1. Introduction
The global production of fisheries and aquaculture has notably increased, which may be primarily attributed to the substantial expansion of aquaculture production on a global scale, with Asia emerging as a prominent contributor to this rise [
1]. Consequently, there has been a substantial surge in the demand for feed ingredients, notably fishmeal. In order to ensure the continued sustainability of aquaculture, it is imperative to incorporate alternative proteins that can either complement or replace fishmeal in aquafeeds [
2]. Cottonseed meal emerges as a highly attractive alternative in comparison to other plant protein sources owing to its notable protein content, extensive accessibility, and cost-effectiveness [
3]. Nevertheless, the presence of gossypol and the insufficient levels of critical amino acids in cottonseed meal pose restrictions on its application in aquaculture feeds [
4]. The intestine plays a crucial role in the digestive system of fish, serving as the primary organ responsible for the process of digestion and the absorption of nutrients [
5]. Substituting fishmeal with a large amount of plant proteins in fish feed might cause harm to the intestines of carnivorous fish because of the elevated presence of antinutritional substances found in plant protein sources [
6]. Many studies have previously shown that dietary gossypol addition can reduced intestinal immunity and aggravated inflammation in growing grass carp (
Ctenopharyngodon idella) [
7]. The hepatic antioxidant defense system plays a crucial role in maintaining the proper physiological function and overall health of an animal body [
8].The Keap1–Nrf2 system is currently recognized as the major cellular defense mechanism under oxidative stress [
9]. It has been reported that a high-gossypol acetic diet induced excessive hepatocyte injures in carp (
Carassius auratus gibelio) [
10]. Previous studies have shown that replacing 70% of dietary fishmeal with cottonseed protein concentrate produced negative effects on the growth performance and flesh quality of largemouth bass (
Micropterus salmoides) [
11]. Fermentation is characterized by the absence of free water inside the fermentation medium, since water is assimilated into the solid substrate to facilitate microbial growth and metabolic processes. Fermented components provide the ability to be incorporated directly into formulations or employed as additions in animal feed [
12]. Fermentation with
Bacillus subtilis can digest free cotton phenol and improve the nutritional quality of cottonseed meal [
13]. Therefore, fermented cottonseed meal was selected as a feasible substitute for fishmeal. Previous studies have shown that the FI (feed intake) and WGR (weight gain rate) of crucian carp (
Carassius auratus) increased as the proportion of fermented cottonseed meal was gradually increased compared with the control group [
14].
Animal tissues are amply populated with taurine, a sulfonic acid [
15]. In recent years, several studies have demonstrated the indispensability of dietary taurine for various economically significant species, with a special emphasis on marine teleost fish [
16]. As a result, the elimination of dietary components that are high in taurine, such as fishmeal, can lead to deficits characterized by various symptoms, including diminished growth and survival rates, heightened vulnerability to diseases, and hindered development [
12]. In cases when taurine is insufficiently provided by feed, its deficiency mostly manifests as sluggish growth, diminished survival rates, and compromised antioxidant and immunological capabilities [
17]. When taurine was added to the diet of golden pompano, there were significant improvements in growth performance and antioxidant status, as well as a significant reduction in lipid peroxidation [
18]. Adding taurine at a dosage of 0.8% has been shown to significantly improve carbohydrate synthesis, protein digestion and absorption, and fat deposition in tilapia. Therefore, this supplementation enhances the general growth and development of tilapia [
19]. Taurine is an essential β-amino acid that serves several biological functions and has significant importance in the growth and development of fish [
20].
