Abstract
The study aimed to evaluate the effect of inorganic mercury (iHg) on the sperm quality, embryos, and larvae of Prochilodus magdalenae. Two experiments were carried out. In experiment 1, semen was activated with contaminated water at different iHg concentrations (0.0, 25, 50, 100 µg/L) and sperm kinetics were analyzed with a computer-assisted semen analysis (CASA) system. In the second trial, 2 g of oocytes were fertilized with 50 µL of milt, activated with contaminated water at different iHg concentrations (0.0, 25, 50, 100 µg/L), and maintained that way during the first hour of incubation. Samples were taken every 3–4 h until hatching to identify embryo deformations. Fertilization and hatching rates were estimated. The survival of newly hatched larvae (1 day) and larvae eight days post-hatching (dph) was also assessed. The results show that the quality parameters of semen treated with contaminated water at different iHg concentrations (25, 50, 100 µg/L) were altered and showed significant differences (p < 0.05) from the control group (0 µg/L). Total motility decreased (9.8–13.2%) and the amount of immotile sperm increased (3-fold) relative to values in the control group. A significant increase in oocyte diameter and a decrease in fertilization and hatching rates were observed with the higher iHg concentrations (50–100 µg/L). Embryo deformations (>40%) such as scoliosis, lordosis, and microcephaly were observed, as well as premature hatchings. The larval survival at 1 dph was reduced between 25% (25 µg/L) and 97.2% (100 µg/L) relative to that in the control group. The results suggest that bocachico semen, embryos, and larvae are sensitive to contamination with iHg from 25 µg/L.
Key Contribution:
The study demonstrated that for externally fertilizing fish, such as the bocachico Prochilodus magdalenae, when gametes are released in waters polluted with inorganic mercury (25–100 µg/L), the seminal quality is altered and the fertilizing capacity is decreased. In embryonic development, deformations such as scoliosis, lordosis and microcephaly are registered, and the survival of newly hatched larvae is reduced between 25% (25 µg/L) and 97% (100 µg/L). Bocachico is highly sensitive to the toxic effects of inorganic mercury in the early stages of its life cycle.
1. Introduction
The main waste generated by gold mining is mercury (Hg). This heavy metal is considered highly toxic and ubiquitous, with diverse natural and anthropogenic sources [1]. Once discharged, mercury is dispersed through the air and stored in water, soil, and the atmosphere, redistributing among these environments depending on environmental parameters, emission source, and dumping site. In the soil, mercury has a long retention time; once accumulated, it continues to be released into surface waters and other environments for prolonged periods [2]. The contamination of the aquatic environment with heavy metals threatens the recruitment and survival of aquatic fauna due to detrimental effects on metabolism, growth, and reproduction [3,4,5,6,7].
Colombia is one of the countries that pollutes the most with mercury, and in 2021 was considered the fourth largest gold producer in Latin America, with a production of 76.8 tons, representing an increase of 17% compared to production in 2020 [8]; it was also considered to hold the first place in per capita pollution in the world [9]. Concern about inappropriate practices and excessive use of mercury in informal and illegal mining and their threat to Colombian fish biodiversity is growing daily [10,11,12,13].
In Colombia, more than 100 tons of inorganic mercury is discharged into the Magdalena River annually [14], and the contamination by inorganic mercury in the waters of rivers such as the Magdalena (0.06–0.12 µg/L), Cauca (0.004–0.240 µg/L), and Nechí (0.0015–2.580 µg/L) [14,15] reaches concentrations that affect aquatic life. It is important to note that in the Cauca River, where gold mining occurs, direct discharge of mercury can reach very high concentrations that affect fish survival, mainly when it occurs in spawning and early development areas of the fish [15,16]. Likewise, there are records of mercury occurring in tissues of fish species from this basin such as Hoplias malabaricus, Ageneiosus pardalis, Prochilodus magdalenae, Triportheus magdalenae, and Megaleporinus muyscorum [17,18].
According to the USEPA [19], for aquatic life, Hg levels of 1.4 µg/L imply acute toxicity and 0.8 µg/L is considered chronic toxicity [20]; however, Boyd [21] points out that concentrations >0.1 µg/L in natural waters affect aquatic life.
Fish absorb heavy metals mainly through the gills, digestive tract, and, to a lesser extent, the skin. Toxicity depends on metallic form, speciation, bioavailability, toxicokinetics (absorption, distribution, biotransformation, and excretion), and toxicodynamics (interactions with ligands) [22]. Most of the Hg found in water, soil, sediments, and biota is in the form of inorganic Hg (Hg+2 or Hg22+) and organic Hg, particularly monomethylmercury (MeHg) and dimethylmercury (DMHg) [23,24,25]. The methylation of mercury is a key step in the entrance of mercury into food chains. The biotransformation of inorganic mercury species to methylated organic species in water bodies can occur in the sediment and in the water column [25]. Factors such as concentration, pH, temperature, presence of organic matter, microbial activity, dissolved oxygen, and type of sediments can affect the toxicity and speciation of mercury in the aquatic environment [13,24]. The intake of heavy metals by fish and accumulation in the organism result in alterations in the structure and function of different tissues and vital organs [26].
Vital fish organs like the gills, liver, and kidney are susceptible to heavy metal toxicants; these organs respond differently to various heavy metals and are considered important bio-monitoring tools in evaluating the toxic effects of mercury on different fish species [27,28,29]. Prolonged exposure to heavy metals induces cytotoxicity and causes degenerative changes in the vital organs of the fish [30]. Also, the exposure of fish to heavy metals is manifested by multiple changes in hematological (red blood cells, hemoglobin, leucocytes, and lymphocytes) and biochemical parameters (glucose, glycogen, cholesterol, albumin, transferrin, ceruloplasmin) [29,30,31].
