Exploring the Dual Benefits of Fermented and Non-Fermented Garlic Powder on Growth, Antioxidative Capacity, Immune Responses, and Histology in Gray Mullet (Liza ramada)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and Experimental Setup
2.2. Source and Preparation of Garlic Forms
2.3. Formulation of Experimental Diets
2.4. Sample Collection and Performance Metrics
2.5. Digestive Enzyme Activity
2.6. Blood Biochemical Parameters
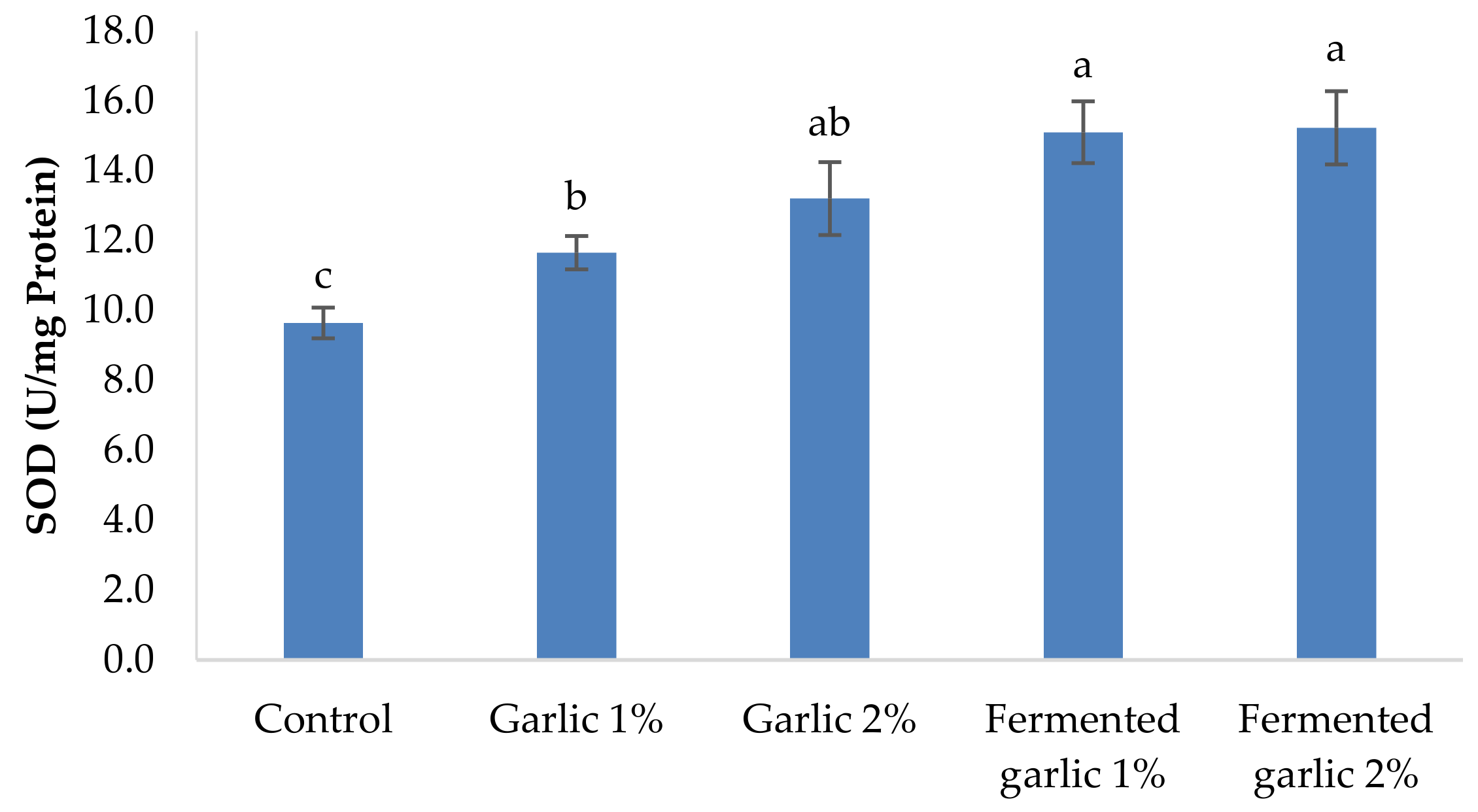
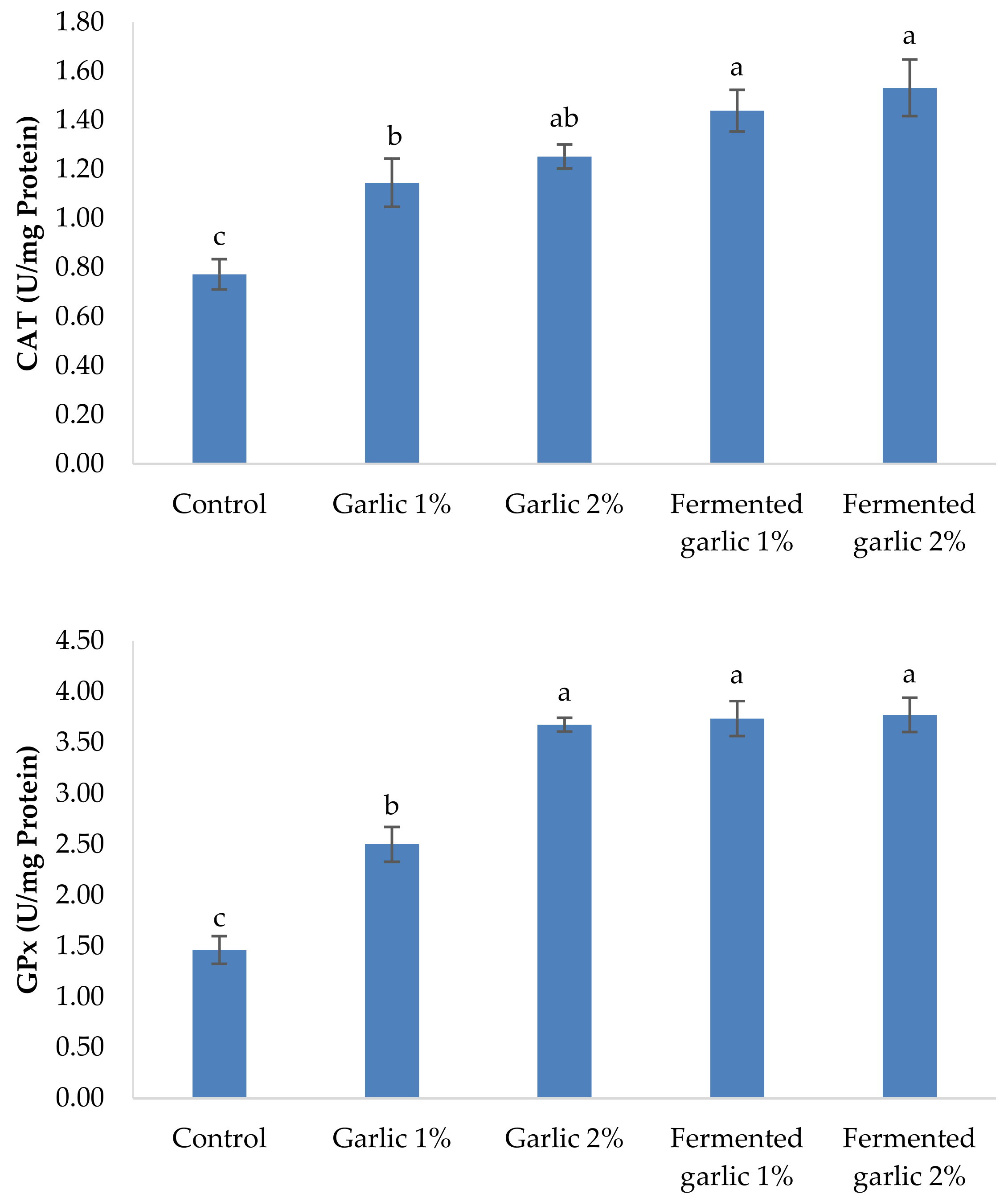
2.7. Antioxidant Activity Assessment
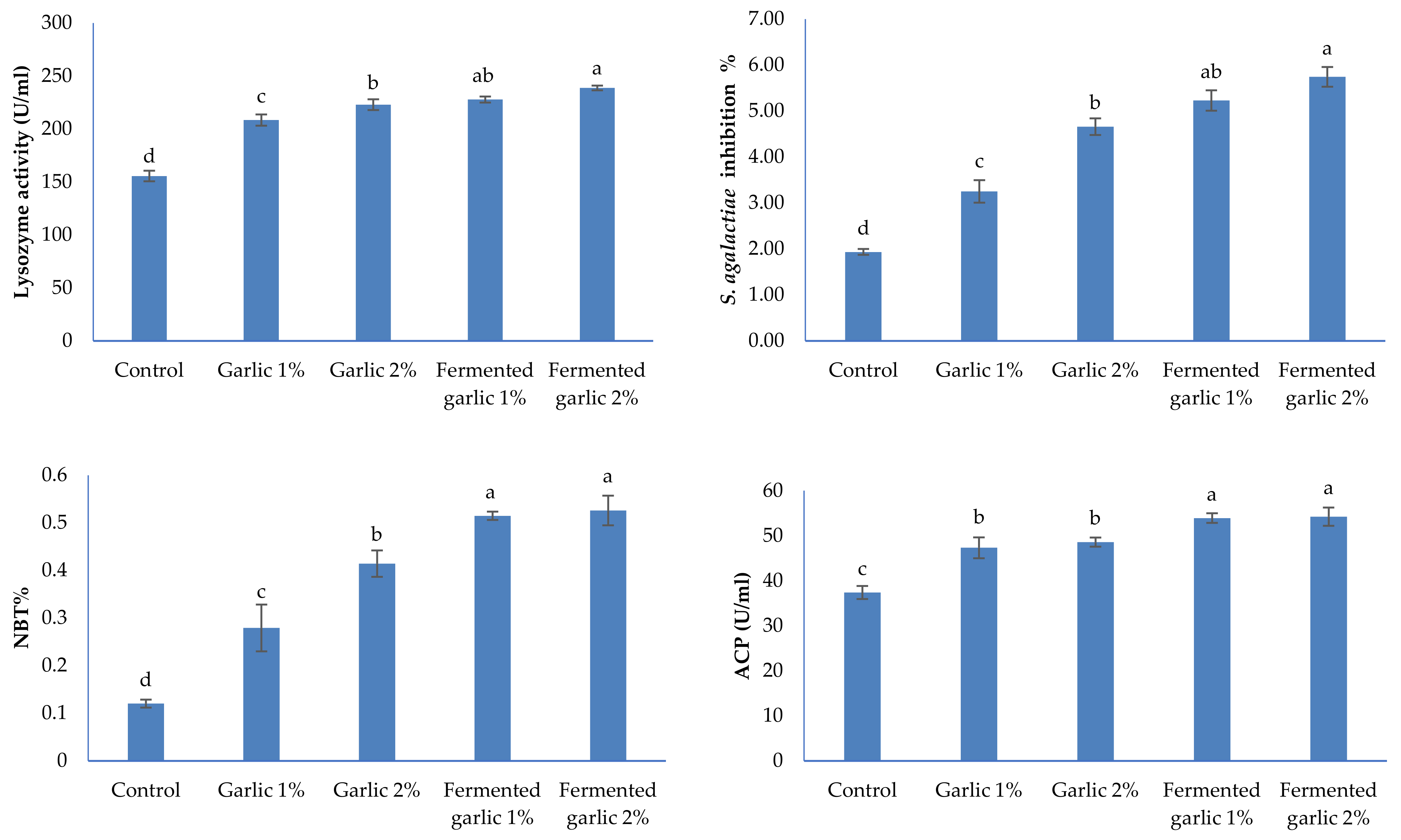
2.8. Immune Function Tests
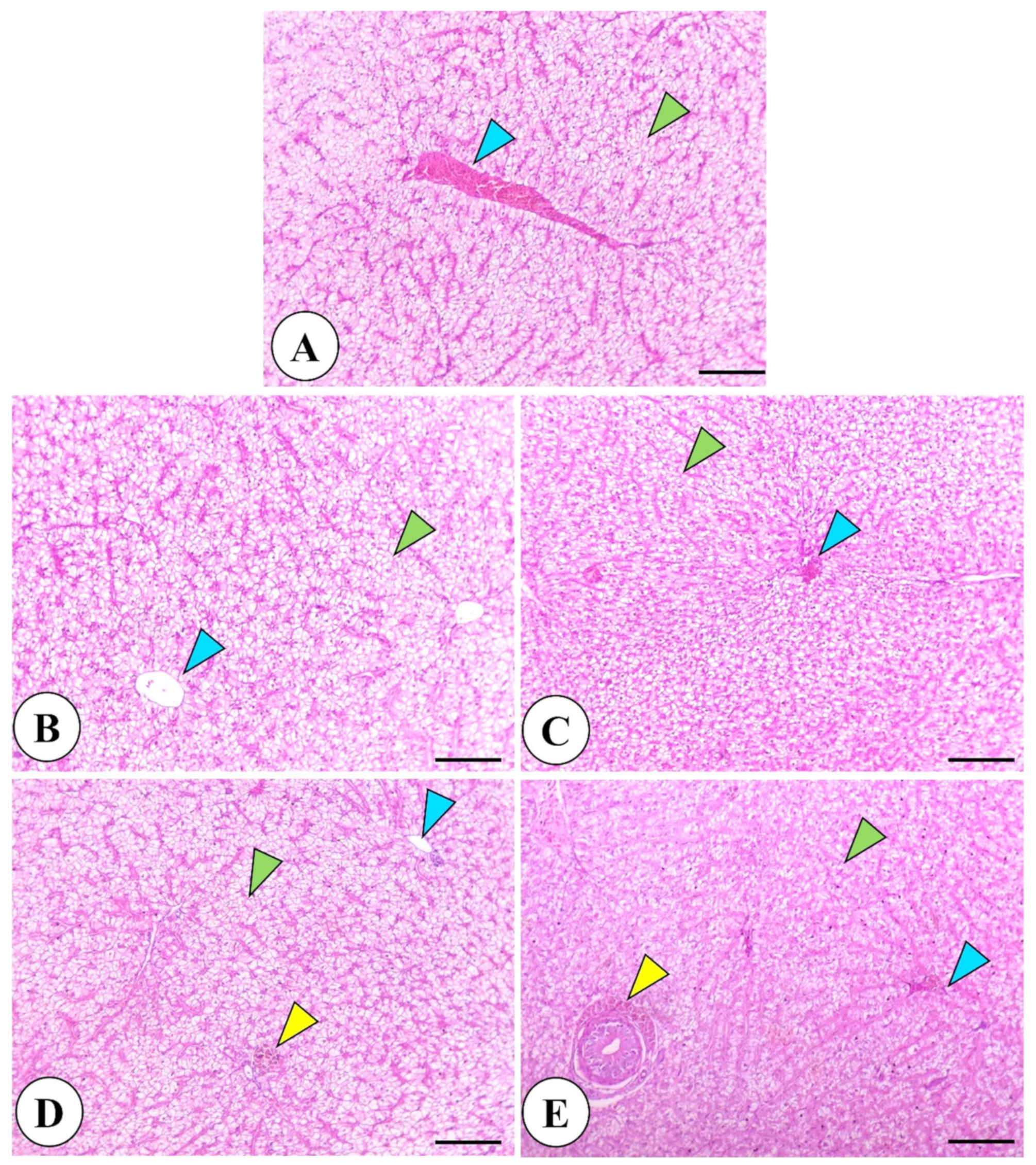
2.9. Histological Examination of Intestine and Liver
2.10. Data Collection and Statistical Analysis
3. Results
3.1. Garlic Organosulfur Compounds
3.2. Growth, Feed Utilization, and Survival Rate
3.3. Digestive Enzymes
3.4. Intestine and Liver Histology
3.5. Blood Biochemical Parameters
3.6. Immunity
3.7. Antioxidants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- El Basuini, M.F.; Abdel Fattah, A.M.; El-Hais, A.M.; Soliman, A.A.; Amer, A.A.; Gewaily, M.; Zaki, M.A.A.; Zaineldin, A.I.; Dossou, S.; Teiba, I.I.; et al. Dietary co-enzyme Q10 boosted the growth performance, antioxidative capacity, immune responses, and intestinal and hepatic histomorphology of grey mullet (Liza ramada). Aquac. Rep. 2024, 36, 102147. [Google Scholar] [CrossRef]
- Shehata, A.I.; Shahin, S.A.; Elmaghraby, A.M.; Alhoshy, M.; Soliman, A.A.; Amer, A.A.; Habib, Y.J.; Gewaily, M.S.; El Basuini, M.F. Synergistic benefits of dietary silymarin and selenium on growth, immune functions, antioxidants, and gut/liver health of Thinlip mullet (Liza ramada) juveniles. Ann. Anim. Sci. 2024. [Google Scholar] [CrossRef]
- Shaalan, M.; El-Mahdy, M.; Saleh, M.; El-Matbouli, M. Aquaculture in Egypt: Insights on the Current Trends and Future Perspectives for Sustainable Development. Rev. Fish. Sci. Aquac. 2018, 26, 99–110. [Google Scholar] [CrossRef]
- Shehata, A.I.; Shahin, S.A.; Elmaghraby, A.M.; Alhoshy, M.; Toutou, M.M.; Soliman, A.A.; Amer, A.A.; Habib, Y.J.; Gewaily, M.S.; Teiba, I.I.; et al. Stevioside mitigates lead toxicity in thinlip mullet juveniles: Impacts on growth, metabolism, and immune function. Aquatic Toxicol. 2024, 271, 106910. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso Jr, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Araujo, G.S.; Silva, J.W.A.d.; Cotas, J.; Pereira, L. Fish Farming Techniques: Current Situation and Trends. J. Mar. Sci. Eng. 2022, 10, 1598. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed Additives in Animal Health. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 345–362. [Google Scholar]
