A Critical Assessment of the Process and Logic Behind Fish Production in Marine Recirculating Aquaculture Systems
Abstract
1. Introduction
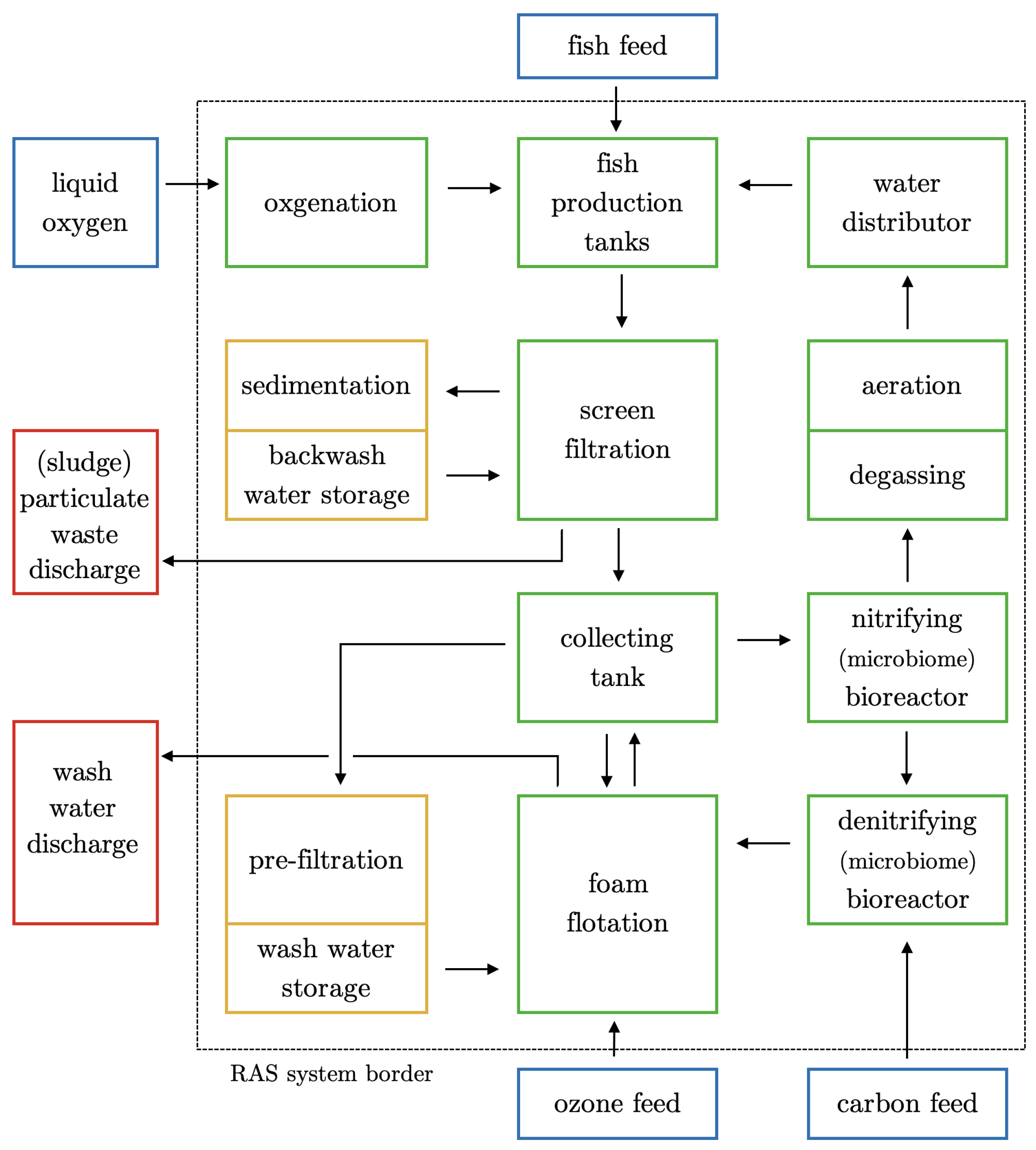
2. The Process Chain and Components of a Marine RAS
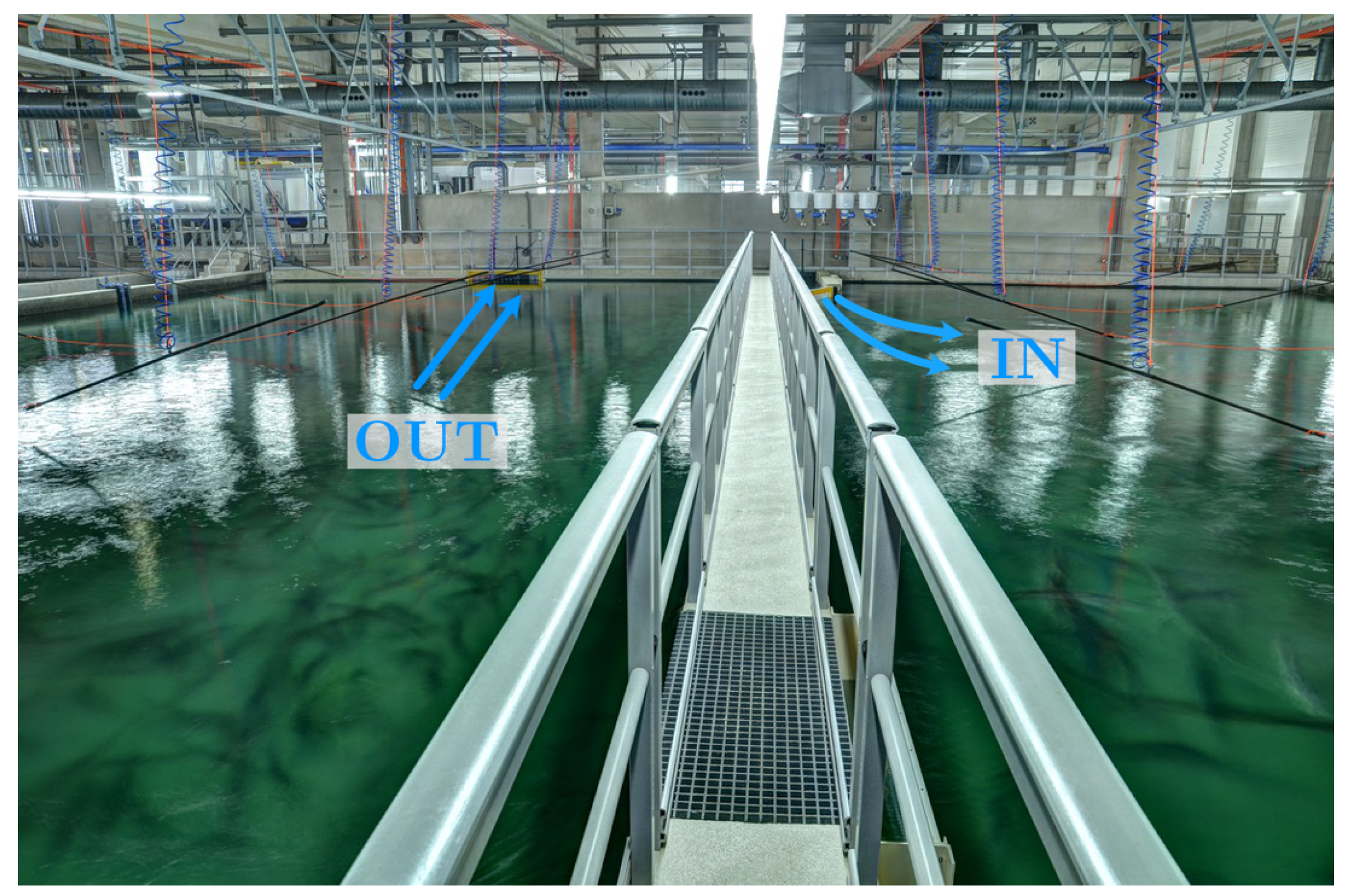
2.1. Fish Production Tanks
2.2. Water Distributor
2.3. Screen Filtration
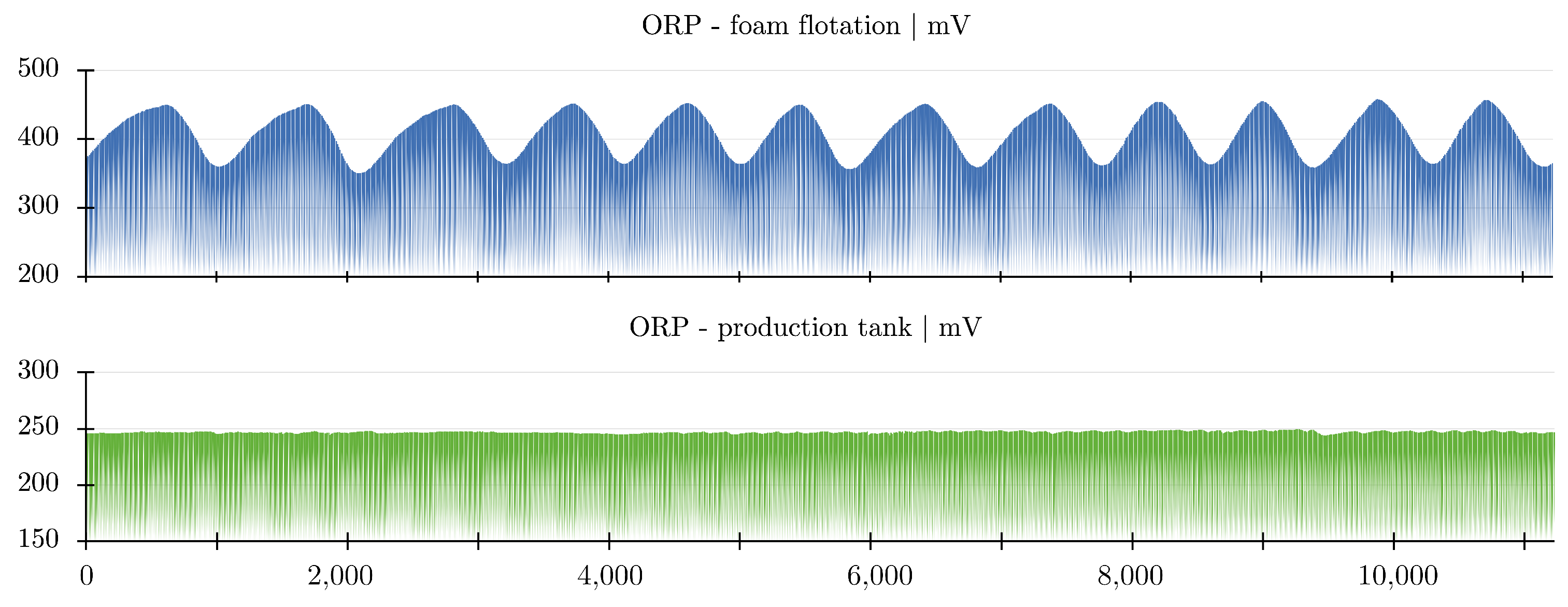