In reproduction, the quality of the gametes plays a key role in fertilization and embryonic development [5]. Parameters such as total motility, sperm velocity, and the duration of motility are considered critical to the fertilizing capacity of semen [23,24]. So, exposure of sperm to toxic substances could affect its quality and fertilization capacity [32,33].
The fertilization capacity of fish that freely release their gametes into the environment (external fertilization), such as the bocachico Prochilodus magdalenae, can be affected by contaminants such as heavy metals [32]. Mercury affects the quality of spermatozoa and therefore impedes fertilization and embryo development, either by accumulation in the gonads or by exposure in the aquatic environment during external fertilization [33].
Externally fertilized fish, such as the bocachico, simultaneously release their gametes into the riverbed during spawning, and this can occur in mining areas with high discharges of inorganic mercury. The chorion does not fully protect the embryo against the penetration of metals, particularly during the egg’s hydration (swelling) phase; therefore, metals can accumulate in the egg [26,34,35]. During embryonic development, a strong influence of metals has been reported immediately after fertilization and during the hydration phase of the egg. In Cyprinus carpio, it was reported that the chorion is permeable to metal ions such as cadmium and lead, which can change the structure and permeability of the chorion [26]. The permeability of the chorion decreases markedly after hydration and fertilization [36,37]. In neotropical migratory fish, the hydration phase of the eggs takes between one and two hours after fertilization [38]. Heavy metals can affect embryonic development processes, resulting in reduced quantity and quality of offspring [26].
Fish embryonic and larval deformations disturb population dynamics because they affect survival, growth, well-being, morphology, and ability to interact with habitats [39,40]. Among the damages reported in fish by heavy metals are deformations in the spine (lordosis, scoliosis, kyphosis), cephalic region, fins, and lateral line [40,41].
Prochilodus magdalenae, commonly known as bocachico, is the main species of Colombian freshwater fisheries, with great importance for the food security of the riverside county inhabitants of the Magdalena, Cauca, Atrato, and Sinú rivers [42]. Catches of this species have drastically decreased from 40,000 tons in 1975 to 4517 tons in 2020 [43,44]. Due to the drastic decrease in its catches, it has been categorized as vulnerable to extinction [45]. It is a potamodromous species whose population dynamics depend on annual migrations and the interactions between the rivers where they reproduce and the swamps where they feed and grow [46]. This species spawns in the main channels of the rivers, where its embryo development also takes place; then, the larvae enter the flood plains (swamps) to continue their development and growth [47,48].
Mercury in gold mining is a general problem that could affect the availability, distribution, genetics, and development of native fish species and, therefore, human populations consuming contaminated fish [13,24]. This study aimed to evaluate the effect of inorganic mercury on semen quality, embryos, and larval development of bocachico to provide basic information for managing native fish species contaminated by this xenobiotic.
2. Materials and Methods
2.1. Semen and Egg Collection
The gametes were obtained by induced reproduction of two-year-old bocachico broodstock (0.35 ± 0.5 kg) kept in captivity at the Institute of Fishculture Research of the University of Córdoba—CINPIC (Montería, Colombia). The females were injected with carp pituitary extract at a dose of 5 mg/kg of live weight in two doses (10% and 90% of the total dose in 12 h), and the males received a single dose of 4 mg/kg of live weight [49]. The gametes were collected six hours after the second injection of the females [49].
2.2. Experiment 1
In a Makler chamber (Sefi Medical, Haifa, Israel), 0.25 µL of semen was activated with 75 µL of water contaminated with different concentrations of inorganic mercury (Hg2+, iHg): 0.0, 25, 50, and 100 µg/L. Total motility, types of motility (rapid, medium, and slow), progressivity, curvilinear velocity (VCL), and straight linear velocity (VSL) were evaluated with a computer-assisted sperm analysis (CASA) system (Microptic, SCA-Vet, Barcelona, Spain) and a contrast microscope of phase (Nikon, Eclipse 50i, Tokio, Japan). Rapid motility was considered the percentage of spermatozoa with velocities greater than 100 μm/s, medium motility described spermatozoa with velocities less than 100 μm/s but greater than 50 μm/s, and slow motility described the percentage of spermatozoa with velocities less than 50 μm/s [49]. The duration of motility was estimated as the time until around 90% of the spermatozoa stopped moving [42]. In addition, the straightness index (STR), linearity index (LIN), oscillation index (WOB), and parameters of angularity and oscillation of the spermatozoa heads, such as lateral width of the head (ALH) and beat cross frequency (BCF), were estimated.
2.3. Experiment 2
Fertilization was performed in vitro by mixing 2 g of oocytes with 50 µL of semen, which was activated and maintained during the first hour of incubation with water contaminated with different concentrations of inorganic mercury (Hg2+, iHg) in the form of Hg(NO₃)₂: 0.0 (control) 25, 50, and 100 µg/L. For the preparation of the contaminated water, a standard solution of mercury Hg(NO₃)₂ (1000 mg/L Hg) (Certipur®, USA) in HNO₃ (2 mol/L) was used. Aliquots of 50, 100, and 200 μL of this standard solution were taken to prepare 2 L of solutions with concentrations of 25, 50, and 100 µg/L; distilled water was used to complete this volume and maintain the pH of these solutions between 6.5 and 7.5. The gametes were kept in the contaminated water at different iHg concentrations for one hour during the fertilized egg hydration period [43]. Then, the embryos were transferred to experimental incubators (2.5 L of volume) with an upflow of uncontaminated water until hatching, approximately 14 h post-fertilization (hpf).
