Systematic Review of Multi-Species Models in Fisheries: Key Features and Current Trends
Abstract
1. Introduction
2. Material and Methods
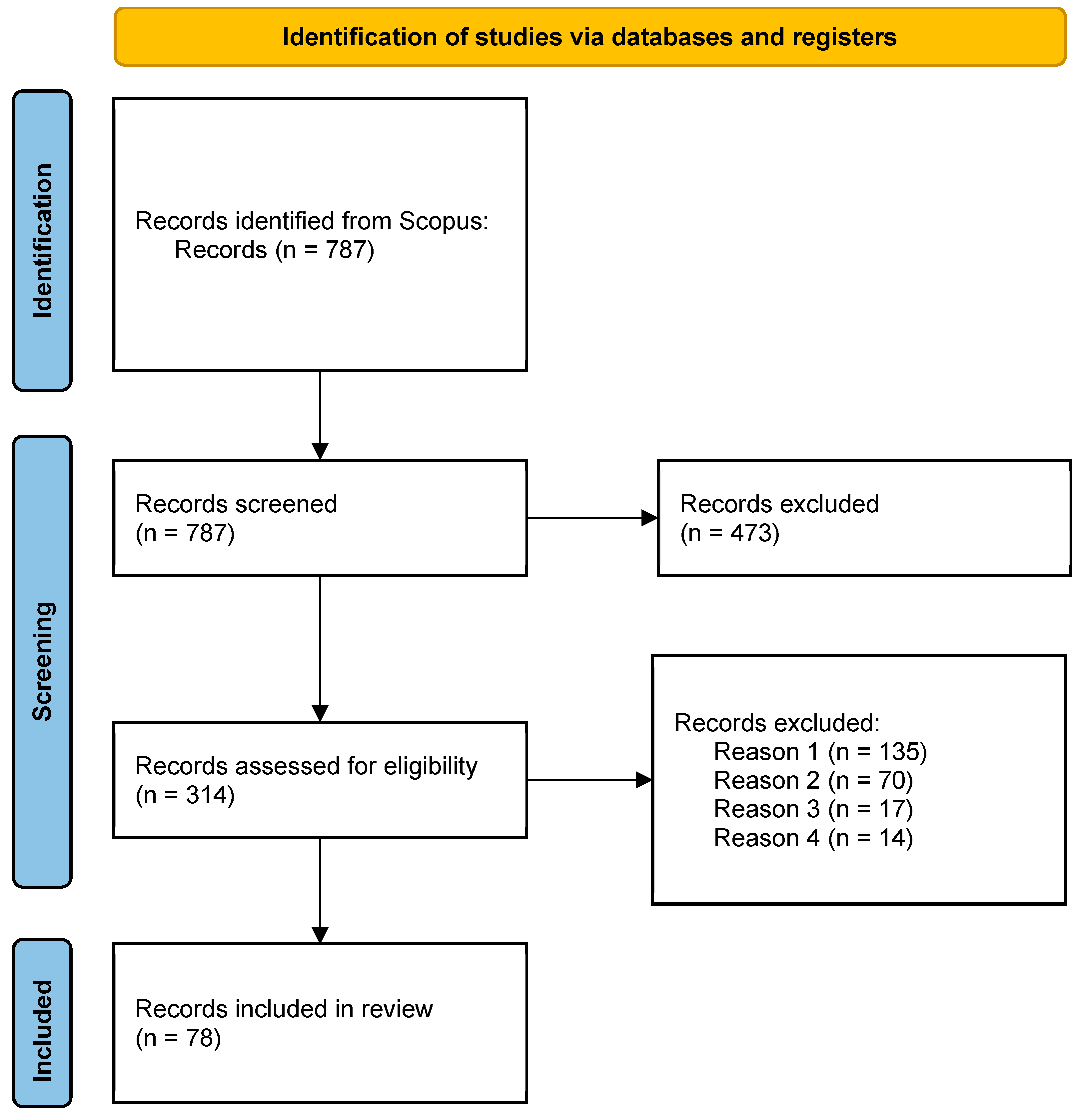
2.1. PRISMA Method
2.2. Eligibility Criteria
2.3. Features of Multi-Species Models
- Conceptual and qualitative models: Such methods focus on identifying specific, large-scale stressors and their effects on natural systems, rather than assessing a particular species or multiple species [37,38]. These models do not incorporate the precise quantitative estimates of the magnitude or strength of the species interactions. Instead, these models only account for the qualitative sign (+, −, 0) of the species interactions [39]. These types of models were outside the scope of the present review and were, therefore, not included.
- Extensions of single-species models (EXT): They focus on the dynamics of a single species, but include the effects of interactions with different species as fixed effects. These models can explicitly include predation mortality, although it is usually treated as another type of fishery rather than being estimated as part of natural mortality [7,40].
- Dynamic multi-species models (DYN): These models use a limited number of species or functional groups that are likely to have relevant interactions with the target species. They are built upon single-species theory to understand the dynamics of multi-species fisheries, but do not address the ecosystem as a whole. They can include diverse environmental variables depending on the scenario [41,42,43].
- Aggregated ecosystem models (AGG): These models consider all trophic levels (producers and consumers) in the ecosystem to explore energy flow among the levels. They include both top-down and bottom-up processes, which allows for the development of trade-off relationships between prey harvest and predator biomass [44]. The most representative example is Ecopath with Ecosim (EwE) [45,46] and its spatial form, Ecospace. Ecopath creates a mass-balanced snapshot, while Ecosim uses Ecopath parameters as initial conditions to produce time-dynamic simulations [45,47].
- End-to-end models (E2E): These models track nutrient flows through the ecosystem components, simulating annual cycles of nutrients and feeding into a representation of lower trophic levels, higher trophic levels, and even anthropogenic effects. E2E models can be coupled to different submodels, such as NEMURO [48,49,50]. The use of E2E models has been focused on strategic management, such as performing management strategy evaluations (MSEs) [15]. In this context, models such as Atlantis [51,52] can be employed as operating models to represent the impacts of fishing and other anthropogenic effects and capable of simulating the full trophic spectrum and considering physical, biochemical, and human components in a spatially resolved area.
- Coupled and hybrid model platforms (C&H): These models incorporate interactions by coupling or combining different types of model platforms. Unlike EwE and Atlantis, this framework is specifically designed for the coupling or combination of diverse model types [35]. Individual-based (IBM) and agent-based models fall within this category, employing multiple submodels to integrate the complexity of individual behavior, which can influence system dynamics. Models such as OSMOSE (Object-oriented Simulator of Marine Ecosystems Exploitation) [53] can incorporate IBM-based age-structured fish or predator population and trophic interaction models, biogeochemical plankton production models, hydrodynamic and environmental models, habitat models, representations of human activities, and potentially even representations of their social and economic drivers [35].
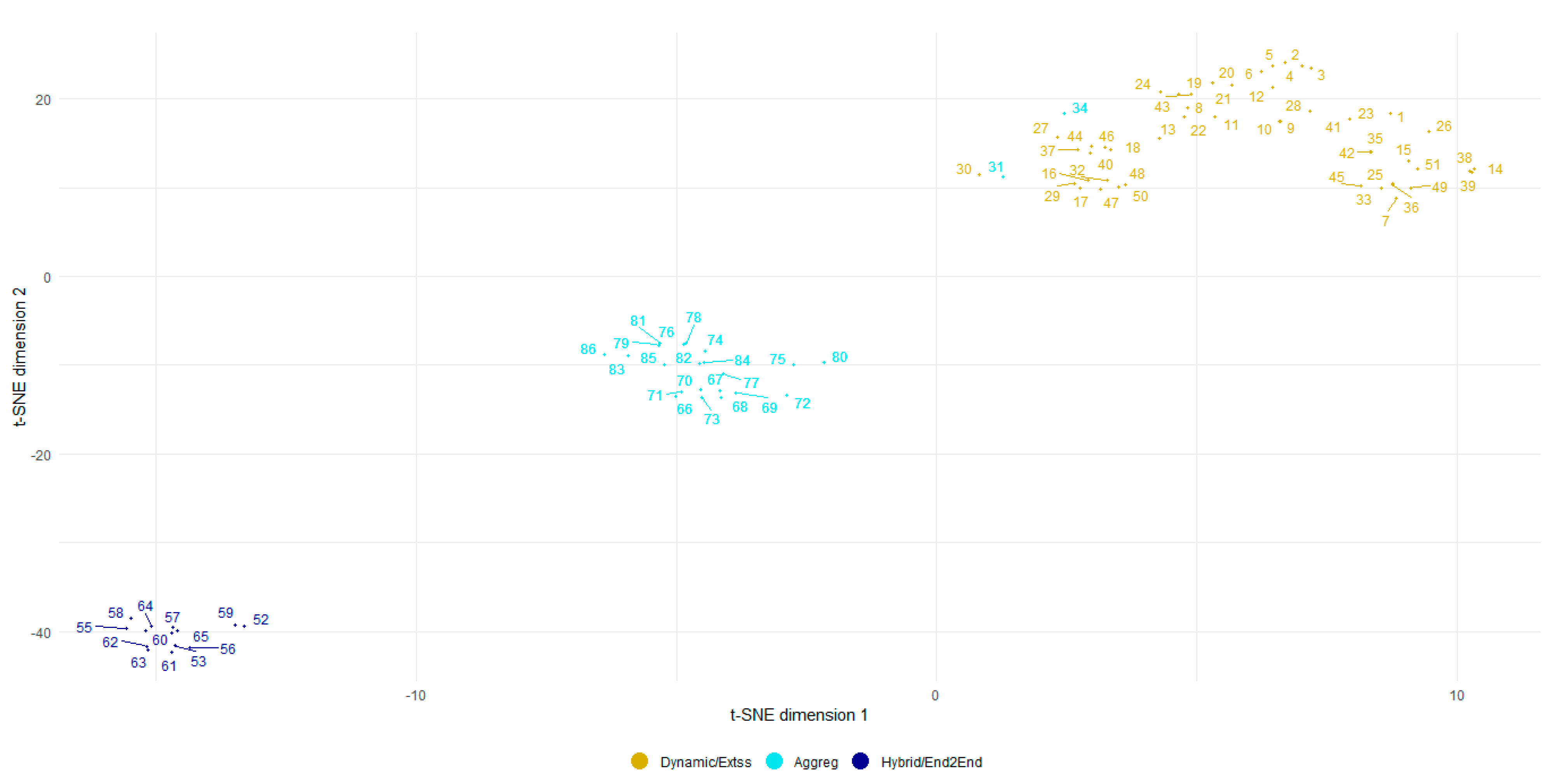
2.4. Clustering of Multi-Species Models
3. Results
3.1. Identification of Multi-Species Models
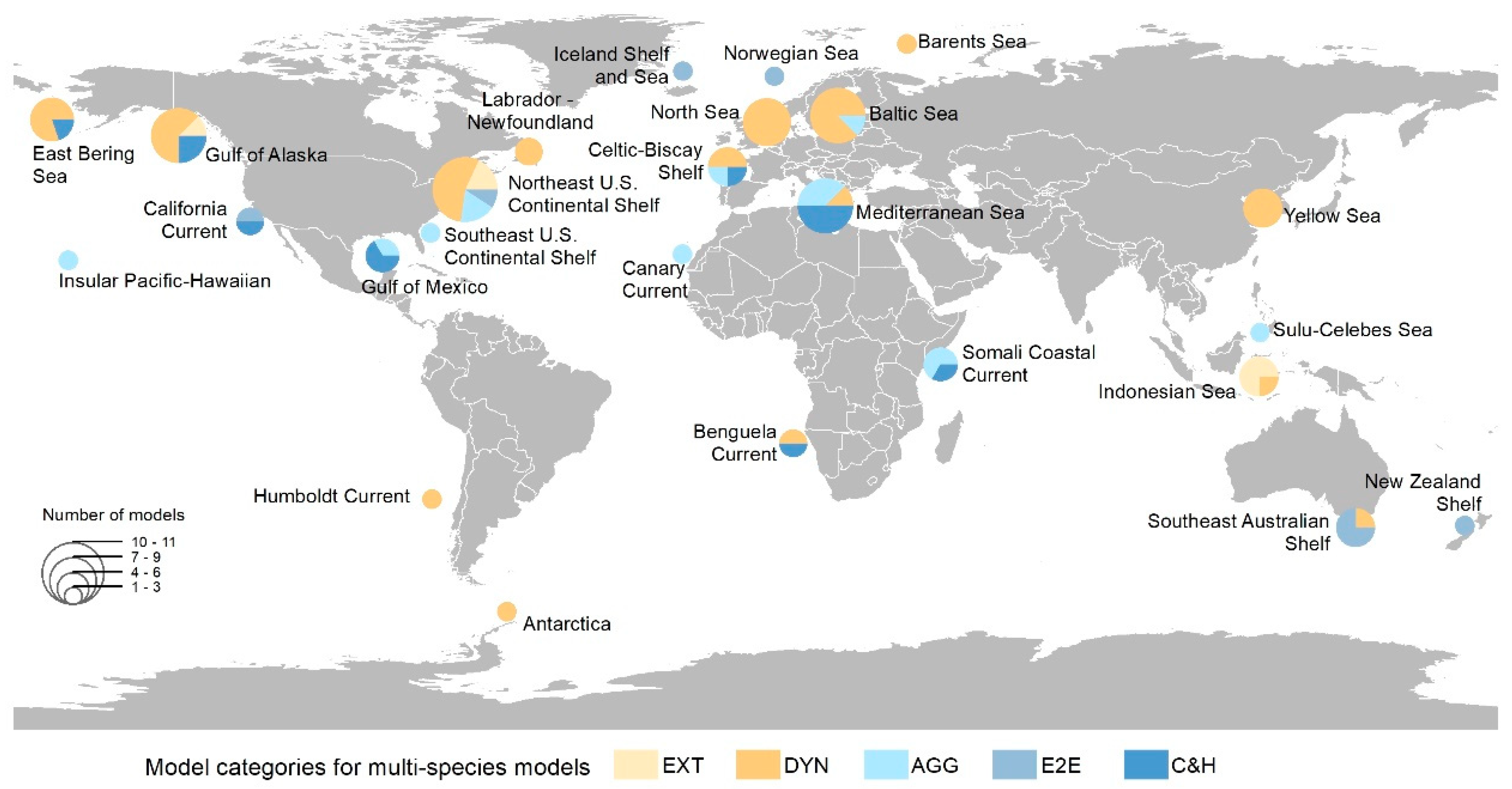
3.2. Categorization and Geographical Distribution of Multi-Species Models
- -
- Northeast U.S.: Atlantic cod (Gadus morhua), Atlantic herring (Clupea harengus), and Atlantic menhaden (Brevoortia tyrannus).
- -
- Baltic Sea: Atlantic cod, Atlantic herring, and sprat (Sprattus sprattus).
- -
- Gulf of Alaska: Arrowtooth flounder (Atheresthes stomias), Pacific cod (Gadus macrocephalus), and pollock (Theragra chalcogramma).
- -
- Mediterranean Sea: European hake (Merluccius merluccius), sardine (Sardina pilchardus), and European anchovy (Engraulis encrasicolus).
- -
- North Sea: Haddock (Melanogrammus aeglefinus), Atlantic herring, and Atlantic cod.
3.3. Multi-Species Models’ Clustering
4. Discussion
4.1. Regional Interest in Multi-Species Models
4.1.1. North Sea
4.1.2. Baltic Sea
4.1.3. Gulf of Alaska
4.1.4. Mediterranean Sea
4.1.5. Gulf of Maine