2.4. Foam Flotation
2.5. Nitrifying Bioreactor
2.6. Denitrifying Bioreactor
2.7. Degassing, Aeration, and Oxygenation
3. Discussion
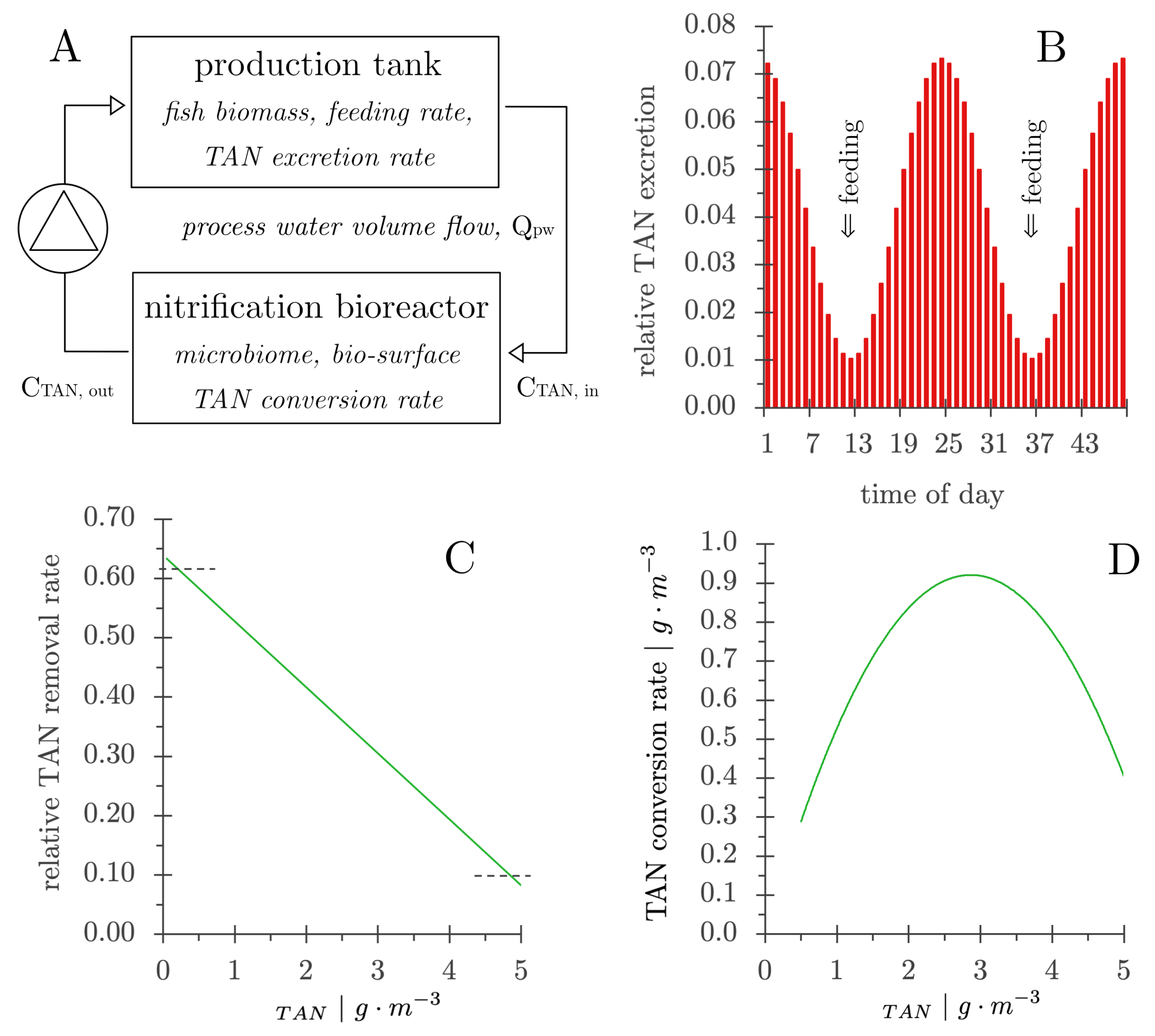
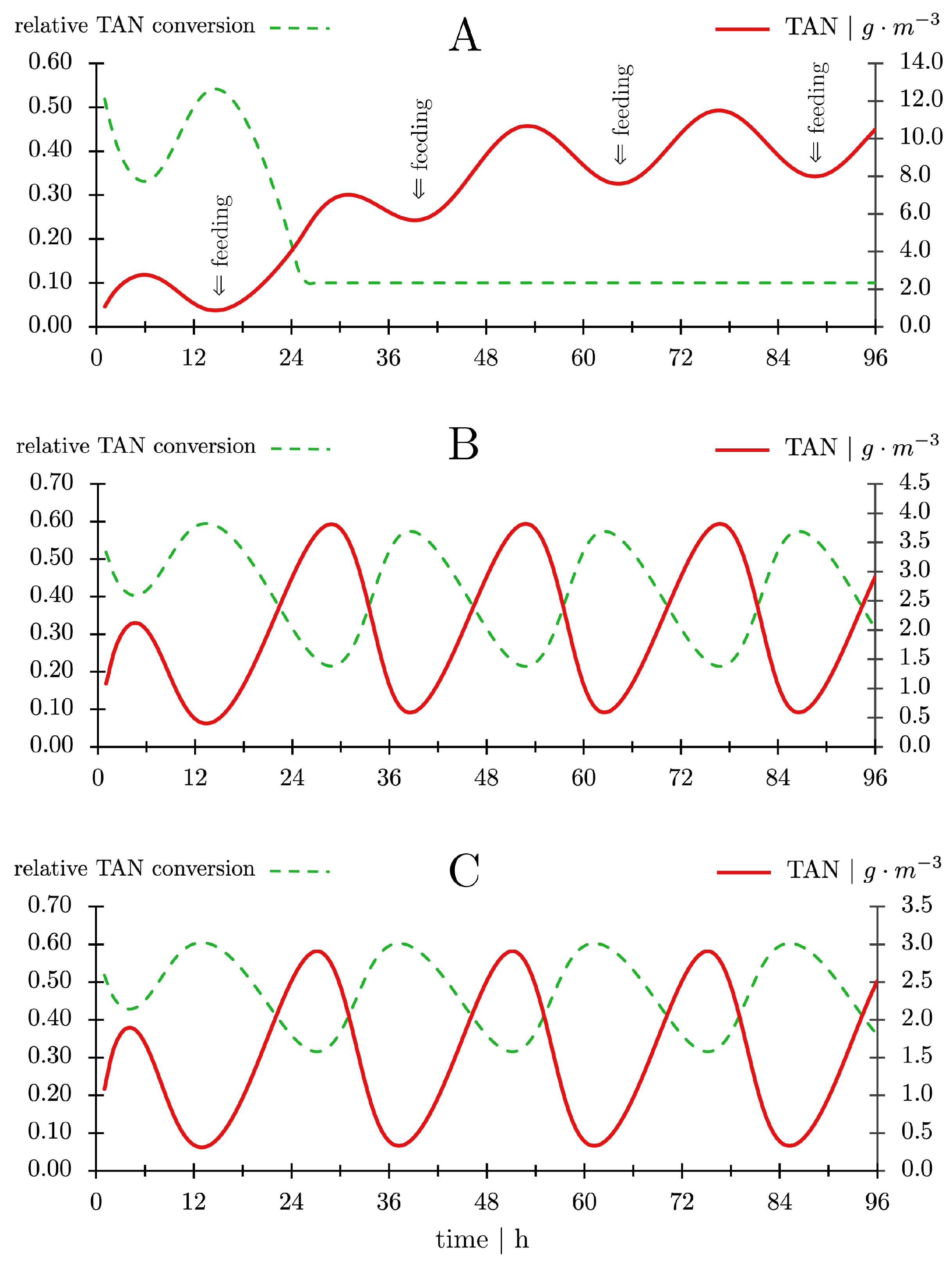
- The TAN concentration in the production tank was calculated from the TAN concentration in the bioreactor effluent (CTAN,out) and the TAN excretion by the fish [105].
