Tissue-Specific Toxicity in Common Carp (Cyprinus carpio) Caused by Combined Exposure to Triphenyltin and Norfloxacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Chemicals
2.2. Experimental Animals and Experimental Design
2.3. Sample Collection
2.4. Biochemical Analysis
2.5. Quantitative Real-Time PCR (qPCR) Assay
2.6. Statistical Analysis
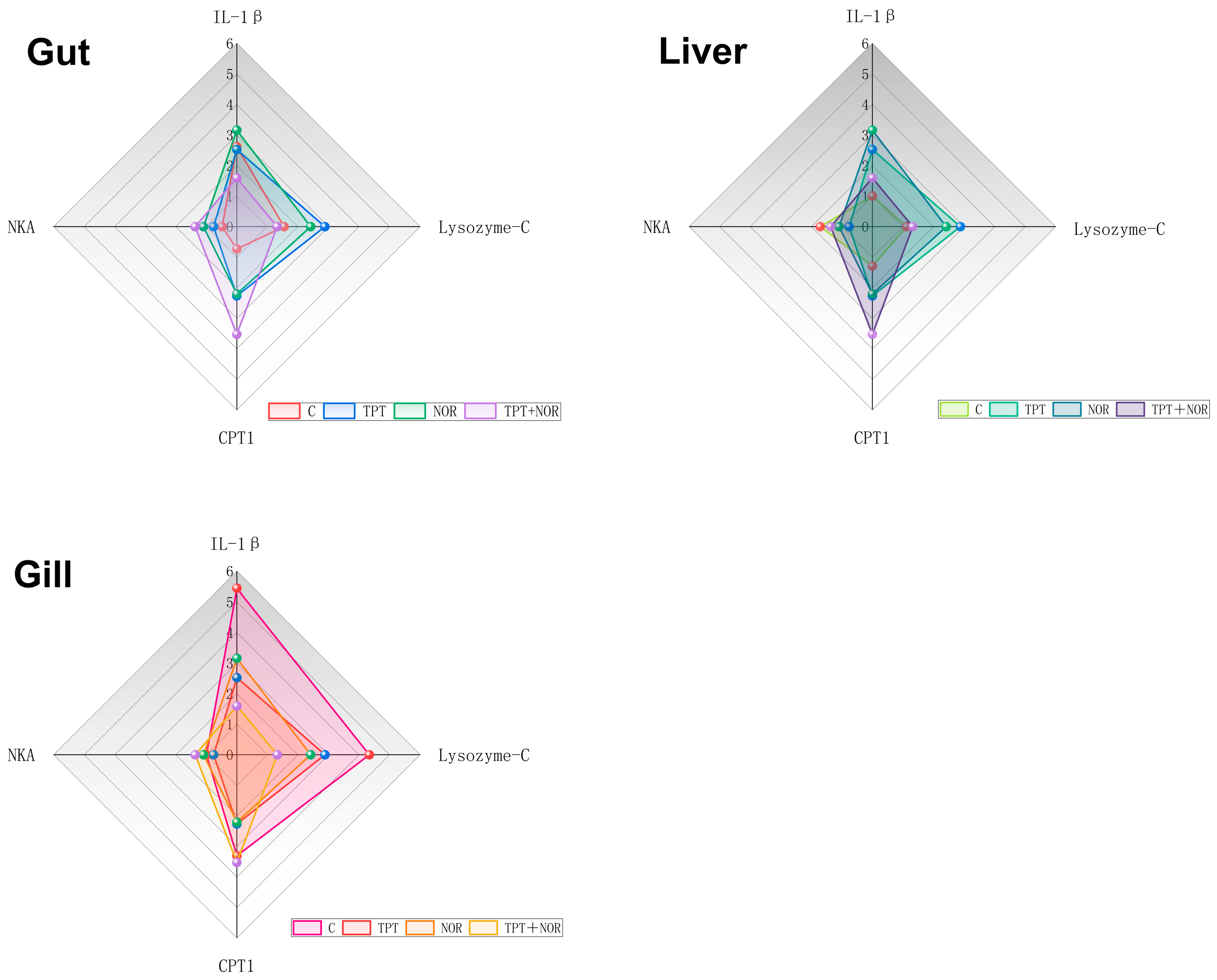
3. Results
3.1. The Change of Biochemical Index
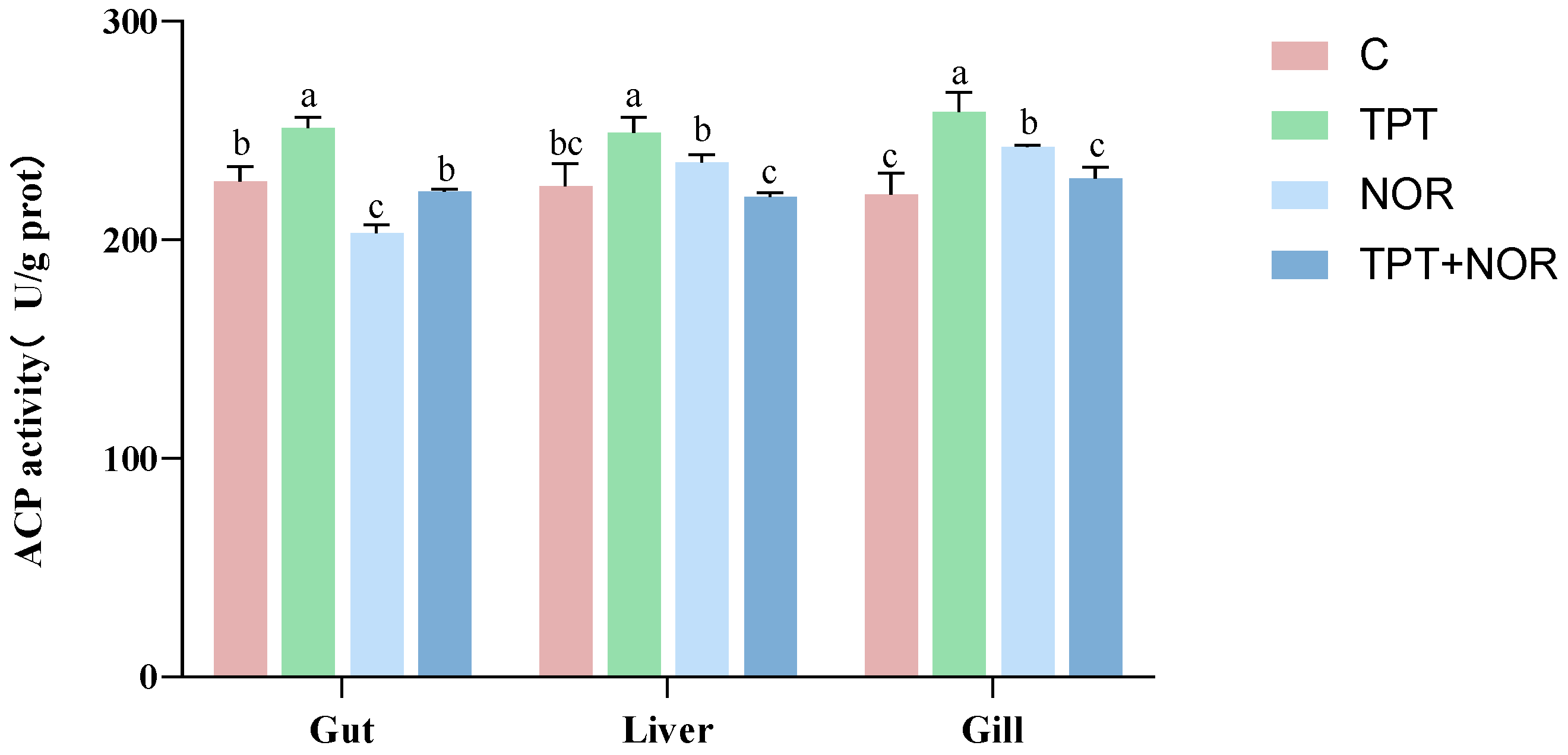
3.1.1. Effect on ACP Activity
3.1.2. Effect of Interleukin
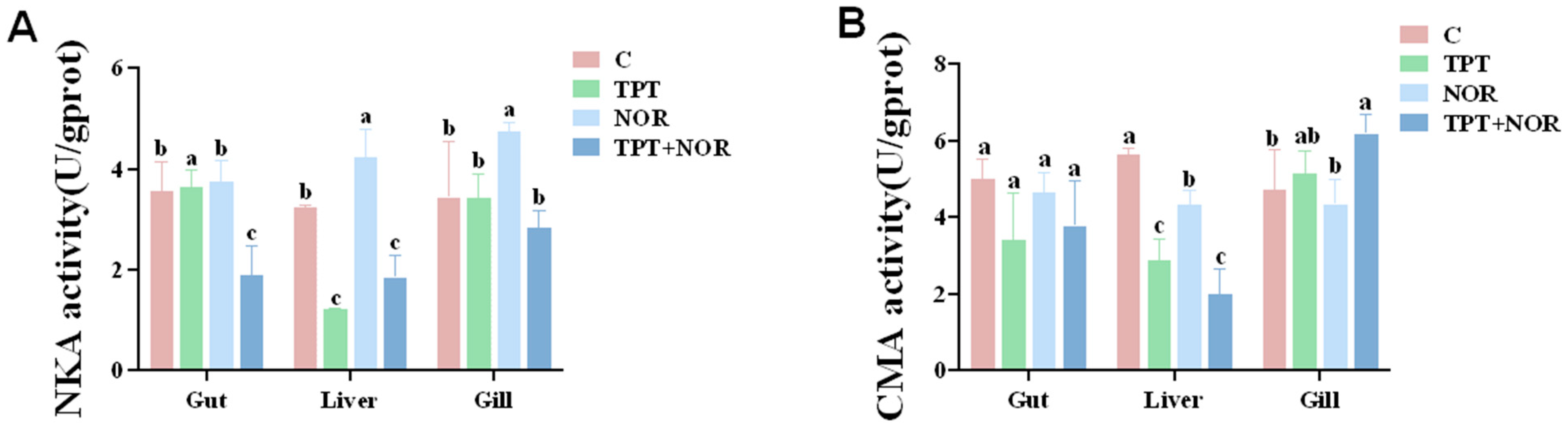
3.1.3. Effects on ATPase Activity
3.2. Analysis of Changes Expression Levels of Related Genes
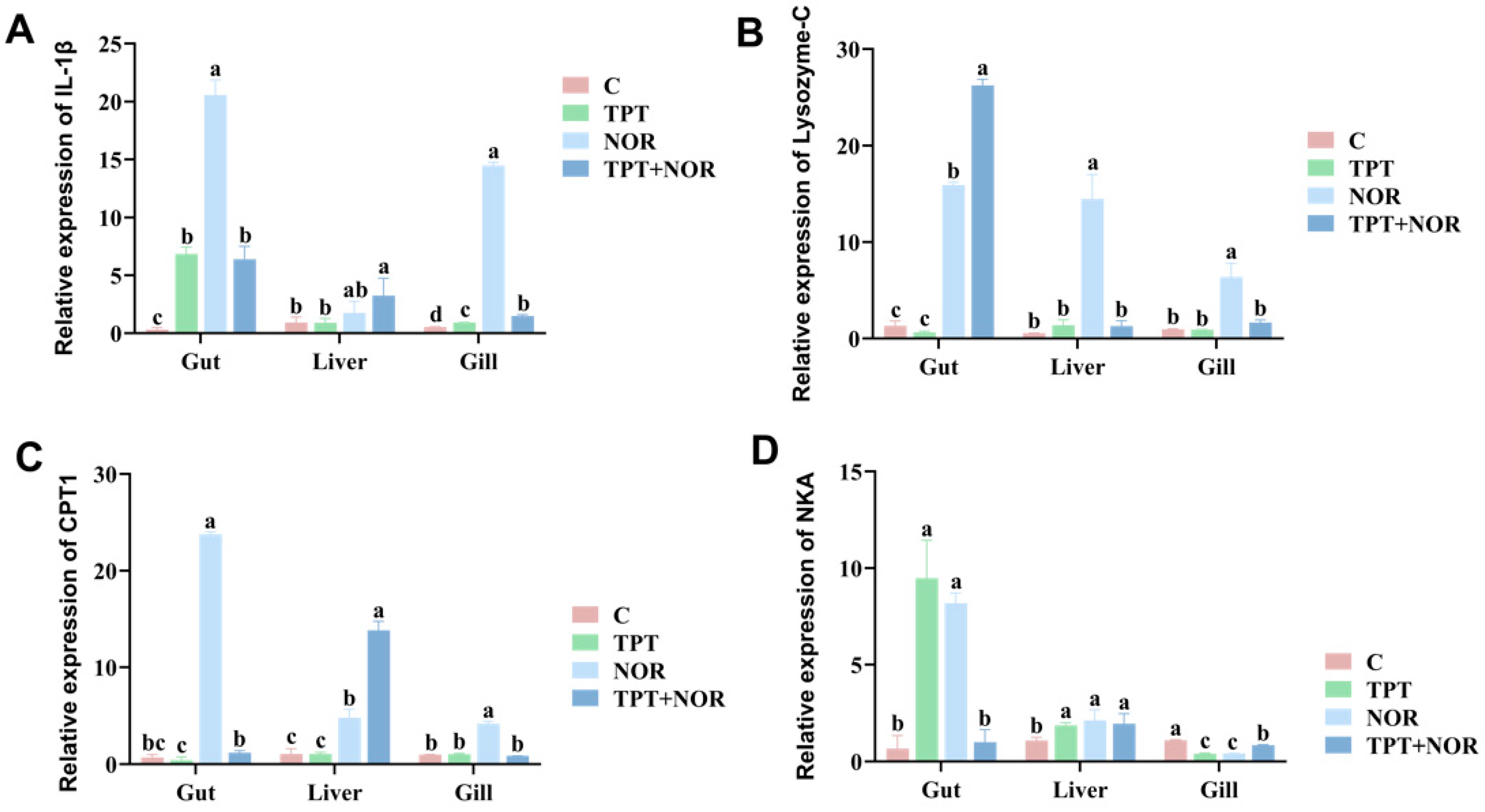
3.2.1. Differences in Expression Levels of Related Genes
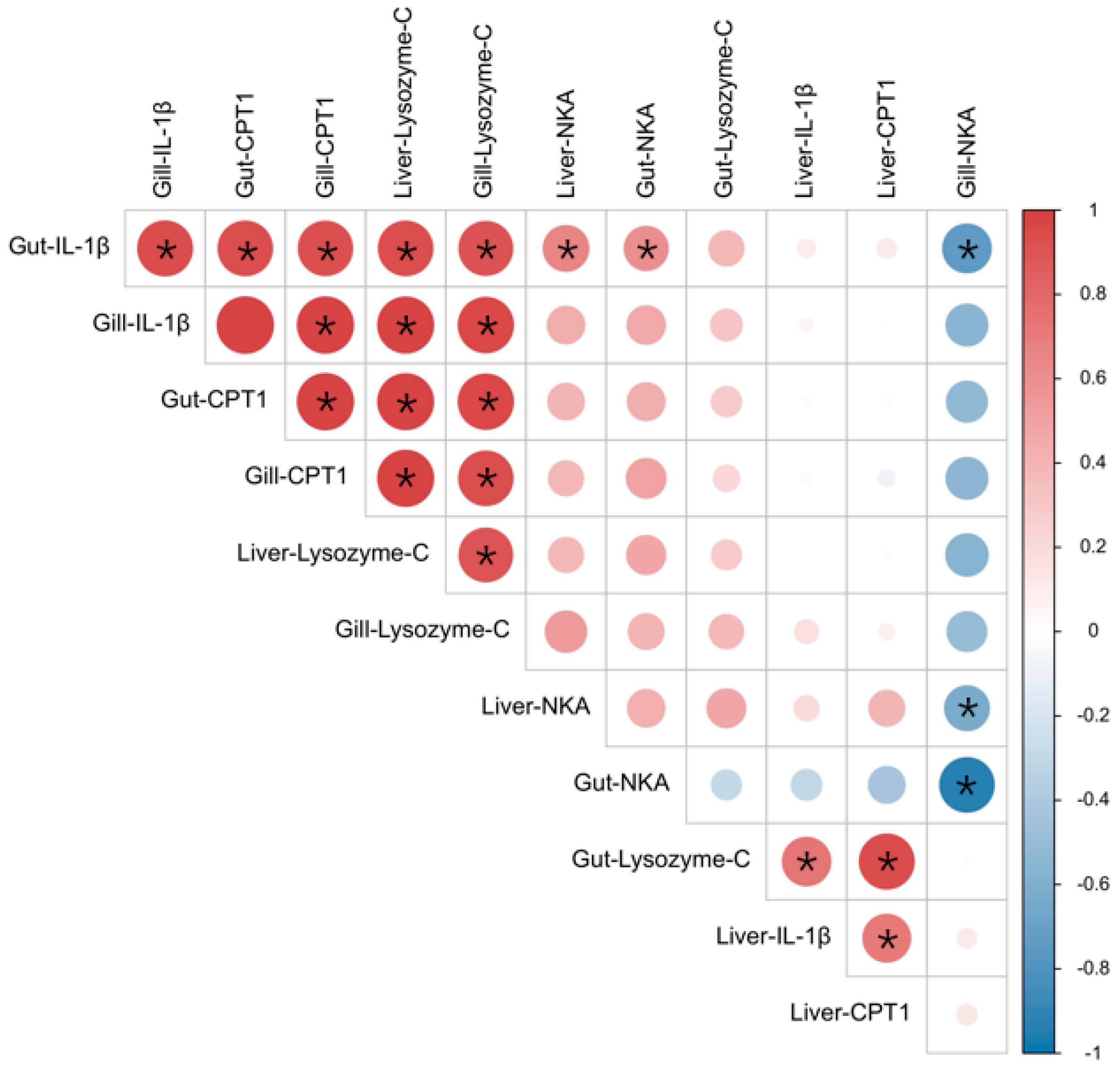
3.2.2. Analysis of Related Gene Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastasiou, T.I.; Chatzinikolaou, E.; Mandalakis, M.; Arvanitidis, C. Imposex and organotin compounds in ports of the Mediterranean and the Atlantic: Is the story over? Sci. Total Environ. 2016, 569–570, 1315–1329. [Google Scholar] [CrossRef]
- Snoeij, N.J.; Penninks, A.H.; Seinen, W. Biological activity of organotin compounds—An overview. Environ. Res. 1987, 44, 335–353. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Huang, Q.; Chen, Z.; Zhang, W. Organotin contamination in commercial and wild oysters from China: Increasing occurrence of triphenyltin. Sci. Total Environ. 2019, 650, 2527–2534. [Google Scholar] [CrossRef]
- Guérin, T.; Sirot, V.; Volatier, J.L.; Leblanc, J.C. Organotin levels in seafood and its implications for health risk in high-seafood consumers. Sci. Total Environ. 2007, 388, 66–77. [Google Scholar] [CrossRef]
- Liu, L.; Du, R.Y.; Jia, R.L.; Wang, J.X.; Chen, C.Z.; Li, P.; Li, Z.H.; Kong, L.M. Micro(nano)plastics in marine medaka: Entry pathways and cardiotoxicity with triphenyltin. Environ. Pollut. 2024, 342, 123079. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Hu, J.Y.; Zhen, H.J.; Wu, X.Q.; Huang, C. Reproductive Inhibition and Transgenerational Toxicity of Triphenyltin on Medaka (Oryzias latipes) at Environmentally Relevant tip Levels. Environ. Sci. Technol. 2008, 42, 8133–8139. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, X.; Yu, L.; Sun, Z.; Zhu, P.; Wang, X.; Shi, H. Stage-specific malformations and phenotypic changes induced in embryos of amphibian (Xenopus tropicalis) by triphenyltin. Ecotoxicol. Environ. Saf. 2011, 74, 1960–1966. [Google Scholar] [CrossRef]