Cottonseed protein concentrate (CPC) is a recently developed form of cottonseed protein that is obtained through a process of extraction from cottonseed meal by solvent through a pressing process extraction occurring during cottonseed meal production [
11]. CPC has the advantage of containing low levels of free gossypol and a high crude protein content ranging from 60 to 70% [
11]. Research indicates that cottonseed protein antimicrobial peptides have the ability to cause harm to the cell membrane by interacting with its surface. Additionally, cottonseed protein hydrolysate shows great potential as a natural source of antimicrobial agents [
21]. Studies suggest that replacing fishmeal with enzymatic cottonseed protein has a positive effect on the growth, immunity, and intestinal health of Chinese Soft-Shelled Turtles (
Pelodiscus sinensis) [
22]. Studies suggest that replacing fishmeal with enzymatic cottonseed protein has a positive effect on the liver antioxidant capacity and immune status of largemouth bass (
Micropterus salmoides) [
23]. Therefore, we selected taurine and enzymatic cottonseed protein concentrate to assess the impact of low-fishmeal and high-fermented cottonseed meal diets on the growth performance and feed utilization of golden pompano.
Trachinotus ovatus (
T. ovatus), also referred to as the golden pompano, possesses a soft and fatty flesh, devoid of intermuscular spines. This species holds significant economic value in the field of marine aquaculture and enjoys widespread consumer popularity [
24]. In this experiment, golden pompano was fed with 0.5% taurine (CSM-T), 2% enzymatic cottonseed protein (CSM-C), 1% enzymatic cottonseed protein, and 0.25% taurine (CSM-TC) diets. Therefore, the present study aimed at evaluating the effect of ECPC and taurine supplementation to a plant-based diet on growth performance, body composition, antioxidant capacity, and intestinal flora in golden pompano. The findings of this study can serve as a theoretical basis for the appropriate use of these additives in
T. ovatus diets.
4. Discussion
Taurine assumes a significant role in the nutritional aspects, metabolic processes, immune control, as well as growth and developmental mechanisms in fish [
34,
35]. The addition of taurine can improve the performance of channel catfish (
Ictalurus punetaus) [
36], senegalese sole (
Solea senegalensis) [
37], and rainbow trout (
Oncorhynchus mykiss) [
38] by increasing feed conversion, protein efficiency, fish protein, and growth performance. A study investigating the use of ECPC as a replacement for fishmeal found that adding moderate amounts of this concentrate did not have any noticeable effect on the growth of largemouth bass [