- The TAN concentration in the influent to the nitrifying bioreactor (CTAN,in) was calculated from the TAN concentration in the production tank and the water exchange rate.
- The TAN concentration in the nitrification bioreactor effluent (CTAN,out) was calculated after one pass through the nitrifying bioreactor using the rate of decrease in concentration as shown in Figure 10C.
- -
- During the project planning, the fundamentals and feasibility were not sufficiently questioned. Independent opinions would probably have revealed the risks.
- -
- When the RAS was completed and put into operation, well-trained personnel with specialization in the operation of RASs should have taken over the system maintenance.
- -
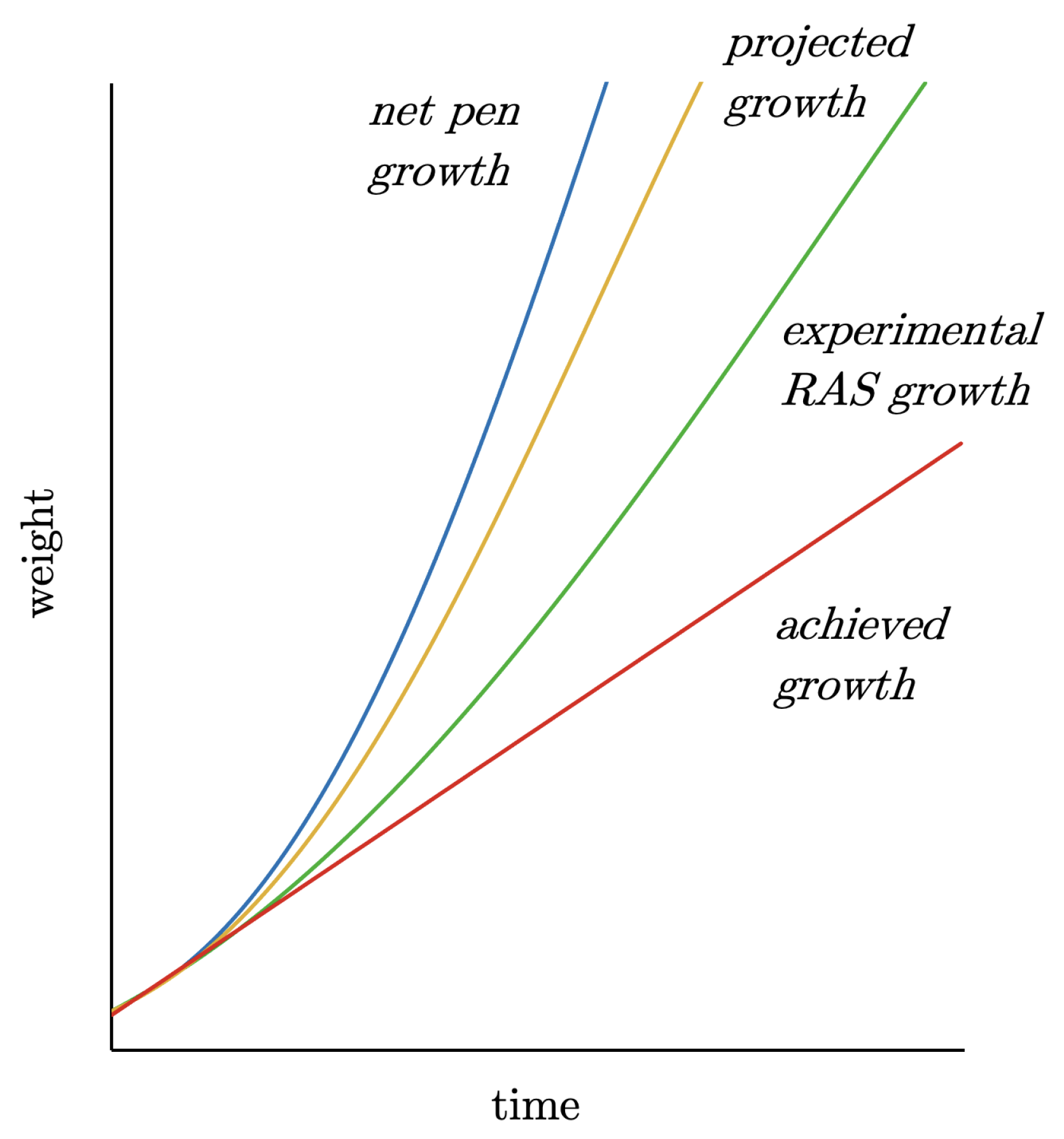
- When the first problems arose on the farm, as can be seen in Figure 13 from the linear growth, measures should have been taken immediately. This would have included reducing the stocking density until the problems that were already emerging had been resolved.
- -
- The management should have worked cooperatively with the employees and experts to find solutions. Ultimately, management’s insistence on the manufacturer’s planning documents was the real cause of the problems.