- Gao, J.M.; Zhang, Y.; Guo, J.S.; Jin, F.; Zhang, K. Occurrence of organotins in the Yangtze River and the Jialing River in the urban section of Chongqing, China. Environ. Monit. Assess. 2013, 185, 3831–3837. [Google Scholar] [CrossRef]
- Liu, L.-L.; Wang, J.-T.; Chung, K.-N.; Leu, M.-Y.; Meng, P.-J. Distribution and accumulation of organotin species in seawater, sediments and organisms collected from a Taiwan mariculture area. Mar. Pollut. Bull. 2011, 63, 535–540. [Google Scholar] [CrossRef]
- Li, Y.; Mu, D.; Wu, H.-Q.; Liu, H.-J.; Wang, Y.-H.; Ma, G.-C.; Duan, X.-M.; Zhou, J.-J.; Zhang, C.-M.; Lu, X.-H.; et al. Derivation of copper water quality criteria in Bohai Bay for the protection of local aquatic life and the ecological risk assessment. Mar. Pollut. Bull. 2023, 190, 114863. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.H.; Zhong, L. Effects of low concentrations of triphenyltin on neurobehavior and the thyroid endocrine system in zebrafish. Ecotoxicol. Environ. Saf. 2019, 186, 109776. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Li, P.; He, S.W.; Xing, S.Y.; Cao, Z.H.; Zhao, X.L.; Sun, C.C.; Li, Z.H. Combined effect of microplastic and triphenyltin: Insights from the gut-brain axis. Environ. Sci. Ecotechnol. 2023, 16, 100266. [Google Scholar] [CrossRef]
- Morcillo, Y.; Porte, C. Interaction of tributyl- and triphenyltin with the microsomal monooxygenase system of molluscs and fish from the Western Mediterranean. Aquat. Toxicol. 1997, 38, 35–46. [Google Scholar] [CrossRef]
- Harino, H.; Fukushima, M.; Kawai, S. Accumulation of butyltin and phenyltin compounds in various fish species. Arch. Environ. Contam. Toxicol. 2000, 39, 13–19. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Zuo, Z.; Chen, Y.; Wang, X.; Huang, X.; Wang, C. Influence of triphenyltin exposure on the hypothalamus–pituitary–gonad axis in male Sebastiscus marmoratus. Aquat. Toxicol. 2011, 104, 263–269. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Li, P.; Zhao, X.-L.; He, S.-W.; Xing, S.-Y.; Cao, Z.-H.; Zhang, H.-Q.; Li, Z.-H. Hepatotoxicity in carp (Cyprinus carpio) exposed to environmental levels of norfloxacin (NOR): Some latest evidences from transcriptomics analysis, biochemical parameters and histopathological changes. Chemosphere 2021, 283, 131210. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T. A comprehensive review on quinolone contamination in environments: Current research progress. Environ. Sci. Pollut. Res. Int. 2023, 30, 48778–48792. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.-R.; Zhou, G.-J.; Liu, S.-S.; Yue, W.-Z.; Sun, K.-F.; Ying, G.-G. Antibiotics in the coastal environment of the Hailing Bay region, South China Sea: Spatial distribution, source analysis and ecological risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef]