- Shehata, A.I.; Rasheed, M.; Rafiq, H.; Khalid, N.; Rafique, A.; Alhoshy, M.; Habib, Y.J.; El Basuini, M.F. Multi-functional application of octacosanol as a feed additive in animal and aquaculture: A review. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1595–1603. [Google Scholar] [CrossRef]
- Hossain, M.S.; Small, B.C.; Kumar, V.; Hardy, R. Utilization of functional feed additives to produce cost-effective, ecofriendly aquafeeds high in plant-based ingredients. Rev. Aquac. 2024, 16, 121–153. [Google Scholar] [CrossRef]
- Eroldoğan, O.T.; Glencross, B.; Novoveska, L.; Gaudêncio, S.P.; Rinkevich, B.; Varese, G.C.; de Fátima Carvalho, M.; Tasdemir, D.; Safarik, I.; Nielsen, S.L.; et al. From the sea to aquafeed: A perspective overview. Rev. Aquac. 2023, 15, 1028–1057. [Google Scholar] [CrossRef]
- Alagawany, M. Antibiotic Alternatives in Poultry and Fish Feed; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Yamamoto, F.Y. Chapter 11—Nutritional supplements and fish health. In Fish Nutrition, 4rth ed.; Hardy, R.W., Kaushik, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 745–773. [Google Scholar]
- Gupta, A.J.; Mainkar, P.; Mahajan, V. Exploring the Nutritional-Nutraceutical Composition and Phytochemical Potential of Garlic Agents in Preclinical and Clinical Studies with a Focus on Drug Likeness. J. Herb. Med. 2024, 46, 100911. [Google Scholar] [CrossRef]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the garlic (Allium sativum) properties for fish aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Rasheed, R.; Hussain, M.; Aamir, M.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Anjum, F.M. Nutritional, biological, and therapeutic properties of black garlic: A critical review. Int. J. Food Prop. 2021, 24, 1387–1402. [Google Scholar] [CrossRef]
- Shah, A.M.; Tarfeen, N.; Mohamed, H.; Song, Y. Fermented Foods: Their Health-Promoting Components and Potential Effects on Gut Microbiota. Fermentation 2023, 9, 118. [Google Scholar] [CrossRef]
- Ozma, M.A.; Abbasi, A.; Ahangarzadeh Rezaee, M.; Hosseini, H.; Hosseinzadeh, N.; Sabahi, S.; Noori, S.M.A.; Sepordeh, S.; Khodadadi, E.; Lahouty, M.; et al. A Critical Review on the Nutritional and Medicinal Profiles of Garlic’s (Allium sativum L.) Bioactive Compounds. Food Rev. Int. 2023, 39, 6324–6361. [Google Scholar] [CrossRef]