In the incubation and larviculture water, iHg concentration was estimated, and iHg concentration in the oocytes after exposure to the different concentrations was evaluated. The iHg was estimated as total mercury using a direct mercury analyzer (Milestone, DMA-80, Sorisole, Italy) with the following sequence: thermal decomposition, catalytic conversion, amalgamation, and atomic absorption spectrophotometry (EPA 7473). Each measurement was performed in triplicate for each experimental unit (incubator).
2.4. Embryonic Deformations
Embryo samples (n = 50) were taken in triplicate at 1 (cleavage), 5 (gastrula), 8 (segmentation), and 11 h post-fertilization (hpf) (pharyngula) to identify embryonic deformations in each incubator. Bocachico eggs and larvae were photographed with a stereoscope (Zeiss, Stemi 508, Jena, Germany) with a built-in camera (Canon, G10, Japan) and then measured with image analysis software (Zeiss, Axiovision 4.8, Jena, Germany).
2.5. Fertilization and Hatching Rates
The fertilization rate was estimated at 5 hpf (gastrulation stage), and the hatching rate was measured at 10 hpf (pharyngulation stage). Viable embryos were considered translucent, and non-viable embryos were opaque, with detachment of the cellular material and without a chorion (premature hatching). Fertilization and hatching rates were estimated by dividing the number of viable embryos by the total number of embryos analyzed [49].
2.6. Larval Survival
The newly hatched larvae from each experimental incubator were transferred to 5 L tanks with water exchange and permanent aeration. Larval survival was estimated on the first day post-hatching (1 dph) and at 8 dph based on the number of larvae obtained at 1 dph.
2.7. Water Quality
Twice during incubation (~14 h) and daily during larviculture (8 days), water quality parameters such as temperature, dissolved oxygen (YSI, 550A, Yellow Springs, OH, USA), and pH (YSI, 100, Yellow Springs, OH, USA) were measured. Likewise, hardness, total alkalinity, non-ionized ammonium, and nitrites were measured with a photometer (YSI, 9500, Yellow Springs, OH, USA). The temperature (26.0–27.6 °C), dissolved oxygen (4.2–7.5 mg/L), pH (7.2–8.4), total alkalinity (40.0–70.0 mg CaCO3/L), total hardness (50.0–60.0 mg CaCO3/L), non-ionized ammonia (0.08–0.11 mg/L), and nitrite (0.02–0.04 mg/L) levels ranged within normal values for the embryonic and larval development of neotropical fish species [50].
2.8. Statistical Analysis
A completely randomized design was used, with four treatments evaluated in triplicate. All variables were submitted to normality (Kolmogorov–Smirnov test) and homoscedasticity (Levene test) tests. The percentage data were normalized using the arcsine square root transformation and then were analyzed using ANOVA followed by Tukey’s multiple range test. In all cases, p < 0.05 was considered statistically significant. The analysis was performed with the Statgraphics Centurion XVI program. All results were expressed as mean ± standard deviation.
3. Results
3.1. Semen Quality
Table 1 shows the semen quality of bocachico activated with water contaminated at different concentrations of iHg (0, 25, 50, and 100 µg/L). The results show that the quality parameters of semen treated with contaminated water at different concentrations of iHg (25, 50, and 100 µg/L) were altered and showed significant differences (p < 0.05) from those of the control group (0 µg/L), except VSL, STR, and LIN. In addition, the values of the different parameters analyzed from semen activated with contaminated water at iHg concentrations from 25 to 100 µg/L did not show statistical differences among these treatments (p > 0.05).

Table 1.
The sperm quality of bocachico Prochilodus magdalenae activated with water contaminated with different concentrations of inorganic mercury (iHg). VCL, curvilinear velocity; VSL, straight linear velocity; STR, straightness index; LIN, linearity index; WOB, wobbling index; ALH, amplitude of lateral head displacement; BCF, beat cross frequency.
3.2. Fertilization and Hatching Rates
The highest fertilization rates were recorded in the control (72.6 ± 9.8%) and 25 µg/L (68.8 ± 5.3%) groups, with no significant difference between these values (p > 0.05). The lowest fertilization rates were obtained in contaminated water at doses of 50 µg/L (53.4 ± 6.7%) and 100 µg/L (56.1 ± 8.4%), without observing a significant difference between these values (p > 0.05). Also, the highest percentage of hatching was recorded in the control group (67.1 ± 9.4%), with a significant difference (p < 0.05) from the percentages obtained when the water was contaminated with iHg. Hatching rates in the treatments with iHg-contaminated water ranged between 48.5 ± 11.2% (100 µg/L) and 55.8 ± 6.8% (25 µg/L), without a statistical difference between these values (p > 0.05) (Figure 1).

Figure 1.
Fertilization and hatching rates of bocachico Prochilodus magdalenae were obtained with gametes activated and incubated during the first hour with water contaminated with different inorganic mercury (iHg) concentrations. Different letters indicate significant differences (p < 0.05).
3.3. Larval Survival
At 1 dph, the lowest survival rates were recorded in the treatments with contaminated water at 50 µg/L (6.8%) and 100 µg/L (0.8%), with no significant difference between these values (p > 0.05). The highest survival was recorded in the control group (29.0%) and in gametes treated with 25 µg/L (21.8%) (p > 0.05). At 8 dph, survival oscillated between 96.8% (control) and 98.1% (25 µg/L), with no significant difference between these treatments (p > 0.05) (Figure 2).
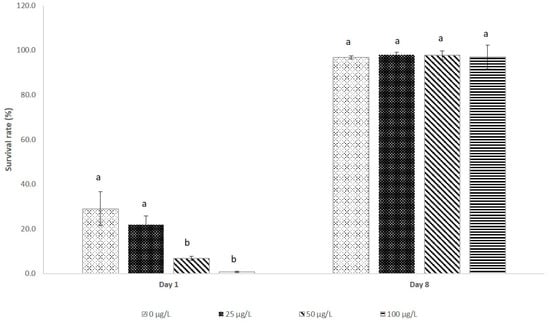
Figure 2.