- Zhao, X.L.; Li, P.; Zhang, S.Q.; He, S.W.; Xing, S.Y.; Cao, Z.H.; Lu, R.; Li, Z.H. Effects of environmental norfloxacin concentrations on the intestinal health and function of juvenile common carp and potential risk to humans. Environ. Pollut. 2021, 287, 117612. [Google Scholar] [CrossRef]
- Shi, H.; Yang, Y.; Liu, M.; Yan, C.; Yue, H.; Zhou, J. Occurrence and distribution of antibiotics in the surface sediments of the Yangtze Estuary and nearby coastal areas. Mar. Pollut. Bull. 2014, 83, 317–323. [Google Scholar] [CrossRef]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef]
- Diwan, V.; Tamhankar, A.J.; Aggarwal, M.; Sen, S.; Khandal, R.K.; Lundborg, C.S. Detection of antibiotics in hospital effluents in India. Curr. Sci. 2009, 97, 1752–1755. [Google Scholar]
- Lindberg, R.; Jarnheimer, P.-Å.; Olsen, B.; Johansson, M.; Tysklind, M. Determination of antibiotic substances in hospital sewage water using solid phase extraction and liquid chromatography/mass spectrometry and group analogue internal standards. Chemosphere 2004, 57, 1479–1488. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, S.Q.; Pan, J.L.; Zhu, F.; Wu, M.H.; Xu, G. Antibiotics in urine of the general population: Exposure, health risk assessment, and food factors. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2022, 57, 1–12. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Zhou, G.J.; Xu, E.G.B.; Wang, X.; Leung, K.M.Y. Long-term spatio-temporal trends of organotin contaminations in the marine environment of Hong Kong. PLoS ONE 2016, 11, e0155632. [Google Scholar] [CrossRef]
- Ebert, I.; Bachmann, J.; Kühnen, U.; Küster, A.; Kussatz, C.; Maletzki, D.; Schlüter, C. Toxicity of the fluoroquinolone antibiotics enrofloxacin and ciprofloxacin to photoautotrophic aquatic organisms. Environ. Toxicol. Chem. 2011, 30, 2786–2792. [Google Scholar] [CrossRef]
- Liang, X.M.; Wang, L.; Ou, R.K.; Nie, X.P.; Yang, Y.F.; Wang, F.; Li, K.B. Effects of norfioxacin on hepatic genes expression of P450 isoforms (CYP1A and CYP3A), GST and P-glycoprotein (P-gp) in Swordtail fish (Xiphophorus Helleri). Ecotoxicology 2015, 24, 1566–1573. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Wu, D.; Yan, Z. A multi-biomarker assessment of single and combined effects of norfloxacin and sulfamethoxazole on male goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2014, 102, 12–17. [Google Scholar] [CrossRef]
