish, modifying the immune system and oxidative stress response.
1. Introduction
Aquaculture has been one of the fastest-growing and economically important activities in the world in recent years [1], bringing economic and social growth. However, with intensive production, the three main species of salmonids (salmon and trout) are farmed in Chile: Atlantic salmon (Salmo salar), by far the most farmed; Pacific salmon or coho salmon (Oncorhynchus kisutch); and rainbow trout (Oncorhynchus mykiss). These fish are affected by multiple factors, ranging from environmental factors to diseases [2,3,4]. In Chile, disease is one of the main problems related to aquaculture activity [4]. This is why diseases pose a significant risk to aquaculture, as they appear at various stages of fish farming and are a leading cause of considerable economic losses. Furthermore, the dense populations within culture cages facilitate the rapid spread of infectious outbreaks caused by harmful microorganisms. The use of antimicrobials has made it possible to increase production considerably, thus avoiding economic losses [5]. These compounds are usually administered through food to both sick and healthy animals [6,7].
The pharmaceutical industry has biologically active chemical compounds that can inactivate and/or eliminate pathogenic microorganisms to keep disease-causing pathogens under control [8,9]. These encompass bactericides, which are characterized by causing permanent harm to bacteria, leading to their demise. Conversely, bacteriostatics do not kill the bacteria but inhibit their growth or metabolism, eventually causing them to die without reproducing. The leading causes of mortality in salmonids are different bacteria such as Piscirickettsia salmonis (‘SRS’), Renibacterium salmoninarum (BKD), and Tenacibaculum dicentrarchi (Tenacibaculosis). However, the latter has been considered emerging since 2018 [4]. Pathogens are mainly dealt with using ‘oxytetracycline (OTC)’ and ‘florfenicol (FLO)’ [4]. Both antibiotics are broad-spectrum with bacteriostatic action. OTC is a compound that inhibits protein synthesis in the 30S subunit of the bacterial ribosome, and FLO inhibits protein synthesis in the 50S subunit of the bacteria, preventing their replication and then their death [10,11,12].
The use of antibiotics in Chile is quite elevated, with Chilean farms using a total of 463.4 tons of antimicrobials, and an annual consumption of 0.47 kg of antimicrobials per ton of harvested salmon [13], resulting in bacteria that are becoming more resistant. Bacteria possess various resistance mechanisms, such as acquired resistance through mutations, acquiring mobile genetic elements (plasmids or transposons), or producing enzymes that hydrolyze and inactivate the antibiotic, among other intracellular and extracellular mechanisms [14,15,16].
Overusing antibiotics may lead to side effects like liver damage and a weakened immune response. According to Nakano et al. [17], excessive doses of OTC influence stress-related biomarkers and redox status in coho salmon, causing tissue damage, especially to the liver. Similarly, Rodrigues [18] considers that OTC can cause a potential health risk to aquatic organisms, reporting that OTC can interfere with biochemical pathways in Oncorhynchus mykiss, causing oxidative effects in tissues and, in turn, generating genotoxicity as an indirect result of oxidative stress.
Immunosuppression will affect the activation cascade of the innate immune system at the cellular level, which is carried out by a few PRRs (Pattern Recognition Receptors) that recognize the PAMPs (Pathogen-Associated Molecular Patterns) of invading microbes. The recognition and binding of PAMPs by specific TLRs (Toll-like receptors) activate cell signaling cascades through MyD88 (primary myeloid differentiation response 88)-dependent and MyD88-independent pathways. This MyD88 binds to the TIR domain of the receptor, is recruited, and activates cytoplasmic kinases, which leads to the activation of several cytosolic enzymes to induce the production of proinflammatory cytokines such as IFN, IL1β, IL6, IL12, and TNFα [19,20].
According to Nakano et al. [17], liver damage will affect oxidative processes, such as inducing oxidative stress as a result of a cyclic oxidation-reduction process (redox cycle) [21]. Antioxidants come in ‘enzymatic’ and ‘non-enzymatic’ forms. Among the primary enzymatic antioxidants are the enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (Gpx); however, another series of specialized enzymes with indirect antioxidant functions must be taken into account, such as glutathione reductase (GR), whose function is to regenerate glutathione to its reduced form (GSH), and glutathione-S-transferase (GST), which is involved in the transport and removal of reactive components and the transport system of conjugates with glutathione [22,23].