The larval survival rate of bocachico Prochilodus magdalenae on the first and eighth day post-hatching. The larvae were obtained using gametes activated and treated during the first hour of incubation with water contaminated at different inorganic mercury (iHg) concentrations. Different letters indicate significant differences (p < 0.05).
3.4. Egg Diameters, Mercury Absorption by Eggs and Larvae, and Size of Newly Hatched Larvae
The diameter of the oocytes was greatest in the treatments with contaminated water at 50 µg/L (4.9 ± 0.4 mm) and 100 µg/L (4.9 ± 0.5 mm) iHg, with a statistical difference (p < 0.05) from oocyte diameter in the control group (4.3 ± 0.3 mm) and the 25 µg/L group (4.5 ± 0.3 mm). Also, dose-dependent uptake of iHg was observed in eggs and newly hatched larvae. The higher the concentration of iHg in the water, the higher the absorption by embryos and larvae. However, despite the changes in the diameter of the oocytes, the size of the newly hatched larvae (day 1) did not show significant differences between the different treatments (p > 0.05) (Table 2).

Table 2.
Egg diameters, inorganic mercury absorption by eggs and larvae, and total length of newly hatched bocachico Prochilodus magdalenae larvae (1 day old). The larvae were obtained with gametes activated and incubated through the first hour with water contaminated at different inorganic mercury concentrations. tL, total length.
3.5. Deformation of Embryos and Larvae Exposed to Inorganic Mercury
In the treatments with water contaminated with iHg, the embryos’ deformations were significantly greater than those in the control group (p < 0.05). The deformations occurred in all stages of embryonic development and became more evident as embryo development advanced. At the pharyngula stage, the embryo deformation rates were significantly higher in all iHg-treated groups than in the control (Table 3, Figure 3).

Table 3.
Deformation percentages at different stages of the embryonic development of bocachico Prochilodus magdalenae. The embryos were obtained with gametes activated and incubated through the first hour with water contaminated with different inorganic mercury concentrations. HPF, hours post-fertilization.

Figure 3.
Microphotographs of deformed embryos of the bocachico Prochilodus magdalenae. The gametes were activated and incubated with water contaminated with inorganic mercury (iHg) through the first hour. (a) Normal embryo in the cleavage stage; (b) embryo with anomalies in the blastomeres; (c) embryo with yolk deformation; (d) normal embryo in the gastrula stage; (e) embryo with deformation of the blastoderm; (f) embryo with deformation of the blastoderm and detachment of cellular material; (g) normal embryo in the segmentation phase; (h) embryo with deformation in the cephalic region; (i) embryo with deformation in the cephalic and caudal regions; (j) normal hatching embryo; (k) embryo with deformation in the dorsal spine (lordosis); (l) embryo with head deformation (microcephaly). The arrow indicates the injury.
4. Discussion
The results of trial 1 showed a decrease in the semen quality of bocachico when milt was exposed to water contaminated with iHg. Parameters such as total motility (9.8–13.2%), duration of motility (8.5–14.7%), WOB (8.2–10.2%), ALH (12.5–18-8%), BCF (14.4–18-3%%), VCL (18–26%), and rapid motility (38–48.2%) decreased when milt was activated with water contaminated with iHg at a dosage between 25 to 100 µg/L; in contrast, the quantity of immotile sperm increased 3-fold relative to that in the control (Table 1).
In studies carried out with other species of fish, alterations have been reported when milt is activated with water contaminated with inorganic mercury. Hayati et al. [51] reported a 20% decrease in motility duration of sperm from the common carp Cyprinus carpio when they activated semen with mercuric chloride contaminated water at dosages between 1000 and 5000 µg/L. Dietrich et al. [33] also reported decreased total motility below 50% and a significant reduction of VSL but not VCL in the semen of rainbow trout Oncorhynchus mykiss when they activated the sperm with inorganic-mercury-contaminated water (Hg2+, iHg) at 10,000 µg/L. Pataki et al. [52] suggested that the total motility and VCL of zebrafish Danio rerio semen decreased when the sperm was activated with Hg-contaminated water between 500 and 5000 µg/L; however, they found that with older fish (7, 12, and 18 months) these parameters suffered more remarkable alterations. In the present study, the semen evaluated was extracted from bocachico breeders of the same age (24 months). It has been reported that semen tolerance to mercury contamination is species-specific and depends on the characteristics of the spermatozoa and the physiology and biochemistry of the seminal plasma of each species [32,53]. Therefore, considering the results of this study and those obtained for other fish species, we can suggest that bocachico semen is sensitive to mercury contamination (iHg) from a concentration of 25 µg/L.
In general, a decrease in sperm kinetics suggests a disruption in energy metabolism and/or damage to the mitochondria [32,54,55]. Mercury in its different forms (organic and inorganic) in high concentrations induces cytotoxicity mediated by reactive oxygen species (ROS) and could be toxic to the male reproductive system of fish, affecting testicular spermatogenic and steroidogenic functions [6,56,57]. In addition, Hg has been shown to delay spermatogenesis through inhibitory actions of pituitary gonadotropins, decreases in the gonadosomatic index, or inhibition of the spermatid-to-sperm transformation [6,25].
The results of trial 2 showed that the lowest fertilization and hatching rates were obtained at the highest doses of iHg (50 µg/L and 100 µg/L). These treatments showed a significant decrease in fertilization (23–26%) and hatching (27–28%) rates relative to those in the control group. In the common carp Cyprinus carpio, a decreased fertilization rate from exposure to 500 µg/L Hg was reported [51]. In the rainbow trout Oncorhynchus mykiss, when eggs were exposed to Hg at 10,000 µg/L for four hours, the hatching rate was reduced to one-third of that obtained in the group control [26]. These results suggest that the inorganic mercury intoxication of bocachico gametes and embryos, particularly in the permeability stage of the chorion, reduces fertilization and hatching rates at concentrations above 50 µg/L, which indicates a greater sensitivity of this species to the effects of mercury.