- Janecko, N.; Pokludova, L.; Blahova, J.; Svobodova, Z.; Literak, I. Implications of fluoroquinolone contamination for the aquatic environment—A review. Environ. Toxicol. Chem. 2016, 35, 2647–2656. [Google Scholar] [CrossRef]
- Zhang, C.-N.; Zhang, J.-L.; Ren, H.-T.; Zhou, B.-H.; Wu, Q.-J.; Sun, P. Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Rahman, M.F.; Siddiqui, M.K.J. Biochemical effects of vepacide (from Azadirachta indica) on Wistar rats during subchronic exposure. Ecotoxicol. Environ. Saf. 2004, 59, 332–339. [Google Scholar] [CrossRef]
- Enan, E.E.; Enan, O.H.; El-Sebae, A.E. Biochemical targets affected by sublethal doses of organophosphorus insecticides. J. Int. Pest Control 1982, 24, 120. [Google Scholar]
- Cajaraville, M.P.; Bebianno, M.J.; Blasco, J.; Porte, C.; Sarasquete, C.; Viarengo, A. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. Sci. Total Environ. 2000, 247, 295–311. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Leung, K.M.Y. Imposex status associated with organotin contamination in Reishia clavigera after reciprocal transplantation between clean and polluted sites in Hong Kong. Reg. Stud. Mar. Sci. 2016, 8, 480–486. [Google Scholar] [CrossRef]
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. Spatio-temporal variation of organotin compounds in seawater and sediments from Cape Town harbour, South Africa using gas chromatography with flame photometric detector (GC-FPD). Arab. J. Chem. 2016, 9, 95–104. [Google Scholar] [CrossRef]
- Le Page, G.; Gunnarsson, L.; Snape, J.; Tyler, C.R. Integrating human and environmental health in antibiotic risk assessment: A critical analysis of protection goals, species sensitivity and antimicrobial resistance. Environ. Int. 2017, 109, 155–169. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Corripio-Miyar, Y.; Bird, S.; Tsamopoulos, K.; Secombes, C.J. Cloning and expression analysis of two pro-inflammatory cytokines, IL-1β and IL-8, in haddock (Melanogrammus aeglefinus). Mol. Immunol. 2007, 44, 1361–1373. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Li, P.; He, S.-W.; Xing, S.-Y.; Cao, Z.-H.; Zhao, X.-L.; Sun, C.; Li, Z.-H. Assessing the ecotoxicity of combined exposure to triphenyltin and norfloxacin at environmental levels: A case study of immunotoxicity and metabolic regulation in carp (Cyprinus carpio). Chemosphere 2023, 313, 137381. [Google Scholar] [CrossRef]
