Abstract
The aquaculture production of pikeperch has reached commercial scale in a number of European countries, but the high mortality of early life cycle stages and minor understanding of nutritional requirements are still major bottlenecks. To investigate the fate of fatty acids during early development, weaning and rearing, pikeperch larvae and juveniles from a commercial recirculating aquaculture system (RAS) were sampled over 2 months for morphometric data, as well as fatty acid composition, with a total of 6 sampling days, with four to five replicates per sampling day and between 1 and 25 pikeperch larvae per individual sample, depending on larval biomass. The biomass of sampled pikeperch larvae varied from 0.1 to 420 mg (dry mass DM), depending on the age of the larvae, and the initial length of the pikeperch larvae was about 4.5 mm. Our data confirm that, accompanied by an exponential increase in dry mass, total fatty acids (TFAs) in larval tissues increased with the beginning of exogenous feed uptake and were depleted between days 13 and 25 post hatch, most likely associated with the weaning and metamorphosis of the larvae. We conclude that all fatty acid classes may serve as metabolic fuel during metamorphosis, but the ultimate fatty acid composition is strongly impacted by the available feed. The chosen diet probably caused a lack of alpha-linolenic (18:3n-3; ALA) and docosahexaenoic acid (22:6n-3; DHA) during larval development and a shortage of vaccenic (18:1n-7), alpha-linolenic (18:3n-3; ALA) and arachidonic acid (20:4n-6; ARA) in juvenile pikeperch. This led to low DHA/EPA ratios 13 days post hatch, a high EPA/ARA ratio at days 41 and 56 post hatch and a fluctuating ratio of alpha-linolenic acid to linoleic acid (18:2n-6; LA). A temporary lack of essential fatty acids can cause dysfunctions and eventually mortalities in pikeperch larvae and juveniles. Despite high larval growth rates, the biochemical composition of the first fed Artemia and microdiets was most likely not sufficient and in need of improvement. We suggest that deficiencies must be compensated, e.g., through the substitution of the offered Artemia with more suitable live feed organisms, such as freshwater rotifers, and the enrichment of current microdiets in order to prevent high mortalities during pikeperch rearing and weaning.
Keywords:
Sander lucioperca; commercial aquaculture; feeding regime; fatty acid composition; larviculture management Key Contribution:
The focus of this study was on the fatty acid composition of pikeperch larvae and their dynamics during development. By investigating the fatty acids of the pikeperch larvae over a period of 56 days post hatch, the importance of the nutrient supply during the metamorphosis of the pikeperch larvae, as well as the species-specific nutrient composition, could be demonstrated.
1. Introduction
Pikeperch (Sander lucioperca L., (1758)) is a fast-growing and valuable fish species of European freshwater systems [1,2,3] and a promising candidate for aquaculture in Northern Europe, including Germany. In a number of countries, pikeperch rearing has already reached commercial scale, but sufficient production of fry is still a bottleneck [4]. Survival of pikeperch in early life stages under hatchery conditions depends strongly on the amount and quality of the feed. Summerfelt (1996) [5] identified the end of the post-larval and the beginning of the juvenile stages as most critical during early development. At temperatures below 20 °C, walleye (Sander vitreus) larvae performed metamorphosis between days 16 and 19 post hatch [5]. Pikeperch was successfully weaned with artificial diets [6,7], and, despite the fragility of the larvae, Ostaszewska et al. (2005) [8] suggested that pikeperch larvae can be adapted to high-quality microdiets already at day 5 post hatch, without significant disorders in the digestion and absorption of dry feeds. Kestemont et al. (2007) [7] demonstrated that the addition of microdiets to larval diets from day 12 post hatch onward caused significantly lower deformities than exclusive feeding with Artemia and that early weaning reduced cannibalism.
Brine shrimp (Artemia spp.) are still the most commonly used first feed for pikeperch to date. Numerous studies have shown that pikeperch larvae can be reared with Artemia [7,8,9,10,11,12,13,14] under aquaculture conditions but still with unsatisfactory mortality rates. It should thus be investigated whether Artemia can completely satisfy the nutritional requirements of pikeperch larvae.
Already in 1988, Heming and Buddington [15] suggested that the composition of larval yolk reserves could be a helpful tool in the identification of larval requirements and the development of suitable diets for first-feeding larvae. Palm and co-workers [16] assumed that a high sum of monounsaturated fatty acid (ΣMUFA) contents and high levels of eicosapentaenoic acid 20:5n-3 (EPA) in combination with the absence of 22:6n-3 (docosahexaenoic acid; DHA) in brine shrimp are unfavorable for the early development of pikeperch. This leads to a high EPA/ARA ratio and especially a low DHA/EPA ratio, which might be possible reasons for high mortality rates in the conventional rearing process of the fry. Inside their natural habitats, freshwater fish species are generally characterized by higher levels of C18 polyunsaturated fatty acids (PUFAs) and the highly unsaturated fatty acid (HUFA) 20:4n-6 arachidonic acid (ARA) compared to marine species [17,18,19]. Nevertheless, freshwater species also contain substantial amounts of n-3 (omega-3) HUFAs, especially 20:5n-3 (EPA) and 22:6n-3 (DHA) [19], and several studies confirmed the importance of HUFAs for pikeperch larvae [7,10,12].
The aim of the present study was to analyze the fatty acid composition and its dynamics in pikeperch larvae and juveniles under commercial aquaculture conditions until day 56 post hatch. We hypothesized that the most important fatty acids for the early life cycle development reach their maximum in newly hatched yolk-sac larvae and that the fatty acid ratios can serve as a reference point also in the later larval and juvenile development. We further hypothesized that the fatty acid patterns in pikeperch larvae are highly influenced by the diet and may not necessarily meet the actual larval requirements.
2. Materials and Methods
For a comprehensive representative and reproducible analysis of the effects of live feed on the development of pikeperch larvae and juveniles, further parameters such as mortality, deformations or the effects of stress would be helpful and necessary in addition to the fatty acid and growth data (specific growth rate (SGR)), which were used here. However, due to limited financial and personnel capacities, not all these parameters could be determined in the course of this experiment. This is also due to the fact that the thematic priorities of the Institute for Fisheries of the State Research Centre for Agriculture and Fishery Mecklenburg-Western Pomerania (LFA MV) in Hohen Wangelin at that time were the investigation of breeding and hatching rates, as well as growth parameters, but not yet the above-mentioned parameters.