Different research models encompass fish used in aquaculture, ornamental fish, and, though less frequently, wild fish. Certain species, like salmonids, exhibit significant adaptability. Recently, the principles of ‘animal welfare’ and the 3Rs rule have aimed to enhance procedures. This optimization can be achieved through primary cell cultures or cell cultures, such as Atlantic salmon head kidney cells (SHK-1), which are highly effective for obtaining direct cellular responses [24,25].
Because of this, we set the following objective: ‘to determine the innate immune and oxidative response of SHK-1 cells when challenged with different concentrations of OTC and FLO’. To monitor these responses, we will use qRT-PCR determinations of TLR1, MyD88, IFNγ and NFκb, catalase (CAT), and glutathione peroxidase (GPx) genes and the quantification of NFκb proteins by dot blot.
2. Materials and Methods
The cells were cultured at 18 °C in T-25 flasks (Corning, Sigma-Aldrich, Burlington, MA, USA) in Leibovitz L-15 medium supplemented with 5% fetal bovine serum (FBS) (Invitrogen, Waltham, MA, USA). The confluent flasks were subcultured by distributing the medium uniformly between them and adding fresh medium to complete the volume, following the Pontigo and Vargas-Chacoff and Nualart et al. protocol [24,25].
The experimental stimuli were carried out at 0.5, 1, 3, 6, 12, 24, and 48 h and with 0.25, 0.5, and 1.5 µg/mL of OTC or 1, 10, and 20 µg/mL of FLO in supplemented L-15 medium. Phosphate-buffered saline (PBS) alone was used as a control. The OTC doses chosen are similar to those described by Tafalla et al. [26], and the FLO doses chosen are described by Martinsen et al. [27], following which the doses used were calculated from the pharmacokinetic study of OTC and FLO in Atlantic salmon in seawater using as a reference plasma levels of antibiotics [14,27]. The experiment was assayed in duplicates and by two technical replicates for RT-qPCR analyses.
2.1. Gene Expression Analyses
RNA was isolated from cell pellets using TRIzol reagent (Invitrogen) according to the manufacturer’s guidelines and then processed with amplification grade DNase I (1 U/μg RNA, Invitrogen). SuperScript III RNase H Reverse Transcriptase (Invitrogen) synthesized first-strand cDNA with oligo-dT18 primers and 1 μg of total RNA at 50 °C for 50 min. Experiments were conducted with an AriaMx Real-Time PCR System. For qRT-PCR analyses, cDNA diluted to 100 ng served as the template, and the Brilliant SYBR® Green qPCR Master Mix (Stratagene, La Jolla, CA, USA) was utilized. Primers were designed for TLR1, MyD88, INFδ, NFκb, catalase (CAT), glutathione peroxidase (GPx), and 18S (housekeeping gene) (Table 1). All reactions were performed in triplicate and in a total volume of 14 μL, which contained 6 μL SYBR® Green, 2 μL cDNA template, 1.08 μL of each primer, and 4.92 μL of PCR-grade water. The applied PCR protocol was as follows: 95 °C for 10 min, then 40 cycles at 95 °C for 15 s, 60 °C for 1 min, and lastly 95 °C for 15 s. After each PCR, a melting curve analysis of the amplification products was conducted to verify the presence and amplification of only one product. TLR1, MyD88, IFNγ, NFκb, catalase (CAT), and glutathione peroxidase (GPx) were analyzed using the comparative Ct (∆∆Ct) method [28]. PCR products were visualized on a 2% agarose gel, purified using the E.Z.N.A Gel Extraction Kit (Omega Biotek, Norcross, GA, USA), and sequenced by Macrogen Inc (Seoul, Republic of Korea). The primers’ efficiency was checked between 100 and 103% (Table 1).

Table 1.
Primer sequences for expression analysis.
2.2. Immunodetection of NFκb in SHK-1 Cells (Dot Blot for NFκb)
Total protein extraction was performed from SHK-1 cells and subsequently quantified using the PierceTM BCA protein assay kit (Thermo Scientific, Waltham, MA, USA). NFκb was immunodetected using the dot blot technique, loading 30 µg of total protein per spot. The membrane was blocked with 6% albumin and incubated with primary anti-NFκb (Santa Cruz, Starr County, TX, USA) and anti-Actin (Cell Signaling, Danvers, MA, USA) antibodies. It was then incubated with a secondary anti-mouse antibody (Cell Signaling) coupled to the HRP enzyme for its subsequent detection. Images were captured on the G: BOX unit using Genesys Software 4.3.8.0 (SynGene, model Chemi XRQ) and quantified with ImageJ Software 1.45.