- Adi, A.C.; Susanti, Y.; Rachmawati, H.; Ishaura, E.R.; Farapti, F.; Salisa, W.; Rasyidi, M.F.; Sutthiwong, N. Effects of fermented and non-fermented garlic as an anti-diabetic on blood glucose levels of wistar rat. J. Public Health Afr. 2023, 15, 2541. [Google Scholar] [CrossRef]
- Lee, K.; Lee, K.; Kim, G.; Kim, J.; Yeon, J.; Cho, S.; Chang, B.; Kim, S. Effects of dietary fermented garlic on the growth performance, relative organ weights, intestinal morphology, cecal microflora and serum characteristics of broiler chickens. Rev. Bras. Ciência Avícola 2016, 18, 511–518. [Google Scholar] [CrossRef]
- Lee, W.-D.; Kothari, D.; Moon, S.-G.; Kim, J.; Kim, K.-I.; Ga, G.-W.; Kim, Y.-G.; Kim, S.-K. Evaluation of Non-Fermented and Fermented Chinese Chive Juice as an Alternative to Antibiotic Growth Promoters of Broilers. Animals 2022, 12, 2742. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Yin, Y.; Ma, X. The nutritional applications of garlic (Allium sativum) as natural feed additives in animals. PeerJ 2021, 9, e11934. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Yang, Y.; Song, Q.; Ding, J.; Han, B.; Zhao, C. Dietary allicin improves behavior, physiology, growth, and disease resistance in the sea cucumber Apostichopus japonicus. Aquaculture 2024, 593, 741321. [Google Scholar] [CrossRef]
- Metwally, M. Effects of garlic (Allium sativum) on some antioxidant activities in tilapia nilotica (Oreochromis niloticus). World J. Fish Mar. Sci. 2009, 1, 56–64. [Google Scholar]
- Setijaningsih, L.; Setiadi, E.; Taufik, I. The effect of garlic Allium sativum addition in feed to the growth performance and immune response of tilapia Oreochromis niloticus. IOP Conf. Ser. Earth Environ. Sci. 2021, 744, 012072. [Google Scholar] [CrossRef]
- Ajiboye, O.O.; Qari, R. Short-term evaluation of graded levels of dietary garlic powder (Allium sativum L.) as growth promoter on growth, survival and feed utilization of redbelly Tilapia, Tilapia zillii reared in glass aquaria tanks. Int. J. Mar. Sci. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Ayoola, S.; Uzoamaka, O. Effect of Allium sativum on growth, feed utilization and haematological parameters of Clarias gariepinus juvenile. Afr. J. Livest. Ext. 2013, 12, 1–7. [Google Scholar]
- Gabriel, N.N.; Wilhelm, M.R.; Habte-Tsion, H.-M.; Chimwamurombe, P.; Omoregie, E. Dietary garlic (Allium sativum) crude polysaccharides supplementation on growth, haematological parameters, whole body composition and survival at low water pH challenge in African catfish (Clarias gariepinus) juveniles. Sci. Afr. 2019, 5, e00128. [Google Scholar] [CrossRef]