These parameters have already been collected for subsequent experiments and will now also be collected for future experiments, as long as this is appropriate for the thematic priority of the research.
2.1. Hatching and Rearing of Pikeperch in a Commercial Recirculating Aquaculture System (RAS)
The pikeperch nests were obtained from a pond production in Saxony, Germany. Fish used as broodstock originated from wild catches and had a minimum total wet mass of 4 kg, and they were therefore assumed to spawn repeatedly. After spawning, the nests, including the fertilized eggs, were transferred to the facilities of the Institute for Fisheries of the State Research Centre for Agriculture and Fishery Mecklenburg-Western Pomerania (LFA MV) in Hohen Wangelin, which is operated as a commercial hatchery with research tasks. There, the nests were inserted in conical 500 L breeding tanks. Each breeding tank was part of a freshwater recirculating system. Pikeperch larvae hatched at a temperature of 19 °C and were immediately transferred to separate conical 500 L larval rearing tanks of another recirculating system, to avoid a decline in water quality due to the hatching process. Fish larvae were reared in freshwater at an average temperature of 21.01 ± 0.99 °C under dim light with a light intensity between 20 and 40 Lux. Within the first thirty days post hatch, the tanks were permanently illuminated (24 h); thereafter, a light cycle of 11L:13D (light:dark) was chosen. The larval rearing tanks were gently aerated. The nitrogen compounds were analyzed regularly and were 0.11 ± 0.05 mg·L−1 for ammonium (NH4+), 0.58 ± 0.84 mg·L−1 for nitrite (NO2−) and 57.72 ± 9.69 mg·L−1 for nitrate (NO3−). The recorded average pH was 8.21 ± 0.09.
2.2. Feed and Feeding Regime
Pikeperch larvae had an initial length of 4.5 ± 0.1 mm and an initial biomass of 0.1 ± 0.0 mg (DM). External feeding started four days post hatch (dph). Following a set feeding regime; Micro Artemia (Ocean Nutrition Micro Artemia Cysts AF430, Rijkmakerlaan 15, 2910 Essen, Belgium) were first given to the larvae over a period of four days (dph 4–7). Micro Artemia are small Artemia selected by mechanical separation, which can hatch faster. Due to the earlier hatching, Micro Artemia are smaller than conventional Artemia and can therefore be taken up by small and sensitive fish larvae. The feeding regime was continued with a mixture of Micro Artemia and Artemia sp. (Coppens, Premium Artemia Cysts (GSL), Dwarsdijk 4, 5705 DM Helmond, The Netherlands) at day 8 post hatch and a gradual adaption to dry feeds (weaning period) by feeding a combination of different-sized Artemia and microdiets (O.range Start, INVE, Dendermonde, Belgium) between days 9 and 20 post hatch. Larvae were fed ad libitum by adding Artemia four times a day to the fish-rearing tanks. After the weaning period, pikeperch juveniles were exclusively fed microdiets (dph 21–56) of increasing particle size (100–200 µm, 200–300 µm and 300–500 µm) until the end of the sampling period (Table 1).

Table 1.
The applied feeding regime in a pikeperch hatchery in a commercial recirculating aquaculture system (RAS) over the course of the first 56 days post hatch.
Cysts of Artemia were incubated in 25 L cylindrical-conical polyethylene tanks (PE), at a salinity of 30–35 psu and a temperature of 27 °C. Hatching of Artemia eggs was performed under intense continuous aeration. Artemia nauplii were given to the fish larvae without additional food or enrichments to the culture flasks. The cysts of larger-sized Artemia were decapsulated before adding them to the rearing tanks. Fatty acid compositions of all applied feeds were analyzed to study their effects on the fatty acid composition of pikeperch larvae over the period of dph 4–56 (Table 2).

Table 2.
Fatty acid contents (means ± SD in µg·mg−1 DM of n = 4 replicates for Micro Artemia and Artemia spp. and n = 3 for the microdiet) and fatty acid ratios of live feeds and microdiet utilized in a commercial recirculating aquaculture system (RAS); ΣSFA = sum of saturated fatty acids, ΣMUFA = sum of monounsaturated fatty acids, ΣPUFA = sum of polyunsaturated fatty acids, HUFA = highly unsaturated fatty acids (i.e., PUFA ≥ 20 carbon chain length), TFAs = total fatty acids.
2.3. Determination of Fish Larvae Mass
Samples of fish larvae were collected according to the protocol below in the course of the fatty acid analysis (see Section 2.4 Sampling and fatty acid analyses). After lyophilization for 48 h, dry mass (DM) of the fish larvae was determined using a Sartorius micro balance (MC21 S, ± 2 µg).
Specific growth rate (SGR) [%∙day−1] of the pikeperch larvae was calculated on dry matter basis modified after Jorgensen (1990), applying the formula
where MtE and MtI are the average dry mass of the larvae at time tE (end of the growth interval) and time tI (beginning of the interval).
Additionally, for the dry mass, which was recorded for each sampling day, total length was only recorded at days 1, 7 and 13 post hatch. Each sample encompassed 4 to 16 individuals. Total length of the fish larvae was measured using a stereo light microscope (SZX10 Olympus, Hamburg, Germany) connected to a UC30 digital camera (Olympus, Hamburg, Germany) and the software package cellSens Dimension 1.6 (Olympus Soft Imaging Solutions, Hamburg, Germany). Therefore, fish larvae were individually placed under the stereo light microscope, and the longest distance between the tip of the head and the tip of the tail was recorded.
The data on dry mass and total length were used to determine Fulton’s condition index (kc) according to the formula
where Mi is the average dry mass, and Li is the total length of the corresponding sampling days.
2.4. Sampling and Fatty Acid Analyses
For the fatty acid analyses of the feeds, all Artemia samples consisted of approximately 500 individuals per replicate sample. Respective to culture density, a volume of 10 to 20 mL was filtered through gauze (mesh net size 23 µm) and rinsed three times with saltwater (3 psu) to remove small particles. Pooled samples were subsequently collected in pre-combusted (12 h at 400 °C) and pre-cooled glass vials and stored at −80 °C until analysis.