23]. In the current investigation, the inclusion of taurine and ECPC did not negatively affect golden pompano. The results indicated that compared with other groups, the addition of 1% ECPC and 0.25% taurine has been found to enhance the WGR, SGR, and CF. The variation may be attributable to the simultaneous addition of taurine and ECPC, the fish species, or the experimental conditions. The underlying mechanisms in fish species need further detailed studies.
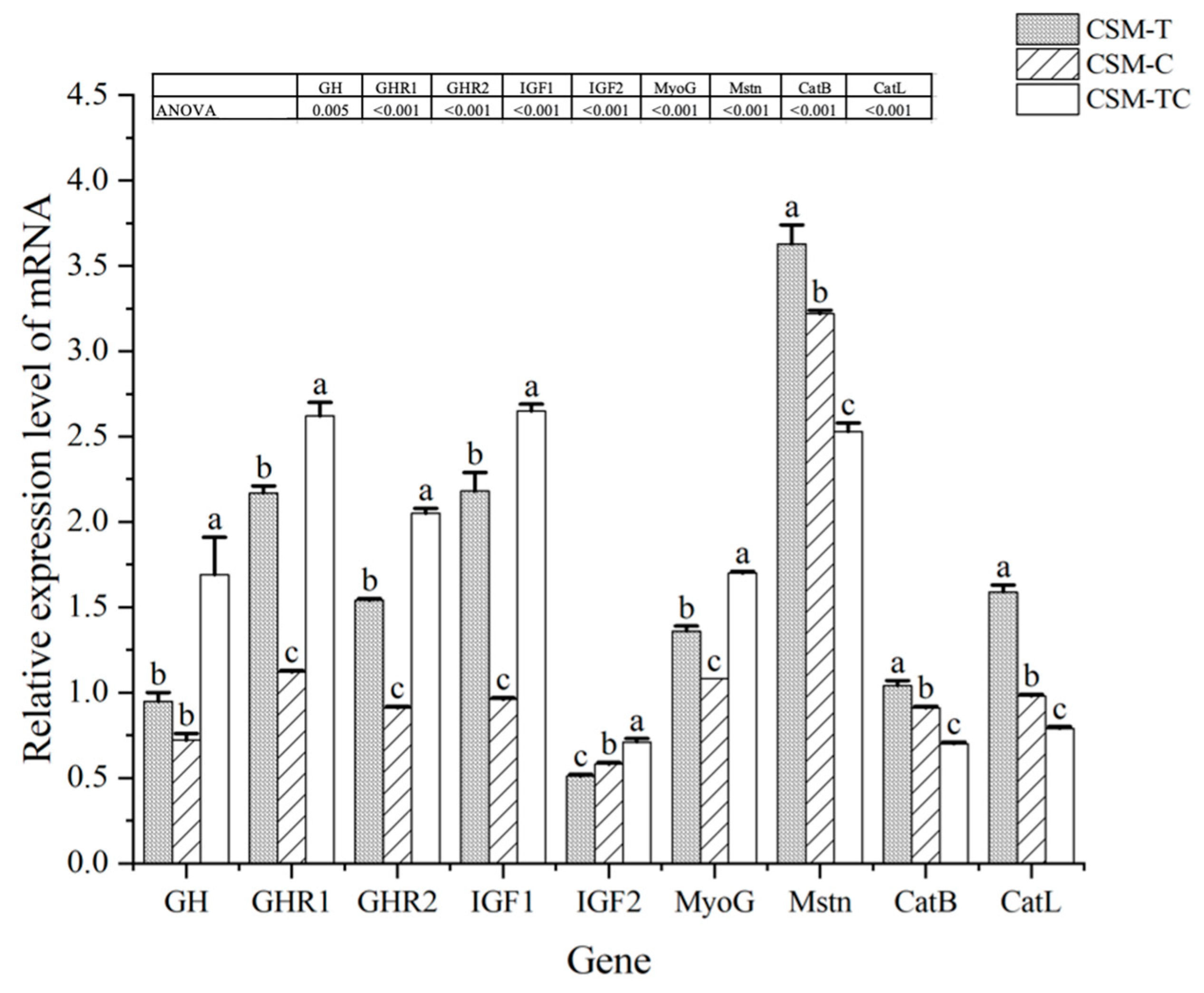
The primary consumable component of fish is muscle tissue, and research has demonstrated that the quality of fish muscle can be enhanced by nutritional management [
39]. According to the research conducted by Yan Liangchao, it was found that taurine has the potential to enhance the muscular characteristics of fish. The enhancement in muscle quality was correlated with an augmentation in muscle nutrition, taste amino acids, and healthy fatty acids, with improvements in muscle physical qualities such as shear, water holding capacity, pH, and antioxidant capacity [
40]. In the present study, the relative gene expression of
GH,
GHR1,
GHR2,
IGF1,
IGF2, and
MyoG in fish fed with CSM-TC were significantly higher than those of the other groups. The trait of growth is determined by multiple genes and is also influenced by environmental factors. Among these genes, the most significant ones are those responsible for
growth hormone (GH) and
insulin-like growth factor-I (IGF-I), as they play a central role in the
hypothalamic–pituitary–somatotropic (HPS) axis [
41]. The primary control of somatic growth is regulated by the
growth hormone (GH)/
insulin-like growth factor (IGF) axis. This crucial process is facilitated by a singular transmembrane receptor known as the
growth hormone receptor (GHR). These subtypes are regulated by distinct genes, leading to divergent physiological outcomes. This review centers on the examination of
IGF-I, a crucial component of the
GH/IGF-I axis that plays a significant role in the process of growth [
41]. The gene expressions of
growth hormone (GH),
growth hormone receptor 1 (GHR1),
growth hormone receptor 2 (GHR2),
insulin-like growth factor 1 (IGF1), and
insulin-like growth factor 2 (IGF2) in the CSM-TC group exhibited greater levels compared with other groups. This suggests that the inclusion of 1% ECPC and 0.25% taurine in the diet may enhance fish growth. MRFs are transcription factors that belong to the basic helix–loop–helix (bHLH) family. These factors play a crucial role in controlling muscle hyperplasia and hypertrophy, which include the gene
Myog [
42]. The mechanism by which
MSTN exerts its effects is widely accepted to involve the suppression of satellite cell activation, self-renewal, and proliferation [
43].