2.3. Statistical Analyses
All data are expressed as the mean ± standard error of the mean (S.E.M.). PCR efficiency was assessed through linear regression analysis of sample data using LinRegPCR. Gene expression was evaluated by conducting a two-way ANOVA, with various stimuli and time serving as factors for variance. The data were previously checked for normality and homoscedasticity. Tukey’s HSD test was carried out after ANOVA as a posterior analysis test.
3. Results
3.1. Oxytetracycline (OTC)
The expression of innate immune genes like TLR-1 was highest at 6 and 12 h at 1.5 µg/mL and 1.0 µg/mL after 3 h of exposure, compared to the control group (Figure 1A). MyD88 gene expression was highest at 0.5 and 6.0 h at 1.5 µg/mL, and at 3 h at 0.25 and 0.5 µg/mL. During the last hours of the experiment, 24 and 48 h, the expression was downregulated (Figure 1B). Meanwhile, INFγ presented upregulation at 12 h in 0.25 µg/mL (Figure 1C). NFκb gene expression showed upregulation at 6 h with 1.5 µg/mL (Figure 1D).
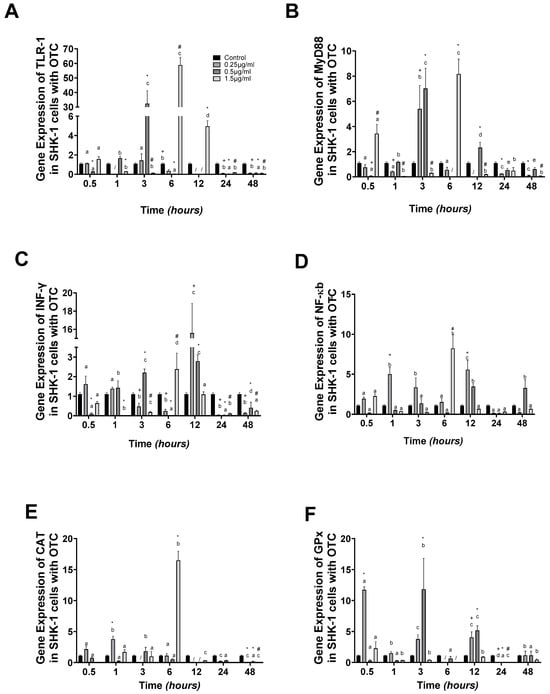
Figure 1.
SHK-1 cells treated with oxytetracycline: gene expression. (A) TLR-1, (B) MyD88, (C) INFγ, (D) NFκb, (E) CAT, and (F) GPx in SHK-1 cells treated with oxytetracycline (OTC). The relative expression of genes was calculated by the comparative Ct method (2−ΔΔCT) using the 18s ribosomal protein as the internal reference gene. Each value represents the mean ± S.E.M. (n = 3). Different letters indicate statistical differences within the same treatment between time points. Symbols (*, /, #, and +) over the bars indicate statistical differences among treatments (control and different doses of antibiotic) at the same time. Two-way ANOVA followed by Tukey’s test (p < 0.05).
Our research thoroughly investigated the oxidative stress response, as determined by the gene expression of catalase (CAT). We found that CAT was upregulated at 6 h in 1.5 µg/mL, almost 9-fold compared to the control group. After this time, the expression was downregulated at 12, 24, and 48 h (Figure 1E). In the case of the GPx transcript, upregulation was observed at 0.5 and 3 h in 0.25 and 0.5 µg/mL, respectively (Figure 1F).
The dot blot results of NFκb proteins provide further evidence of the reliability of our data. Compared to the control group, protein concentrations decreased at 1, 6, and 48 h (Figure 2A,B).
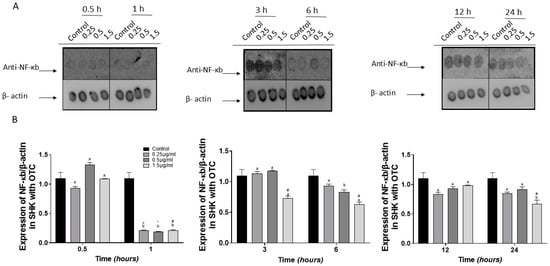
Figure 2.