For the fatty acid analyses of the pikeperch larvae and juveniles, 4 to 5 replicate samples were taken in regular intervals from the rearing tanks at days 1, 7, 13, 25, 41 and 56 post hatch. One sample encompassed 5 to 25 individual pikeperch larvae, with a reduced number of individuals per sample with increasing body size and mass. At days 25, 41 and 56 post hatch, only two individuals and one individual, respectively, were taken per replicate sample. Immediately after sampling, the larvae were sorted in pre-combusted (12 h at 400 °C) and pre-cooled glass vials. Thereafter, the samples were stored at −80 °C until analysis. Dry mass (DM) was determined using a Sartorius micro balance (MC21 S, ±2 µg).
Samples were analyzed according to the method described by Fink [20] and Kattner and Fricke [21]. Lipid extraction was conducted by immersing homogenized tissue in a dichloromethane and methanol solution (2:1/v:v) for at least 12 h. Thereafter, fish and zooplankton samples were sonicated and centrifuged for 5 min (4500× g). After taking up the supernatant quantitatively, the solvent was evaporated to dryness under a N2 atmosphere at 40 °C. In the following, total fatty acids were transesterified with 5 mL of 3 N methanolic HCl (SUPELCO) at 70 °C for 20 min to their respective fatty acid methyl esters (FAMEs) [20]. FAMEs were extracted with 2 × 2 mL iso-hexane, and after evaporation, the samples were finally dissolved in 100 µL iso-hexane. For gas chromatographic analyses, 1 µL of each sample was injected splitless into an Agilent 6890N GC System (Agilent Technologies. Waldbronn, Germany) equipped with a DB-225 capillary column (30 m length, 0.25 mm inner diameter, 0.25 µm film thickness) and an FID detector. Helium was used as the carrier gas at a flow rate of 1.5 mL min−1 and an oven temperature gradient as described elsewhere [20]. FAMEs were identified by comparing the retention times with those of reference compounds and quantified using two internal standards (nonadecanoic (C19:0 ME) and tricosanoic acid (C23:0 ME) methyl esters) and previously established calibration functions for each individual FAME [20].
2.5. Statistical Analyses
All statistical analyses were performed by applying the software IBM SPSS Statistics, Version 22 (IBM Corp, Armonk, NY, USA). For the test of normal distribution, the Shapiro–Wilk test was applied. To test the homogeneity of variance, the Levene test was used. To analyze differences between means, an analysis of variance (ANOVA) was performed. In case significant results were obtained, post hoc tests followed, either Tukey–Kramer (with variance homogeneity) or Dunnett T3 post hoc tests (without variance homogeneity). As non-parametric tests either a Mann–Whitney U-Test or a Kruskal–Wallis analysis of variance (ANOVA) was chosen. All significance levels α were set to 0.05.
3. Results
3.1. Growth
The early development of the pikeperch larvae was characterized by exponential growth between days 1 and 56 post hatch (Figure 1 and Table 3). Pikeperch larvae were fed according to the feeding protocol in Table 1.
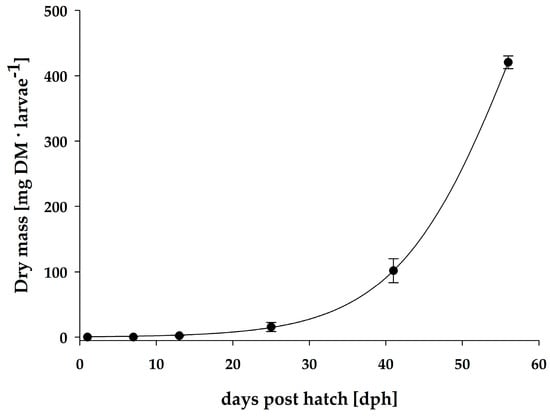
Figure 1.
Individual dry mass of pikeperch larvae from the RAS over a course of 56 days.

Table 3.
Individual pikeperch dry mass, specific growth rate (SGR) and mass-specific and individual fatty acids on day 1 post hatch (dph 1, n = 5; dph 7, n = 5; dph 13, n = 5; dph 25, n = 4; dph 41, n = 4; dph 56, n = 5) and Fulton’s condition index on day 1 post hatch (dph 1, n = 5; dph 7, n = 5; dph 13).
3.2. Fatty Acid Composition of Pikeperch Larvae
The total fatty acid (TFA) content per individual pikeperch larvae increased significantly (ANOVA, p = 0.005) within the first 13 days post hatch compared to the initial content (Table 3). By contrast, the TFA content in relation to dry mass did not change significantly (ANOVA, p = 0.714) at the same time (Table 3). Between day 13 and day 25 post hatch, the TFA content per individual larva, as well as in relation to dry mass, decreased significantly (ANOVA, p = 0.009 and p = 0.001) by 72% and 58%, respectively. After day 25 post hatch, again, a distinct increase in the TFA level per individual fish larva was observed, with significant (ANOVA, p = 0.023) differences between days 25 and 56 post hatch. The TFA content per dry mass also increased, but the differences were not significant (ANOVA, p = 0.134), despite an increase in the TFA content per individual larva by a factor of 36 over the first 56 days post hatch. The final TFA content per dry mass 56 days post hatch was not significantly (ANOVA, p = 0.751) different compared to the initial content of newly hatched larvae.
In terms of fatty acid classes, PUFAs were the dominant fatty acids in pikeperch larvae one day post hatch, with a content twice as high as the initial MUFA content and five times higher compared to the initial SFA level (Table 4). Each fatty acid class, SFAs, MUFAs and PUFAs, increased per individual after the beginning of exogenous feeding within the first seven days of development. Due to only a slight absolute increase in PUFAs compared to SFAs and MUFAs, the PUFA content in relation to dry mass decreased significantly (ANOVA, p < 0.001) from day 1 to day 7 post hatch. In terms of fatty acid composition, 18:1n-9, 22:6n-3 (DHA), 18:2n-6 (LA), 16:0 and 18:3n-3 (ALA) (>7 µg·mg−1 DM and ≥1 µg·Ind−1) were the dominant fatty acids in larvae one day post hatch. Despite an absolute increase, the content of each fatty acid class in relation to dry mass did not change significantly (ANOVA, p = 0.860).