Cathepsin B (
CatB) and
Cathepsin L (
CatL) are significant constituents of the cysteine protease family, predominantly localized within lysosomes and implicated in a diverse range of physiological processes. Upon liberation from lysosomes, they possess the capability to impair the structural integrity of muscle proteins and expedite the process of muscle softening [
44]. There is limited literature available regarding the potential effects of incorporating taurine and ECPC on muscle quality. In the present study, the relative gene expressions of
Mstn,
CatB, and
CatL in fish fed with the CSM-TC diet were significantly lower than those of the other groups. This study’s findings that the addition of 1% ECPC and 0.25% taurine has increased muscle development and reduced quality degradation in fish.
AKP holds significant regulatory importance and is closely linked to various critical processes. It plays a crucial role in facilitating the absorption of important nutrients such as lipids, glucose, calcium, and inorganic phosphates [
45]. In the present study, CSM-TC significantly improved plasma AKP activity, which indicated that the process of nutrient absorption in golden pompano improved [
46]. C4 is a globulin found in fish that displays zymogenic activity, enabling it to effectively eliminate or eliminate harmful bacteria [
47]. In the present study, compared with the other groups, plasma AKP, C3, and C4 activity in the CSM-TC group were significantly higher. These findings suggest that the appropriate inclusion of taurine can enhance the fish’s immune response, which may be compromised when fishmeal is replaced with FCSM. This is similar to the findings of Zhang et al. [
48]. Hence, the findings of this study demonstrated that the incorporation of 1% ECPC and 0.25% taurine enhanced plasma nutritional absorption mechanisms when FCSM replaced 50% of fishmeal.
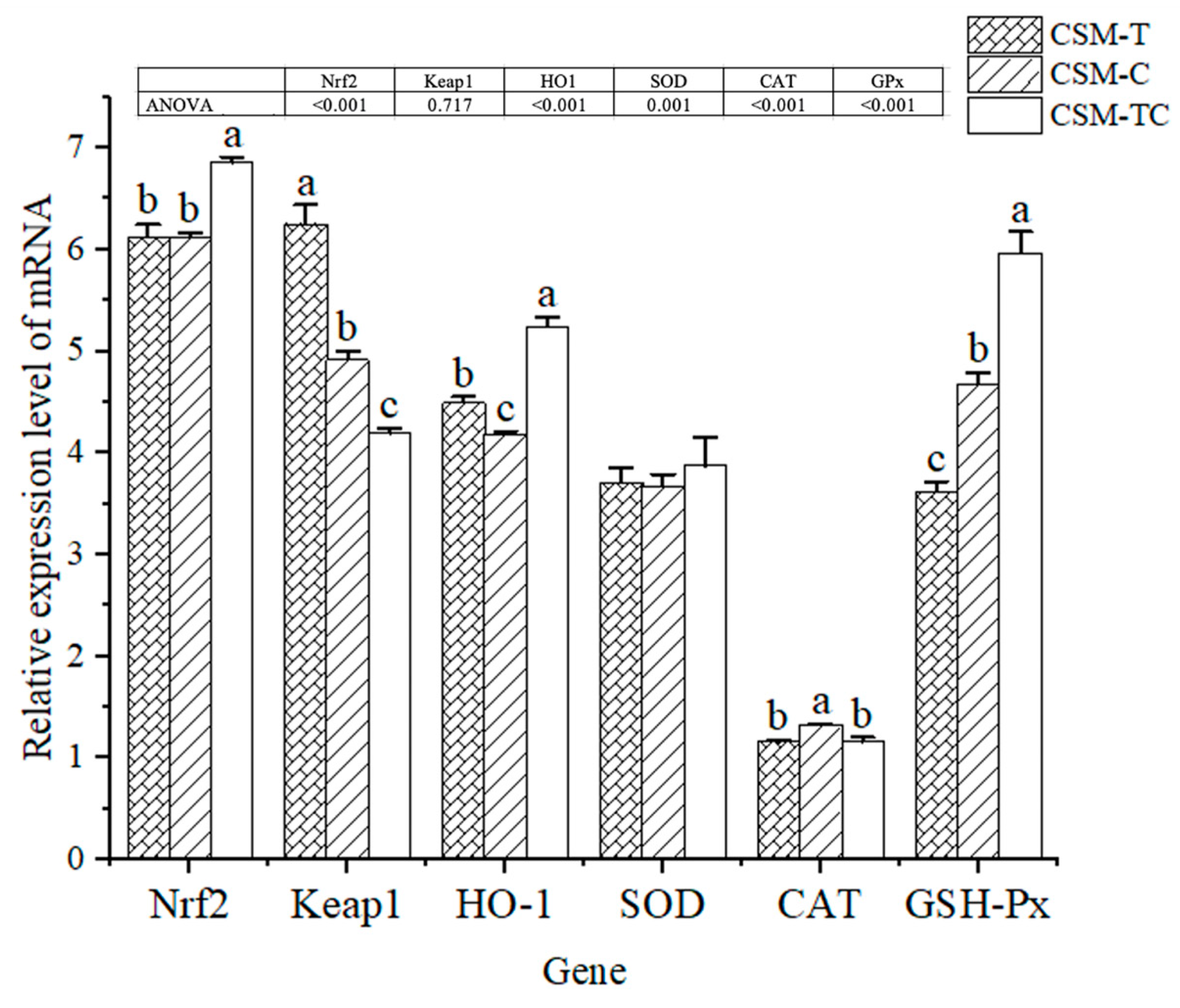