Immunodetection of NFkb in SHK-1 cells treated with oxytetracycline (OTC). (A) a representative image of the dot blot assay and (B) its respective graph. The quantification of expression of NFκb used b-actin as the internal reference control. Each value represents the mean ± S.E.M. (n = 3). Different letters indicate statistical differences within the same treatment between time points. Symbols (*, #, and +) over the bars indicate statistical differences among treatments (control and different doses of antibiotic) at the same time. Two-way ANOVA followed by Tukey’s test (p < 0.05).
3.2. Florfenicol (FLO)
The gene expression results of TLR-1 indicated that it was in line with OTC concentration, with the highest gene expression at 20 µg/mL at 0.5 h. At 1 h, it presented the highest levels of expression at 1 and 10 µg/mL, whereas at 20 µg/mL, it was similar to the control group. At 12, 24, and 48 h, the experimental groups presented downregulation compared to the control group (Figure 3A). MyD88 gene expression was highest at 0.5 and 12 h at 20 µg/mL and 1 µg/mL, respectively (Figure 3B). Meanwhile, INFγ showed upregulation at 12 and 48 h at 10 µg/mL (Figure 3C). The NFκb gene expression presented upregulation at 0.5 h at 10 and 20 µg/mL, but after 3 h of experimentation, the NFκb gene expression was downregulated (Figure 3D).
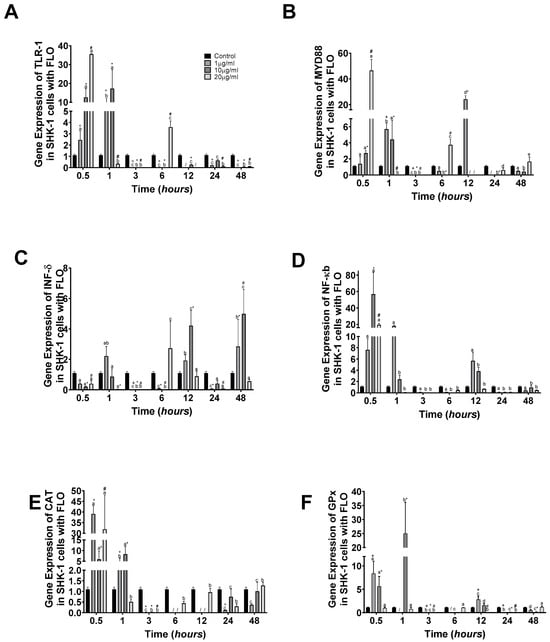
Figure 3.
SHK-1 cells treated with florfenicol: gene expression. (A) TLR-1, (B) MyD88, (C) INFγ, (D) NFκb, (E) CAT, and (F) GPx in SHK-1 cells treated with florfenicol (FLO). the relative expression of genes was calculated by the comparative Ct method (2−ΔΔCT) using the 18s ribosomal protein as the internal reference gene. Each value represents the mean ± S.E.M. (n = 3). Different letters indicate statistical differences within the same treatment between time points. Symbols (*, /, #, and +) over the bars indicate statistical differences among treatments (control and different doses of antibiotic) at the same time. Two-way ANOVA followed by Tukey’s test (p < 0.05).
The oxidative stress response was determined by CAT gene expression, which was upregulated at 0.5 h at 1 and 20 µg/mL almost 40-fold compared to the control group. After this time, at 1 h at 1 and 10 µg/mL, it showed upregulation, but 4-fold compared to the control group (Figure 3E). Meanwhile, GPx gene expression showed upregulation at 0.5 and 1 h at 1 and 10, and again at 10 µg/mL, respectively (Figure 3F).
The dot blot results of NFκb proteins show that at 1, 6, and 12 h, protein concentrations increased compared to the control group, but at different FLO concentrations (Figure 4A,B).
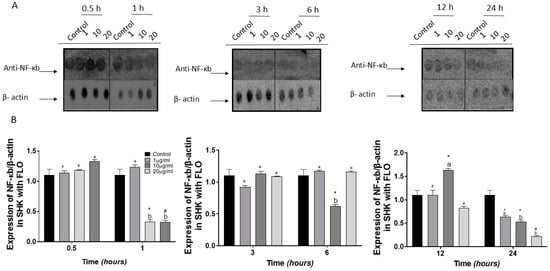
Figure 4.
Immunodetection of NFkb in SHK-1 cells treated with florfenicol (FLO). (A) a representative image of the dot blot assay and (B) its respective graph. The quantification of expression of NFκb used b-actin as the internal reference control. Each value represents the mean ± S.E.M. (n = 3). Different letters indicate statistical differences within the same treatment between time points. Symbols (*, #, and +) over the bars indicate statistical differences among treatments (control and different doses of antibiotic) at the same time. Two-way ANOVA followed by Tukey’s test (p < 0.05).