Table 4.
Fatty acid contents (means ± SD in µg·mg−1 DM of n = 5 replicates at dph 1, 7, 13 and 56 as well as n = 4 at dph 25 and 41) during early development of pikeperch in a commercial recirculating aquaculture system under conventional rearing conditions over 56 days post hatch with a feeding period from day 4 to day 56 post hatch. One replicate sample for dry masses and fatty acids encompassed 5 to 25 individual larvae with a reduced number of individuals per sample with increasing body mass. At days 25, 41 and 56 post hatch, only two individuals and one individual, respectively, were taken per replicate sample. ΣSFA = sum of saturated fatty acids, ΣMUFA = sum of monounsaturated fatty acids, ΣPUFA = sum of polyunsaturated fatty acids, HUFA = highly unsaturated fatty acids (i.e., PUFA ≥ 20 carbon chain length), TFAs = total fatty acids. Data in a row with different characters indicate statistical differences over the course of the experiment.
Similarly, the level of each fatty acid per individual increased in larval tissues, with the highest increase in 18:1n-9, 18:3n-3 (ALA), 16:0 and 18:1n-7 (in that order). Despite the fact that the content of 18:1n-9 was similar in both types of Artemia, 18:1n-9 was accumulated to the highest extent between days 7 and 13 post hatch.
After 13 days, the content per individual larva increased by a factor of about 10, and also, the content per dry mass was higher than the initial content. In relation to dry mass, decreasing levels of 16:1n-7, 18:1n-7, 16:3n-4, 20:4n-6 (ARA), 20:5n-3 (EPA) and 22:6n-3 (DHA) were seen. On the other hand, the content of 18:3n-3 (ALA) per individual increased by a factor of 57.
Between day 13 and day 25 post hatch, the absolute amount of each fatty acid class and the amount of each principal fatty acid per individual larva also decreased, with the exception of 22:6n-3 (DHA). The 22:6n-3 (DHA) level per individual increased by a factor of about 6 between days 13 and 25 post hatch. While the content of MUFAs and PUFAs in relation to dry mass also decreased significantly (Kruskal–Wallis, p = 0.007) between days 13 and 25 post hatch, the relative amount of SFAs per dry mass did not change significantly (Kruskal–Wallis, p = 0.415). Compared to the initial contents, the SFA level per dry mass was higher, while the PUFA content was significantly (ANOVA, p < 0.001) lower compared to the initial content. The most pronounced decrease was detected for the levels of 18:1n-9, 18:3n-3 (ALA), 16:0, 18:1n-7 and 16:1n-7. While the contents of 16:0 and 18:0 in relation to dry mass were higher compared to the initial proportions 25 days post hatch, the relative content of any other principal fatty acid was reduced compared to the starting values.
Between days 25 and 41 post hatch, the level of fatty acid classes, as well as the content of each dominant fatty acid, increased per individual as well as per dry mass. While the PUFA level per dry mass increased significantly (ANOVA, p = 0.001) at this time, the accumulation of SFAs and MUFAs was statistically not significant (Kruskal–Wallis, p = 0.168 and p = 1.000) in relation to dry mass. The highest increase per individual was observed for 16:0, 18:1n-9, 18:2n-6 (LA) and 22:6n-3 (DHA). On the other hand, a low increase per individual was observed for 18:1n-7, 20:1n-9, 22:1n-11, 18:3n-3 (ALA), 18:4n-3 and 20:4n-6 (ARA), and the level of 16:3n-4 per individual even decreased. In relation to dry mass, 20:4n-6 (ARA) was the only fatty acid with a decreasing amount relative to dry mass from days 25 to 41 post hatch.
Between days 41 and 56 post hatch, the level of fatty acid classes per dry mass did not change significantly (Kruskal–Wallis, p = 1.000) at this time. Nevertheless, compared to the initial content, total SFAs, as well as the level of both dominant SFAs, 16:0 and 18:0, in relation to dry mass, increased significantly (Mann–Whitney U-Test, p = 0.016) at 56 days post hatch. On the other hand, total MUFAs, as well as the levels of 16:1n-7, 18:1n-9, 18:2n-6 (LA) and 20:5n-3 (EPA) per dry mass, were not significantly (Mann–Whitney U-Test, p = 0.421 and p = 0.151) different compared to the starting values, while total PUFAs and the level of 18:1n-7, 18:3n-3 (ALA), 20:4n-6 (ARA) and 22:6n-3 (DHA) decreased significantly (Mann–Whitney U-Test, p < 0.001) within the first 56 days of pikeperch development.
In accordance with the highest n-3/n-6 ratio in Artemia fed between days 4 and 20, the highest n-3/n-6 ratio in pikeperch larvae was observed on days 13 and 25 post hatch. Concerning the DHA/EPA ratio, the proportion of DHA was about four times higher in larvae one day post hatch. Due to the absence of DHA in Micro Artemia, as well as in Artemia, the proportion of EPA in relation to DHA increased during early development to the lowest DHA/EPA ratio 13 days post hatch. Associated with the presence of DHA in microdiets, the proportion of DHA was higher compared to EPA from day 25 post hatch onward. Whereas the EPA/ARA ratio was balanced already one day post hatch, the proportion of EPA increased during early development and was twice as high as the ARA level until 25 days post hatch. Associated with the highest EPA/ARA ratio in microdiets, the EPA proportion in pikeperch juveniles was six times higher in relation to ARA at 41 and 56 days post hatch. Caused by the highest ALA level in Artemia, the highest ALA/LA ratio was detected in larvae on day 13 post hatch. On the other hand, accompanied by comparable levels of LA but distinctly lower levels of ALA in microdiets compared to Artemia, the lowest ALA/LA ratio was detected in pikeperch 41 and 56 days post hatch.
The proportion of MUFAs was generally higher than SFAs. Exceptions were days 25 and 41 post hatch, where either the proportion of SFAs was higher or a balanced SFA/MUFA ratio was observed. Further, the PUFA level was higher compared with SFAs at all sampling times, with the highest proportion of PUFAs in relation to SFAs in newly hatched larvae. The MUFA/PUFA ratio was the lowest in newly hatched larvae and 25 days post hatch, while the MUFA proportion compared to PUFA was higher 7, 13 and 56 days post hatch.