- Akbary, P.; Negahdari Jafarbeigi, Y.; Sondakzehi, A. Effects of garlic (Allium sativum L) extract on growth, feed utilization and carcass composition in Mugil cephalus (Linnaeus, 1758) larvae. Iran. J. Fish. Sci. 2016, 15, 552–557. [Google Scholar]
- Talpur, A.D.; Ikhwanuddin, M. Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 2012, 364–365, 6–12. [Google Scholar] [CrossRef]
- Nya, E.J.; Austin, B. Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2009, 32, 963–970. [Google Scholar] [CrossRef]
- Güroy, D.; Emre, N.; Yalım, F.B.; Karadal, O.; Kaya, D.; Arifoğlu, N. Interaction of dietary garlic (Allium sativum), onion (Allium cepa), and probiotic on the growth performance and health status of juvenile rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2024, 32, 4515–4528. [Google Scholar] [CrossRef]
- Karimi Pashaki, A.; Ghasemi, M.; ZorriehZahra, M.J.; Shrif Rohani, M.; Hosseini, S.M. Effects of dietary garlic (Allium sativum) extract on survival rate, blood and immune parameters changes and disease resistance of Common carp (Cyprinus carpio carpio Linnaeus, 1758) against Spring Viremia of Carp (SVC). IFRO 2020, 19, 1024–1039. [Google Scholar]
- Ahmadniaye Motlagh, H.; Safari, O.; Selahvarzi, Y.; Baghalian, A.; Kia, E. Non-specific immunity promotion in response to garlic extract supplemented diets in female Guppy (Poecilia reticulata). Fish Shellfish Immunol. 2020, 97, 96–99. [Google Scholar] [CrossRef]
- Ghehdarijani, M.S.; Hajimoradloo, A.; Ghorbani, R.; Roohi, Z. The effects of garlic-supplemented diets on skin mucosal immune responses, stress resistance and growth performance of the Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immunol. 2016, 49, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Das, B.K.; Mishra, B.K.; Pradhan, J.; Sarangi, N. Effect of Allium sativum on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. J. Appl. Ichthyol. 2007, 23, 80–86. [Google Scholar] [CrossRef]
- Sharma, N.; Behl, T.; Singh, S.; Bansal, A.; Singh, S.K.; Zahoor, I. Expatiating the therapeutic profile of garlic (Allium sativum): A bench to bedside approach. Biointerface Res. Appl. Chem. 2021, 11, 14225–14239. [Google Scholar] [CrossRef]
- Chitmanat, C.; Tongdonmuan, K.; Nunsong, W. The use of crude extracts from traditional medicinal plants to eliminate Trichodina sp. in tilapia (Oreochromis niloticus) fingerlings. Songklanakarin J. Sci. Technol. 2005, 27, 359–364. [Google Scholar]
- Adineh, H.; Harsij, M.; Jafaryan, H.; Asadi, M. The effects of microencapsulated garlic (Allium sativum) extract on growth performance, body composition, immune response and antioxidant status of rainbow trout (Oncorhynchus mykiss) juveniles. J. Appl. Anim. Res. 2020, 48, 372–378. [Google Scholar] [CrossRef]