The early period of embryo development just after fertilization and the hatching period are the most sensitive to metal poisoning. Alterations during these periods reduce the quantity and quality of larvae [26]. The decrease in fertilization capacity due to exposure to contaminants is not only related to the inhibition or suppression of the onset of motility and sperm velocity due to a decrease in ATP content but also to damage to DNA integrity due to oxidative stress produced by reactive oxygen species (ROS) [32,58,59,60].
In the present study, the percentage of embryo deformations was higher in the treatments exposed to iHg than in the control group. Although deformations were observed in all stages of embryonic development, the abnormalities were more expressed as development progressed. At the pharyngula stage, the embryonic deformation rate in iHg-treated groups was four times higher than that estimated in the control group. The malformations were expressed in different parts of the embryo (blastomeres, blastoderm, yolk).
In the blastomeration state, abnormalities in blastomere distribution, asymmetry in size, and disturbances during cell division were observed; yolk alterations were also observed. In the gastrulation state, deformations were observed in the blastoderm, and detachment of the cellular material was noted. In the segmentation stage, deformations were observed in the caudal and cephalic regions. In the pharyngulation stage, malformations were observed in the head (microcephaly) and the spinal column (scoliosis or lordosis). Deformation percentages were highest in the final stage of embryo development (pharyngulation). In common carp, egg agglomerations and embryo malformations were reported after inorganic mercury exposure (12 and 24 h) at much higher doses (500 μg/L) than in the present study. These damages were attributed to the toxic nature of mercury and the increase in reactive oxygen species (ROS), which affected fish fertility and embryo development [51].
Exposure of bocachico eggs to different iHg concentrations (25, 50, and 100 µg/L) during the first hour of hydration caused damage to the embryos. In the oocytes exposed to different iHg concentrations, an increase in diameter was recorded relative to that the control group. The diameter of the eggs increased significantly, with values between 4.7% (25 µg/L) and 14.0% (50 and 100 µg/L) greater than control values.
Moreover, eggs absorbed more inorganic mercury at higher heavy metal concentrations in the water; this suggests that mercury absorption by eggs depends on the metal concentration in the water. The oocytes exposed to 100 µg/L absorbed 16 times the concentration to which they were treated (1603.3 ± 766.1 µg/kg); eggs subjected to 50 µg/L absorbed approximately nine times the dose to which they were exposed (447.6 ± 367.9 µg/kg), and the eggs exposed to 25 µg/L absorbed about 2.5 times the exposed concentration (64.5 ± 3.5 µg/kg). The oocytes of the control treatment (0 µg/L) presented an iHg concentration of 1.1 ± 0.2 µg/kg (p < 0.05). The iHg concentrations in the eggs of the control group may be attributed to the volatility of mercury, which may have been distributed throughout the experimental trial environment, despite the measures taken to prevent this effect.
The increase in the diameter of the eggs could be attributed to the absorption of iHg, which caused a high osmolarity and, therefore, a high intra-oocyte osmotic pressure that, in some cases, caused premature hatching or abortion in the contaminated treatments at the highest iHg concentrations (50 and 100 µg/kg). Nevertheless, it was also observed that the deformations impeded the movements of the embryos required to break the chorion before hatching (weak embryos), especially in the treatments contaminated with the highest doses of iHg evaluated in the present study (50 and 100 µg/L). Likewise, these treatments showed high deformations in the dead larvae on the first day post-hatching (dph). It should be noted that the larvae that survived after 1 dph were resistant, without evident malformations. Mortality was low between 1 and 8 dph, and survival in that period was similar between the different treatments.
It is convenient to note that more research is necessary to understand the chemical interactions of chorion components with different xenobiotics and the ability of contaminants to cross the chorion. It is necessary to deepen the knowledge of the composition and dynamics of the chorion pores (permeability), the only route of hydration for the eggs. It is known that the permeability of the chorion varies during development and is affected by environmental conditions. However, there is no clarity about the types of molecules for which the chorion is permeable or acts as a barrier [36,61]. Therefore, more studies on the permeability of the chorion of the eggs of migratory fish such as bocachico is recommended, allowing for more precise identification of the causes of embryonic and larval deformations and the high mortality caused by heavy metals.
5. Conclusions
The results of the present study reveal that during the reproduction of bocachico in contaminated waters, inorganic mercury affects sperm quality and embryonic development from 25 µg/L, as well as and fertilization and hatching rates from 50 µg/L. In addition, the results suggest that bocachico Prochilodus magdalenae is highly sensitive to the toxic effects of mercury in the early stages of its life cycle.
Author Contributions
Conceptualization, methodology, validation, formal analysis, investigation, visualization, writing—review and editing, supervision, and project administration, V.A.-G. and J.M.-N.; formal analysis, investigation, visualization, writing—original draft preparation, data curation, D.M.-M.; funding acquisition, V.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by an agreement between the Empresas Públicas de Medellín (EPM) and the University of Córdoba (CT 2019-000636). This research is part of the Project ex situ conservation strategies for the main migratory fish species of the Middle and Lower Cauca River Basin (Project 3).