4. Discussion
4.1. Effect of Feeding on the Growth of Pikeperch Larvae and Juveniles
The present study analyzed the growth and fatty acid composition of conventionally reared pikeperch larvae during their first 56 days post hatch. The observed growth was comparable to growth rates described in the literature, such as Colchen et al. (2020) [22], who described SGRs of 16.9 ± 1.7%·d−1, or Péter et al. (2023) [23], who described 17.8 ± 0.7%·d−1, but exceeding other studies where SGRs were ranging between 0.8 and 3.0%·d−1 (Ballesteros et al., 2023) [24] and 0.9 and 4.1%·d−1 (Bischoff and Kubitz et al., 2022) [25]. Consequently, the sampled pikeperch larvae performed according to other studies, also mainly based on Artemia as first life feed. However, this data comparison should be considered carefully and together with the survival rates as well as other parameters, such as the condition index, which shows already a strong variation for the first 13 days of larval development, which indicates a possible lack of nutrients very early post hatch. The survival rates of 7.9 ± 3.5% per tank described by Colchen et al. (2020) [22] and 11.3% by Péter et al. (2023) [23] were rather low survival rates compared to the survival rates ranging from 64.0 to 93.7% by Ballesteros et al. (2023) [24] and up to 94.0% by Bischoff and Kubitz et al. (2022) [25]. Therefore, we hypothesize that a low survival rate might lead to growth rates that do not represent the whole population but rather the small group of survivors, which are strong and fast-growing. In terms of ecologically and economically sustainable aquaculture, the best possible performance should be a good compromise between high survival rates and good growth rates, and the focus should not be on only one of the two factors. Consequently, a lower growth rate can be compensated by a significantly higher number of individuals.
4.2. Effect of Feed on Pikeperch Fatty Acid Composition
4.2.1. Period 1–7 dph
Larvae were fed exclusively Micro Artemia from dph 4 to dph 7.
For the cultivated pikeperch larvae, the FAs are described as FA classes and single FAs in the order of PUFAs, MUFAs and SFAs.
While PUFAs and MUFAs were, in that order, the dominant fatty acids in larvae one day post hatch, SFAs increased at most during the first seven days post hatch.
Within the PUFAs, the absolute contents of LA, ALA and DHA per individual decreased within the first seven days. This could be due to a reduced concentration or the absence within the microdiets or an increased demand during the early development. The level of C18 PUFAs also seemed to be highly related to the diet. These observations could be confirmed by previous studies, where the level of C18 PUFAs of pikeperch larvae and juveniles was highly influenced by the concentration in the corresponding diet [15,24]. Low contents of LA and especially ALA in Micro Artemia might cause the detected depletion of C18 PUFAs within the first seven days of larval development. In a previous study, we supposed that the availability of both C18 PUFA LA and ALA in the diet is important during the early development of pikeperch [16]. The highest survival rates could be observed in pikeperch larvae with a final content of LA, as well as ALA, of about 3 µg mg−1 DM.
The larval EPA content increased during the first feeding period. This reflected that the larvae fed on Micro Artemia. However, LA and DHA decreased to low levels. DHA contents on dph 7 were lower than those reported by Bischoff et al. (2018) [26] on dph 10 under starvation. Ballesteros-Redondo et al. (2023) [24] showed that the provision of dietary LA is particularly important during this period. Consequently, our data indicate that Micro Artemia is not adequate for this period and that EPA should not be considered an essential fatty acid for pikeperch larvae during this early life stage.
As Micro Artemia are rich in each principal MUFA, they had the highest net increase in pikeperch larvae. These results can be confirmed by observations made in a previous feeding study, where the principal MUFAs, 16:1n-7, 18:1n-7 and 18:1n-9, were highly accumulated in pikeperch larvae within the first ten days post hatch, if high amounts were available in the diet [16].
Due to depletions without a minimal threshold when being less abundant in the diet, we already concluded 16:1n-7 and 18:1n-7 as non-essential fatty acids for pikeperch larvae. On the other hand, the structural importance of 18:1n-9 could be confirmed by a minimal threshold of this MUFA in pikeperch larval tissues. We hypothesize that if non-essential MUFAs, such as 16:1n-7 and 18:1n-7, are highly available in the diet during early development, they will be stored as energy for the upcoming metamorphosis. If non-essential fatty acids are not available, probably any other alternative energy source, like proteins, has to be utilized during metamorphosis.
Concerning the ratios of the FAs, the initial SFA:MUFA:PUFA ratio in yolk-sac larvae was 1:3:5 and changed to a ratio of 1:2:2 after 7 days. In a previous feeding study, we hypothesized that an increasing level of SFAs during early larval development might be an indicator for a good feeding constitution of pikeperch larvae [16]. In that study, a similar SFA:MUFA:PUFA ratio of 1:3:4 was observed in newly hatched larvae, and after a ten-day feeding period, the level of fatty acid classes (SFA:MUFA:PUFA ratios) differed between the feeding groups. While the proportion of SFAs was higher in relation to MUFAs in pikeperch larvae fed Brachionus calyciflorus and Eurytemora affinis, the proportion of MUFAs exceeded total SFAs in larvae fed conventional Artemia.
A balanced ALA/LA ratio ten days post hatch was described by [16]. A similar ALA/LA ratio of 1.0:1.4 could be observed in newly hatched larvae of the present study and therefore could be a kind of recommended value. Sargent et al. [27] already revealed that freshwater fish species, unlike most marine fish species, have high requirements for both n-3 and n-6 PUFAs. In perch breeders, Henrotte et al. [28] observed that an n-3/n-6 ratio between 0.8:1.0 and 2.5:1.0 was optimal for the reproduction success of the perch breeder, while a higher proportion of n-3 PUFAs decreased the fertilization and the spawning rate, and too high levels of n-6 PUFAs, on the other hand, can cause inflammations and diseases in fish [28].
4.2.2. Period 7–13 dph
During this period, the pikeperch larvae were still fed Micro Artemia until dph 10, which was followed by a co-feeding of regular Artemia and microdiets.
Accompanied by the exponential growth of the pikeperch dry mass during the experiment, a sigmoidal increase in TFAs per individual was observed, and the TFAs showed the highest increase between days 7 and 13 post hatch.