- Muahiddah, N.; Diamahesa, W.A. The use of garlic (Allium sativum) as an immunostimulant in aquaculture. J. Fish Health 2023, 3, 11–18. [Google Scholar] [CrossRef]
- Magouz, F.; Abu-Ghanima, H.; Zaineldin, A.I.; Gewaily, M.S.; Soliman, A.; Amer, A.A.; Moustafa, E.M.; Younis, E.M.; Abdel-Warith, A.-W.A.; Davies, S.J.; et al. Dietary Bacillus subtilis relieved the growth retardation, hepatic failure, and antioxidative depression induced by ochratoxin A in Thinlip Mullet (Liza ramada). Aquac. Rep. 2022, 22, 100984. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Rockville, MD, USA, 2008. [Google Scholar]
- Gisbert, E.; Giménez, G.; Fernández, I.; Kotzamanis, Y.; Estévez, A. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 2009, 287, 381–387. [Google Scholar] [CrossRef]
- Au–Cupp-Enyard, C. Sigma’s Non-specific Protease Activity Assay–Casein as a Substrate. JoVE 2008, e899. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Zalat, R.Y.I.; El-Hais, A.M.; Soliman, A.A.; Amer, A.A.; Gewaily, M.; Gabr, S.A.; Zaineldin, A.I.; Dossou, S.; Teiba, I.I.; et al. Bee venom enhances performance and immune function in thinlip mullet: A promising approach for sustainable aquaculture. Fish Shellfish Immunol. 2024, 151, 109713. [Google Scholar] [CrossRef]
- Paoletti, F.; Mocali, A. [18] Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oOxidation. Methods Enzymol. 1990, 186, 209–220. [Google Scholar] [PubMed]
- Carlberg, I.; Mannervik, B. [59] Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Lygren, B.; Hjeltnes, B.; Waagbø, R. Immune response and disease resistance in Atlantic salmon (Salmo salar L.) fed three levels of dietary vitamin E and the effect of vaccination on the liver status of antioxidant vitamins. Aquac. Int. 2001, 9, 401–411. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Zaki, M.A.A.; El-Hais, A.M.; Elhanafy, M.G.; El-Bilawy, E.H.; Zaineldin, A.I.; Abdel-Aziz, M.F.A.; Abouelsaad, I.A.; El-Ratel, I.T.; Mzengereza, K.; et al. Microbial, immune and antioxidant responses of Nile tilapia with dietary nano-curcumin supplements under chronic low temperatures. Aquac. Fish. 2024, 9, 57–65. [Google Scholar] [CrossRef]
- Anderson, D.; Siwicki, A. Basic Hematology and Serology for Fish Health Programs. 1995. Available online: https://api.semanticscholar.org/CorpusID:68117060 (accessed on 24 September 2024).
- Yano, T. Assay of hemolytic complement activity. Tech. Fish Immunol. 1992, 131–141. Available online: https://cir.nii.ac.jp/crid/1574231875535834880 (accessed on 24 September 2024).