4.2. Multi-Species Model Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, A.J.; Stephenson, R.L. Options for integrating ecological, economic, and social objectives in evaluation and management of fisheries. Fish Fish. 2018, 19, 40–56. [Google Scholar] [CrossRef]
- Collie, J.S. Fisheries: Multispecies dynamics. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, UK, 2001; pp. 371–378. [Google Scholar] [CrossRef]
- Plaganyi, E. Models for an Ecosystem Approach to Fisheries; FAO: Rome, Italy, 2007; 108p. [Google Scholar]
- Geary, W.L.; Bode, M.; Doherty, T.S.; Fulton, E.A.; Nimmo, D.G.; Tulloch, A.I.T.; Tulloch, V.J.D.; Ritchie, E.G. A guide to ecosystem models and their environmental applications. Nat. Ecol. Evol. 2020, 4, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Plagányi, É.E.; Blamey, L.K.; Rogers, J.G.; Tulloch, V.J. Playing the detective: Using multispecies approaches to estimate natural mortality rates. Fish. Res. 2022, 249, 106229. [Google Scholar] [CrossRef]
- Tyrrell, M.; Link, J.; Moustahfid, H. The importance of including predation in fish population models: Implications for biological reference points. Fish. Res. 2011, 108, 1–8. [Google Scholar] [CrossRef]
- Hollowed, A.B.; Bax, N.; Beamish, R.; Collie, J.; Fogarty, M.; Livingston, P.; Pope, J.; Rice, J.C. Are multispecies models an improvement on single-species models for measuring fishing impacts on marine ecosystems? ICES J. Mar. Sci. 2000, 57, 707–719. [Google Scholar] [CrossRef]
- Fitzpatrick, K.B.; Weidel, B.C.; Connerton, M.J.; Lantry, J.R.; Holden, J.P.; Yuille, M.J.; Lantry, B.F.; LaPan, S.R.; Rudstam, L.G.; Sullivan, P.J.; et al. Balancing prey availability and predator consumption: A multispecies stock assessment for Lake Ontario. Can. J. Fish. Aquat. Sci. 2022, 79, 1529–1545. [Google Scholar] [CrossRef]
- Gislason, H. Single and multispecies reference points for Baltic fish stocks. ICES J. Mar. Sci. 1999, 56, 571–583. [Google Scholar] [CrossRef]
- Punt, A.E.; Butterworth, D.S.; de Moor, C.L.; De Oliveira, J.A.A.; Haddon, M. Management strategy evaluation: Best practices. Fish Fish. 2016, 17, 303–334. [Google Scholar] [CrossRef]
- Garcia, S.M.; Zerbi, A.; Aliaume, C.; Do Chi, T.; Lasserre, C.L. The Ecosystem Approach to Fisheries. Issues, Terminology, Principles, Institutional Foundations, Implementation and Outlook; FAO Fisheries Technical Paper, No. 443; FAO: Rome, Italy, 2003; 71p. [Google Scholar]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B.; et al. Ecosystem-Based Fishery Management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef]
- Howell, D.; Schueller, A.M.; Bentley, J.W.; Buchheister, A.; Chagaris, D.; Cieri, M.; Drew, K.; Lundy, M.G.; Pedreschi, D.; Reid, D.G.; et al. Combining Ecosystem and Single-Species Modeling to Provide Ecosystem-Based Fisheries Management Advice within Current Management Systems. Front. Mar. Sci. 2021, 7, 607831. [Google Scholar] [CrossRef]
- Patrick, W.S.; Link, J.S. Myths that Continue to Impede Progress in Ecosystem-Based Fisheries Management. Fisheries 2015, 40, 155–160. [Google Scholar] [CrossRef]
- Karp, M.A.; Link, J.S.; Grezlik, M.; Cadrin, S.; Fay, G.; Lynch, P.; Townsend, H.; Methot, R.D.; Adams, G.D.; Blackhart, K.; et al. Increasing the uptake of multispecies models in fisheries management. ICES J. Mar. Sci. 2023, 80, 243–257. [Google Scholar] [CrossRef]
- Anstead, K.A.; Drew, K.; Chagaris, D.; Schueller, A.M.; McNamee, J.E.; Buchheister, A.; Nesslage, G.; Uphoff, J.H., Jr.; Wilberg, M.J.; Sharov, A.; et al. The Path to an Ecosystem Approach for Forage Fish Management: A Case Study of Atlantic Menhaden. Front. Mar. Sci. 2021, 8, 607657. [Google Scholar] [CrossRef]
- Bentley, J.W.; Lundy, M.G.; Howell, D.; Beggs, S.E.; Bundy, A.; de Castro, F.; Fox, C.J.; Heymans, J.J.; Lynam, C.P.; Pedreschi, D.; et al. Refining Fisheries Advice with Stock-Specific Ecosystem Information. Front. Mar. Sci. 2021, 8, 602072. [Google Scholar] [CrossRef]
- Chagaris, D.; Drew, K.; Schueller, A.; Cieri, M.; Brito, J.; Buchheister, A. Ecological Reference Points for Atlantic Menhaden Established Using an Ecosystem Model of Intermediate Complexity. Front. Mar. Sci. 2020, 7, 606417. [Google Scholar] [CrossRef]
- ICES. Working Group on Integrative, Physical-Biological and Ecosystem Modelling (WGIPEM). ICES Sci. Rep. 2021, 3, 45–56. [Google Scholar] [CrossRef]
- ICES. Working Group on Multispecies Assessment Methods (WGSAM; outputs from 2022 meeting). ICES Sci. Rep. 2023, 5, 7–32. [Google Scholar] [CrossRef]
- Lewy, P.; Vinther, M. A stochastic age-length-structured multispecies model applied to North Sea stocks. In Proceedings of the ICES CM 2004, Vigo, Spain, 22–25 September 2004; pp. 1–33. Available online: https://orbit.dtu.dk/en/publications/62f59400-9068-46d7-975c-e2359ecfa355 (accessed on 13 November 2023).