4. Conclusions
5. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, G.G.; Sajid, Z.; Mather, C. Quantitative analysis of mass mortality events in salmon aquaculture shows increasing scale of fish loss events around the world. Sci. Rep. 2008, 14, 3763. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS). Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Nilav, A.; Suman, N.; Abhilipsa, B.; Tapas, P. A review on recirculating aquaculture systems: Challenges and opportunities for sustainable aquaculture. Inno. Farm. 2020, 5, 017–024. [Google Scholar]
- Golfand, I. Economics of growing salmon in recirculating aquaculture systems (RAS). J. Aquac. Mar. Biol. 2023, 12, 99–102. [Google Scholar] [CrossRef]
- Schreier, H.J.; Mirzoyan, N.; Saito, K. Microbial diversity of biological filters in recirculating aquaculture systems. Curr. Opin. Biotechnol. 2010, 21, 318–325. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. A review of influencing factors on a recirculating aquaculture system: Environmental conditions, feeding strategies, and disinfection methods. J. World Aquac. Soc. 2023, 54, 566–602. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Colson, V.; Sadoul, B.; Valotaire, C.; Prunet, P.; Gaumé, M.; Labbé, L. Welfare assessment of rainbow trout reared in a Recirculating Aquaculture System: Comparison with a Flow-Through System. Aquaculture 2015, 436, 151–159. [Google Scholar] [CrossRef]
- Waller, U. Tank culture and recirculating systems. In Environmental Impact of Aquaculture, 1st ed.; Black, K.D., Ed.; Sheffield Academic Press: Sheffield, UK, 2001; pp. 99–127. [Google Scholar]
- Coombs, S.; Bak-Coleman, J.; Montgomery, J. Rheotaxis revisited: A multi-behavioral and multisensory perspective on how fish orient to flow. J. Exp. Biol. 2020, 223, jeb223008. [Google Scholar] [CrossRef]
- Davidson, J.; Summerfelt, S. Solids flushing, mixing, and water velocity profiles within large (10 and 150 m) circular ‘Cornell-type’ dual-drain tanks. Aquac. Eng. 2004, 32, 245–271. [Google Scholar] [CrossRef]
- Timmerhaus, G.; Lazado, C.C.; Cabillon, N.A.R.; Reiten, B.K.M.; Johansen, L.-H. The optimum velocity for Atlantic salmon post-smolts in RAS is a compromise between muscle growth and fish welfare. Aquaculture 2021, 532, 736076. [Google Scholar] [CrossRef]
- Waller, U.; Black, E.; Burt, D.; Groot, C.; Rosenthal, H. The reaction of young coho Oncorhynchus kisutch to declining oxygen levels during long-term exposure. J. Appl. Ichthyol. 2000, 16, 14–19. [Google Scholar] [CrossRef]
- Akaarir, M.; Martorell-Barceló, M.; Morro, B.; Suau, M.; Alós, J.; Aspillaga, E.; Gamundí, A.; Grau, A.; Lana, A.; Nicolau, M.C.; et al. Measuring activity-rest rhythms under different acclimation periods in a marine fish using automatic deep learning-based video tracking. Chronobiol. Int. 2024, 41, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Marius, C.B.; Kiemer, M.C.B.; Black, K.D.; Lussot, L.; Bullock, A.M.; Ezzi, I. The effects of chronic and acute exposure to hydrogen sulfide on Atlantic salmon (Salmo salar L.). Aquaculture 1995, 135, 311–327. [Google Scholar]
- Ciani, E.; Kvæstad, B.; Stormoen, M.; Mayer, I.; Gupta, S.; Ribičić, D.; Netzer, R. Early warning through video monitoring: Dissolved hydrogen sulfide (H2S) affects Atlantic salmon swimming behavior in recirculating aquaculture systems. Aquaculture 2024, 581, 740201. [Google Scholar] [CrossRef]
- Bergstedt, J.H.; Skov, P.V. Acute hydrogen sulfide exposure in post-smolt Atlantic salmon (Salmo salar): Critical levels and recovery. Aquaculture 2023, 570, 739405. [Google Scholar] [CrossRef]
- Lazado, C.C.; Stiller, K.T.; Timmerhaus, G.; Megård Reiten, B.K.; Nicolaysen, I.L.; Carletto, D.; Alipio, H.R.; Bergstedt, J.H.; Andersen, Ø. Mucosal and systemic physiological changes underscore the welfare risks of environmental hydrogen sulphide in post-smolt Atlantic salmon (Salmo salar). Ecotoxicol. Environ. Saf. 2024, 270, 115897. [Google Scholar] [CrossRef]
- Kraul, S.; Szyper, J.P.; Burke, B. Practical Formulas for Computing Water Exchange Rates. Progress. Fish-Cult. 1985, 47, 69–70. [Google Scholar] [CrossRef]
- Schram, E.; Verdegem, M.C.; Widjaja, R.; Kloet, C.J.; Foss, A.; Schelvis-Smit, R.; Roth, B.; Imsland, A.K. Impact of increased flow rate on specific growth rate of juvenile turbot (Scophthalmus maximus, Rafinesque 1810). Aquaculture 2009, 292, 46–52. [Google Scholar] [CrossRef]
- Pedersen, P.B.; von Ahnen, M.; Fernandes, P.; Naas, C.; Pedersen, L.-F.; Dalsgaard, J. Particle surface area and bacterial activity in recirculating aquaculture systems. Aquac. Eng. 2017, 78, 18–23. [Google Scholar] [CrossRef]
- Branson, E. Environmental Aspects of Aquaculture. In Aquaculture for Veterinarians; Brown, L., Ed.; Pergamon Press: Oxford, UK, 1993; pp. 57–67. [Google Scholar]
- Klontz, G.W. Environmental Requirements and Environmental Diseases of Salmonids. In Fish Medicine; Stoskopf, M.K., Ed.; W. B. Saunders Company: Philadelphia, PA, USA, 1993; pp. 333–358. [Google Scholar]
- Dahle, S.W.; Bakke, I.; Birkeland, M.; Nordøy, K.; Dalum, A.S.; Attramadal, K.J.K. Production of lumpfish (Cyclopterus lumpus L.) in RAS with distinct water treatments: Effects on fish survival, growth, gill health and microbial communities in rearing water and biofilm. Aquaculture 2020, 522, 735097. [Google Scholar] [CrossRef]
- Becke, C.; Schumann, M.; Steinhagen, D.; Geist, J.; Brinker, A. Physiological consequences of chronic exposure of rainbow trout (Oncorhynchus mykiss) to suspended solid load in recirculating aquaculture systems. Aquaculture 2018, 484, 228–241. [Google Scholar] [CrossRef]
- Bruton, M.N. The effects of suspensoids on fish. Hydrobiologia 1985, 125, 221–241. [Google Scholar] [CrossRef]
- Moretto, J.A.; Freitas, P.N.N.; Souza, J.P.; Oliveira, T.M.; Brites, I.; Pinto, E. off-flavors in Aquacultured Fish: Origins and Implications for Consumers. Fishes 2022, 7, 34. [Google Scholar] [CrossRef]
- Azaria, S.; van Rijn, J. off-flavor compounds in recirculating aquaculture systems (RAS): Production and removal processes. Aquac. Eng. 2018, 83, 57–64. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Pedersen, L.-F.; Pedersen, P.B. Daily micro particle distribution of an experimental recirculating aquaculture system—A case study. Aquac. Eng. 2014, 60, 28–34. [Google Scholar] [CrossRef]
- Langer, J.; Efthimiou, S.; Rosenthal, H.; Bronzi, P. Drum filter performance in a recirculating eel culture unit. J. Appl. Ichthyol. 1996, 12, 61–65. [Google Scholar] [CrossRef]
- Lal, D. The Oceanic Microcosm of Particles. Science 1977, 198, 997–1009. [Google Scholar] [CrossRef]
- Sander, E. Skimmers in the marine aquarium. Petfish Mon. 1967, 2, 49–51. [Google Scholar]
- Waller, U.; Orellana, J.; Sander, M. The Control of Water Quality and Hygienic Conditions in Aquaculture Recirculation Systems (RAS): The Use of Foam Fractionation and Ozone. In Proceedings of the IOA/IUVA Joint World Congress on Ozone and Ultraviolet Technologies, Los Angeles, CA, USA, 27–29 August 2007; Volume 4, pp. 1916–1936. [Google Scholar]
- Fabio Brambilla, F.; Antonini, M.; Ceccuzzi, P.; Terova, G.; Saroglia, M. Foam fractionation efficiency in particulate matter and heterotrophic bacteria removal from a recirculating seabass (Dicentrarchus labrax) system. Aquac. Eng. 2008, 39, 37–42. [Google Scholar] [CrossRef]
- Burghoff, B. Foam fractionation applications. J. Biotechnol. 2012, 161, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Majumder, S.K.; Singh, A. Flotation technique: Its mechanisms and design parameters. Chem. Eng. Process. Process. Intensif. 2018, 127, 249–270. [Google Scholar] [CrossRef]