- Wen, J.; Cui, X.; Gibson, M.; Li, Z. Water quality criteria derivation and ecological risk assessment for triphenyltin in China. Ecotoxicol. Environ. Saf. 2018, 161, 397–401. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary arginine supplementation on growth, biochemical, and immunological responses of common carp (Cyprinus carpio L.), stressed by stocking density. Aquaculture 2019, 503, 452–459. [Google Scholar] [CrossRef]
- Yuan, C.; Pan, X.; Gong, Y.; Xia, A.; Wu, G.; Tang, J.; Han, X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int. Immunopharmacol. 2008, 8, 51–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Cai, J.; Yang, J.; Shen, Q.; Xu, S. Chlorpyrifos exposure in common carp (Cyprinus carpio L.) leads to oxidative stress and immune responses. Fish Shellfish Immunol. 2017, 67, 604–611. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, C.; Huang, Y.; Dai, Y.; Desouky, H.E. A comparative study on intestinal morphology and function of normal and injured intestines of Jian carp (Cyprinus carpio var. Jian). Aquaculture 2020, 528, 735496. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, V.; Blanco, A.; Kelly, S.P.; Unniappan, S. Ion-poor water and dietary salt deprivation upregulate the ghrelinergic system in the goldfish (Carassius auratus). J. Fish Biol. 2021, 99, 1100–1109. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Li, P.; Wang, W.-B.; Li, Z.-H. Response of growth performance, serum biochemical parameters, antioxidant capacity, and digestive enzyme activity to different feeding strategies in common carp (Cyprinus carpio) under high-temperature stress. Aquaculture 2022, 548, 737636. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Qian, S.; Liu, P.; Li, X.; Li, J. Exposure to nitrate induces alterations in blood parameter responses, liver immunity, and lipid metabolism in juvenile turbot (Scophthalmus maximus). Aquat. Toxicol. 2022, 251, 106280. [Google Scholar] [CrossRef]
- Vandenberghe, G. The role of the liver in metabolic homeostasis—Implications for inborn-errors of metabolism. J. Inherit. Metab. Dis. 1991, 14, 407–420. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Huang, W.-B.; Wang, Q.-W.; Wu, M.-L.; Liu, J.; Wang, K.-J. Effects of tributyltin and benzo[a]pyrene on the immune-associated activities of hemocytes and recovery responses in the gastropod abalone, Haliotis diversicolor. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 120–128. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, Z.; Jin, Y. Immunotoxic effects of atrazine and its main metabolites at environmental relevant concentrations on larval zebrafish (Danio rerio). Chemosphere 2017, 166, 212–220. [Google Scholar] [CrossRef]