- Pope, J.; Gislason, H.; Rice, J.; Daan, N. Scrabbling around for understanding of natural mortality. Fish. Res. 2021, 240, 105952. [Google Scholar] [CrossRef]
- Holling, C.S. The Components of Predation as Revealed by a Study of Small-Mammal Predation of the European Pine Sawfly. Can. Èntomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Solomon, M.E. The Natural Control of Animal Populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Kinzey, D.; Punt, A.E. Multispecies and single-species models of fish population dynamics: Comparing parameter estimates. Nat. Resour. Model. 2009, 22, 67–104. [Google Scholar] [CrossRef]
- Plagányi, É.E. Fitting the puzzle—Modeling species interactions in marine ecosystems. Bull. Mar. Sci. 2013, 89, 397–417. [Google Scholar] [CrossRef]
- Berger, A.M.; Goethel, D.R.; Lynch, P.D.; Quinn, T.; Mormede, S.; McKenzie, J.; Dunn, A. Space oddity: The mission for spatial integration. Can. J. Fish. Aquat. Sci. 2017, 74, 1698–1716. [Google Scholar] [CrossRef]
- Skern-Mauritzen, M.; Ottersen, G.; Handegard, N.O.; Huse, G.; Dingsør, G.E.; Stenseth, N.C.; Kjesbu, O.S. Ecosystem processes are rarely included in tactical fisheries management. Fish Fish. 2016, 17, 165–175. [Google Scholar] [CrossRef]
- Marshall, K.N.; Koehn, L.E.; Levin, P.S.; Essington, T.E.; Jensen, O.P. Inclusion of ecosystem information in US fish stock assessments suggests progress toward ecosystem-based fisheries management. ICES J. Mar. Sci. 2019, 76, 1–9. [Google Scholar] [CrossRef]
- Perryman, H.A.; Hansen, C.; Howell, D.; Olsen, E. A Review of Applications Evaluating Fisheries Management Scenarios through Marine Ecosystem Models. Rev. Fish. Sci. Aquac. 2021, 29, 800–835. [Google Scholar] [CrossRef]
- ICES. Report of the Workshop on Guidelines for Management Strategy Evaluations. 2013. Available online: https://ices-library.figshare.com/articles/_/19255196 (accessed on 4 June 2024).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 264–269. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Espinoza-Tenorio, A.; Wolff, M.; Taylor, M.H.; Espejel, I. What model suits ecosystem-based fisheries management? A plea for a structured modeling process. Rev. Fish Biol. Fish. 2012, 22, 81–94. [Google Scholar] [CrossRef]
- Fulton, E.A. Approaches to end-to-end ecosystem models. J. Mar. Syst. 2010, 81, 171–183. [Google Scholar] [CrossRef]
- O’fArrell, H.; Grüss, A.; Sagarese, S.R.; Babcock, E.A.; Rose, K.A. Ecosystem modeling in the Gulf of Mexico: Current status and future needs to address ecosystem-based fisheries management and restoration activities. Rev. Fish Biol. Fish. 2017, 27, 587–614. [Google Scholar] [CrossRef]
- Cook, G.S.; Fletcher, P.J.; Kelble, C.R. Towards marine ecosystem based management in South Florida: Investigating the connections among ecosystem pressures, states, and services in a complex coastal system. Ecol. Indic. 2014, 44, 26–39. [Google Scholar] [CrossRef]
- Ogden, J.C.; Davis, S.M.; Jacobs, K.J.; Barnes, T.; Fling, H.E. The use of conceptual ecological models to guide ecosystem restoration in South Florida. Wetlands 2005, 25, 795–809. [Google Scholar] [CrossRef]
- Dambacher, J.M.; Rothlisberg, P.C.; Loneragan, N.R. Qualitative mathematical models to support ecosystem-based management of Australia’s Northern Prawn Fishery. Ecol. Appl. 2015, 25, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Livingston, P.; Methot, R. Incorporation of Predation into a Population Assessment Model of Eastern Bering Sea Walleye Pollock. In Fishery Stock Assessment Models; Alaska Sea Grant: Anchorage, AK, USA, 1998; pp. 663–678. [Google Scholar] [CrossRef]
- Holsman, K.K.; Ianelli, J.; Aydin, K.; Punt, A.E.; Moffitt, E.A. A comparison of fisheries biological reference points estimated from temperature-specific multi-species and single-species climate-enhanced stock assessment models. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 134, 360–378. [Google Scholar] [CrossRef]
- Howell, D.; Bogstad, B. A combined Gadget/FLR model for management strategy evaluations of the Barents Sea fisheries. ICES J. Mar. Sci. 2010, 67, 1998–2004. [Google Scholar] [CrossRef]
- Plagányi, É.E.; Punt, A.E.; Hillary, R.; Morello, E.B.; Thebaud, O.; Hutton, T.; Pillans, R.D.; Thorson, J.T.; Fulton, E.A.; Smith, A.D.M.; et al. Multispecies fisheries management and conservation: Tactical applications using models of intermediate complexity. Fish Fish. 2014, 15, 1–22. [Google Scholar] [CrossRef]
- Drew, K.; Cieri, M.; Schueller, A.M.; Buchheister, A.; Chagaris, D.; Nesslage, G.; McNamee, J.E.; Uphoff, J.H.J. Balancing Model Complexity, Data Requirements, and Management Objectives in Developing Ecological Reference Points for Atlantic Menhaden. Front. Mar. Sci. 2021, 8, 608059. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Walters, C.; Christensen, V.; Pauly, D. Structuring dynamic models of exploited ecosystems from trophic mass-balance assessments. Rev. Fish Biol. Fish. 1997, 7, 139–172. [Google Scholar] [CrossRef]
- Ito, S.-I.; Megrey, B.A.; Kishi, M.J.; Mukai, D.; Kurita, Y.; Ueno, Y.; Yamanaka, Y. On the interannual variability of the growth of Pacific saury (Cololabis saira): A simple 3-box model using NEMURO.FISH. Ecol. Model. 2007, 202, 174–183. [Google Scholar] [CrossRef]
- Kishi, M.J.; Kashiwai, M.; Ware, D.M.; Megrey, B.A.; Eslinger, D.L.; Werner, F.E.; Noguchi-Aita, M.; Azumaya, T.; Fujii, M.; Hashimoto, S.; et al. NEMURO—A lower trophic level model for the North Pacific marine ecosystem. Ecol. Model. 2007, 202, 12–25. [Google Scholar] [CrossRef]
- Rose, K.A.; Fiechter, J.; Curchitser, E.N.; Hedstrom, K.; Bernal, M.; Creekmore, S.; Haynie, A.; Ito, S.-I.; Lluch-Cota, S.; Megrey, B.A.; et al. Demonstration of a fully-coupled end-to-end model for small pelagic fish using sardine and anchovy in the California Current. Prog. Oceanogr. 2015, 138, 348–380. [Google Scholar] [CrossRef]
- Fulton, E.A.; Smith, A.D.M.; Johnson, C.R. Biogeochemical marine ecosystem models I: IGBEM—A model of marine bay ecosystems. Ecol. Model. 2004, 174, 267–307. [Google Scholar] [CrossRef]
- Fulton, E.A.; Link, J.S.; Kaplan, I.C.; Savina-Rolland, M.; Johnson, P.; Ainsworth, C.; Horne, P.; Gorton, R.; Gamble, R.J.; Smith, A.D.M.; et al. Lessons in modelling and management of marine ecosystems: The Atlantis experience. Fish Fish. 2011, 12, 171–188. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Cury, P. Using an individual-based model of fish assemblages to study the response of size spectra to changes in fishing. Can. J. Fish. Aquat. Sci. 2004, 61, 414–431. [Google Scholar] [CrossRef]
- Yates, K.L.; Bouchet, P.J.; Caley, M.J.; Mengersen, K.; Randin, C.F.; Parnell, S.; Fielding, A.H.; Bamford, A.J.; Ban, S.; Barbosa, A.M.; et al. Outstanding Challenges in the Transferability of Ecological Models. Trends Ecol. Evol. 2018, 33, 790–802. [Google Scholar] [CrossRef]
- Rastetter, E.B. Modeling for Understanding v. Modeling for Numbers. Ecosystems 2017, 20, 215–221. [Google Scholar] [CrossRef]
- Brown, B.E. Regional fishery management organizations and large marine ecosystems. Environ. Dev. 2016, 17, 202–210. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System [Internet]. Open Source Geospatial Foundation Project. 2022. Available online: https://www.qgis.org (accessed on 16 August 2024).