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Gewaily, M.S.; Abumandour, M.M.A. Gross morphological, histological and scanning electron specifications of the oropharyngeal cavity of the hooded crow (Corvus cornix pallescens). Anat. Histol. Embryol. 2021, 50, 72–83. [Google Scholar] [CrossRef]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Vet. J. 2020, 73, 2. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Gao, Y. Review of the Application of Garlic, Allium sativum, in Aquaculture. J. World Aquac. Soc. 2012, 43, 447–458. [Google Scholar] [CrossRef]
- Bisen, P.; Emerald, M. Nutritional and therapeutic potential of garlic and onion (Allium sp.). Curr. Nutr. Food Sci. 2016, 12, 190–199. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef]
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical Composition, Antibacterial and Antioxidant Activities of Six Essentials Oils from the Alliaceae Family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef]
- Gong, X.; Su, X.; Liu, H. Diallyl Trisulfide, the Antifungal Component of Garlic Essential Oil and the Bioactivity of Its Nanoemulsions Formed by Spontaneous Emulsification. Molecules 2021, 26, 7186. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, B.; Qin, G.; Liang, S.; Yin, J.; Jiang, H.; Liu, M.; Li, X. Therapeutic potentials of allicin in cardiovascular disease: Advances and future directions. Chin. Med. 2024, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.; El-L, S. Effect of probiotics and some spices as feed additives on the performance and behaviour of the Nile tilapia, Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2008, 12, 63–80. [Google Scholar] [CrossRef]
- Zare, M.; Tran, H.Q.; Prokešová, M.; Stejskal, V. Effects of Garlic Allium sativum Powder on Nutrient Digestibility, Haematology, and Immune and Stress Responses in Eurasian Perch Perca fluviatilis Juveniles. Animals 2021, 11, 2735. [Google Scholar] [CrossRef]
- Inoue, L.A.K.A.; Oliveira Maciel, P.; Gusmão Affonso, E.; de Lima Boijink, C.; Tavares-Dias, M. Growth, parasitic infection and hematology in Colossoma macropomum Cuvier, 1818 fed diets containing Allium sativum. J. Appl. Ichthyol. 2016, 32, 901–905. [Google Scholar] [CrossRef]
- Samson, J. Effect of garlic (Allium sativum) supplemented diets on growth, feed utilization and survival of red tilapia (Oreochromis sp.). Int. J. Agric. Technol. 2019, 15, 637–644. [Google Scholar]
- Ndong, D.; Fall, J. The effect of garlic (Allium sativum) on growth and immune responses of hybrid tilapia (Oreochromis niloticus x Oreochromis aureus). J. Clin. Immnunol. Immunopathol. Res. 2011, 3, 1–9. [Google Scholar]
- Onomu, A.J. Growth and haematological response of Clarias gariepinus to garlic (Allium sativum) supplemented diet. Sustain. Agric. Res. 2018, 8, 67–73. [Google Scholar] [CrossRef][Green Version]
- Mattos, D.C.; Manhães, J.; Cardoso, L.; Aride, P.; Lavander, H.; Oliveira, A.; Radael, M.; Azevedo, R.; Junior, V. Influence of garlic extract on larval performance and survival of juvenile angelfish Pterophyllum scalare during transport. Braz. J. Biol. 2021, 83, e244480. [Google Scholar] [CrossRef]
- Breidt, F.; McFeeters, R.F.; Perez-Diaz, I.; Lee, C.-H. Fermented Vegetables. Food Microbiol. 2012, 841–855. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1128/9781555818463.ch33 (accessed on 24 September 2024).
- Nya, E.J.; Austin, B. Dietary modulation of digestive enzymes by the administration of feed additives to rainbow trout, Oncorhynchus mykiss Walbaum. Aquac. Nutr. 2011, 17, e459–e466. [Google Scholar] [CrossRef]
- Platel, K.; Rao, A.; Saraswathi, G.; Srinivasan, K. Digestive stimulant action of three Indian spice mixes in experimental rats. Food/Nahr. 2002, 46, 394–398. [Google Scholar] [CrossRef]
- Esmaeili, N.; Abedian Kenari, A.; Rombenso, A.N. Effects of fish meal replacement with meat and bone meal using garlic (Allium sativum) powder on growth, feeding, digestive enzymes and apparent digestibility of nutrients and fatty acids in juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Aquac. Nutr. 2017, 23, 1225–1234. [Google Scholar] [CrossRef]
- Shehata, A.I.; Soliman, A.A.; Ahmed, H.A.; Gewaily, M.S.; Amer, A.A.; Shukry, M.; Abdel-Latif, H.M.R. Evaluation of different probiotics on growth, body composition, antioxidant capacity, and histoarchitecture of Mugil capito. Sci. Rep. 2024, 14, 7379. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Soliman, A.A.; Gewaily, M.S.; Amer, A.A.; Shukry, M.; Khalil, R.H.; Shehata, A.I. Dietary effects of Saccharomyces cerevisiae and Allium sativum on growth, antioxidant status, hepatic and intestinal histoarchitecture, expression of growth- and immune-related genes, and resistance of Oreochromis niloticus to Aeromonas sobria. Fish Shellfish Immunol. 2024, 148, 109493. [Google Scholar] [CrossRef]
- Adibmoradi, M.; Navidshad, B.; Seifdavati, J.; Royan, M. Effect of Dietary Garlic Meal on Histological Structure of Small Intestine in Broiler Chickens. J. Poult. Sci. 2006, 43, 378–383. [Google Scholar] [CrossRef]
- Agbebi, O.; Ogunmuyiwa, T.; Herbert, S. Effect of dietary garlic source on feed utilization, growth and Histopathology of the African catfish (Clarias gariepinus). J. Agric. Sci. 2013, 5, 26. [Google Scholar] [CrossRef][Green Version]
- Mohammad, M.A. Effect of adding garlic Allium sativum powder in diet on hematological, biochemical and histopathological criteria of common carp Cyprinus carpio L. Iraqi J. Agric. Sci. 2023, 54, 1040–1049. [Google Scholar] [CrossRef]
- Camargo, M.M.; Martinez, C.B. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef]
- Öz, M.; Inanan, B.E.; Üstüner, E.; Karagoz, B.; Dikel, S. Effects of dietary garlic (Allium sativum) oil on growth performance, haemato-biochemical and histopathology of cypermethrin-intoxicated Nile tilapia (Oreochromis niloticus). Vet. Med. Sci. 2024, 10, e1449. [Google Scholar] [CrossRef] [PubMed]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, S.R.; Ra, C.S.; Kim, J.D. Effects of Dietary Garlic Extracts on Whole Body Amino Acid and Fatty Acid Composition, Muscle Free Amino Acid Profiles and Blood Plasma Changes in Juvenile Sterlet Sturgeon, Acipenser ruthenus. Asian-Austral. J. Anim. Sci. 2012, 25, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Ali, M. Garlic [Allium sativum]: A review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 2003, 3, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.R.; Reddy, K.P. Reduced nociceptive responses in mice with alloxan induced hyperglycemia after garlic (Allium sativum Linn.) treatment. Indian J. Exp. Biol. 1999, 37, 662–666. [Google Scholar]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G.; Owoloye, T.R.; Agbebi, O.J. Modulatory effects of dietary inclusion of garlic (Allium sativum) on gentamycin–induced hepatotoxicity and oxidative stress in rats. Asian Pac. J. Trop. Biomed. 2013, 3, 470–475. [Google Scholar] [CrossRef]
- Vazquez-Prieto, M.A.; Rodriguez Lanzi, C.; Lembo, C.; Galmarini, C.R.; Miatello, R.M. Garlic and Onion Attenuates Vascular Inflammation and Oxidative Stress in Fructose-Fed Rats. J. Nutr. Metab. 2011, 1, 475216. [Google Scholar] [CrossRef]
- Gholipour Kanani, H.; Nobahar, Z.; Kakoolaki, S.; Jafarian, H. Effect of ginger- and garlic-supplemented diet on growth performance, some hematological parameters and immune responses in juvenile Huso huso. Fish Physiol. Biochem. 2014, 40, 481–490. [Google Scholar] [CrossRef]
- Ravi, S.; Jitender, K. Fish Biology and Ecology Theory: Circulatory System of Fish; University College of Sciences, Osmania University, Hyderabad, India, 2005; p. 347.