4. Discussion
Antibiotics affect bacterial diseases, but their use and abuse modify resistance and alter the environment [9], influencing fish physiological responses, as Nakano et al. [17] indicated. OTC and FLO have therapeutic applications in Atlantic salmon culture, and their effects on the fish’s different immune and oxidative stress parameters must be elucidated. Here, we studied the innate immune system and oxidative stress response. TLRs are type I transmembrane proteins with numerous LRRs (leucine-rich repeats) in their extracellular domain. They possess an intracellular signaling domain known as TIR (toll/interleukin-1 receptor), expected to both Toll and IL-1 receptors. This TIR domain can bind to and activate molecules such as MyD88 (myeloid differentiation factor 88) [31,32], while catalase and GPx are part of the detoxification process.
In our study, the OTC and FLO have different effects on the innate immune system; the OTC has a chained effect between 3 and 12 hours, where there was an increase in TLR1 (which is a broad-spectrum receptor), then MyD88, to then activate NFκb, which will then translocate to the nucleus to activate IFNγ, generating a “false positive” response because OTC is not a pathogen but can act as a PAMP. On the other hand, FLO has two responses: the first is the activation of TLR1, MyD88, and NFκb during the first minutes (0.5 to 1 h), while IFNγ increases its expression at 6 h of the experiment. Unlike OTC, FLO may be affected by an epigenetic modification from the binding to regimens that promote genes associated with the immune response with IFNγ, which stops immediate signaling (as in the case of OTC) to the nucleus through IFNγ, taking more than five hours of difference [33,34,35,36].
Rijker et al. [37] described that OTC dramatically suppresses the immune system in carp (Cyprinus carpio). Also, Guardiola et al. [38] found that the dietary intake of OTC leads to suppressed humoral and activated cellular innate immune responses in gilthead seabream (Sparus aurata). This also causes immune-related genes to be upregulated or downregulated, depending on the OTC concentration in the diet. The results of Guardiola et al. [38] are consistent with our results for both antibiotics, OTC and FLO, which depend on concentration and time (TLR1-MyD88-IFNγ and NFκb). On the contrary, Lundén et al. [39] showed that florfenicol does not significantly influence the immune parameters in rainbow trout, while Tafalla et al. [26] observed the head kidney macrophage respiratory burst and phagocytosis were inhibited by the in vitro treatment in Scophthalmus maximus. They were dose-dependent on OTC; our dot blots of NFκb show similar results, where dose-dependent OTC and FLO inhibited protein production.
The innate immune system drives the activation of different cellular components such as leukocytes, phagocytes, macrophages, or nets, among others; if a false immune response is promoted against something that is not pathogenic or a PAMP, it causes these to promote the activation of oxidative stress, increasing reactive oxygen species, “ROS”. In our study, as in the immune system, there are two responses according to the antibiotic, with OTC generating an increase in catalase at 6 h, while GPx had three peaks. This indicates the formation of peroxide that will be eliminated as water and oxygen. At the same time, GPx catalyzes the reduction of hydrogen peroxide (H2O2) or lipoperoxide (L-OOH), using reduced glutathione (GSH) as a reducing agent. Regarding FLO, like the immune system, it presented its highest expression at 0.5 and 1 h of treatment, indicating that both catalase and GPx are aligned with the immune system, helping to eliminate excess peroxide [40].
Antibiotics have been reported to activate the production of reactive oxygen species (ROS) and cause oxidative damage [41,42,43]. Caipang et al. [44] presented a differential transcription of the oxidative stress-related gene, catalase, in the blood during the oral administration of antibiotics in cod (Gadus morhua). They showed a significant upregulation of this gene in the oxolinic-fed group, while a downregulation of gene expression was observed in the florfenicol-fed group. There are two potential responses: “oxolinic acid can penetrate cells and tissue and produce oxidative stress” or “type of metabolites that are produced by each antibiotic that could result in downregulation of antioxidant defense in the fish” [45]. Meanwhile, other studies have demonstrated that the oral administration of OTC induces the production of ROS (reactive oxygen species) and DNA damage in different fish species.
These fish species include rainbow trout and silver catfish, Rhamdia quelen [18,46,47,48]. Our results show two responses: very high at the initial experiment with upregulation, but at the end of the experiment, there was downregulation, indicating the incapacity to eliminate the antibiotics or metabolites, as was mentioned before.