- van der Maaten, L.; Hinton, G.E. Visualizing Data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- van der Maaten, L. Learning a Parametric Embedding by Preserving Local Structure. In Proceedings of the Twelfth International Conference on Artificial Intelligence & Statistics (AI-STATS), Clearwater Beach, FL, USA, 16–18 April 2009; JMLR W&CP. pp. 384–391. Available online: https://lvdmaaten.github.io/tsne (accessed on 16 August 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 May 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Krijthe, H.J. Rtsne: T-Distributed Stochastic Neighbor Embedding Using a Barnes-Hut Implementation. 2015. Available online: https://github.com/jkrijthe/Rtsne (accessed on 16 August 2024).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions [Internet]. R Package Version 2.1.3. 2022. Available online: https://CRAN.R-project.org/package=cluster (accessed on 23 January 2023).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses [Internet]. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 January 2023).
- A’mAr, Z.T.; Punt, A.E.; Dorn, M.W. Incorporating ecosystem forcing through predation into a management strategy evaluation for the Gulf of Alaska walleye pollock (Theragra chalcogramma) fishery. Fish. Res. 2010, 102, 98–114. [Google Scholar] [CrossRef]
- Wijayanto, D.; Bambang, A.N.; Kurohman, F. The multi-species fisheries model of fringescale sardinella and largehead hairtail in rembang regency, Indonesia. AACL Bioflux 2020, 13, 2312–2319. [Google Scholar]
- Wijayanto, D.; Bambang, A.N.; Kurohman, F. The multi-species competition model of Bali sardinella and fringescale sardinella in Pati Regency, Indonesia. AACL Bioflux 2021, 14, 2335–2342. [Google Scholar]
- Wijayanto, D.; Kurohman, F. Multi Species Model (Anchovy, Yellowstripe Scad and Narrow-Barred Spanish Mackerel) in Semarang Coastal [Internet]. 2022, Volume 15. Available online: http://www.bioflux.com.ro/aacl (accessed on 23 November 2022).
- Richards, R.A.; Jacobson, L.D. A simple predation pressure index for modeling changes in natural mortality: Application to Gulf of Maine northern shrimp stock assessment. Fish. Res. 2016, 179, 224–236. [Google Scholar] [CrossRef]
- Plagányi, É.E.; Butterworth, D.S. The Scotia Sea krill fishery and its possible impacts on dependent predators: Modeling localized depletion of prey. Ecol. Appl. 2012, 22, 748–761. [Google Scholar] [CrossRef]
- Heikinheimo, O. Interactions between cod, herring and sprat in the changing environment of the Baltic Sea: A dynamic model analysis. Ecol. Model. 2011, 222, 1731–1742. [Google Scholar] [CrossRef]
- Voss, R.; Quaas, M.; Neuenfeldt, S. Robust, ecological–economic multispecies management of Central Baltic fishery resources. ICES J. Mar. Sci. 2022, 79, 169–181. [Google Scholar] [CrossRef]
- Horbowy, J.; Luzeńczyk, A. Effects of multispecies and density-dependent factors on MSY reference points: Example of the Baltic Sea sprat. Can. J. Fish. Aquat. Sci. 2017, 74, 864–870. [Google Scholar] [CrossRef]
- Norrström, N.; Casini, M.; Holmgren, N.M.A. Nash equilibrium can resolve conflicting maximum sustainable yields in multi-species fisheries management. ICES J. Mar. Sci. 2017, 74, 78–90. [Google Scholar] [CrossRef]
- Bauer, B.; Horbowy, J.; Rahikainen, M.; Kulatska, N.; Müller-Karulis, B.; Tomczak, M.T.; Bartolino, V. Model uncertainty and simulated multispecies fisheries management advice in the Baltic Sea. PLoS ONE 2019, 14, e0211320. [Google Scholar] [CrossRef] [PubMed]
- Kulatska, N.; Woods, P.J.; Elvarsson, B.Þ.; Bartolino, V. Size-selective competition between cod and pelagic fisheries for prey. ICES J. Mar. Sci. 2021, 78, 1872–1886. [Google Scholar] [CrossRef]
- Blamey, L.K.; Plagányi, É.E.; Branch, G.M. Was overfishing of predatory fish responsible for a lobster-induced regime shift in the Benguela? Ecol. Model. 2014, 273, 140–150. [Google Scholar] [CrossRef]
- Spence, M.A.; Thorpe, R.B.; Blackwell, P.G.; Scott, F.; Southwell, R.; Blanchard, J.L. Quantifying uncertainty and dynamical changes in multi-species fishing mortality rates, catches and biomass by combining state-space and size-based multi-species models. Fish Fish. 2021, 22, 667–681. [Google Scholar] [CrossRef]
- Spence, M.A.; Dolder, P.J.; Nash, R.; Thorpe, R.B. The Use of a Length-Structured Multispecies Model Fitted Directly to Data in Near-Real Time as a Viable Tool for Advice. Front. Mar. Sci. 2021, 8, 700534. [Google Scholar] [CrossRef]
- Uchiyama, T.; Mueter, F.J.; Kruse, G.H. Multispecies biomass dynamics models reveal effects of ocean temperature on predation of juvenile pollock in the eastern Bering Sea. Fish. Oceanogr. 2020, 29, 10–22. [Google Scholar] [CrossRef]
- Uchiyama, T.; Kruse, G.H.; Mueter, F.J. A multispecies biomass dynamics model for investigating predator–prey interactions in the Bering Sea groundfish community. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 134, 331–349. [Google Scholar] [CrossRef]
- Van Kirk, K.F.; Quinn, T.J.; Collie, J.S. A multispecies age-structured assessment model for the Gulf of Alaska. Can. J. Fish. Aquat. Sci. 2010, 67, 1135–1148. [Google Scholar] [CrossRef]
- Gaichas, S.; Gamble, R.; Fogarty, M.; Benoît, H.; Essington, T.; Fu, C.; Koen-Alonso, M.; Link, J. Assembly rules for aggregate-species production models: Simulations in support of management strategy evaluation. Mar. Ecol. Prog. Ser. 2012, 459, 275–292. [Google Scholar] [CrossRef]
- Van Kirk, K.F.; Quinn, T.J.; Collie, J.S.; A’Mar, Z.T. Assessing uncertainty in a multispecies age-structured assessment framework: The effects of data limitations and model assumptions. Nat. Resour. Model. 2015, 28, 184–205. [Google Scholar] [CrossRef]
- Thorson, J.T.; Adams, G.; Holsman, K. Spatio-temporal models of intermediate complexity for ecosystem assessments: A new tool for spatial fisheries management. Fish Fish. 2019, 20, 1083–1099. [Google Scholar] [CrossRef]
- Adams, G.D.; Holsman, K.K.; Barbeaux, S.J.; Dorn, M.W.; Ianelli, J.N.; Spies, I.; Stewart, I.J.; Punt, A.E. An ensemble approach to understand predation mortality for groundfish in the Gulf of Alaska. Fish. Res. 2022, 251, 106303. [Google Scholar] [CrossRef]
- Jurado-Molina, J.; Gatica, C.; Arancibia, H.; Neira, S.; Alarcón, R. A multispecies virtual population analysis for the southern chilean demersal fishery. Mar. Coast. Fish. 2016, 8, 350–360. [Google Scholar] [CrossRef]
- Carvalho, P.G.; Humphries, A. Gear restrictions create conservation and fisheries trade-offs for management. Fish Fish. 2022, 23, 183–194. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Howell, D.; Casas, M.; Saborido-Rey, F.; Avila-de Melo, A. Dynamic of the Flemish Cap commercial stocks: Use of a Gadget multispecies model to determine the relevance and synergies among predation, recruitment, and fishing. Can. J. Fish. Aquat. Sci. 2017, 74, 582–597. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Umar, I.; Goto, D.; Howell, D.; Mosqueira, I.; González-Troncoso, D. Evaluation of harvest control rules for a group of interacting commercial stocks using a multispecies MSE framework. Can. J. Fish. Aquat. Sci. 2022, 79, 1302–1320. [Google Scholar] [CrossRef]
- Angelini, S.; Hillary, R.; Morello, E.B.; Plagányi, É.E.; Martinelli, M.; Manfredi, C.; Isajlović, I.; Santojanni, A. An Ecosystem Model of Intermediate Complexity to test management options for fisheries: A case study. Ecol. Model. 2016, 319, 218–232. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, Y.; Zhu, J. Simulating the impacts of fishing on central and eastern tropical Pacific ecosystem using multispecies size-spectrum model. Acta Oceanol. Sin. 2022, 41, 34–43. [Google Scholar] [CrossRef]
- Lagarde, A.; Doyen, L.; Claudet, J.; Thebaud, O. Stochastic Multi-species MSY to Achieve Ecological-Economic Sustainability of a Coral Reef Fishery System in French Polynesia. Environ. Model. Assess. 2022, 27, 771–789. [Google Scholar] [CrossRef]