Institutional Review Board Statement
The study was conducted in accordance with the Rules of Conduct for the Use of Animals in Teaching and Research of the Faculty of Veterinary Medicine and Zootechnics of the University of Córdoba (Colombia) and approved by the Ethics Committee (Act 009 of 30 November 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data provided in this manuscript were appropriately cited in the tables, figures, and reference section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaioli, M.; Amoedo, D.; González, D. Impact of mercury on human health and the environment. Arch. Argent. Pediatr. 2012, 110, 259–264. [Google Scholar] [CrossRef]
- Casas, I.C.; Gómez, E.; Rodríguez, L.M.; Girón, S.L.; Mateus, J.C. Hacia un plan nacional para el control de los efectos en salud del mercurio en Colombia. Biomedica 2015, 35, 30–37. [Google Scholar] [CrossRef][Green Version]
- Bera, T.; Kumar, S.V.; Devi, M.S.; Kumar, V.; Behera, B.K.; Das, B.K. Effect of heavy metals in fish reproduction: A review. J. Environ. Biol. 2022, 43, 631–642. [Google Scholar] [CrossRef]
- Chen, Q.; An, J.; Xie, D.; Gong, S.; Lian, X.; Liu, Z.; Shen, Y.; Li, Y. Suppression and recovery of reproductive behavior induced by early life exposure to mercury in zebrafish. Comp. Biochem. Physiol. Pt. C Toxicol. Pharmacol. 2021, 239, 108876. [Google Scholar] [CrossRef] [PubMed]
- Brraich, O.S.; Jangu, S. Some aspects of reproductive biology on effect of heavy metal pollution on the histopathological structure of gonads in Labeo rohita (Hamilton-Buchanan) from Harike wetland, India. Int. J. Fish. Aquac. 2015, 7, 9–14. [Google Scholar] [CrossRef][Green Version]
- Crump, K.L.; Trudeau, V.L. Mercury-induced reproductive impairment in fish. Environ. Toxicol. Chem. 2009, 28, 895–907. [Google Scholar] [CrossRef]
- Telmer, K.H.; Veiga, M.M. World emissions of mercury from artisanal and small scale gold mining. In Mercury Fate and Transport in the Global Atmosphere; Springer US: Boston, MA, USA, 2009; pp. 131–172. [Google Scholar]
- ANM (Agencia Nacional de Minería). Boletín Estadístico Minero: 2020–2021; ANM, Ministerio de Minas y Energía: Bogotá, Colombia, 2021. [Google Scholar]
- Cordy, P.; Veiga, M.; Salih, I.; Al-Saadi, S.; Console, S.; García, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world’s highest per capita mercury pollution. Sci. Total Environ. 2011, 410–411, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.J.S. All that glitters is not gold or platinum: Institutions and the use of mercury in mining in Chocó, Colombia. Extr. Ind. Soc. 2018, 5, 308–318. [Google Scholar] [CrossRef]
- Londoño Franco, L.F.; Londoño Muñoz, P.T.; Muñoz García, F.G. Los riesgos de los metales pesados en la salud humana y animal. Biotecnol. Sect. Agropecu. Agroind. 2016, 14, 145–153. [Google Scholar] [CrossRef]
- Díaz Arriaga, F.A. Mercurio en la minería del oro: Impacto en las fuentes hídricas destinadas para consumo humano. Rev. Salud Pública 2015, 16, 947–957. [Google Scholar] [CrossRef]
- Enamorado-Montes, G.; Reino-Causil, B.; Urango-Cardenas, I.; Marrugo-Madrid, S.; Marrugo-Negrete, J. Mercury Accumulation in Commercial Varieties of Oryza sativa L. Cultivated in Soils of La Mojana Region, Colombia. Toxics 2021, 9, 304. [Google Scholar] [CrossRef]
- Gutiérrez, F.; de la Parra, A. Contaminación del agua en la cuenca del rio Magdalena (Colombia) y su relación con los peces. In Peces de la Cuenca del río Magdalena, Colombia: Diversidad, Conservación y Uso Sostenible; Jiménez-Segura, L., Lasso, C.A., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2020; pp. 239–269. [Google Scholar] [CrossRef]
- Atencio-García, V.J.; Madariaga-Mendoza, D.L.; Ortiz-Bedoya, A.; Guerrero-Durango, H.; Marrugo-Negrete, J.L. Fish health in the Cauca River (Colombia). In Proceedings of the IX International Symposium on Aquatic Animal Health: Enhancing Aquatic Animal Health towards One Health, Santiago, Chile, 5–8 September 2022. [Google Scholar]
- Moreno-Arias, C.; López-Casas, S.; Rogeliz-Prada, C.; Jiménez-Segura, L. Protection of spawning habitat for potamodromous fish, an urgent need for the hydropower planning in the Andes. Neotrop. Ichthyol. 2021, 19, e210027. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Navarro-Frómeta, A.; Ruiz-Guzmán, J. Total mercury concentrations in fish from Urrá reservoir (Sinú river, Colombia): Six years of monitoring. Rev. MVZ Córdoba 2015, 20, 4754–4765. [Google Scholar] [CrossRef][Green Version]
- Marrugo-Negrete, J.; Lans, E.; Benítez, L. Hallazgo de mercurio en peces de la Ciénaga de Ayapel, Córdoba, Colombia. Rev. MVZ Córdoba 2007, 12, 878–886. [Google Scholar] [CrossRef]
- USEPA (US Environmental Protection Agency). Updates 1995: Water Quality Criteria Documents for the Protection of Aquatic Life in Ambient Water; EPA-820-B-96-001; Office of Water: Washington, DC, USA, 1996. [Google Scholar]
- Porthro, M.G. Office of Water Policy and Technical Guidance on Interpretation and Implementation of Aquatic Life Metal Criteria. 1993. Available online: https://www.epa.gov/wqc/office-water-policy-and-technical-guidance-interpretation-and-implementation-aquatic-life-metals (accessed on 21 November 2022).