From dph 7 onward, the C18 PUFA level also increased in pikeperch larvae, and the content of ALA in relation to dry mass after thirteen days post hatch was between six and nine times higher than during the rest of the sampling period.
Due to a lack of DHA in both Micro Artemia and Artemia, combined with the highest growth of pikeperch larvae between days 7 and 13 post hatch, we conclude a shortage of DHA in pikeperch larvae at this stage. Moreover, although regular Artemia are rich in ALA, a depletion of DHA within this period indicated that a lack of synthesis from C18 PUFAs to their higher homologous n-3 and n-6 HUFAs occurred, which was also observed in other freshwater species [28,29,30]. Lund et al. [12] made similar observations in pikeperch larvae, revealing that, in spite of DHA deprivation in the diet, ALA was not converted to their higher homologous HUFAs. Instead, they obtained elevated stress sensitivity in pikeperch larvae fed on a diet lacking DHA. The authors consequently hypothesized that pikeperch, like most marine fish species and some strict piscivorous freshwater species such as pike (Esox lucius), are unable to synthesize essential HUFAs from their C18 omega-3 precursors. Nevertheless, Mourente [31] found the DHA levels detected in neural tissues of marine fish to be mainly of dietary origin and independent of the capability of in vivo DHA synthesis. These findings emphasize the need for sufficient supplies of dietary DHA. Bischoff and Kubitz et al. (2022) [25] and Ballesteros-Redondo et al. (2023) [24] have shown recently that rotifers are adequate feed for pikeperch larvae since they apparently help the larvae to maintain sufficient DHA levels. Nonetheless, DHA has to be provided for the later life stages. Our data demonstrate that the microdiets were either eaten or digested by the larvae, and thus, DHA should be offered via alternative live feeds such as copepods (Ballesteros-Redondo et al. submitted) for this life stage.
As a result of the high importance of DHA for the development of neural tissues, such as the retina of the eye and the brain [10,12] and, as already discussed, the general importance of HUFAs in larvae of other carnivorous fish [22], we expect that the absence of DHA in different-sized Artemia probably caused dysfunctions, a high stress sensitivity and/or probably mortalities in our experiment.
Due to significant increases in ALA and 18:4n-3 and concurrent decreases in 16:1n-7, ARA and EPA in the larvae between days 7 and 13 post hatch, we assume a higher influence of Artemia compared to Micro Artemia at this time. Moreover, due to an ongoing reduction in the DHA content relative to dry mass, we conclude that the impact of microdiets is minor at this life stage.
Furthermore, the absence of DHA in the first feeds in combination with the highest EPA contents in Micro Artemia probably led to the lowest DHA/EPA ratio in pikeperch larvae 13 days post hatch. Henrotte et al. [32] already revealed the importance of an adequate DHA/EPA ratio in Eurasian perch. In this study, the best hatching rates, as well as the highest stress resistance in larvae, were observed for perch eggs with the highest DHA/EPA ratio. In a previous study, we assumed a DHA/EPA ratio of 5:1 in larval tissues as optimal during early development [16], which is comparable to the DHA/EPA ratio observed in newly hatched larvae [16] as well as in juvenile pikeperch [33] as well as perch [32].
4.2.3. Period 13–25 dph
During the period between dph 13 and 20, the pikeperch larvae were fed with a combination of regular Artemia and microdiets, which was followed by exclusively microdiets from dph 21 onward.
The constant increase in TFAs in the larvae was intermitted between days 13 and 25 post hatch, where a significant decrease in TFAs per individual larvae, as well as per dry mass, was observed. During this period, the TFA content in relation to dry mass was about three times lower at day 25 post hatch than in larvae one day post hatch. Such a similar decrease in the TFA content within the first 21 days of pikeperch development was also detected by Lund and Steenfeldt [10], where the TFA content per dry mass was three to six times lower after a 21-day feeding period with different-enriched Artemia compared to newly hatched larvae.
During the feeding period dph 13–25, the level of LA in pikeperch larvae fed with microdiets was comparable to larvae fed Artemia, but the ALA content was markedly lower, leading to an ALA/LA ratio of approx. 1:7. The differences in the diet probably caused high variations in the ALA/LA ratio in pikeperch fry, with the highest ALA/LA ratio at day 13 post hatch. DHA was the only fatty acid with increasing amounts between days 13 and 25 post hatch.
As explained before, probably influenced by the fatty acid composition of the diet, 16:0, 18:0, 16:1n-7, 18:1n-7, 18:1n-9 and ALA were the dominant fatty acids before metamorphosis and also the most depleted during metamorphosis. These results provide evidence that the influence of bigger-sized Artemia was the highest before metamorphosis and that each abundant fatty acid in the diet can principally be stored and utilized during metamorphosis. Nevertheless, the lowest final level per dry mass after 25 days post hatch could be observed for 16:1n-7, 18:1n-7, ALA and ARA, while 16:0, DHA, 18:1n-9 and 18:0 were the most abundant fatty acids. One reason could be that these fatty acids were low-abundant in microdiets, but another reason could be the higher structural importance of SFAs and 18:1n-9 compared to 16:1n-7, 18:1n-7 and ALA.
As mentioned for the previous period, despite an increase in SFAs, the highest net increase was observed concerning MUFAs and PUFAs, and the levels per larvae were almost twice as high as the level of SFAs before metamorphosis. Due to the fact that MUFAs and PUFAs were depleted to the same extent between days 13 and 25 post hatch, we conclude that these fatty acids were consumed for growth and during metamorphosis. These results provide evidence that not only MUFAs but also PUFAs are generally utilized for metabolic purposes, as already observed in early pikeperch larvae [16].
As no DHA could be detected in both types of Artemia, this possibly caused critical DHA levels in larval tissues with a subsequent need for the accumulation (instead of utilization) of dietary DHA during metamorphosis as earlier mentioned by Imentai et al. (2020 and 2022) [34,35]. This resulted in DHA being the only fatty acid that increased in larval tissues during metamorphosis.