- Kempf, A.; Dingsør, G.E.; Huse, G.; Vinther, M.; Floeter, J.; Temming, A. The importance of predator–prey overlap: Predicting North Sea cod recovery with a multispecies assessment model. ICES J. Mar. Sci. 2010, 67, 1989–1997. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Andersen, K.H.; Scott, F.; Hintzen, N.T.; Piet, G.; Jennings, S. Evaluating targets and trade-offs among fisheries and conservation objectives using a multispecies size spectrum model. J. Appl. Ecol. 2014, 51, 612–622. [Google Scholar] [CrossRef]
- Pope, J.G. T-ONS a swift transportable and user friendly integrative model of the North Sea for decision support. Fish. Res. 2019, 215, 9–20. [Google Scholar] [CrossRef]
- Speirs, D.; Guirey, E.; Gurney, W.; Heath, M. A length-structured partial ecosystem model for cod in the North Sea. Fish. Res. 2010, 106, 474–494. [Google Scholar] [CrossRef]
- Speirs, D.C.; Greenstreet, S.P.; Heath, M.R. Modelling the effects of fishing on the North Sea fish community size composition. Ecol. Model. 2016, 321, 35–45. [Google Scholar] [CrossRef]
- Thorpe, R.B.; Dolder, P.J.; Reeves, S.; Robinson, P.; Jennings, S. Assessing fishery and ecological consequences of alternate management options for multispecies fisheries. ICES J. Mar. Sci. 2016, 73, 1503–1512. [Google Scholar] [CrossRef]
- Trijoulet, V.; Fay, G.; Miller, T.J. Performance of a state-space multispecies model: What are the consequences of ignoring predation and process errors in stock assessments? J. Appl. Ecol. 2020, 57, 121–135. [Google Scholar] [CrossRef]
- Garrison, L.P.; Link, J.S.; Kilduff, D.P.; Cieri, M.D.; Muffley, B.; Vaughan, D.S.; Sharov, A.; Mahmoudi, B.; Latour, R.J. An expansion of the MSVPA approach for quantifying predator–prey interactions in exploited fish communities. ICES J. Mar. Sci. 2010, 67, 856–870. [Google Scholar] [CrossRef]
- Curti, K.L.; Collie, J.S.; Legault, C.M.; Link, J.S. Evaluating the performance of a multispecies statistical catch-at-age model. Can. J. Fish. Aquat. Sci. 2013, 70, 470–484. [Google Scholar] [CrossRef]
- Collie, J.; Minto, C.; Worm, B.; Bell, R. Predation on prerecruits can delay rebuilding of depleted COD stocks. Bull. Mar. Sci. 2013, 89, 107–122. [Google Scholar] [CrossRef]
- Novaglio, C.; Blanchard, J.L.; Plank, M.J.; van Putten, E.I.; Audzijonyte, A.; Porobic, J.; Fulton, E.A. Exploring trade-offs in mixed fisheries by integrating fleet dynamics into multispecies size-spectrum models. J. Appl. Ecol. 2022, 59, 715–728. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Ren, Y. An evaluation of implementing long-term MSY in ecosystem-based fisheries management: Incorporating trophic interaction, bycatch and uncertainty. Fish. Res. 2016, 174, 179–189. [Google Scholar] [CrossRef]
- Wo, J.; Zhang, C.; Pan, X.; Xu, B.; Xue, Y.; Ren, Y. Modeling the Dynamics of Multispecies Fisheries: A Case Study in the Coastal Water of North Yellow Sea, China. Front. Mar. Sci. 2020, 7, 524463. [Google Scholar] [CrossRef]
- Wo, J.; Zhang, C.; Ji, Y.; Xu, B.; Xue, Y.; Ren, Y. A multispecies TAC approach to achieving long-term sustainability in multispecies mixed fisheries. ICES J. Mar. Sci. 2022, 79, 218–229. [Google Scholar] [CrossRef]
- Xia, S.; Yamakawa, T.; Zhang, C.; Ren, Y. A multispecies size-structured matrix model incorporating seasonal dynamics. Ecol. Model. 2021, 453, 109612. [Google Scholar] [CrossRef]
- Mendoza, J.C.; de la Cruz-Modino, R.; Dorta, C.; Sosa, P.M.; Hernández, J.C. Ecosystem modeling to evaluate the ecological sustainability of small-scale fisheries: A case study from El Hierro, Canary Islands. Ocean Coast. Manag. 2022, 228, 106297. [Google Scholar] [CrossRef]
- Lassalle, G.; Lobry, J.; Le Loc’h, F.; Bustamante, P.; Certain, G.; Delmas, D.; Dupuy, C.; Hily, C.; Labry, C.; Le Pape, O.; et al. Lower trophic levels and detrital biomass control the Bay of Biscay continental shelf food web: Implications for ecosystem management. Prog. Oceanogr. 2011, 91, 561–575. [Google Scholar] [CrossRef]
- Sagarese, S.R.; Lauretta, M.V.; Walter, J.F. Progress towards a next-generation fisheries ecosystem model for the northern Gulf of Mexico. Ecol. Model. 2017, 345, 75–98. [Google Scholar] [CrossRef]
- Weijerman, M.; Gove, J.M.; Williams, I.D.; Walsh, W.J.; Minton, D.; Polovina, J.J. Evaluating management strategies to optimise coral reef ecosystem services. J. Appl. Ecol. 2018, 55, 1823–1833. [Google Scholar] [CrossRef]
- Moutopoulos, D.K.; Libralato, S.; Solidoro, C.; Stergiou, K.I. Toward an ecosystem approach to fisheries in the Mediterranean Sea: Multi-gear/multi-species implications from an ecosystem model of the Greek Ionian Sea. J. Mar. Syst. 2013, 113–114, 13–28. [Google Scholar] [CrossRef]
- Halouani, G.; Abdou, K.; Hattab, T.; Romdhane, M.S.; Lasram, F.B.R.; Le Loc’h, F. A spatio-temporal ecosystem model to simulate fishing management plans: A case of study in the Gulf of Gabes (Tunisia). Mar. Policy 2016, 69, 62–72. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Tsagarakis, K.; Sylaios, G.; Tsikliras, A.C. Ecosystem trophic structure and fishing effort simulations of a major fishing ground in the northeastern Mediterranean Sea (Thermaikos Gulf). Estuar. Coast. Shelf Sci. 2022, 264, 107667. [Google Scholar] [CrossRef]
- Rehren, J.; Wolff, M.; Jiddawi, N. Holistic assessment of Chwaka Bay’s multi-gear fishery—Using a trophic modeling approach. J. Mar. Syst. 2018, 180, 265–278. [Google Scholar] [CrossRef]
- Tuda, P.M.; Wolff, M. Comparing an ecosystem approach to single-species stock assessment: The case of Gazi Bay, Kenya. J. Mar. Syst. 2018, 184, 1–14. [Google Scholar] [CrossRef]
- Luczkovich, J.J.; Johnson, J.C.; Deehr, R.A.; Hart, K.J.; Clough, L.; Griffith, D.C. Linking Fishing Behavior and Ecosystem Dynamics Using Social and Ecological Network Models. Front. Ecol. Evol. 2021, 9, 662412. [Google Scholar] [CrossRef]
- Bacalso, R.T.M.; Wolff, M. Trophic flow structure of the Danajon ecosystem (Central Philippines) and impacts of illegal and destructive fishing practices. J. Mar. Syst. 2014, 139, 103–118. [Google Scholar] [CrossRef]
- Kaplan, I.C.; Hansen, C.; Morzaria-Luna, H.N.; Girardin, R.; Marshall, K.N. Ecosystem-Based Harvest Control Rules for Norwegian and US Ecosystems. Front. Mar. Sci. 2020, 7, 652. [Google Scholar] [CrossRef]
- Sturludottir, E.; Desjardins, C.; Elvarsson, B.; Fulton, E.A.; Gorton, R.; Logemann, K.; Stefansson, G. End-to-end model of Icelandic waters using the Atlantis framework: Exploring system dynamics and model reliability. Fish. Res. 2018, 207, 9–24. [Google Scholar] [CrossRef]
- McGregor, V.L.; Fulton, E.A.; Dunn, M.R. Spawning stock recruitment creates misleading dynamics under predation release in ecosystem and multi-species models. PeerJ 2019, 7, e7308. [Google Scholar] [CrossRef]
- Link, J.S.; Fulton, E.A.; Gamble, R.J. The northeast US application of ATLANTIS: A full system model exploring marine ecosystem dynamics in a living marine resource management context. Prog. Oceanogr. 2010, 87, 214–234. [Google Scholar] [CrossRef]
- Fulton, E.A.; Smith, A.D.M.; Smith, D.C.; Johnson, P. An integrated approach is needed for ecosystem based fisheries management: Insights from ecosystem-level management strategy evaluation. PLoS ONE 2014, 9, e84242. [Google Scholar] [CrossRef] [PubMed]
- Audzijonyte, A.; Kuparinen, A. The role of life histories and trophic interactions in population recovery. Conserv. Biol. 2016, 30, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Fulton, E.A.; Punt, A.E.; Dichmont, C.M.; Harvey, C.J.; Gorton, R. Ecosystems say good management pays off. Fish Fish. 2019, 20, 66–96. [Google Scholar] [CrossRef]
- Briton, F.; Shannon, L.; Barrier, N.; Verley, P.; Shin, Y.-J. Reference levels of ecosystem indicators at multispecies maximum sustainable yield. ICES J. Mar. Sci. 2019, 76, 2070–2081. [Google Scholar] [CrossRef]
- Travers-Trolet, M.; Bourdaud, P.; Genu, M.; Velez, L.; Vermard, Y. The Risky Decrease of Fishing Reference Points under Climate Change. Front. Mar. Sci. 2020, 7, 568232. [Google Scholar] [CrossRef]