5. Conclusions
Our results indicate that both antibiotics affect the innate response from transcriptional expression until protein production. Meanwhile, the oxidative stress markers presented two responses, “upregulation” and “downregulation”, at the beginning and end of the experiment, with dose-dependency and time as change factors. More studies are needed to clarify the effect of antibiotics on physiological response, achieve a holistic view, and improve tools in aquaculture handling.
Author Contributions
Conceptualization, J.L.M. and L.V.-C.; methodology, D.F., D.N. and L.V.-C.; investigation D.F., D.N., J.L.M. and L.V.-C.; resources, L.V.-C. and J.L.M.; data curation, D.F. and D.N.; writing—original draft preparation D.F., D.N., J.L.M. and L.V.-C.; writing—review and editing, D.F., D.N., J.L.M. and L.V.-C.; visualization D.F., D.N., J.L.M. and L.V.-C.; supervision, L.V.-C.; project administration, J.L.M. and L.V.-C.; funding acquisition, J.L.M. and L.V.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fondecyt Regular (1180957), Fondap-Ideal 15150003, ANID-Millennium Science Initiative Program-Center code “ICN2021_002”. D. Nualart was awarded the scholarship ANID-Millennium Science Initiative Program-Center code “ICN2021_002”.
Institutional Review Board Statement
The study was approved by Audit Bioetical Aspects Research Projects, Los Lagos University, Approval Code: 01/2023, Approval Date: 12 January 2023.
Data Availability Statement
Data Availability Statements are available by requirement.
Acknowledgments
The authors also acknowledge the support provided by the Vicerrectoría de Investigación, Desarrollo y Creación Artística (VIDCA) of the Universidad Austral de Chile.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; p. 288.
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Lagos, C.; Pontigo, J.P.; Oyarzún, R.; Soto-Dávila, M.; Morera, F.; Yáñez, A.; Vargas-Chacoff, L. Intestinal incomplete process on the osmoregulation system during Salmo salar smoltification in a productive conditions. Aquaculture 2018, 491, 121–127. [Google Scholar] [CrossRef]
- SERNAPESCA. Report with Health Background of Freshwater and Sea Year 1st Semester 2023; Department of Animal Health Sub-Directorate of Aquaculture National Fisheries and Aquaculture Service: Valparaiso, Chile, 2023; p. 55.
- Bravo, S.; Dolz, H.; Silva, M.T.; Lagos, C.; Millanao, A.; Urbina, M. Informe Final. Diagnostico del uso de Fármacos y Otros Productos Químicos en la Acuicultura; Casilla 1327; Proyecto No. 2003-28; Instituto de Acuicultura, Facultad de Pesquerias y Oceanografia, Universidad Austral de Chile: Puerto Montt, Chile, 2005. [Google Scholar]
- Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Rodgers, C.; Furones, M. Antimicrobial agents in aquaculture: Practice, needs and issues. In The Use of Veterinary Drugs and Vaccines in Mediterranean Aquaculture; Rogers, C., Basurco, B., Eds.; Zaragoza-CIHEAM: Zaragoza, España, 2009; p. 41e59. [Google Scholar]
- Cabello, F.C. Antibiotics and Aquaculture. An Analysis of Their Potential Impact Upon the Environment, Human and Animal Health in Chile. Fundacion Terram. Analisis de Politicas Publicas No. 17. 2003. pp. 1–16. Available online: http://www.terram.cl/docs/App17_Antibioticos_y_Acuicultura.pdf (accessed on 24 November 2024).