- Li, Z.H.; Xu, H.Y.; Zheng, W.L.; Lam, S.H.; Gong, Z.Y. RNA-Sequencing Analysis of TCDD-Induced Responses in Zebrafish Liver Reveals High Relatedness to Mammalian Models and Conserved Biological Pathways. PLoS ONE 2013, 8, e77292. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, D.; Ge, R.; Bao, Z. Fecal transplantation of young mouse donors effectively improves enterotoxicity in elderly recipients exposed to triphenyltin. Ecotoxicol. Environ. Saf. 2024, 273, 116140. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Li, W.; Li, X. Hepatotoxicity of paraquat on common carp (Cyprinus carpio L.). Sci. Total Environ. 2018, 616–617, 889–898. [Google Scholar] [CrossRef]
- Horiguchi, T.; Kojima, M.; Kaya, M.; Matsuo, T.; Shiraishi, H.; Morita, M.; Adachi, Y. Tributyltin and triphenyltin induce spermatogenesis in ovary of female abalone, Haliotis gigantea. Mar. Environ. Res. 2002, 54, 679–684. [Google Scholar] [CrossRef]
- Ghelichpour, M.; Taheri Mirghaed, A.; Hoseinifar, S.H.; Khalili, M.; Yousefi, M.; Van Doan, H.; Perez-Jimenez, A. Expression of immune, antioxidant and stress related genes in different organs of common carp exposed to indoxacarb. Aquat. Toxicol. 2019, 208, 208–216. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Song, G. Immunotoxicological effects of insecticides in exposed fishes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 247, 109064. [Google Scholar] [CrossRef]
- Joshi, P.K.; Bose, M.; Harish, D. Changes in certain haematological parameters in a siluroid cat fish Clarias batrachus (Linn) exposed to cadmium chloride. Pollut. Res. 2002, 21, 129–131. [Google Scholar]
- Rajalakshmi, S.; Mohandas, A. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicol. Environ. Saf. 2005, 62, 140–143. [Google Scholar] [CrossRef]
- McClellan-Green, P.; Robbins, J. Effects of TBT and 3-MC co-exposure on cytochrome P450 expression and activity in marine organisms. Mar. Environ. Res. 2000, 50, 243. [Google Scholar] [CrossRef]
- Zhou, L.; Limbu, S.M.; Shen, M.; Zhai, W.; Qiao, F.; He, A.; Du, Z.-Y.; Zhang, M. Environmental concentrations of antibiotics impair zebrafish gut health. Environ. Pollut. 2018, 235, 245–254. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Hwang, P.-P.; Lee, T.-H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Sun, H.J.; Cao, L.; Wu, Z.Y.; Leng, B.; Bian, J.S. Role of Na+/K+-ATPase in ischemic stroke: In-depth perspectives from physiology to pharmacology. J. Mol. Med.-JMM 2022, 100, 395–410. [Google Scholar] [CrossRef]