- Reum, J.C.P.; Blanchard, J.L.; Holsman, K.K.; Aydin, K.; Hollowed, A.B.; Hermann, A.J.; Cheng, W.; Faig, A.; Haynie, A.C.; Punt, A.E. Ensemble Projections of Future Climate Change Impacts on the Eastern Bering Sea Food Web Using a Multispecies Size Spectrum Model. Front. Mar. Sci. 2020, 7, 124. [Google Scholar] [CrossRef]
- Fu, C.; Perry, R.I.; Shin, Y.-J.; Schweigert, J.; Liu, H. An ecosystem modelling framework for incorporating climate regime shifts into fisheries management. Prog. Oceanogr. 2013, 115, 53–64. [Google Scholar] [CrossRef]
- Guo, C.; Fu, C.; Forrest, R.E.; Olsen, N.; Liu, H.; Verley, P.; Shin, Y.-J. Ecosystem-based reference points under varying plankton productivity states and fisheries management strategies. ICES J. Mar. Sci. 2019, 76, 2045–2059. [Google Scholar] [CrossRef]
- Campbell, M.D.; Rose, K.; Boswell, K.; Cowan, J. Individual-based modeling of an artificial reef fish community: Effects of habitat quantity and degree of refuge. Ecol. Model. 2011, 222, 3895–3909. [Google Scholar] [CrossRef]
- Grüss, A.; Harford, W.J.; Schirripa, M.J.; Velez, L.; Sagarese, S.R.; Shin, Y.-J.; Verley, P. Management strategy evaluation using the individual-based, multispecies modeling approach OSMOSE. Ecol. Model. 2016, 340, 86–105. [Google Scholar] [CrossRef]
- Halouani, G.; Lasram, F.B.R.; Shin, Y.-J.; Velez, L.; Verley, P.; Hattab, T.; Oliveros-Ramos, R.; Diaz, F.; Ménard, F.; Baklouti, M.; et al. Modelling food web structure using an end-to-end approach in the coastal ecosystem of the Gulf of Gabes (Tunisia). Ecol. Model. 2016, 339, 45–57. [Google Scholar] [CrossRef]
- Moullec, F.; Barrier, N.; Drira, S.; Guilhaumon, F.; Marsaleix, P.; Somot, S.; Ulses, C.; Velez, L.; Shin, Y.-J. An End-to-End Model Reveals Losers and Winners in a Warming Mediterranean Sea. Front. Mar. Sci. 2019, 6, 345. [Google Scholar] [CrossRef]
- Halouani, G.; Le Loc’H, F.; Shin, Y.-J.; Velez, L.; Hattab, T.; Romdhane, M.S.; Lasram, F.B.R. An end-to-end model to evaluate the sensitivity of ecosystem indicators to track fishing impacts. Ecol. Indic. 2019, 98, 121–130. [Google Scholar] [CrossRef]
- Moullec, F.; Velez, L.; Verley, P.; Barrier, N.; Ulses, C.; Carbonara, P.; Esteban, A.; Follesa, C.; Gristina, M.; Jadaud, A.; et al. Capturing the big picture of Mediterranean marine biodiversity with an end-to-end model of climate and fishing impacts. Prog. Oceanogr. 2019, 178, 102179. [Google Scholar] [CrossRef]
- Wilson, R.J.; Sailley, S.F.; Jacobs, Z.L.; Kamau, J.; Mgeleka, S.; Okemwa, G.M.; Omukoto, J.O.; Osuka, K.E.; Samoilys, M.; Sauer, W.; et al. Large projected reductions in marine fish biomass for Kenya and Tanzania in the absence of climate mitigation. Ocean Coast. Manag. 2021, 215, 105921. [Google Scholar] [CrossRef]
- Scott, F.; Blanchard, J.L.; Andersen, K.H. mizer: An R package for multispecies, trait-based and community size spectrum ecological modelling. Methods Ecol. Evol. 2014, 5, 1121–1125. [Google Scholar] [CrossRef]
- Syed, S.; Aodha, L.N.; Scougal, C.; Spruit, M. Mapping the global network of fisheries science collaboration. Fish Fish. 2019, 20, 830–856. [Google Scholar] [CrossRef]
- Aksnes, D.W.; Browman, H.I. An overview of global research effort in fisheries science. ICES J. Mar. Sci. 2016, 73, 1004–1011. [Google Scholar] [CrossRef]
- Dornelas, M.; Antão, L.H.; Moyes, F.; Bates, A.E.; Magurran, A.E.; Adam, D.; Akhmetzhanova, A.A.; Appeltans, W.; Arcos, J.M.; Arnold, H.; et al. BioTIME: A database of biodiversity time series for the Anthropocene. Glob. Ecol. Biogeogr. 2018, 27, 760–786. [Google Scholar] [CrossRef]
- Tennant, J.P. Web of Science and Scopus are not global databases of knowledge. Eur. Sci. Ed. 2020, 46, e51987. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Fulton, E.A.; Smith, A.D.; Punt, A.E. Which ecological indicators can robustly detect effects of fishing? ICES J. Mar. Sci. 2005, 62, 540–551. [Google Scholar] [CrossRef]
- Punt, A.E.; Butterworth, D.S. The effects of future consumption by the Cape fur seal on catches and catch rates of the Cape hakes. 4. Modelling the biological interaction between Cape fur seals Arctocephalus pusillus pusillus and the Cape hakes Merluccius capensis and M. paradoxus. S. Afr. J. Mar. Sci. 1995, 16, 255–285. [Google Scholar] [CrossRef]
- Mori, M.; Butterworth, D.S. Consideration of multispecies interactions in the Antarctic: A preliminary model of the minke whale—Blue whale—Krill interaction. Afr. J. Mar. Sci. 2004, 26, 245–259. [Google Scholar] [CrossRef]
- ICES. Greater North Sea Ecoregion—Fisheries Overview; ICES Advice: Fisheries Overviews. 2022. Available online: https://ices-library.figshare.com/articles/report/Greater_North_Sea_ecoregion_fisheries_overview/21641360/1 (accessed on 20 January 2023).
- ICES. Baltic Sea Ecoregion—Fisheries Overview; ICES Advice: Fisheries Overviews. 2022. Available online: https://ices-library.figshare.com/articles/report/Baltic_Sea_ecoregion_fisheries_overview/21646934/1 (accessed on 20 January 2023).
- Bartolino, V.; Margonski, P.; Lindegren, M.; Linderholm, H.W.; Cardinale, M.; Rayner, D.; Wennhage, H.; Casini, M. Forecasting fish stock dynamics under climate change: Baltic herring (Clupea harengus) as a case study. Fish. Oceanogr. 2014, 23, 258–269. [Google Scholar] [CrossRef]
- Ojaveer, H.; Olenin, S.; Narščius, A.; Florin, A.-B.; Ezhova, E.; Gollasch, S.; Jensen, K.R.; Lehtiniemi, M.; Minchin, D.; Normant-Saremba, M.; et al. Dynamics of biological invasions and pathways over time: A case study of a temperate coastal sea. Biol. Invasions 2017, 19, 799–813. [Google Scholar] [CrossRef]
- Voss, R.; Quaas, M.F.; Stiasny, M.H.; Hänsel, M.; Pinto, G.A.S.J.; Lehmann, A.; Reusch, T.B.; Schmidt, J.O. Ecological-economic sustainability of the Baltic cod fisheries under ocean warming and acidification. J. Environ. Manag. 2019, 238, 110–118. [Google Scholar] [CrossRef]
- National Marine Fisheries Service. Fisheries of the United States, 2020. U.S. Department of Commerce, NOAA Current Fishery Statistics No. 2020. 2022. Available online: https://www.fisheries.noaa.gov/national/sustainable-fisheries/fisheries-united-states (accessed on 9 March 2023).
- Lindeberg, M.R.; Baker, M.; Dickson, D.M.; Kimmel, D.G.; Ormseth, O.A.; Strom, S.L.; Suryan, R.M. Long-term monitoring and integrated research—Understanding ecosystem processes in the Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2022, 206, 105203. [Google Scholar] [CrossRef]
- Surma, S.; Pakhomov, E.A.; Pitcher, T.J. Pacific herring (Clupea pallasii) as a key forage fish in the southeastern Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2022, 196, 105001. [Google Scholar] [CrossRef]
- McQuaw, K.; Hilborn, R. Why are catches in mixed fisheries well below TAC? Mar. Policy 2020, 117, 103931. [Google Scholar] [CrossRef]
- Hollowed, A.B.; Ianelli, J.N.; Livingston, P.A. Including predation mortality in stock assessments: A case study for Gulf of Alaska walleye pollock. ICES J. Mar. Sci. 2000, 57, 279–293. [Google Scholar] [CrossRef]
- Maynou, F. Trade-offs between employment and profitability in a Mediterranean Sea mixed bottom trawl fishery. Reg. Stud. Mar. Sci. 2021, 48, 102020. [Google Scholar] [CrossRef]
- Piroddi, C.; Colloca, F.; Tsikliras, A.C. The living marine resources in the Mediterranean Sea Large Marine Ecosystem. Environ. Dev. 2020, 36, 100555. [Google Scholar] [CrossRef]
- Verdura, J.; Linares, C.; Ballesteros, E.; Coma, R.; Uriz, M.J.; Bensoussan, N.; Cebrian, E.; Verdura, J.; Linares, C.; Ballesteros, E.; et al. Biodiversity loss in a Mediterranean ecosystem due to an extreme warming event unveils the role of an engineering gorgonian species. Sci. Rep. 2019, 9, 5911. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Osio, G.C.; Scarcella, G. Mediterranean sea: A failure of the European fisheries management system. Front. Mar. Sci. 2017, 4, 72. [Google Scholar] [CrossRef]
- FAO. The State of Mediterranean and Black Sea Fisheries 2022; FAO: Rome, Italy, 2022; Available online: http://www.fao.org/documents/card/en/c/cc3370en (accessed on 18 January 2023).