4.2.4. Period from dph 25 Onward
On the basis of the morphological analyses of the sampling material, we concluded that pikeperch larvae had completed their morphogenesis to juveniles at 25 days post hatch. In percid larvae, the metamorphosis usually occurs 29 days post hatch and at a total length of about 25 mm, and the completed metamorphosis comes along with the complete functionality of the stomach and pepsin activity. This early development of the digestive system is comparable to other carnivorous species, like the European sea bass (Dicentrarchus labrax) [36]. In Asian seabass (Lates calcarifer), Dhert et al. [37] already demonstrated the high importance of HUFAs in the diet with the onset of and especially during metamorphosis for the stress resistance and survival of seabass fry. Due to a high TFA reduction during metamorphosis in the present study, we conclude a significant consumption of fatty acids as, e.g., metabolic energy at that time, and it indicates that the feed either did not provide the right fatty acid composition or was not well assimilated (digestion and absorption) by the larvae. Consequently, especially during the early life stages in preparation for metamorphosis, fatty acids must be taken up and accumulated from an appropriate diet.
Due to the lowest net depletion during metamorphosis compared to other fatty acid classes, our results provide evidence that SFAs played a minor role as an energy source not only post hatch but also during metamorphosis. We conclude a substantial role as structural fatty acids and potential storage fatty acids during early development, due to an early accumulation in larval tissues and the fact that the relative SFA content per dry mass at each point in time was higher compared to the initial content, even after metamorphosis. Due to a continuous supply of SFAs from the diet, it is difficult to conclude whether SFAs were accumulated from exogenous food or synthesized de novo. Following metamorphosis, each fatty acid class highly increased again until day 41 post hatch. Due to an ongoing accumulation of MUFAs between days 41 and 56, MUFAs were the dominant fatty acids at the end of this study.
An ongoing deficiency of DHA in the applied diet after metamorphosis might be associated with increased mortalities in the present study, but no survival data are available, and therefore this can only be speculated. Due to increasing amounts of DHA during metamorphosis, we can conclude that the surviving pikeperch were already successfully weaned on microdiets.
The observed general trend of fatty acids also provides evidence that principally each fatty acid is able to serve as an energy source during metamorphosis, except an essential fatty acid, like DHA, that was missing in the first feeds. On the basis of our results, we conclude that the extent of fatty acid utilization was highly dependent on the fatty acid composition of the diet. If important fatty acids are absent in the diet during early life cycle stages and especially metamorphosis, the respective fatty acids will be depleted inside the fish tissues, as observed for ALA and ARA.
After metamorphosis, pikeperch were exclusively fed with fatty acid rich microdiets. Consequently, high amounts of fatty acids accumulated in fish tissues in this phase; thus, the fatty acid composition of the larvae reflects the composition of the feed. Similar fatty acid compositions between fish and their diets were found for 16:1n-7, 18:1n-9, LA and EPA, sometimes even significantly higher within the larvae, like for 16:0 and 18:0. On the other hand, accompanied by low amounts in the diet, only low levels of 18:3n-3 (ALA) and 20:4n-6 (ARA) could be found in pikeperch. We hypothesize that, if higher amounts of these fatty acids were available in the diet, they would be accumulated in pikeperch and that the initial level could be a kind of reference. In a previous study, a physiological ARA threshold of 1.7 µg mg−1 DM was identified for pikeperch larvae [16]. The low ARA content in the microdiet in our study suppressed the ARA content in pikeperch tissues below this threshold and thus possibly reached a critical point for their ongoing development. The lowest level in relation to dry mass was not observed after metamorphosis (dph 25) but at day 56 post hatch, leading to the lowest EPA/ARA ratios at days 41 and 56 post hatch. Henrotte et al. [32] found a high EPA/ARA ratio in the diet of perch to negatively influence reproductivity as well as larval quality. In their study, the highest fertilization and hatching rates, as well as the highest survival of larvae exposed to osmotic stress, could be achieved with a dietary DHA:EPA:ARA ratio of 3:2:2, while, on the other hand, egg and larval quality was degraded if the EPA content was higher compared to DHA and ARA. Therefore, the dietary ARA supply in our study may not have been sufficient to increase its relative content in fish tissues. Furthermore, a conversion of dietary LA to ARA seems unlikely, as already discussed for the n-3 HUFAs. These results provide evidence that ARA supplementation to larval diets is of high importance and that microdiets probably caused a shortage in pikeperch.
In summary, the final (dry-mass-specific) contents of 16:0, 18:0, 16:1n-7, 18:1n-9, LA and EPA were not significantly different from the initial levels or even higher after 56 days post hatch. Therefore, we conclude that these fatty acids were present in sufficient amounts in the respective diets and that these levels could be representative for well-supplied pikeperch. On the other hand, the relative final contents of 18:1n-7, ALA, ARA and DHA were lower than the initial values, suggesting a shortage of these fatty acids in the offered diets relative to the larval demands.
5. Conclusions
All offered feeds were ingested by larval and juvenile pikeperch, and dietary fatty acids were incorporated into larval tissues. Increasing levels of certain fatty acids in the larvae, which were highly abundant in the diet at this time, indicate that the fatty acid pattern of pikeperch is highly influenced by the diet. However, the supply of some specific PUFAs through the applied first feeds was apparently insufficient, which might have caused dysfunctions and mortalities in pikeperch larvae and juveniles. In the future, more efforts should be made to incorporate parameters such as mortality, deformities and the effects of stress into data collection. On the other hand, the observed relationship between feeds and larval deficiencies should be easy to compensate by different enrichments that may ultimately prevent mortalities during pikeperch rearing and weaning.
In general, the contents of fatty acids in pikeperch seemed to be highly dependent on their respective availability in the diet. Conversion of HUFAs from precursors appears to be impossible, at least at sufficient rates. As a consequence, an adequate exogenous supply of essential fatty acids is necessary. Artemia provided critically low DHA levels and hence unfavorable DHA/EPA ratios for pikeperch larvae. Our results further suggest that the ARA content in Artemia and microdiets is insufficient or unbalanced to fulfill the requirements of developing pikeperch.
Overall, our study emphasizes the importance of suitable dietary fatty acid compositions in aquaculture feeds that are well-tailored to the specific requirements of fishes and their larval stages.