- Boyd, C.E. Water Quality, 2nd ed.; Springer: London, UK, 2015; Volume 2, pp. 277–311. [Google Scholar] [CrossRef]
- Kennedy, C.J. The toxicology of metals in fishes. In Encyclopedia of Fish Physiology: From Genome to Environment; Academic Press: San Diego, CA, USA, 2011. [Google Scholar] [CrossRef]
- Kotnik, J.; Horvat, M.; Begu, E.; Shlyapnikov, Y.; Sprovieri, F.; Pirrone, N. Dissolved gaseous mercury (DGM) in the Mediterranean Sea: Spatial and temporal trends. Mar. Chem. 2017, 193, 8–19. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Žagar, D. Mercury transport and fate models in aquatic systems: A review and synthesis. Sci. Total Environ. 2018, 639, 538–549. [Google Scholar] [CrossRef]
- USEPA (US Environmental Protection Agency). Mercury Study Report to Congress; EPA-452/R-97-003; Office of Water: Washington, DC, USA, 1997; Volume I. [Google Scholar]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625–649. [Google Scholar] [CrossRef]
- Drąg-Kozak, E.; Łuszczek-Trojnar, E.; Socha, M.; Bojarski, B. Effects of melatonin on cadmium accumulation and haematological parameters in cadmium intoxicated Prussian carp (Carassius gibelio B.). Ann. Anim. Sci. 2021, 21, 899–923. [Google Scholar] [CrossRef]
- Naz, S.; Hussain, R.; Ullah, Q.; Chatha, A.M.; Shaheen, A.; Khan, R.U. Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ. Sci. Pollut. Res. 2021, 28, 6533–6539. [Google Scholar] [CrossRef]
- Suchana, S.A.; Ahmed, M.S.; Islam, S.M.; Rahman, M.L.; Rohani, M.F.; Ferdusi, T.; Ahmmad, A.K.; Fatema, M.K.; Badruzzaman, M.; Shahjahan, M. Chromium exposure causes structural aberrations of erythrocytes, gills, liver, kidney, and genetic damage in striped catfish Pangasianodon hypophthalmus. Biol. Trace Elem. Res. 2021, 199, 3869–3885. [Google Scholar] [CrossRef]
- Shahjahan, M.D.; Taslima, K.; Rahman, M.S.; Al-Emran, M.D.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Rohani, M.F.; Zabed, S.A.; Islam, M.T.; Jannat, R.; Akter, Y.; Shahjahan, M. Acute effects of chromium on hemato-biochemical parameters and morphology of erythrocytes in striped catfish Pangasianodon hypophthalmus. Toxicol. Rep. 2020, 7, 664–670. [Google Scholar] [CrossRef]
- Hatef, A.; Alavix, S.M.H.; Golshan, M.; Linhart, O. Toxicity of environmental contaminants to fish spermatozoa function in vitro—A review. Aquat. Toxicol. 2013, 140-141, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, G.J.; Dietrich, M.; Kowalski, R.K.; Dobosz, S.; Karol, H.; Demianowicz, W.; Glogowski, J. Exposure of rainbow trout milt to mercury and cadmium alters sperm motility parameters and reproductive success. Aquat. Toxicol. 2010, 10, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Coward, K.; Bromage, N.K.; Hibbit, O.; Parrington, J. Gamete physiology, fertilization and activation in teleost fish. Rev. Fish Biol. Fish. 2002, 12, 33–58. [Google Scholar] [CrossRef]
- Rawson, D.W.; Zhang, T.; Kalicharan, D.; Jongebloed, W.L. Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachydanio rerio: A consideration of the structural and functional relationships with respect to cryoprotectant penetration. Aquac. Res. 2000, 31, 325–336. [Google Scholar] [CrossRef]
- Jaramillo, R.; Goicoechea, O.; Garrido, O.; Molinari, E. Salmo salar: Morfología ultraestructural de la pared del corión en ovas normales y con problemas de eclosión. Arch. Med. Vet. 2009, 41, 67–71. [Google Scholar] [CrossRef]
- Villalobos, S.A.; Hamm, J.T.; Teh, S.J.; Hinton, D.E. Thiobencarb-induced embryotoxicity in medaka (Oryzias latipes): Stage-specific toxicity and the protective role of chorion. Aquat. Toxicol. 2000, 48, 309–326. [Google Scholar] [CrossRef]
- Atencio-García, V.J.; Arabia, R.F.; Aristizábal, R.J. Desarrollo embrionario y larvario de dorada Brycon sinuensis. In Memorias del IV Congreso Iberoamericano Virtual de Acuicultura CIVA; Universidad de Zaragoza: Zaragoza, Spain, 2007. [Google Scholar]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.; Moren, M.; Fontagn, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev. Aquac. 2013, 5 (Suppl. S1), 121–167. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Renieri, E.; Kentouri, M.; Tsatsakis, A.M. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- Favaloro, E.; Mazzola, A. Meristic variation and skeletal anomalies of wild and reared sharpsnout seabream juveniles (Diplodus puntazzo, Cetti 1777) off coastal Sicily, Mediterranean Sea. Aquac. Rev. 2003, 34, 575–579. [Google Scholar] [CrossRef]
- Yepes-Blandón, J.A.; Bian, C.; Benítez-Galeano, M.J.; Aristizabal-Regino, J.L.; Estrada-Posada, A.L.; Mir, D.; Vásquez-Machado, G.; Atencio-García, V.J.; Shi, Q.; Rodríguez-Osorio, N. Draft genome assembly for the Colombian freshwater bocachico fish, Prochilodus magdalenae. Front. Genet. 2023, 13, 989788. [Google Scholar] [CrossRef] [PubMed]
- Lasso, C.; Agudelo, E.; Jiménez-Segura, L.F.; Ramírez-Gil, H.; Morales-Betancourt, M.; Ajiaco-Martínez, R.; Gutiérrez, F.; Usma, J.; Muñoz, S.; Sanabria, A. Catalogo de Los Recursos Pesqueros Continentales; Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia; Instituto de Investigacion de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2011. [Google Scholar]
- Duarte, L.; Cuervo, C.; Vargas, O.; Gil-Manrique, B.; Cuello, F.; De León, G.; Isaza, E.; Tejada, K.; Manjarrez-Martínez, L.; Reyes-Ardila, H. Estadísticas de Desembarco y Esfuerzo de las Pesquerías Artesanales de Colombia, Santa Marta, Colombia. 2020. Available online: http://sepec.aunap.gov.co/Archivos/Boletines-2020/SEPEC_Boletin_Pesca_Artesanal_2020.pdf (accessed on 10 October 2022).