- Lu, W.; Long, L.; Zhao, P.; Zhang, X.; Yan, C.; Dong, S.; Huang, Q. Perfluorinated compounds disrupted osmoregulation in Oryzias melastigma during acclimation to hypoosmotic environment. Ecotoxicol. Environ. Saf. 2021, 223, 112613. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, X.; Xue, X.; Zhu, X.; Chen, Y.; Yang, Z. Mitigation of nitrite toxicity by increased salinity is associated with multiple physiological responses: A case study using an economically important model species, the juvenile obscure puffer (Takifugu obscurus). Environ. Pollut. 2018, 232, 137–145. [Google Scholar] [CrossRef]
- Ngugi, C.C.; Oyoo-Okoth, E.; Mugo-Bundi, J.; Orina, P.S.; Chemoiwa, E.J.; Aloo, P.A. Effects of dietary administration of stinging nettle (Urtica dioica) on the growth performance, biochemical, hematological and immunological parameters in juvenile and adult Victoria Labeo (Labeo victorianus) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 44, 533–541. [Google Scholar] [CrossRef]
- Marcon, L.; Lopes, D.S.; Mounteer, A.H.; Goulart, A.M.A.; Leandro, M.V.; dos Anjos Benjamin, L. Pathological and histometric analysis of the gills of female Hyphessobrycon eques (Teleostei:Characidae) exposed to different concentrations of the insecticide Dimilin®. Ecotoxicol. Environ. Saf. 2016, 131, 135–142. [Google Scholar] [CrossRef]
- Crestani, M.; Menezes, C.; Glusczak, L.; Santos Miron, D.d.; Spanevello, R.; Silveira, A.; Gonçalves, F.F.; Zanella, R.; Loro, V.L. Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 2007, 67, 2305–2311. [Google Scholar] [CrossRef]
- Paulino, M.G.; Souza, N.E.S.; Fernandes, M.N. Subchronic exposure to atrazine induces biochemical and histopathological changes in the gills of a Neotropical freshwater fish, Prochilodus lineatus. Ecotoxicol. Environ. Saf. 2012, 80, 6–13. [Google Scholar] [CrossRef]
- Boran, H.; Capkin, E.; Altinok, I.; Terzi, E. Assessment of acute toxicity and histopathology of the fungicide captan in rainbow trout. Exp. Toxicol. Pathol. 2012, 64, 175–179. [Google Scholar] [CrossRef]
- Sonne, C.; Bach, L.; Søndergaard, J.; Rigét, F.F.; Dietz, R.; Mosbech, A.; Leifsson, P.S.; Gustavson, K. Evaluation of the use of common sculpin (Myoxocephalus scorpius) organ histology as bioindicator for element exposure in the fjord of the mining area Maarmorilik, West Greenland. Environ. Res. 2014, 133, 304–311. [Google Scholar] [CrossRef]
- Lutfi, E.; Riera-Heredia, N.; Córdoba, M.; Porte, C.; Gutiérrez, J.; Capilla, E.; Navarro, I. Tributyltin and triphenyltin exposure promotes in vitro adipogenic differentiation but alters the adipocyte phenotype in rainbow trout. Aquat. Toxicol. 2017, 188, 148–158. [Google Scholar] [CrossRef]