If the reactive oxygen radicals (ROS) that are produced during animal metabolism are not effectively eliminated, they can cause the oxidation of lipids, specifically ω-3 and ω-6 fatty acids. This oxidation process results in the formation of lipid oxidation products, such as MDA [
49]. In severe cases, this can lead to apoptosis. The level of reactive oxygen species (ROS) can be used as an indirect measure of the amount of reactive oxygen radicals and the degree of lipid peroxidation in tissues and cells [
50]. In the present study, MDA in fish fed with CSM-TC was lower than other groups. Consequently, the inclusion of 1% ECPC and 0.25% taurine can effectively mitigate the occurrence of lipid oxidation. Similar results were also found in marine carnivorous fish,
Scophthalmus maximus L. [
51]. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) are essential intracellular enzymes that play a critical role in antioxidant defense systems [
52]. SOD is responsible for scavenging superoxide anion radicals, while CAT eliminates hydrogen peroxide within the organism [
50]. The activity of these enzymes can serve as an indicator of the organism’s capacity to counteract oxygen radicals. In the present study, SOD, GSH-Px, and T-AOC in fish fed with the CSM-TC diet were significantly higher than those of the other groups. Similar results were also found in
Litopenaeus vannamei [
53]. The transcription factor
Nrf-2, which acts as a mediator in the
Keap1-Nrf2-ARE signaling pathway, plays a substantial role in modulating inflammatory responses [
54]. The primary line of enzymatic defense against free radicals in organisms consists of the antioxidant enzymes
CAT,
SOD, and
GSH-Px [
45]. Previous studies showed that taurine binds to hypochlorous acid to form tauroch loramine, which activates the Nrf2/ARE signaling pathway and upregulates the relative gene expression of antioxidant enzymes [
55]. In the present study, the relative gene expression of
Nrf-2,
HO-1, and
GSH-Px in fish fed with CSM-TC were significantly higher than those of the other groups. Similar results were also found in
Rhynchocypris lagowskii Dybowski [
56].