- Vasilakopoulos, P.; Maravelias, C.D.; Tserpes, G. The alarming decline of mediterranean fish stocks. Curr. Biol. 2014, 24, 1643–1648. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, E.; Vivas, M.; Bellido, J.M.; Esteban, A.; Ángeles Torres, M. Ontogenetic shifts and feeding strategies of 7 key species of Gadiformes in the western Mediterranean Sea. Fish. Bull. 2021, 119, 50–65. [Google Scholar] [CrossRef]
- Mellon-Duval, C.; Harmelin-Vivien, M.; Métral, L.; Loizeau, V.; Mortreux, S.; Roos, D.; Fromentin, J.M. Ecología trófica de la merluza europea en el golfo de león, mediterráneo noroccidental. Sci. Mar. 2017, 81, 7–18. [Google Scholar] [CrossRef]
- Modica, L.; Bozzano, A.; Velasco, F. The influence of body size on the foraging behaviour of European hake after settlement to the bottom. J. Exp. Mar. Biol. Ecol. 2013, 444, 46–54. [Google Scholar] [CrossRef]
- Coll, M.; Albo-Puigserver, M.; Navarro, J.; Palomera, I.; Dambacher, J.M. Who is to blame? Plausible pressures on small pelagic fish population changes in the northwestern Mediterranean Sea. Mar. Ecol. Prog. Ser. 2019, 617–618, 277–294. [Google Scholar] [CrossRef]
- Cury, P.; Bakun, A.; Crawford, R.J.M.; Jarre, A.; Quiñones, R.A.; Shannon, L.J.; Verheye, H.M. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- McQuinn, I.H. Pelagic fish outburst or suprabenthic habitat occupation: Legacy of the Atlantic cod (Gadus morhua) collapse in eastern Canada. Can. J. Fish. Aquat. Sci. 2009, 66, 2256–2262. [Google Scholar] [CrossRef]
- Walter, J.F.; Overton, A.S.; Ferry, K.H.; Mather, M.E. Atlantic coast feeding habits of striped bass: A synthesis supporting a coast-wide understanding of trophic biology. Fish. Manag. Ecol. 2003, 10, 349–360. [Google Scholar] [CrossRef]
- Butle, C.M.; Rudershausen, P.J.; Buckel, J.A. Feeding ecology ofAtlantic bluefin tuna (Thunnus thynnus) in North Carolina: Diet, daily ration, and consumption of Atlantic menhaden (Brevoortia tyrannus). Fish Bull. 2010, 108, 56–69. [Google Scholar]
- Gannon, D.P.; Waples, D.M. Diets of coastal bottlenose dolphins from the U.S. mid-Atlantic coast differ by habitat. Mar. Mammal Sci. 2004, 20, 527–545. [Google Scholar] [CrossRef]
- Koen-Alonso, M.; Pepin, P.; Fogarty, M.J.; Kenny, A.; Kenchington, E. The Northwest Atlantic Fisheries Organization Roadmap for the development and implementation of an Ecosystem Approach to Fisheries: Structure, state of development, and challenges. Mar. Policy 2019, 100, 342–352. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Overholtz, W.J.; Link, J.S. Aggregate surplus production models for demersal fishery resources of the Gulf of Maine. Mar. Ecol. Prog. Ser. 2012, 459, 247–258. [Google Scholar] [CrossRef]
- Craig, J.K.; Link, J.S. It is past time to use ecosystem models tactically to support ecosystem-based fisheries management: Case studies using Ecopath with Ecosim in an operational management context. Fish Fish. 2023, 24, 381–406. [Google Scholar] [CrossRef]
- Hunsicker, M.E.; Ciannelli, L.; Bailey, K.M.; Buckel, J.A.; White, J.W.; Link, J.S.; Essington, T.E.; Gaichas, S.; Anderson, T.W.; Brodeur, R.D.; et al. Functional responses and scaling in predator-prey interactions of marine fishes: Contemporary issues and emerging concepts. Ecol. Lett. 2011, 14, 1288–1299. [Google Scholar] [CrossRef]
- Ahrens, R.N.M.; Walters, C.J.; Christensen, V. Foraging arena theory. Fish Fish. 2012, 13, 41–59. [Google Scholar] [CrossRef]
- Mackinson, S.; Blanchard, J.L.; Pinnegar, J.K.; Scott, R. Consequences of alternative functional response formulations in models exploring whale-fishery interactions. Mar. Mammal Sci. 2003, 19, 661–681. [Google Scholar] [CrossRef]
- Heikinheimo, O. Average salinity as an index for environmental forcing on cod recruitment in the Baltic Sea. Boreal Environ. Res. 2008, 13, 457–464. [Google Scholar]
- Moustahfid, H.; Tyrrell, M.C.; Link, J.S.; Nye, J.A.; Smith, B.E.; Gamble, R.J. Functional feeding responses of piscivorous fishes from the northeast US continental shelf. Oecologia 2010, 163, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Carozza, D.A.; Bianchi, D.; Galbraith, E.D. Metabolic impacts of climate change on marine ecosystems: Implications for fish communities and fisheries. Glob. Ecol. Biogeogr. 2019, 28, 158–169. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Eddy, T.D.; Lotze, H.K.; Galbraith, E.D.; Cheung, W.; Barange, M.; Blanchard, J.L.; Bopp, L.; Bryndum-Buchholz, A.; Büchner, M.; et al. A protocol for the intercomparison of marine fishery and ecosystem models: Fish-MIP v1.0. Geosci. Model Dev. 2018, 11, 1421–1442. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, P.; Karmakar, M.; Leta, J.; Mayr, P. The journal coverage of Web of Science, Scopus and Dimensions: A comparative analysis. Scientometrics 2021, 126, 5113–5142. [Google Scholar] [CrossRef]
- Pranckutė, R. Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today’s Academic World. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- Punt, A.E.; Dunn, A.; Elvarsson, B.Þ.; Hampton, J.; Hoyle, S.D.; Maunder, M.N.; Methot, R.D.; Nielsen, A. Essential features of the next-generation integrated fisheries stock assessment package: A perspective. Fish. Res. 2020, 229, 105617. [Google Scholar] [CrossRef]
| id | Model Name | Large Marine Ecosystem (LME) | Specific Location | Reference | |
|---|---|---|---|---|---|
| Extension of singles-species models | |||||
| 1. | MSSCAA | Statistical catch-at-age model with predation | Gulf of Alaska | Gulf of Alaska | [65] |
| 2. | PS-mod | Schaefer surplus production model modified | Indonesian Sea | Java sea, Rembang Regency | [66] |
| 3. | PS-mod | Schaefer surplus production model modified | Indonesian Sea | Java sea, Pati Regency | [67] |
| 4. | PS-mod | Schaefer surplus production model modified | Indonesian Sea | Java sea, Semarang city | [68] |
| 5. | PPI-CSA | Predation pressure index for Collie–Sissenwine catch-survey analysis | Northeast U.S. Continental Shelf | Gulf of Maine | [69] |
| 6. | SPM-SH | Steele–Henderson surplus production model with predation | Northeast U.S. Continental Shelf | Northwest Atlantic coastal ecosystem | [44] |
| Dynamic multi-species models | |||||
| 7. | SMOM | Spatial multispecies operating model | Antarctica | Scotia Sea | [70] |
| 8. | CHS | Age-structured dynamic model | Baltic Sea | Baltic Sea | [71] |
| 9. | EE MSM | Ecological–economic multispecies model | Baltic Sea | Central Baltic Sea | [72] |
| 10. | SMS | Stochastic multispecies model | Baltic Sea | Baltic Sea | [73] |
| 11. | MSI-SOM | Multispecies interaction stochastic operative model | Baltic Sea | Baltic Sea | [74] |
| 12. | MSPM | Multispecies stock production model | Baltic Sea | Baltic Sea | [75] |
| 13. | Gadget | Globally Applicable Area-Disaggregated General Ecosystem Toolbox | Baltic Sea | Baltic Sea | [75] |
| 14. | Gadget | Globally Applicable Area-Disaggregated General Ecosystem Toolbox | Baltic Sea | Baltic Sea | [76] |
| 15. | Gadget | Globally Applicable Area-Disaggregated General Ecosystem Toolbox | Barents Sea | Barents Sea | [42] |
| 16. | ASPM | Model of intermediate complexity for ecosystem assessment | Benguela Current | Benguela | [77] |
| 17. | MSM | Multispecies size-spectrum model | Celtic-Biscay Shelf | Celtic Sea, 7e-j | [78] |
| 18. | LeMans | Length-based multispecies analysis by numerical simulation | Celtic-Biscay Shelf | Irish Sea | [79] |
| 19. | MBD | Multispecies biomass dynamics model | East Bering Sea | Bering Sea | [80] |
| 20. | MDD | Multispecies delay difference model | Eastern Bering Sea | Eastern Bering Sea | [81] |
| 21. | MBD | Multispecies biomass dynamics model | Eastern Bering Sea | Eastern Bering Sea | [81] |
| 22. | CEATTLE | Climate-enhanced, age-based model with temperature-specific trophic linkages and energetics | Eastern Bering Sea | Eastern Bering Sea | [41] |
| 23. | MS-ASA | Multispecies age-structured assessment model | Gulf of Alaska | Gulf of Alaska | [82] |