- Lubrano, C.; Genovesi, G.; Specchia, P.; Costantini, D.; Mariani, S.; Petrangeli, E.; Lenzi, A.; Gnessi, L. Obesity and metabolic comorbidities: Environmental diseases? Oxidative Med. Cell. Longev. 2013, 2013, 640673. [Google Scholar] [CrossRef]
- Soler-Vázquez, M.C.; Romero, M.d.M.; Todorcevic, M.; Delgado, K.; Calatayud, C.; Benitez-Amaro, A.; La Chica Lhoëst, M.T.; Mera, P.; Zagmutt, S.; Bastías-Pérez, M.; et al. Implantation of CPT1AM-expressing adipocytes reduces obesity and glucose intolerance in mice. Metab. Eng. 2023, 77, 256–272. [Google Scholar] [CrossRef]
- Tang, Y.M.; Zhang, W.Y.; Wang, Y.G.; Li, H.Y.; Zhang, C.H.; Wang, Y.; Lin, Y.Q.; Shi, H.B.; Xiang, H.; Huang, L.; et al. Expression Variation of CPT1A Induces Lipid Reconstruction in Goat Intramuscular Precursor Adipocytes. Int. J. Mol. Sci. 2023, 24, 13415. [Google Scholar] [CrossRef]

| Gene | Sequences of Primers (5′−3′) | Accession No. | qPCR Efficiency |
|---|---|---|---|
| IL-1β | F: ACCAGCTGGATTTGTCAGAAG R: ACATACTGAATTGAACTTTG | AB010701.1 | 98.7190% |
| Lysozyme-C | F: GTGTCTGATGTGGCTGTGCT R: TTCCCCAGGTATCCCATGAT | AB027305 | 98.0435% |
| CPT1 | F: CAGATGGAAAGTGTTGCTAATGAC R: TGTGTAGAAGTTGCTGTTGACCA | JF728839 | 98.9315% |
| NKA | F: TGCCAGAACTTCTCCACA R: AGCGATACCCATAGCCAC | JN032759 | 95.7576% |
| β-actin | F: GGCTGTGCTGTCCCTGTA R: GGCGTAACCCTCGTAG | M25013 | 97.5814% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, L.; Zhang, S.; Yin, M.; Li, T.; Zeng, B.; Liu, L.; Li, P.; Li, Z. Tissue-Specific Toxicity in Common Carp (Cyprinus carpio) Caused by Combined Exposure to Triphenyltin and Norfloxacin. Fishes 2024, 9, 415. https://doi.org/10.3390/fishes9100415
Liu Y, Li L, Zhang S, Yin M, Li T, Zeng B, Liu L, Li P, Li Z. Tissue-Specific Toxicity in Common Carp (Cyprinus carpio) Caused by Combined Exposure to Triphenyltin and Norfloxacin. Fishes. 2024; 9(10):415. https://doi.org/10.3390/fishes9100415
Chicago/Turabian StyleLiu, Yiwei, Luoxin Li, Siqi Zhang, Minghao Yin, Tengzhou Li, Bianhao Zeng, Ling Liu, Ping Li, and Zhihua Li. 2024. "Tissue-Specific Toxicity in Common Carp (Cyprinus carpio) Caused by Combined Exposure to Triphenyltin and Norfloxacin" Fishes 9, no. 10: 415. https://doi.org/10.3390/fishes9100415
APA StyleLiu, Y., Li, L., Zhang, S., Yin, M., Li, T., Zeng, B., Liu, L., Li, P., & Li, Z. (2024). Tissue-Specific Toxicity in Common Carp (Cyprinus carpio) Caused by Combined Exposure to Triphenyltin and Norfloxacin. Fishes, 9(10), 415. https://doi.org/10.3390/fishes9100415