- Mojica, J.; Castellanos, C.; Usma, J.; Álvarez, R.; Lasso, C. Libro Rojo de Peces Dulceacuícolas de Colombia; Serie Libros Rojos de Especies Amenazadas de Colombia; Universidad Nacional de Colombia, WWF: Manizales, Colombia, 2012. [Google Scholar]
- Atencio-García, V. Impactos de la Hidroeléctrica Urra en los peces migratorios del río Sinú. Rev. Temas Agrar. 2000, 5, 29–40. [Google Scholar]
- Ochoa-Orrego, L.; Jiménez-Segura, L.F.; Palacio, J. Ictioplancton en la ciénaga de Ayapel, Río San Jorge (Colombia): Cambios espacio-temporales. Bol. Cient. Mus. Hist. Nat. 2015, 19, 103–114. [Google Scholar] [CrossRef]
- Jiménez-Segura, L.F. Aspectos Diferenciales de las Comunidades de Peces en Grandes Ríos Tropicales y Sus Lagunas Marginales; Seminario de ecología de comunidades; Universidad Federal de Minas Gerais: Belo Horizonte, Brazil, 1998. [Google Scholar]
- Atencio-García, V.J.; Espinosa, J.A.; Martínez, J.G.; Pardo-Carrasco, S.C. Insemination of bocachico fish (Prochilodus magdalenae) with fresh or cryopreserved semen: Effect of spermatozoa/oocyte ratio. Rev. Colomb. Cienc. Pecu. 2015, 28, 347–355. [Google Scholar] [CrossRef]
- Atencio, V.; Kerguelén, E.; Naar, E.; Petro, R. Desempeño reproductivo del bocachico Prochilodus magdalenae inducido dos veces en un mismo año. Rev. MVZ Córdoba 2013, 18, 3304–3310. [Google Scholar] [CrossRef]
- Hayati, A.; Wulansari, E.; Armando, D.S.; Sofiyanti, A.; Amin, M.H.; Pramudy, M. Effects of in vitro exposure of mercury on sperm quality and fertility of tropical fish Cyprinus carpio L. Egypt. J. Aquat. Res. 2019, 45, 189–195. [Google Scholar] [CrossRef]
- Pataki, B.; Roberta, B.I.; Gazsi, G.; Urbányi, B.; Kollár, T.; Horváth, A. Effect of age on the mercury sensitivity of zebrafish (Danio rerio) sperm. Fish Physiol. Biochem. 2021, 47, 687–695. [Google Scholar] [CrossRef]
- Hatef, A.; Alavi, S.M.; Butts, A.E.; Policar, T.; Linhart, O. Mechanism of action of mercury on sperm morphology, adenosine triphosphate content, and motility in Perca fluviatilis (Percidae; Teleostei). Environ. Toxicol. Chem. 2011, 30, 905–914. [Google Scholar] [CrossRef]
- Xin, M.; Niksirat, H.; Shaliutina-Kolešová, A.; Siddique, M.; Sterba, J.; Boryshpolets, S.; Linhart, O. Molecular and subcellular cryoinjury of fish spermatozoa and approaches to improve cryopreservation. Rev. Aquac. 2020, 12, 909–924. [Google Scholar] [CrossRef]
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.J.; Riesco, M.F.; Valcarce, D.G.; Sarasquete, C.; Herráez, M.P.; Robles, V. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.S. Reproductive, developmental, and neurobehavioral effects of methylmercury in fishes. J. Environ. Sci. Health Part C 2009, 27, 212–225. [Google Scholar] [CrossRef]
- Linhart, O.; Alavi, S.M.H.; Rodina, M.; Gela, D.; Cosson, J. Comparison of sperm velocity, motility and fertilizing ability between firstly and secondly activated spermatozoa of common carp (Cyprinus carpio). J. Appl. Ichthyol. 2008, 24, 386–392. [Google Scholar] [CrossRef]
- Martínez, C.S.; Escobar, A.G.; Torres, J.G.; Brum, D.S.; Santos, W.F.; Alonso, M.J.; Salaices, M.; Vassallo, D.V.; Peçanha, F.M.; Leivas, F.G.; et al. Chronic exposure to low doses of mercury impairs sperm quality and induces oxidative stress in rats. J. Toxicol. Environ. Health A 2014, 77, 143–154. [Google Scholar] [CrossRef]
- Pieterse, G.M. Histopathological Changes in the Testis of Oreochromis Mossambicus (Cichlidae) as a Biomarker of Heavy Metal Pollution. Ph.D. Thesis, Rand Afrikaans University, Johannesburg, South Africa, 2004. [Google Scholar]
- Monsalvo-Spencer, P.; Salinas-Zavala, C.A.; Reynoso-Granados, T. Morfología de la membrana coriónica de los huevos de Octopus bimaculoides y Octopus hubbsorum (Cephalopoda: Octopodidae). Hidrobiologica 2013, 23, 124–129. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).