- Cabello, F.C. Antibiotics and aquaculture in Chile: Implications for human and animal health. Rev. Med. Chil. 2004, 132, 1001–1006. [Google Scholar]
- Brodersen, D.E.; Clemons, W.M.; Carter, A.P.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B, on the 30S ribosomal subunit. Cell 2000, 103, 1143–1154. [Google Scholar] [CrossRef]
- Pioletti, M. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 2001, 20, 1829–1839. [Google Scholar] [CrossRef]
- Christensen, A.M.; Ingerslev, F.; Baun, A. Ecotoxicity of mixtures of antibiotics used in aquacultures. Environ. Toxicol. Chem. 2006, 25, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R.; Mancilla, M.; Miranda, C.D. Use of antimicrobials in Chilean Salmon farming: Facts, myths and perspectives. Rev. Aquac. 2023, 15, 89–111. [Google Scholar] [CrossRef]
- Elema, M.O.; Hoff, K.A.; Kristensen, H.G. Bioavailability of oxytetracycline from medicated feed administered to Atlantic salmon (Salmo salar L.) in seawater. Aquaculture 1996, 143, 7–14. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Troncoso, C.; Pavez, M.; Santos, A.; Salazar, R.; Barrientos, L. Structural and Physiological Implications of Bacterial Cell in Antibiotic Resistance Mechanisms. Int. J. Morphol. 2017, 35, 4. [Google Scholar]
- Nakano, T.; Hayashi, S.; Nagamine, N. Effect of excessive doses of oxytetracycline on stress-related biomarker expression in coho salmon. Environ. Sci. Pollut. Res. 2018, 25, 7121–7128. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Nunes, B. Rainbow trout (Oncorhynchus mykiss) pro-oxidant and genotoxic responses following acute and chronic exposure to the antibiotic oxytetracycline. Ecotoxicology 2017, 26, 104–117. [Google Scholar] [CrossRef]
- Vadillo, E.; Pelayo, R. Toll-like receptors in development and function of the hematopoietic system. Rev. Investig. Clin. 2012, 64, 461–476. [Google Scholar]
- Rauta, P.R.; Samanta, M.; Dash, H.R.; Nayak, B.; Das, S. Toll-like receptors (TLRs) in aquatic animals: Signaling pathways, expressions and immune responses. Immunol. Lett. 2014, 158, 14–24. [Google Scholar] [CrossRef]
- Ochoa, D.M.; González, J.F. Estrés oxidativo en peces inducido por contaminantes ambientales. Rev. Med. Vet. Zoot. 2008, 55, 115–126. [Google Scholar]
- Lopez-Galindo, C.; Vargas-Chacoff, L.; Nebot, E.; Casanueva, J.F.; Rubio, D.; Sole, M.; Mancera, J.M. Sublethal effects of the organic antifoulant Mexel (R) 432 on osmoregulation and xenobiotic detoxification in the flatfish Solea senegalensis. Chemosphere 2010, 79, 78–85. [Google Scholar] [CrossRef]
- Lopez-Galindo, C.; Vargas-Chacoff, L.; Nebot, E.; Casanueva, J.F.; Rubio, D.; Sole, M.; Mancera, J.M. Biomarker responses in Solea senegalensis exposed to sodium hypochlorite used as antifouling. Chemosphere 2010, 78, 885–893. [Google Scholar] [CrossRef]
- Pontigo, J.P.; Vargas-Chacoff, L. Growth hormone (GH) and growth hormone release factor (GRF) modulate the immune response in the SHK-1 cell line and leukocyte cultures of head kidney in Atlantic salmon. Gen. Comp. Endocrinol. 2021, 300, 113631. [Google Scholar] [CrossRef]
- Nualart, D.P.; Dann, F.; Oyarzún-Salazar, R.; Morera, F.J.; Vargas-Chacoff, L. Immune Transcriptional Response in Head Kidney Primary Cell Cultures Isolated from the Three Most Important Species in Chilean Salmonids Aquaculture. Biology 2023, 12, 924. [Google Scholar] [CrossRef]
- Tafalla, C.; Novoa, B.; Alvarez, J.M.; Figueras, A. In vivo and in vitro effect of oxytetracycline treatment on the immune response of turbot, Scophthalmus maximus (L.). J. Fish Dis. 1999, 22, 271–276. [Google Scholar] [CrossRef]
- Martinsen, B.; Horsberg, T.E.; Varma, K.J.; Sams, R. Single dose pharmacokinetic study of florfenicol in Atlantic salmon (Salmo salar) in sea water at 118C. Aquaculture 1993, 112, 1–11. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Martínez, D.; Vargas-Lagos, C.; Oyarzún, R.; Loncoman, C.A.; Pontigo, J.P.; Yáñez, A.J.; Vargas-Chacoff, L. Temperature modulates the immunological response of the sub-antarctic notothenioid fish Eleginops maclovinus injected with Piscirickettsia salmonis. Fish Shellfish Immunol. 2018, 82, 492–503. [Google Scholar] [CrossRef]