- Zieminski, S.A.; Hume, R.M.; Durham, R. Rates of oxygen transfer from air bubbles to aqueous NaCl solutions at various temperatures. Mar. Chem. 1976, 4, 333–346. [Google Scholar] [CrossRef]
- Sander, M. Aquarientechnik in Süß- und Sewasser, 1st ed.; Ulmer: Stuttgart, Germany, 1998; 256p. [Google Scholar]
- Rosenthal, H.; Otte, G. Ozonation in an Intensive Fish Culture Recycling System. Ozone Sci. Eng. 1979, 1, 319–327. [Google Scholar] [CrossRef]
- Ernst, A.; Steinbach, C.; Wagner, K.; Waller, U. Virtual Sensing of Nitrite: A Novel Control for Safe Denitrification in Recirculating Aquaculture Systems (RASs). Fishes 2024, 9, 398. [Google Scholar] [CrossRef]
- Spiliotopoulou, A.; Rojas-Tirado, P.; Chhetri, R.K.; Kaarsholm, K.M.S.; Martin, R.; Pedersen, P.B.; Pedersen, L.-F.; Andersen, H.R. Ozonation control and effects of ozone on water quality in recirculating aquaculture systems. Water Res. 2018, 133, 289–298. [Google Scholar] [CrossRef]
- Fukunaga, K.; Suzuki, T.; Arita, M.; Suzuki, S.; Hara, A.; Yamauchi, K.; Shinriki, N.; Ishizaki, K.; Takama, K. Acute toxicity of ozone against morphology of gill and erythrocytes of Japanese charr (Salvelinus leucomaenis). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1992, 101, 331–336. [Google Scholar] [PubMed]
- Reiser, S.; Schroeder, J.P.; Wuertz, S.; Kloas, W.; Hanel, R. Histological and physiological alterations in juvenile turbot (Psetta maxima, L.) exposed to sublethal concentrations of ozone-produced oxidants in ozonated seawater. Aquaculture 2010, 307, 157–164. [Google Scholar] [CrossRef]
- Stiller, K.T.; Kolarevic, J.; Lazado, C.C.; Gerwins, J.; Good, C.; Summerfelt, S.T.; Mota, V.C.; Espmark, Å.M.O. The Effects of Ozone on Atlantic Salmon Post-Smolt in Brackish Water—Establishing Welfare Indicators and Thresholds. Int. J. Mol. Sci. 2020, 21, 5109. [Google Scholar] [CrossRef]
- Kovács, B.D.; de Jesus Gregersen, K.J.; Rüppel, F.; von Danwitz, A.; Pedersen, L.-F. Evaluating protein skimmer performance in a commercial seawater recirculating aquaculture system (RAS). Aquac. Eng. 2023, 103, 102369. [Google Scholar] [CrossRef]
- Lazado, C.C.; Stiller, K.T.; Reiten, B.-K.M.; Osório, J.; Kolarevic, J.; Johansen, L.-H. Consequences of continuous ozonation on the health and welfare of Atlantic salmon post-smolts in a brackish water recirculating aquaculture system. Aquat. Toxicol. 2021, 238, 105935. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Blancheton, J.P.; Liu, Y.; Triplet, S.; Michaud, L. Effect of oxidation–reduction potential on performance of European seabass (Dicentrarchus labrax) in recirculating aquaculture systems. Aquac. Int. 2014, 22, 1263–1282. [Google Scholar] [CrossRef]
- Powell, A.; Chingombea, P.; Lupatsch, I.; Shields, R.J.; Lloyd, R. The effect of ozone on water quality and survival of turbot (Psetta maxima) maintained in a recirculating aquaculture system. Aquac. Eng. 2015, 64, 20–24. [Google Scholar] [CrossRef]
- Schroeder, J.P.; Reiser, S.; Croot, L.P.; Hanel, R. A Comparative Study on the Removability of Different Ozone-Produced Oxidants by Activated Carbon Filtration. Ozone Sci. Eng. 2011, 33, 224–231. [Google Scholar] [CrossRef]
- Adey, W.H.; Loveland, K. Dynamic Aquaria—Building Living Ecosystems, 2nd ed.; Academic Press: New York, NY, USA, 1998; pp. 195–202. [Google Scholar]
- Goncalves, A.A.; Gagnon, G.A. Ozone Application in Recirculating Aquaculture System: An Overview. Ozone Sci. Eng. 2011, 33, 345–367. [Google Scholar] [CrossRef]
- Jyoti, K.K.; Pandit, A.B. Ozone and cavitation for water disinfection. Biochem. Eng. J. 2004, 18, 9–19. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, Z.; Wei, F.; Mao, Y.; Xu, Q.; Li, K.; Lu, Y.; Hu, H.-Y. Identification of surrogates for rapid monitoring of microbial inactivation by ozone for water reuse: A pilot-scale study. J. Hazard. Mater. 2022, 424, 127567. [Google Scholar]
- Qi, W.; Skov, P.V.; de Jesus Gregersen, K.J.; Mousavi, S.; Pedersen, L.; Mota, V.C. Estimating biofilm activity on biofilter elements in recirculating aquaculture systems (RAS) for rearing Atlantic salmon parr (Salmo salar) during operation with ozone and peracetic acid. Aquaculture 2025, 594, 741381. [Google Scholar] [CrossRef]
- Schroeder, J.P.; Klatt, S.F.; Schlachter, M.; Zablotski, Y.; Keuter, S.; Spieck, E.; Schulz, C. Impact of ozonation and residual ozone-produced oxidants on the nitrification performance of moving-bed biofilters from marine recirculating aquaculture systems. Aquac. Eng. 2015, 65, 27–36. [Google Scholar] [CrossRef]
- Bakke, I.; Åm, A.L.; Kolarevic, J.; Ytrestøyl, T.; Vadstein, O.; Attramadal, K.J.K.; Terjesen, B.F. Microbial community dynamics in semi-commercial RAS for production of Atlantic salmon post-smolts at different salinities. Aquac. Eng. 2017, 78, 42–49. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Ammonia Production, Excretion, Toxicity, and Defense in Fish: A Review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.M.; Puglisa, H.J.; Kent, D.B.; Duran, L.J.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems—A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef]
- Clegg, S.; Whitfield, M.A. chemical model of seawater including dissolved ammonia and the stoichiometric dissociation constant of ammonia in estuarine water and seawater from −2 to 40 °C. Geochim. Cosmochim. Acta 1995, 59, 2403–2421. [Google Scholar] [CrossRef]
- Dosdat, A.; Ruyet, J.P.; Covés, D.; Dutto, G.; Gasset, E.; Roux, A.L.; Lemarié, G. Effect of chronic exposure to ammonia on growth, food utilisation and metabolism of the European seabass (Dicentrarchus labrax). Aquat. Living Resour. 2003, 16, 509–520. [Google Scholar] [CrossRef]
- Foss, A.; Imsland, A.K.; Roth, B.; Schram, E.; Stefansson, S.O. Effects of chronic and periodic exposure to ammonia on growth and blood physiology in juvenile turbot (Scophthalmus maximus). Aquaculture 2009, 296, 45–50. [Google Scholar] [CrossRef]
- Wicks, B.J.; Joensen, R.; Tang, Q.; Randall, D.J. Swimming and ammonia toxicity in salmonids: The effect of sub lethal ammonia exposure on the swimming performance of coho salmon and the acute toxicity of ammonia in swimming and resting rainbow trout. Aquat. Toxicol. 2002, 59, 55–69. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.C.; Vinogradov, G.A. Ammonia toxicity to fishes. Effect of pH on the toxicity of the unionized ammonia species. Environ. Sci. Technol. 1981, 15, 837–840. [Google Scholar] [CrossRef]
- Mantoura, R.F.C.; Woodward, E.M.S. Optimization of the indophenol blue method for the automated determination of ammonia in estuarine waters. Estuar. Coast. Shelf Sci. 1983, 17, 219–224. [Google Scholar] [CrossRef]
- Peng, X.; Fuchsman, C.A.; Jayakumar, A.; Oleynik, S.; Martens-Habbena, W.; Devol, A.; Ward, B.B. Ammonia and nitrite oxidation in the Eastern Tropical North Pacific. Glob. Biogeochem. Cycles 2015, 29, 2034–2049. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, D.; Huang, Y.; Ma, J.; Feng, S. A sensitive flow-batch system for on board determination of ultra-trace ammonium in seawater: Method development and shipboard application. Anal. Chim. Acta 2013, 794, 47–54. [Google Scholar] [CrossRef]
- Fivelstad, S.; Schwarz, J.H.; Strømsnes, H.; Olsen, A.B. Sublethal effects and safe levels of ammonia in seawater for Atlantic salmon postsmolts (Salmo salar L.). Aquac. Eng. 1995, 14, 271–280. [Google Scholar] [CrossRef]
- Jia, R.; Han, C.; Lei, J.; Liu, B.; Huang, B.; Huo, H.H.; Yin, S. Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat. Toxicol. 2005, 169, 1–9. [Google Scholar] [CrossRef]
- Park, I.; Lee, J.; Hur, J.W.; Song, Y.; Na, H.; Noh, C.H. Acute Toxicity and Sublethal Effects of Nitrite on Selected Hematological Parameters and Tissues in Dark-banded Rockfish, Sebastes inermis. J. World Aquac. Soc. 2007, 38, 188–199. [Google Scholar] [CrossRef]