It has been reported that the growth performance and enzymatic activity of lipase and amylase in
Anguilla were significantly affected by the addition of a certain amount of taurine [
57]. In the current study, the activities of chymotrypsin and AMY were found to increase in CSM-T and CSM-TC compared to CSM-C. Similarly, the previous analysis on
Lateolabrax maculatus indicated that taurine supplementation resulted in an elevation of lipase activity and a reduction in hepatic fat content [
58]. Research has demonstrated that the reduction in the relative gene expression of proinflammatory factors TNF-a and IL-8, along with the increase in the mRNA level of the anti-inflammatory factor IL-10, can effectively impede inflammation within immunological organs [
59]. In the present investigation, the relative gene expression of IL-10 in fish fed with CSM-TC were significantly higher than those of the other groups. These results suggest that the appropriate inclusion of taurine and ECPC can ameliorate the inflammatory damage caused by the substitution of fishmeal with fermented cotton meal in the intestinal tract. The previous study showed that taurine may possess the ability to mitigate intestinal inflammation induced by high levels of phytoalexins. This is achieved through the modulation of the
TLRs/NF-κB signaling pathway [
60]. The role of
NF-κB, a crucial regulator of gene expression associated with proinflammatory processes, has been documented to have a substantial impact on inflammation [
61].
TNF-α and
IL-1β are recognized as potent proinflammatory factors that play a crucial role in the initiation and progression of inflammation organisms. These molecules exhibit a high sensitivity to changes in tissue damage and are considered the primary mediators of the inflammatory response [
62]. In the present study, the relative gene expression of
NF-κB,
TNF-α, and
IL-1β in fish fed with CSM-TC were significantly lower than those of the other groups. These findings suggest that the inclusion of 1% ECPC and 0.25% taurine in the diet led to an improvement in intestinal inflammation in golden pompano. Intestinal permeability has traditionally been regarded as a reliable measure of the integrity and functionality of the intestinal epithelial barrier. The regulation of this barrier was predominantly governed by a highly organized system of an epithelial junctional complex known as the tight junction [
63,
64].
Occludin and
ZO-1 play pivotal roles in the structural and functional organization of tight junctions, making them very significant components [
65].
Claudin-3 protein is essential for strengthening the integrity of the paracellular intestinal barrier by facilitating the establishment of tight junctions (TJ) [
66].
Claudin-15 is widely present in the tight junctions of both the villi and crypt cells in the small and large intestines [
67]. The relative gene expression of
ZO-1,
Claudin-3,
Claudin-15, and
Occludin in fish fed with CSM-TC were significantly higher than those of the other groups. These results suggest that the addition of 1% ECPC and 0.25% taurine can enhance the fish gut’s physical barrier capacity. This suggests that the inclusion of taurine and ECPC may have a beneficial effect on intestinal protection.