- Pedro, A.V.F.; Martínez, D.; Pontigo, J.P.; Vargas-Lagos, C.; Hawes, C.; Wadsworth, S.; Morera, F.J.; Vargas-Chacoff, L.; Yáñez, A.J. Transcriptional activation of genes involved in oxidative stress in Salmo salar challenged with Piscirickettsia salmonis. Comp. Biochem. Physiol. B 2019, 229, 18–25. [Google Scholar] [CrossRef]
- Kopp, E.B.; Medzhitov, R. The toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 1999, 11, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J.; Keith, F.J. Drosophila toll and IL-1 receptor. Nature 1991, 351, 355–356. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Boltana, S.; Aguilar, A.; Sanhueza, N.; Donoso, A.; Mercado, L.; Imarai, M.; Mackenzie, S. Behavioral fever drives epigenetic modulation of the immune response in fish. Front. Immunol. 2018, 9, 1241. [Google Scholar] [CrossRef]
- Gallo, A.; Landi, R.; Rubino, V.; Di Cerbo, A.; Giovazzino, A.; Palatucci, A.T.; Centenaro, S.; Guidetti, G.; Canello, S.; Cortese, L.; et al. Oxytetracycline induces DNA damage and epigenetic changes: A possible risk for human and animal health? PeerJ 2017, 5, e3236. [Google Scholar] [CrossRef]
- Manríquez, R.A.; Sandoval, M.; Loncoman, C.; Tafalla, C.; Avendaño-Herrera, R.; Cárcamo, J.G. Epigenetic reprogramming around IFN1 and IFNy2 promoters in rainbow trout cells inoculated with infectious pancreatic necrosis virus (IPNV). Fish Shellfish Immunol. 2023, 140, 108947. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.T.; Teunissen, A.G.; Van Oosterom, R.; Van Muiswinkel, W.B. The immune system of cyprinid fish: The immunosuppressive effect of the antibiotic oxytetracycline in carp (Cyprinus carpio L.). Aquaculture 1980, 19, 177–189. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cerezuela, R.; Meseguer, J.; Esteban, M.A. Modulation of the immune parameters and expression of genes of gilthead seabream (Sparus aurata L.) by dietary administration of oxytetracycline. Aquaculture 2012, 334–337, 51–57. [Google Scholar] [CrossRef]
- Lundén, T.; Miettinen, S.; Lönnström, L.-G.; Lilius, E.-M.; Bylund, G. Effect of florfenicol on the immune response of rainbow trout (Oncorhynchus mykiss). Vet. Immunol. Immunopathol. 1999, 67, 317–325. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Wang, X.; Han, C.; Cui, Y.; Geng, Y.; Wei, Y.; Shi, W.; Bao, Y. Florfenicol induces renal toxicity in chicks by promoting oxidative stress and apoptosis. Environ. Sci. Pollut. R. 2021, 28, 936–946. [Google Scholar] [CrossRef]
- Zaki, M.M.; Eissa, A.E.; Saeid, S. Assessment of the immune status in Nile Tilapia (Oreochromis niloticus) experimentally challenged with toxogenic/septicemic bacteria during treatment trial with florfenicol and enrofloxacin. World. J. Fish. Mar. Sci. 2011, 3, 21–36. [Google Scholar]
- Oliveira, R.; McDonough, S.; Ladewig, J.C.L.; Soares, A.M.V.M.; Nogueira, A.J.A.; Domingues, I. Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2013, 36, 903–912. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Lazado, C.C.; Brinchmann, M.F.; Berg, I.; Kiron, V. In vivo modulation of immune response and antioxidant defense in Atlantic cod, Gadus morhua following oral administration of oxolinic acid and florfenicol. Comp. Biochem. Physiol. Part C 2009, 150, 459–464. [Google Scholar] [CrossRef]
- Björklund, H.; Bylund, G. Temperature-related absorption and excretion of oxytetracycline in rainbow trout (Salmo gairdneri R.). Aquaculture 1990, 84, 363–372. [Google Scholar] [CrossRef]
- Yonar, M.E.; Yonar, S.M.; Silici, S. Protective effect of propolis against oxidative stress and immunosupression induced by oxytetracycline in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol. 2011, 31, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Yonar, M.E. The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol. 2012, 32, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Pêsa, T.S.; Saccol, E.M.H.; Londero, E.P.; Bressan, C.A.; Ourique, G.M.; Rizzetti, T.M.; Prestes, O.D.; Zanella, R.; Baldisserotto, B.; Pavanato, M.A. Protective effect of quercetin against oxidative stress induced by oxytetracycline in muscle of silver catfish. Aquaculture 2018, 484, 120–125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).