- Crawford, R.E.; Allen, G.H. Seawater Inhibition of Nitrite Toxicity to Chinook Salmon. Trans. Am. Fish. Soc. 1977, 106, 105–109. [Google Scholar] [CrossRef]
- Kroupova, H.; Machova, J.; Svodobova, J. Nitrite influence on fish: A review article. Vet. Med. Czech 2005, 50, 461–471. [Google Scholar] [CrossRef]
- Ruiz, P.; Vidal, J.M.; Sepulveda, D.; Torres, C.; Villouta, G.; Carrasco, C.; Aguilera, F.; Ruiz-Tagle, N.; Urrutia, H. Overview and future perspectives of nitrifying bacteria on biofilters for recirculating aquaculture systems. Rev. Aquac. 2020, 12, 1478–1494. [Google Scholar] [CrossRef]
- Park, J.; Kim, P.-H.; Park, S.; Daniels, H.V. Effects of two different ozone doses on total residual oxidants, nitrogen compounds and nitrification rate in seawater recirculating systems for black seabream Acanthopagrus schlegelii (Bleeker). Aquac. Eng. 2015, 67, 1–7. [Google Scholar] [CrossRef]
- Ødegaard, H. Innovations in wastewater treatment: The moving bed biofilm process. Water Sci. Technol. 2006, 53, 17–33. [Google Scholar] [CrossRef]
- Madan, S.; Madan, R.; Hussain, A. Advancement in biological wastewater treatment using hybrid moving bed biofilm reactor (MBBR): A review. Appl. Water Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Wik, T. Trickling filters and biofilm reactor modelling. Rev. Environ. Sci. Bio/Technol. 2003, 2, 193–212. [Google Scholar] [CrossRef]
- Colt, J. Dissolved Gas Concentrations in Water, 2nd ed.; Elsevier: London, UK, 2012; pp. 1–289. [Google Scholar]
- Eding, E.H.; Kamstra, A.; Verreth, J.A.J.; Huisman, E.A.; Klapwijk, A. Design and operation of nitrifying trickling filters in recirculating aquaculture: A review. Aquac. Eng. 2006, 34, 234–260. [Google Scholar] [CrossRef]
- Chen, S.; Ling, J.H.; Blancheton, J.P. Nitrification kinetics of biofilm as affected by water quality factors. Aquac. Eng. 2006, 34, 179–197. [Google Scholar] [CrossRef]
- Kinyage, J.P.; Pedersen, L.-F. Impact of temperature on ammonium and nitrite removal rates in RAS moving bed biofilters. Aquac. Eng. 2016, 75, 51–55. [Google Scholar] [CrossRef]
- Kumar, R.V.; Joseph, V.; Philip, R.; Bright Singh, I.S. Nitrification by stringed bed suspended bioreactors at different salinities and substrate concentrations. Indian J. Geo-Mar. Sci. 2014, 43, 1899–1904. [Google Scholar]
- Gonzalez-Silva, B.M.; Jonassen, K.R.; Bakke, I.; Østgaard, K.; Vadstein, O. Nitrification at different salinities: Biofilm community composition and physiological plasticity. Water Res. 2016, 95, 48–58. [Google Scholar] [CrossRef]
- Kinyage, J.P.; Pedersen, P.B.; Pedersen, L.-F. Effects of abrupt salinity increase on nitrification processes in a freshwater moving bed biofilter. Aquac. Eng. 2019, 84, 91–98. [Google Scholar] [CrossRef]
- Suriasni, P.A.; Faizal, F.; Panatarani, C.; Hermawan, W.; Joni, I.M. A Review of Bubble Aeration in Biofilter to Reduce Total Ammonia Nitrogen of Recirculating Aquaculture System. Water 2023, 15, 808. [Google Scholar] [CrossRef]
- Suhr, K.I.; Pedersen, P.B. Nitrification in moving bed and fixed bed biofilters treating effluent water from a large commercial outdoor rainbow trout RAS. Aquac. Eng. 2010, 42, 31–37. [Google Scholar] [CrossRef]
- Qi, W.; Skov, P.V.; de Jesus Gregersen, K.J.; Pedersen, L.-F. Estimation of nitrifying and heterotrophic bacterial activity in biofilm formed on RAS biofilter carriers by respirometry. Aquaculture 2022, 561, 738730. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Skillman, A.D.; Crecelius, E.A. The inhibition of marine nitrification by ocean disposal of carbon dioxide. Mar. Pollut. Bull. 2002, 44, 142–148. [Google Scholar] [CrossRef]
- Díaz, V.; Ibáñez, R.; Gómez, P.; Urtiaga, A.M.; Ortiz, I. Kinetics of nitrogen compounds in a commercial marine Recirculating Aquaculture System. Aquac. Eng. 2012, 50, 20–27. [Google Scholar] [CrossRef]
- Kraft, B.; Tegetmeyer, H.E.; Sharma, R.; Klotz, M.G.; Ferdelman, T.G.; Hettich, R.L.; Geelhoed, J.S.; Strous, M. The environmental controls that govern the end product of bacterial nitrate respiration. Science 2014, 345, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, S. Effects of organic carbon on nitrification rate in fixed film biofilters. Aquac. Eng. 2001, 25, 1–11. [Google Scholar] [CrossRef]
- Ling, J.H.; Chen, S. Impact of organic carbon on nitrification performance of different biofilters. Aquac. Eng. 2005, 33, 150–162. [Google Scholar] [CrossRef]
- Navada, S.; Knutsen, M.F.; Bakke, I.; Vadstein, O. Nitrifying biofilms deprived of organic carbon show higher functional resilience to increases in carbon supply. Sci. Rep. 2020, 10, 7121. [Google Scholar] [CrossRef]
- Rojas-Tirado, P.A.; Aalto, S.L.; Åtland, Å.; Letelier-Gordo, C.O. Biofilters are potential hotspots for H2S production in brackish and marine water RAS. Aquaculture 2021, 536, 736490. [Google Scholar] [CrossRef]
- Li, B.; Irvin, S.I. The comparison of alkalinity and ORP as indicators for nitrification and denitrification in a sequencing batch reactor (SBR). Biochem. Eng. J. 2007, 34, 248–255. [Google Scholar] [CrossRef]
- Mariu, A.; Chatha, A.M.; Naz, S.; Khan, M.F.; Safdar, W.; Ashraf, I. Effect of Temperature, pH, Salinity and Dissolved Oxygen on Fishes. J. Zool. Syst. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Tresguerres, M.; Clifford, A.M.; Harter, T.S.; Roa, J.N.; Thies, A.B.; Yee, D.P.; Brauner, C.J. Evolutionary links between intra- and extracellular acid-base regulation in fish and other aquatic animals. J. Exp. Zool. 2020, 333, 449–465. [Google Scholar] [CrossRef]
- Klas, S.; Mozes, N.; Lahav, O. Development of a single-sludge denitrification method for nitrate removal from RAS effluents: Lab-scale results vs. model prediction. Aquaculture 2006, 259, 342–353. [Google Scholar] [CrossRef]
- Prakash, A.; Khanam, S. Nitrogen Pollution Threat to Mariculture and Other Aquatic Ecosystems: An Overview. J. Pharm. Pharmacol. 2021, 9, 428–433. [Google Scholar] [CrossRef]
- Zakem, E.J.; Al-Haj, A.; Church, M.J.; Van Dijken, G.L.; Dutkiewicz, S.; Foster, S.Q.; Fulweiler, R.W.; Mills, M.M.; Follows, M.J. Ecological control of nitrite in the upper ocean. Nat. Commun. 2018, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Kamykowski, D.; Zentara, S.-J. Predicting plant nutrient concentrations from temperature and sigma-t in the upper kilometer of the world ocean. Deep-Sea Res. 1986, 33, 89–105. [Google Scholar] [CrossRef]
- Bristow, L.A.; Mohr, W.; Ahmerkamp, S.; Kuypers, M.M. Nutrients that limit growth in the ocean. Curr. Biol. 2017, 27, R474–R478. [Google Scholar] [CrossRef]
- Bussel, C.G.; Schroeder, J.P.; Wuertz, S.; Schulz, C. The chronic effect of nitrate on production performance and health status of juvenile turbot (Psetta maxima). Aquaculture 2012, 326–329, 163–167. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Thermal plasticity of the cardiorespiratory system provides cross-tolerance protection to fish exposed to elevated nitrate. Comparative biochemistry and physiology. Toxicol. Pharmacol. 2020, 240, 108920. [Google Scholar]
- Pierce, R.H.; Weeks, J.M.; Prappas, J.M. Nitrate Toxicity to Five Species of Marine Fish. J. World Aquac. Soc. 1993, 24, 105–107. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M. Mass Balance, Loading Rate and Fish Growth. In Recirculating Aquaculture, 3rd ed.; Ithaca Publishing Company, LLC: New York, NY, USA, 2013; p. 69. [Google Scholar]
- Balderston, W.L.; Sieburth, J.M. Nitrate removal in closed-system aquaculture by columnar denitrification. Appl. Environ. Microbiol. 1976, 32, 808–818. [Google Scholar] [CrossRef]