- Nwabueze, A.A. The effect of garlic (Allium sativum) on growth and haematological parameters of Clarias gariepinus (Burchell, 1822). Sustain. Agric. Res. 2012, 1, 222–228. [Google Scholar] [CrossRef]
- Abdelwahab, A.M.; El-Bahr, S.M.; Al-Khamees, S. Influence of Dietary Garlic (Allium sativum) and/or Ascorbic Acid on Performance, Feed Utilization, Body Composition and Hemato-Biochemical Parameters of Juvenile Asian Sea Bass (Lates calcarifer). Animals 2020, 10, 2396. [Google Scholar] [CrossRef] [PubMed]
- Öz, M.; DİKel, S. Effect of garlic (Allium sativum)—Supplemented diet on growth performance, body composition and fatty acid profile of rainbow trout (Oncorhynchus mykiss). Cell. Mol. Biol. 2022, 68, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Hou, Y.; Feng, W.; Nomingerel, M.; Li, B.; Zhu, J. Multi-Omics Analysis to Understand the Effects of Dietary Proanthocyanidins on Antioxidant Capacity, Muscle Nutrients, Lipid Metabolism, and Intestinal Microbiota in Cyprinus carpio. Antioxidants 2023, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Warshafsky, S.; Kamer, R.S.; Sivak, S.L. Effect of garlic on total serum cholesterol: A meta-analysis. Ann. Intern. Med. 1993, 119, 599–605. [Google Scholar] [CrossRef]
- Okoro, B.C.; Dokunmu, T.M.; Okafor, E.; Sokoya, I.A.; Israel, E.N.; Olusegun, D.O.; Bella-Omunagbe, M.; Ebubechi, U.M.; Ugbogu, E.A.; Iweala, E.E.J. The ethnobotanical, bioactive compounds, pharmacological activities and toxicological evaluation of garlic (Allium sativum): A review. Pharmacol. Res. Mod. Chin. Med. 2023, 8, 100273. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef]
- Zaefarian, A.; Yeganeh, S.; Adhami, B. Dietary effects of garlic powder (Allium sativum) on growth, blood indices, carcass composition, and lysozyme activity in brown trout (Salmo caspius) and resistance against Yersinia ruckeri infection. Aquac. Int. 2017, 25, 1987–1996. [Google Scholar] [CrossRef]
- Jahanjoo, V.; Yahyavi, M.; Akrami, R.; Bahri, A.H. Influence of adding garlic (Allium sativum), Ginger (Zingiber officinale), thyme (Thymus vulgaris) and their combination on the growth performance, haematoimmunological parameters and disease resistance to Photobacterium damselae in sobaity sea bream (Sparidentex hasta) Fry. Turk. J. Fish. Aquat. Sci. 2018, 18, 633–645. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.; Samal, J.; Thatoi, H. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72. [Google Scholar] [PubMed]
- Firmino, J.P.; Fernández-Alacid, L.; Vallejos-Vidal, E.; Salomón, R.; Sanahuja, I.; Tort, L.; Ibarz, A.; Reyes-López, F.E.; Gisbert, E. Carvacrol, Thymol, and Garlic Essential Oil Promote Skin Innate Immunity in Gilthead Seabream (Sparus aurata) Through the Multifactorial Modulation of the Secretory Pathway and Enhancement of Mucus Protective Capacity. Front. Immunol. 2021, 12, 633621. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Liu, J.; Guo, W.; Yang, M.; Liu, L.; Huang, S.; Tao, L.; Zhang, F.; Liu, Y. Investigation of the dynamic changes in the chemical constituents of Chinese “Laba” garlic during traditional processing. RSC Adv. 2018, 8, 41872–41883. [Google Scholar] [CrossRef]
- Adegbola, P.I.; Fadahunsi, O.S.; Ajilore, B.S.; Akintola, A.O.; Olorunnisola, O.S. Combined ginger and garlic extract improves serum lipid profile, oxidative stress markers and reduced IL-6 in diet induced obese rats. Obes. Med. 2021, 23, 100336. [Google Scholar] [CrossRef]
- Mohebbi, A.; Nematollahi, A.; Dorcheh, E.E.; Asad, F.G. Influence of dietary garlic (Allium sativum) on the antioxidative status of rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2012, 43, 1184–1193. [Google Scholar] [CrossRef]

| Ingredients | % | Proximate Analysis | |
|---|---|---|---|
| Fish meal (65% CP) | 12 | Crude protein (%) | 33.42 ± 0.21 |
| Soybean meal (44% CP) | 38 | Crude lipids (%) | 7.99 ± 0.27 |
| Gluten | 6 | Fiber (%) | 5.11 ± 0.21 |
| Yellow corn | 20 | Ash (%) | 7.44 ± 0.22 |
| Wheat bran | 11 | Gross energy (MJ/Kg) 2 | 18.22 ± 0.33 |
| Rice bran | 7 | ||
| Fish oil | 4 | ||
| Mineral premix 1 | 0.5 | ||
| Vitamin premix 1 | 0.5 | ||
| Dicalcium phosphate | 1 | ||
| Total | 100 | ||
| Compound (mg/g) | Garlic Powder | Fermented Garlic Powder |
|---|---|---|
| Allicin (mg/g) | 3.2 | 0.1 |
| Diallyl sulfide (DAS) (mg/g) | 0.9 | 2.1 |