| 24. | MS-PROD | Multispecies production model | Gulf of Alaska | Gulf of Alaska | [83] |
| 25. | MSASA | Multispecies age-structured assessment model | Gulf of Alaska | Gulf of Alaska | [84] |
| 26. | MICE-in-space | Spatio-temporal model of intermediate complexity for ecosystem assessments | Gulf of Alaska | Gulf of Alaska | [85] |
| 27. | CEATTLE | Climate-enhanced, age-based model with temperature-specific trophic linkages and energetics | Gulf of Alaska | Gulf of Alaska | [86] |
| 28. | MSVPA | Multispecies virtual population analysis | Humboldt Current | Southern Chilean waters | [87] |
| 29. | LB-MSM | Length-based multispecies fisheries model | Indonesian Sea | Wakatobi National Park | [88] |
| 30. | Gadget | Globally applicable area-disaggregated general ecosystem toolbox | Labrador-Newfoundland | Flemish cap | [89] |
| 31. | GadCap | Globally applicable area-disaggregated general ecosystem toolbox | Labrador-Newfoundland | Flemish cap | [90] |
| 32. | MICE | Model of intermediate complexity for ecosystem assessment | Mediterranean Sea | Pomo pits | [91] |
| 33. | mizer | Multispecies size-spectrum model | No LME | Central and eastern tropical Pacific sea | [92] |
| 34. | MICE | Model of intermediate complexity for ecosystem assessment | No LME | Moorea Island | [93] |
| 35. | SMS | Stochastic multispecies assessment model | North Sea | North Sea | [94] |
| 36. | mizer | Multispecies size-spectrum model | North Sea | North Sea | [95] |
| 37. | T-ONS | Trade-offs North Sea model | North Sea | North Sea | [96] |
| 38. | FishSUMs | Size-structured multispecies model | North Sea | North Sea | [97] |
| 39. | FishSUMs | Size-structured multispecies model | North Sea | North Sea | [98] |
| 40. | LB-MSM | Length-based multispecies analysis by numerical simulation modified | North Sea | North Sea | [99] |
| 41. | AS-MSM | Multispecies age-structured population model | Northeast U.S. Continental Shelf | Georges Bank | [100] |
| 42. | MSVPA-X | Extended multispecies virtual population analysis | Northeast U.S. Continental Shelf | Northeast U.S. Coast | [101] |
| 43. | MS-PROD | Multispecies production model | Northeast U.S. Continental Shelf | Georges Bank | [83] |
| 44. | MSSCAA | Multispecies statistical catch-at-age model | Northeast U.S. Continental Shelf | Georges Bank | [102] |
| 45. | LeMans | Length-based multispecies analysis by numerical simulation | Northeast U.S. Continental Shelf | Georges Bank | [103] |
| 46. | MSSCAA | Multispecies statistical catch-at-age model | Northeast U.S. Continental Shelf | Northwest Atlantic coastal ecosystem | [44] |
| 47. | mizer | Multispecies size-spectrum model | Southeastern Australian Shelf | Australian Southern and Eastern | [104] |
| 48. | mizer | Multispecies size-spectrum model | Yellow Sea | Haizhou Bay | [105] |
| 49. | MSSM | Multispecies size-spectrum model | Yellow Sea | North Yellow Sea | [106] |
| 50. | MSSM | Multispecies size-spectrum model | Yellow Sea | North Yellow Sea | [107] |
| 51. | MSSM | Multispecies size-spectrum model | Yellow Sea | Haizhou Bay | [108] |
| Aggregated ecosystem models | |||||
| 52. | EwE | Ecopath with Ecosim | Baltic Sea | Baltic Sea | [75] |
| 53. | EwE | Ecopath with Ecosim | Canary current | Canary Islands, El Hierro | [109] |
| 54. | EwE | Ecopath with Ecosim | Celtic-Biscay Shelf | Bay of Viscay | [110] |
| 55. | nGoM Ecopath | Ecopath with Ecosim | Gulf of Mexico | Gulf of Mexico | [111] |
| 56. | EwE | Ecopath with Ecosim | Insular Pacific-Hawaiian | Puakō, Hawaii | [112] |
| 57. | EwE | Ecopath with Ecosim | Mediterranean Sea | Greek Ionian Sea | [113] |
| 58. | EwE-Ecospace | Ecopath with Ecosim and Ecospace | Mediterranean Sea | Gulf of Gabes | [114] |
| 59. | EwE | Ecopath with Ecosim | Mediterranean Sea | Thermaikos Gulf | [115] |
| 60. | EwE | Ecopath with Ecosim | Northeast U.S. Continental Shelf | Northwest Atlantic coastal ecosystem | [44] |
| 61. | EwE-MICE | Intermediate complexity Ecopath with Ecosim | Northeast U.S. Continental Shelf | Northwest Atlantic coastal ecosystem | [44] |
| 62. | EwE | Ecopath with Ecosim | Somali Coastal Current | Chwaka Bay | [116] |
| 63. | EwE | Ecopath with Ecosim | Somali Coastal Current | Gazi Bay, Kenya | [117] |
| 64. | EwE | Ecopath with Ecosim | Southeast U.S. Continental Shelf | Core sound | [118] |
| 65. | EwE | Ecopath with Ecosim | Sulu-Celebes Sea | Danajon Bank | [119] |
| End-to-end models | |||||
| 66. | Atlantis | Atlantis | California Current | California currents | [120] |
| 67. | Atlantis | Atlantis | Iceland Shelf and Sea, Faroe Plateau and part of the Greenland Sea | Arctic and Atlantic waters (Iceland) | [121] |
| 68. | Atlantis | Atlantis | New Zealand Shelf | Chatham Rise | [122] |
| 69. | Atlantis | Atlantis | Northeast U.S. Continental Shelf | NEUS (Gulf of Maine to Cape Hatteras) | [123] |
| 70. | Atlantis | Atlantis | Norwegian and Barents Sea | Nordic and Barrent Sea | [120] |
| 71. | Atlantis-SE | Atlantis | South-eastern Australian Shelf | Southern Australian waters | [124] |
| 72. | Atlantis | Atlantis | South-eastern Australian Shelf | Southern Australian waters | [125] |
| 73. | Atlantis-RCC | Atlantis | South-eastern Australian Shelf | Southern, southwest and Eastern central Australian waters | [126] |
| Coupled and hybrid model platforms | |||||
| 74. | OSMOSE-Coupled | Coupled end-to-end model for the southern Benguela | Benguela Current | Benguela | [127] |
| 75. | IBM-Coupled | Coupled end-to-end model for the California Current system | California Current | California current | [50] |
| 76. | OSMOSE-Coupled | Coupled end-to-end model for the Eastern English Channel fish community | Celtic-Biscay Shelf | Eastern English Channel | [128] |
| 77. | MSSM-Coupled | Coupled multispecies size spectrum model for the eastern Bering Sea | East Bering Sea | Eastern Bering Sea | [129] |
| 78. | OSMOSE-SoG | Coupled end-to-end model for the Strait of Georgia | Gulf of Alaska | Strait of Georgia, Canada | [130] |
| 79. | OSMOSE-PNCIMA | Coupled end-to-end model for the Pacific North Coast Integrated Management Area | Gulf of Alaska | PNCIMA off western Canada | [131] |
| 80. | IBM | Spatially explicit individual-based model | Gulf of Mexico | Gulf of Mexico | [132] |
| 81. | OSMOSE-WFS | Coupled end-to-end model for the West Florida Shelf | Gulf of Mexico | West Florida Shelf | [133] |
| 82. | OSMOSE-GoG | Coupled end-to-end model for the Gulf of Gabes | Mediterranean Sea | Gulf of Gabes | [134] |
| 83. | OSMOSE-Coupled | Coupled end-to-end model for the Mediterranean Sea | Mediterranean Sea | Mediterranean Sea | [135] |
| 84. | OSMOSE-GoG | Coupled end-to-end model for the Gulf of Gabes | Mediterranean Sea | Gulf of Gabes | [136] |
| 85. | OSMOSE-MED | Coupled end-to-end model for the Mediterranean Sea | Mediterranean Sea | Mediterranean Sea | [137] |
| 86. | SS-DBEM | Size spectrum dynamic bio-climate envelope model | Somali Coastal Current | EEZs Kenya and Tanzania | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couve, P.; Bahamon, N.; Canales, C.M.; Company, J.B. Systematic Review of Multi-Species Models in Fisheries: Key Features and Current Trends. Fishes 2024, 9, 372. https://doi.org/10.3390/fishes9100372
Couve P, Bahamon N, Canales CM, Company JB. Systematic Review of Multi-Species Models in Fisheries: Key Features and Current Trends. Fishes. 2024; 9(10):372. https://doi.org/10.3390/fishes9100372
Chicago/Turabian StyleCouve, Pablo, Nixon Bahamon, Cristian M. Canales, and Joan B. Company. 2024. "Systematic Review of Multi-Species Models in Fisheries: Key Features and Current Trends" Fishes 9, no. 10: 372. https://doi.org/10.3390/fishes9100372
APA StyleCouve, P., Bahamon, N., Canales, C. M., & Company, J. B. (2024). Systematic Review of Multi-Species Models in Fisheries: Key Features and Current Trends. Fishes, 9(10), 372. https://doi.org/10.3390/fishes9100372