- Sauthier, N.; Grasmick, A.; Blancheton, J.P. Biological denitrification applied to a marine closed aquaculture system. Water Res. 1998, 32, 1932–1938. [Google Scholar] [CrossRef]
- Müller-Belecke, A.; Zienert, S.; Thürmer, C.; Kaufhold, S.; Spranger, U. The self cleaning inherent gas denitrification-reactor for nitrate elimination in RAS for pike perch (Sander lucioperca) production. Aquac. Eng. 2013, 57, 18–23. [Google Scholar] [CrossRef]
- Almeida, P.; Dewasme, L.; Vande Wouwer, A.V. Denitrification Control in a Recirculating Aquaculture System—A Simulation Study. Processes 2020, 8, 1306. [Google Scholar] [CrossRef]
- Stavrakidis-Zachou, O.; Ernst, A.; Steinbach, C.; Wagner, K.; Waller, U. Development of denitrification in semi-automated moving bed biofilm reactors operated in a marine recirculating aquaculture system. Aquac. Int. 2019, 27, 1485–1501. [Google Scholar] [CrossRef]
- Preena, P.G.; Rejish Kumar, V.J.; Singh, I.S. Nitrification and denitrification in recirculating aquaculture systems: The processes and players. Rev. Aquac. 2021, 13, 2053–2075. [Google Scholar] [CrossRef]
- Lahav, O.; Massada, I.B.; Yackoubov, D.; Zelikson, R.; Mozes, N.; Tal, Y.; Tarre, S. Quantification of anammox activity in a denitrification reactor for a recirculating aquaculture system. Aquaculture 2009, 288, 76–82. [Google Scholar] [CrossRef]
- Yogev, U.; Sowers, K.R.; Mozes, N.; Gross, A. Nitrogen and carbon balance in a novel near-zero water exchange saline recirculating aquaculture system. Aquaculture 2017, 467, 118–126. [Google Scholar] [CrossRef]
- Letelier-Gordo, C.O.; Huang, X.; Aalto, S.L.; Pedersen, P.B. Activated sludge denitrification in marine recirculating aquaculture system effluent using external and internal carbon sources. Aquac. Eng. 2020, 90, 102096. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justić, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Servili, A.; Lévêque, E.P.; Mouchel, O.; Devergne, J.; Lebigre, C.; Roussel, S.; Mazurais, D.; Zambonino-Infante, J. Ocean acidification alters the acute stress response of a marine fish. Sci. Total Environ. 2023, 858, 159804. [Google Scholar] [CrossRef]
- Stiller, K.T.; Vanselow, K.H.; Moran, D.; Bojens, G.; Voigt, W.; Meyer, S.; Schulz, C. The effect of carbon dioxide on growth and metabolism in juvenile turbot Scophthalmus maximus L. Aquaculture 2015, 444, 143–150. [Google Scholar] [CrossRef]
- Mota, V.C.; Nilsen, T.O.; Gerwins, J.; Gallo, M.; Ytteborg, E.; Baeverfjord, G.; Kolarevic, J.; Summerfelt, S.T.; Terjesen, B.F. The effects of carbon dioxide on growth performance, welfare, and health of Atlantic salmon post-smolt (Salmo salar) in recirculating aquaculture systems. Aquaculture 2019, 498, 578–586. [Google Scholar] [CrossRef]
- Jafari, L.; Fivelstad, S.; Attramadal, K.; Flataker, H.; Mjaatvedt, P.; Olsen, A.B.; Chierici, M.; Hosfeld, C.D. A descriptive study of Carbon Dioxide Production and Removal in Full-Scale RAS for Atlantic Salmon (Salmo salar L.) Post-Smolt: A Comparison of Two Different Measurement Methods for CO2. Aquac. Eng. 2024, 107, 102442. [Google Scholar] [CrossRef]
- Khan, J.R.; Johansen, D.Y.; Skov, P.V. The effects of acute and long-term exposure to CO2 on the respiratory physiology and production performance of Atlantic salmon (Salmo salar) in freshwater. Aquaculture 2018, 491, 20–27. [Google Scholar] [CrossRef]
- Moran, D. Carbon dioxide degassing in fresh and saline water I: Degassing performance of a cascade column. Aquac. Eng. 2010, 43, 29–36. [Google Scholar] [CrossRef]
- Moran, D. Carbon dioxide degassing in fresh and saline water. II: Degassing performance of an air-lift. Aquac. Eng. 2010, 43, 120–127. [Google Scholar] [CrossRef]
- Kramer, D.L. Dissolved oxygen and fish behavior. Environ. Biol. Fishes 1987, 18, 81–92. [Google Scholar] [CrossRef]
- Chabot, D.; Dutil, J. Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J. Fish Biol. 1999, 55, 472–491. [Google Scholar]
- Stierhoff, K.; Targett, T.E.; Miller, K.L. Ecophysiological responses of juvenile summer and winter flounder to hypoxia: Experimental and modeling analyses of effects on estuarine nursery quality. Mar. Ecol. Prog. Ser. 2006, 325, 255–266. [Google Scholar] [CrossRef]
- Jones, J. The Reactions of Fish to Water of Low Oxygen Concentration. J. Exp. Biol. 1952, 29, 403–415. [Google Scholar] [CrossRef]
- Waller, U.; Rosenthal, H.; Black, E. The respiration of young coho Oncorhynchus kisutch at gradually declining oxygen levels and during recovery from oxygen deprivation. J. Appl. Ichthyol. 1997, 13, 73–78. [Google Scholar] [CrossRef]
- Cerezo, J.; García, B.G. The effects of oxygen levels on oxygen consumption, survival and ventilatory frequency of sharpsnout sea bream (Diplodus puntazzo Gmelin, 1789) at different conditions of temperature and fish weight. J. Appl. Ichthyol. 2004, 20, 488–492. [Google Scholar] [CrossRef]
- Valverde, J.C.; López, F.J.; García, B.G. Oxygen consumption and ventilatory frequency responses to gradual hypoxia in common dentex (Dentex dentex): Basis for suitable oxygen level estimations. Aquaculture 2006, 256, 542–551. [Google Scholar] [CrossRef]
- Seginer, I.; Mozes, N. A note on oxygen supply in RAS: The effect of water temperature. Aquac. Eng. 2012, 50, 46–54. [Google Scholar] [CrossRef]
- Thetmeyer, H.; Waller, U.; Black, K.D.; Inselmann, S.; Rosenthal, H. Growth of European seabass (Dicentrarchus labrax L.) under hypoxic and oscillating oxygen conditions. Aquaculture 1999, 174, 355–367. [Google Scholar] [CrossRef]
- Colt, J. Gas supersaturation—Impact on the design and operation of aquatic systems. Aquac. Eng. 1986, 5, 49–85. [Google Scholar] [CrossRef]
- Pleizier, N.K.; Brauner, C.J. Causes and consequences of gas bubble trauma on fish gill function. J. Comp. Physiol. Biochem. Syst. Environ. Physiol. 2024, 194, 739–747. [Google Scholar] [CrossRef]
- Vinci, B.; Davidson, J.; Naveh, E.; Engler, O. Low head oxygenator performance characterization for marine recirculating aquaculture systems. Aquac. Eng. 2016, 75, 22–28. [Google Scholar] [CrossRef]
- Ji, M.; Ye, Z.; Li, H. Performance evaluation and mathematical model of pipeline diffused aeration for recirculating aquaculture system. Aquac. Eng. 2024, 105, 102410. [Google Scholar] [CrossRef]
- Loyless, J.C.; Malone, R.F. Evaluation of air-lift pump capabilities for water delivery, aeration, and degasification for application to recirculating aquaculture systems. Aquac. Eng. 1998, 18, 117–133. [Google Scholar] [CrossRef]
- Orazem, M.E.; Erickson, L.E. Oxygen-transfer rates and efficiencies in one- and two-stage airlift towers. Biotechnol. Bioeng. 1979, 21, 69–88. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Y.; Zheng, H.; Yang, Q.; Zhu, D.; Xie, J. Effect of the Different Shapes of Air Diffuser on Oxygen Mass Transfer Coefficients in Microporous Aeration Systems. Procedia Eng. 2016, 154, 1079–1086. [Google Scholar] [CrossRef]
- Colt, J.; Watten, B.J. Applications of pure oxygen in fish culture. Aquac. Eng. 1988, 7, 397–441. [Google Scholar] [CrossRef]
- Steinberg, K.; Zimmermann, J.; Meyer, S.; Schulz, C. Start-up of recirculating aquaculture systems: How do water exchange rates influence pikeperch (Sander lucioperca) and water composition? Aquac. Eng. 2018, 83, 151–159. [Google Scholar] [CrossRef]
- Calabrese, S.; Nilsen, T.O.; Kolarevic, J.; Ebbesson, L.O.; Pedrosa, C.; Fivelstad, S.; Hosfeld, A.D.; Stefansson, S.O.; Terjesen, B.F.; Takle, H.; et al. Stocking density limits for post-smolt Atlantic salmon (Salmo salar L.) emphasis on production performance and welfare. Aquaculture 2017, 468, 363–370. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, L.; Liu, Q.; Xiao, Y.; Ma, D.; Xiao, Z.; Xu, S.; Li, J. Effects of stocking density on the growth and immunity of Atlantic salmon salmo salar reared in recirculating aquaculture system (RAS). J. Oceanol. Limnol. 2018, 37, 350–360. [Google Scholar] [CrossRef]