| Diallyl disulfide (DADS) (mg/g) | 1.6 | 4.8 |
| S-allyl cysteine (mg/g) | 4.9 | 5.1 |
| Item | Experimental Diets | ||||
|---|---|---|---|---|---|
| Control | Garlic 1% | Garlic 2% | Fermented Garlic 1% | Fermented Garlic 2% | |
| Initial body weight (IBW, g) | 86.25 ± 0.20 | 86.08 ± 0.31 | 86.08 ± 0.12 | 85.81 ± 0.38 | 85.78 ± 0.21 |
| Final body weight (FBW, g) | 225.43 ± 2.93 b | 243.50 ± 2.08 a | 246.31 ± 4.67 a | 247.35 ± 3.90 a | 248.15 ± 4.33 a |
| Weight gain (WG, %) | 139.18 ± 3.05 b | 157.42 ± 1.95 a | 160.23 ± 4.56 a | 161.53 ± 3.54 a | 162.37 ± 4.40 a |
| Specific growth rate (SGR, %/day) | 1.60 ± 0.03 b | 1.73 ± 0.01 a | 1.75 ± 0.03 a | 1.77 ± 0.02 a | 1.77 ± 0.03 a |
| Feed intake (g) | 228.93 ± 2.37 b | 239.26 ± 1.84 a | 239.64 ± 4.04 a | 239.22 ± 3.73 a | 239.97 ± 3.41 a |
| Feed conversion ratio (FCR) | 1.65 ± 0.05 a | 1.52 ± 0.01 b | 1.50 ± 0.02 b | 1.48 ± 0.03 b | 1.48 ± 0.04 b |
| Survival rate (SR, %) | 97.78 ± 2.22 | 100.00 ± 0.00 | 97.78 ± 2.22 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Enzyme Activity (U/ mg) | Experimental Diets | ||||
|---|---|---|---|---|---|
| Control | Garlic 1% | Garlic 2% | Fermented Garlic 1% | Fermented Garlic 2% | |
| Amylase | 11.71 ± 0.64 b | 15.85 ± 1.09 a | 15.89 ± 1.10 a | 16.35 ± 1.08 a | 16.44 ± 1.21 a |
| Lipase | 18.95 ± 0.94 b | 23.19 ± 0.75 a | 24.34 ± 1.55 a | 24.22 ± 1.08 a | 24.49 ± 1.36 a |
| Protease | 15.77 ± 0.62 b | 20.23 ± 0.82 a | 20.22 ± 1.29 a | 20.04 ± 0.75 a | 20.26 ± 1.36 a |
| Items | Experimental Diets | ||||
|---|---|---|---|---|---|
| Control | Garlic 1% | Garlic 2% | Fermented Garlic 1% | Fermented Garlic 2% | |
| Glucose (mg/dL) | 114.00 ± 5.20 a | 93.67 ± 4.26 b | 92.00 ± 4.00 b | 91.67 ± 2.60 b | 88.00 ± 3.46 b |
| Total protein (g/dL) | 5.10 ± 0.37 b | 6.68 ± 0.70 a | 6.69 ± 0.40 a | 6.76 ± 0.55 a | 6.82 ± 0.25 a |
| Globulin (g/dL) | 3.48 ± 0.25 | 4.97 ± 0.69 | 4.90 ± 0.38 | 5.03 ± 0.57 | 5.09 ± 0.51 |
| Albumin (g/dL) | 1.62 ± 0.31 | 1.71 ± 0.06 | 1.79 ± 0.06 | 1.73 ± 0.03 | 1.74 ± 0.34 |
| Total cholesterol (mg/dL) | 13.33 ± 1.20 | 10.33 ± 1.45 | 10.00 ± 1.15 | 10.00 ± 1.53 | 9.00 ± 1.15 |
| Triglyceride (mg/dL) | 65.67 ± 3.76 | 67.67 ± 3.48 | 68.00 ± 8.19 | 69.33 ± 7.13 | 70.00 ± 7.23 |
| ALT (IU/L) | 54.67 ± 4.26 | 56.67 ± 3.28 | 57.67 ± 4.81 | 61.33 ± 5.46 | 60.67 ± 5.36 |
| AST (IU/L) | 56.00 ± 4.36 | 57.00 ± 4.73 | 56.00 ± 5.86 | 57.67 ± 7.22 | 54.67 ± 5.81 |
| Urea (mg/dL) | 10.00 ± 0.58 | 10.33 ± 0.88 | 11.00 ± 1.53 | 9.33 ± 1.86 | 9.67 ± 1.45 |
| Creatinine (mg/dL) | 0.27 ± 0.07 | 0.27 ± 0.03 | 0.30 ± 0.10 | 0.23 ± 0.09 | 0.30 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basuini, M.F.E.; Shaban, M.M.E.A.; El-Hais, A.M.; Soliman, A.A.; Abu-Elala, N.M.; Teiba, I.I.; Alhoshy, M.; Sallam, G.R.; Shadrack, R.S.; Mzengereza, K.; et al. Exploring the Dual Benefits of Fermented and Non-Fermented Garlic Powder on Growth, Antioxidative Capacity, Immune Responses, and Histology in Gray Mullet (Liza ramada). Fishes 2024, 9, 401. https://doi.org/10.3390/fishes9100401
Basuini MFE, Shaban MMEA, El-Hais AM, Soliman AA, Abu-Elala NM, Teiba II, Alhoshy M, Sallam GR, Shadrack RS, Mzengereza K, et al. Exploring the Dual Benefits of Fermented and Non-Fermented Garlic Powder on Growth, Antioxidative Capacity, Immune Responses, and Histology in Gray Mullet (Liza ramada). Fishes. 2024; 9(10):401. https://doi.org/10.3390/fishes9100401
Chicago/Turabian StyleBasuini, Mohammed F. El, Mahasen M. E. A. Shaban, Abdelaziz M. El-Hais, Ali A. Soliman, Nermeen M. Abu-Elala, Islam I. Teiba, Mayada Alhoshy, Ghada R. Sallam, Ronick Spenly Shadrack, Kumbukani Mzengereza, and et al. 2024. "Exploring the Dual Benefits of Fermented and Non-Fermented Garlic Powder on Growth, Antioxidative Capacity, Immune Responses, and Histology in Gray Mullet (Liza ramada)" Fishes 9, no. 10: 401. https://doi.org/10.3390/fishes9100401
APA StyleBasuini, M. F. E., Shaban, M. M. E. A., El-Hais, A. M., Soliman, A. A., Abu-Elala, N. M., Teiba, I. I., Alhoshy, M., Sallam, G. R., Shadrack, R. S., Mzengereza, K., & Shehata, A. I. (2024). Exploring the Dual Benefits of Fermented and Non-Fermented Garlic Powder on Growth, Antioxidative Capacity, Immune Responses, and Histology in Gray Mullet (Liza ramada). Fishes, 9(10), 401. https://doi.org/10.3390/fishes9100401