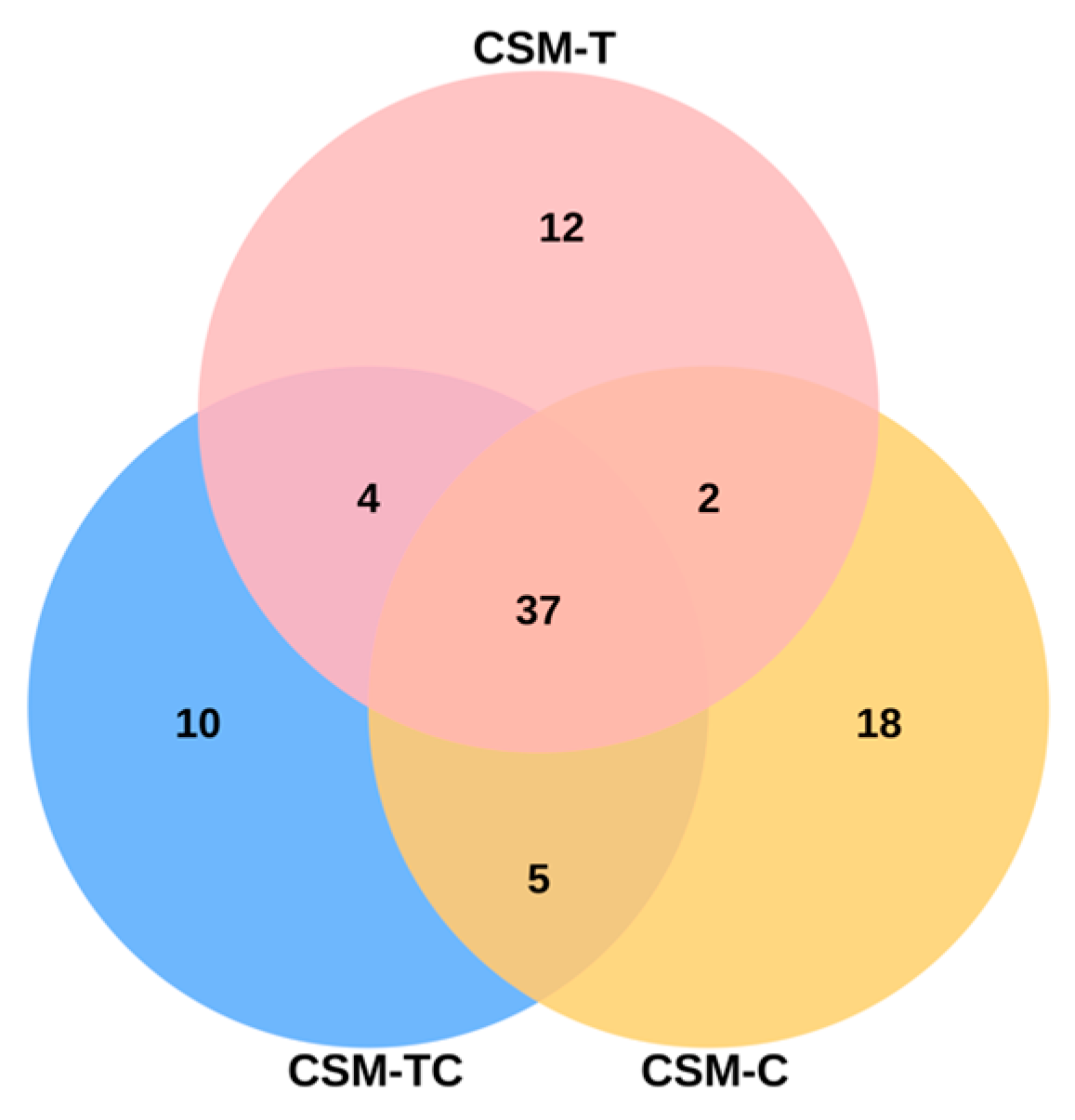
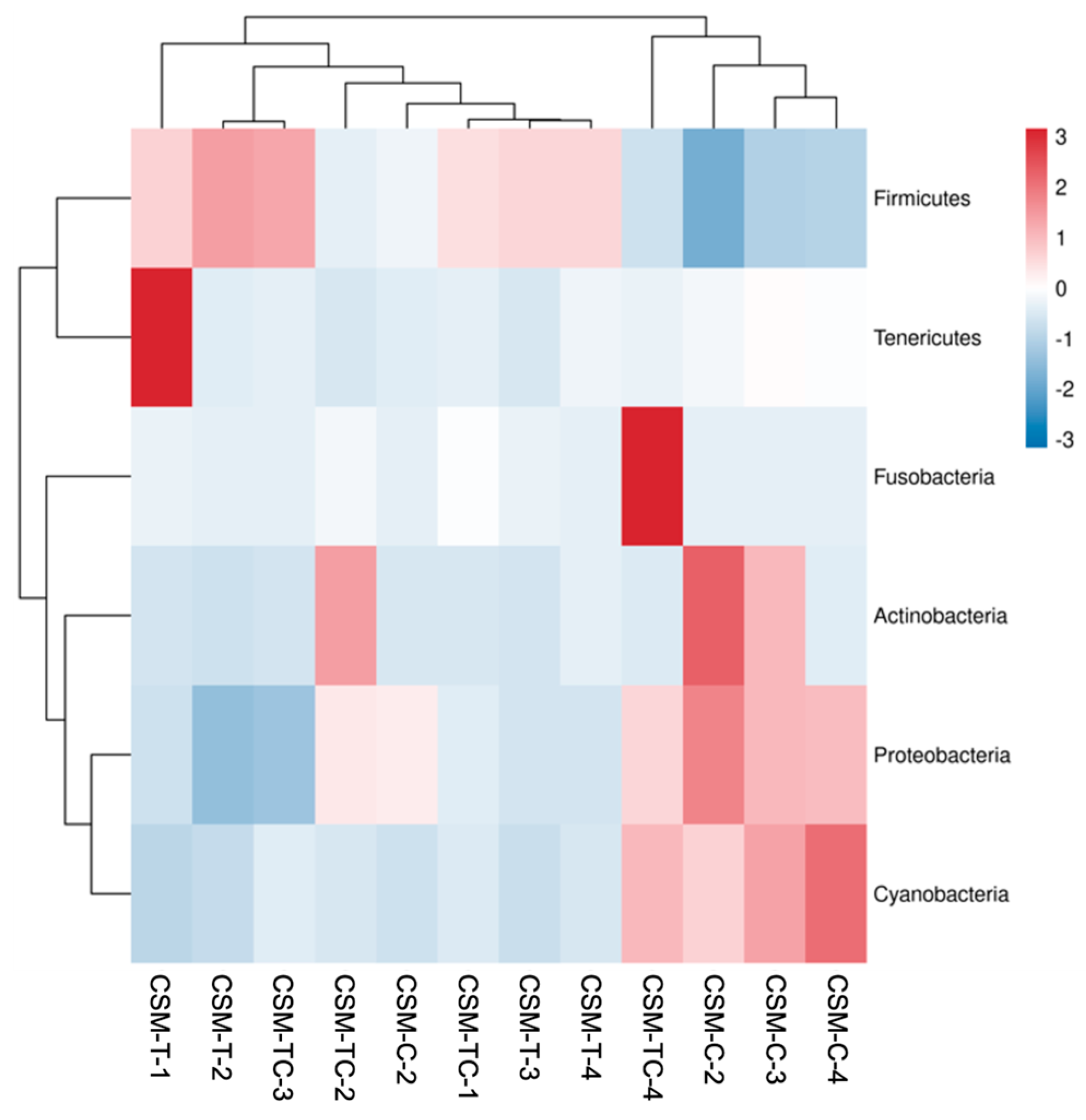
This study examined the effects of adding taurine and ECPC to the diet on the analysis of gut flora’s Alpha diversity. The findings revealed that this dietary intervention effectively regulated the abundance and diversity of intestinal microflora, thereby leading to alterations in the structure of the intestinal flora. The predominant microbial community inhabiting the intestines of golden pompano is composed of Proteobacteria, a finding that aligns with previous research conducted on this species [
18]. The findings of this experiment indicate that the phylum Proteobacteria displayed the highest prevalence within the CSM-TC group. This finding implies that the incorporation of taurine into the diet has the potential to modulate the richness and composition of the gut microbiota. These results align with the research conducted by Ma Qiwei et al. [
68]. The alterations in the makeup of gut microbiota have the potential to play a role in the development of gastrointestinal illnesses. Further research is needed to confirm their impact on the intestinal tract of fish.