- Sammouth, S.; d’Orbcastel, E.R.; Gasset, E.; Lemariéa, G.; Breuil, G.; Marino, G.; Coeurdacier, J.; Fivelstad, S.; Blancheton, J.P. The effect of density on seabass (Dicentrarchus labrax) performance in a tank-based recirculating system. Aquac. Eng. 2009, 40, 72–78. [Google Scholar] [CrossRef]
- Lupatsch, I.; Santos, G.A.; Schrama, J.W.; Verreth, J.A. Effect of stocking density and feeding level on energy expenditure and stress responsiveness in European seabass Dicentrarchus labrax. Aquaculture 2010, 298, 245–250. [Google Scholar] [CrossRef]
- Pitcher, T.J.; Parrish, J.K. Functions of shoaling in teleosts. In Behaviour of Teleost Fishes, 2nd ed.; Pitcher, T.J., Ed.; Chapmann & Hall: London, UK, 1993; pp. 363–439. [Google Scholar]
- Hvas, M.; Folkedal, O.; Solstorm, D.; Vågseth, T.; Fosse, J.O.; Gansel, L.C.; Oppedal, F. Assessing swimming capacity and schooling behaviour in farmed Atlantic salmon Salmo salar with experimental push-cages. Aquaculture 2017, 473, 423–429. [Google Scholar] [CrossRef]
- Orellana, J.; Waller, U.; Wecker, B. Culture of yellowtail kingfish (Seriola lalandi) in a marine recirculating aquaculture system (RAS) with artificial seawater. Aquac. Eng. 2014, 58, 20–28. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Vielma, J. Controlling of geosmin and 2-methylisoborneol induced off-flavours in recirculating aquaculture system farmed fish—A review. Aquac. Res. 2019, 50, 9–28. [Google Scholar] [CrossRef]
- Davidson, J.; Schrader, K.K.; Ruan, E.D.; Swift, B.; Aalhus, J.L.; Juárez, M.; Wolters, W.R.; Burr, G.S.; Good, C.; Summerfelt, S.T. Evaluation of depuration procedures to mitigate the off-flavor compounds geosmin and 2-methylisoborneol from Atlantic salmon Salmo salar raised to market-size in recirculating aquaculture systems. Aquac. Eng. 2014, 61, 27–34. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Aalto, S.L.; Åsnes, H.Ø.; Rojas-Tirado, P.A.; Åtland, Å.; Letelier-Gordo, C.O. Sulphate removal by membrane filtration minimizes the risk of hydrogen sulphide formation in fixed bed biofilters. Aquac. Eng. 2024, 106, 102426. [Google Scholar] [CrossRef]
- Badiola, M.; Basurko, O.C.; Piedrahita, R.H.; Hundley, P.S.; Mendiola, D. Energy use in Recirculating Aquaculture Systems (RAS): A review. Aquac. Eng. 2018, 81, 57–70. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Kikkawa, T.; Hayashi, M.; Lee, K.; Kita, J. Effects of CO2 on Marine Fish: Larvae and Adults. J. Oceanogr. 2004, 60, 731–741. [Google Scholar] [CrossRef]
- Bergstedt, J.H.; Skov, P.V.; Letelier-Gordo, C.O. Efficacy of H2O2 on the removal kinetics of H2S in saltwater aquaculture systems, and the role of O2 and NO3. Water Res. 2022, 222, 118892. [Google Scholar] [CrossRef]
- Lukassen, M.B.; Menanteau-Ledouble, S.; de Jonge, N.; Schram, E.; Nielsen, J.L. Impact of water quality parameters on geosmin levels and geosmin producers in European recirculating aquaculture systems. J. Appl. Microbiol. 2021, 132, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; Pilote, A.J.; Auffret, M.D.; Proulx, É.; Villemur, R.; Deschamps, M.; Vandenberg, G.W. Reducing geosmin off-flavor compounds and waste outputs through dietary phosphorus management in rainbow trout aquaculture. Aquac. Environ. Interact. 2014, 6, 105–117. [Google Scholar] [CrossRef]
- Yuan, Q.; Oleszkiewicz, J.A. Interaction between Denitrification and Phosphorus Removal in a Sequencing Batch Reactor Phosphorus Removal System. Water Environ. Res. 2010, 82, 536–540. [Google Scholar] [CrossRef]
- He, Q.; Song, Q.; Zhang, S.; Zhang, W.; Wang, H. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: Reactor performance, extracellular polymeric substances and microbial successions. Chem. Eng. J. 2018, 331, 841–849. [Google Scholar] [CrossRef]
- Ben-Asher, R.; Zohar, Y.; Stromberg, M.; Lahav, O. A new approach for eliminating off-flavors from RAS fish, as part of the normal grow-out period. Water Res. 2023, 249, 121015. [Google Scholar] [CrossRef]
- Ruyet, J.P.; Mahé, K.; Bayon, N.L.; Delliou, H.L. Effects of temperature on growth and metabolism in a Mediterranean population of European seabass, Dicentrarchus labrax. Aquaculture 2004, 237, 269–280. [Google Scholar] [CrossRef]
- Waller, U. Factors influencing routine oxygen consumption in turbot, Scophthalmus maximus. J. Appl. Ichthyol. 1992, 8, 62–71. [Google Scholar] [CrossRef]
- Rikardsen, A.H.; Righton, D.A.; Strøm, J.F.; Thorstad, E.B.; Gargan, P.G.; Sheehan, T.F.; Økland, F.; Chittenden, C.M.; Hedger, R.D.; Næsje, T.F.; et al. Redefining the oceanic distribution of Atlantic salmon. Sci. Rep. 2021, 11, 12266. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.D.; Sutterlin, A.M. Heat transfer between fish and ambient water. J. Exp. Biol. 1976, 65, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Waversveld, J.V.; Addink, A.D.; Thillart, G.V.; Smit, H.J. Heat production of fish: A literature review. Comp. Biochem. Physiol. Part A Physiol. 1989, 92, 159–162. [Google Scholar] [CrossRef]
- Black, K.D. Environmental Impact of Aquaculture, 1st ed.; Sheffield Academic Press: Sheffield, UK, 2001; 214p. [Google Scholar]
- Waller, U.; Buhmann, A.K.; Ernst, A.; Hanke, V.; Kulakowski, A.; Wecker, B.; Orellana, J.; Papenbrock, J. Integrated multi-trophic aquaculture in a zero-exchange recirculation aquaculture system for marine fish and hydroponic halophyte production. Aquac. Int. 2015, 23, 1473–1489. [Google Scholar] [CrossRef]
- Austreng, E.; Storebakken, T.; Åsgård, T. Growth rate estimates for cultured Atlantic salmon and rainbow trout. Aquaculture 1987, 60, 157–160. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C. Water quality monitoring in recirculating aquaculture systems. Aquac. Fish Fish. 2023, 3, 113–131. [Google Scholar] [CrossRef]
- Grant, E.L.; Laevenworth, R.S. Statistical Quality Control, 6th ed.; McGraw-Hill: New York, NY, USA, 1988; 714p. [Google Scholar]
- Saberioon, M.; Cisar, P. Automated within tank fish mass estimation using infrared reflection system. Comput. Electron. Agric. 2018, 150, 484–492. [Google Scholar] [CrossRef]
- Pargi, M.K.; Bagheri, E.; Shirota, F.R.; Eng Huat, K.; Shishehchian, F.; Nathalie, N. Improving Aquaculture Systems using AI: Employing predictive models for Biomass Estimation on Sonar Images. In Proceedings of the 2022 21st IEEE International Conference on Machine Learning and Applications (ICMLA), Nassau, The Bahamas, 12–14 December 2022; pp. 1629–1636. [Google Scholar]
- Hung, C.; Tsao, S.C.; Huang, K.; Jang, J.; Chang, H.; Dobbs, F.C. A highly sensitive underwater video system for use in turbid aquaculture ponds. Sci. Rep. 2016, 6, 31810. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waller, U. A Critical Assessment of the Process and Logic Behind Fish Production in Marine Recirculating Aquaculture Systems. Fishes 2024, 9, 431. https://doi.org/10.3390/fishes9110431
Waller U. A Critical Assessment of the Process and Logic Behind Fish Production in Marine Recirculating Aquaculture Systems. Fishes. 2024; 9(11):431. https://doi.org/10.3390/fishes9110431
Chicago/Turabian StyleWaller, Uwe. 2024. "A Critical Assessment of the Process and Logic Behind Fish Production in Marine Recirculating Aquaculture Systems" Fishes 9, no. 11: 431. https://doi.org/10.3390/fishes9110431
APA StyleWaller, U. (2024). A Critical Assessment of the Process and Logic Behind Fish Production in Marine Recirculating Aquaculture Systems. Fishes, 9(11), 431. https://doi.org/10.3390/fishes9110431