Author Contributions
Conceptualization, A.A.B., M.K. and H.W.P.; methodology, A.A.B. and H.W.P.; software, A.A.B. and M.K.; validation, A.A.B., M.K, H.W.P., W.H., T.R. and M.S.; formal analysis, A.A.B. and M.K.; investigation, A.A.B. and M.K.; resources, H.W.P., W.H. and P.F.; data curation, A.A.B. and M.K.; writing—original draft preparation, A.A.B., M.K., L.B.-R. and H.W.P.; writing—review and editing, A.A.B., M.K., H.W.P., L.B.-R., W.H., P.F., M.S. and T.R.; visualization, A.A.B. and L.B.-R.; supervision, H.W.P., W.H. and P.F.; project administration, A.A.B. and H.W.P.; funding acquisition, H.W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was part of the project “Entwicklung eines Zooplankton-Reaktors zur Unterstützung der Fischlarvenaufzucht relevanter Zielfischarten in Mecklenburg-Vorpommern” and was partly funded through the European Fisheries Found (VI-560/7308-4).
Institutional Review Board Statement
The animal study protocol was approved by the State Office for Agriculture, Food Safety, and Fisheries Mecklenburg-Western Pomerania—Veterinary Services and Agriculture (protocol code 7221.3-2-021/14; date of approval: 2 May 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Carsten Kühn and Gregor Schmidt from the Institute for Fisheries of the State Research Centre Mecklenburg-West Pomerania for the supply of pikeperch larvae. We also thank Katja Preuß from the Workgroup Aquatic Chemical Ecology at the University of Cologne and Petra Wencke from the Department of Marine Zoology at the University of Bremen for their assistance and support during the fatty analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boisneau, P.; Mennesson-Boisneau, C. Inland commercial fisheries management in France. Fish. Manag. Ecol. 2001, 8, 303–310. [Google Scholar] [CrossRef]
- Wedekind, H.; Hilge, V.; Steffens, W. Present status, and social and economic significance of inland fisheries in Germany. Fish. Manag. Ecol. 2001, 8, 405–414. [Google Scholar] [CrossRef]
- Schulz, C.; Huber, M.; Ogunji, J.; Rennert, B. Effects of varying dietary protein to lipid ratios on growth performance and body composition of juvenile pike perch (Sander lucioperca). Aquac. Nutr. 2008, 14, 166–173. [Google Scholar] [CrossRef]
- Steenfeldt, S. Culture Methods of Pikeperch Early Life Stages. In Biology and Culture of Percid Fishes: Principles and Practices, 1st ed.; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 295–312. [Google Scholar]
- Summerfelt, R.C. Intensive Culture of Walleye Fry. In Walleye Culture Manual; Summerfelt, R.C., Ed.; NCRAC Culture Series 101; Iowa State University: Ames, IA, USA, 1996. [Google Scholar]
- Ljunggren, L.; Staffan, F.; Falk, S.; Linden, B.; Mendes, J. Weaning of juvenile pikeperch, Stizostedion lucioperca L., and perch, Perca fluviatilis L., to formulated feed. Aquac. Res. 2003, 34, 281–287. [Google Scholar] [CrossRef]
- Kestemont, P.; Xueliang, X.; Hamza, N.; Maboudou, J.; Imorou Toko, I. Effect of weaning age and diet on pikeperch larviculture. Aquaculture 2007, 264, 197–204. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Czuminska, K.; Olech, W.; Olejniczak, M. Rearing of pikeperch larvae using formulated diets-first success with starter feeds. Aquac. Res. 2005, 36, 1167–1176. [Google Scholar] [CrossRef]
- Abi-Ayad, S.-M.E.-A.; Boutiba, Z.; Mélard, C.; Kestemont, P. Dynamics of total body fatty acids during early ontogeny of pikeperch (Sander lucioperca) larvae. Fish Physiol. Biochem. 2004, 30, 129–136. [Google Scholar] [CrossRef]
- Lund, I.; Steenfeldt, S.J. The effects of dietary long-chain essential fatty acids on growth and stress tolerance in pikeperch larvae (Sander lucioperca L.). Aquac. Nutr. 2011, 17, 191–199. [Google Scholar] [CrossRef]
- Hamza, N.; Kestemont, P.; Khemis, I.B.; Mhetli, M.; Cahu, C. Effect of different sources and levels of dietary phospholipids on performances and fatty acid composition of pikeperch (Sander lucioperca) larvae. Aquac. Nutr. 2012, 18, 249–257. [Google Scholar] [CrossRef]
- Lund, I.; Skov, P.V.; Hansen, B.W. Dietary supplementation of essential fatty acids in larval pikeperch (Sander lucioperca); short and long term effects on stress tolerance and metabolic physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 340–348. [Google Scholar] [CrossRef]
- Lund, I.; Höglund, E.; Ebbesson, L.O.E.; Skov, P.V. Dietary LC-PUFA deficiency early in ontogeny induces behavioural changes in pike perch (Sander lucioperca) larvae and fry. Aquaculture 2014, 432, 453–461. [Google Scholar] [CrossRef]
- Kestemont, P.; Henrotte, E. Nutritional Requirements and Feeding of Broodstock and Early Life Stages of Eurasian Perch and Pikeperch. In Biology and Culture of Percid Fishes: Principles and Practices, 1st ed.; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 539–564. [Google Scholar]
- Heming, T.A.; Buddington, R.K. Yolk absorption in embryonic and larval fishes. In Fish Physiology: Vol. XI. The Physiology of Developing Fish Part A: Eggs and Larvae; Hoar, W.S., Randall, D.J., Eds.; Academic Press: London, UK; New York, NY, USA, 1988. [Google Scholar]
- Palm, H.W.; Bischoff, A.A.; Wranik, C.; Augustin, C.; Kubitz, M. Endbericht “Entwicklung Eines Zooplankton-Reaktors zur Unterstützung der Fischlarvenaufzucht Relevanter Zielfischarten in Mecklenburg-Vorpommern (MV)”. Available online: https://www.aquakultur-mv.de/static/AQUA/Dokumente/Forschen/Zooreaktor_Endbericht_2015_Finalversion.pdf (accessed on 27 June 2023).
- Ackman, R.G. Characteristics of the fatty acid composition and biochemistry of some fresh-water fish oils and lipids in comparison with marine oils and lipids. Comp. Biochem. Physiol. 1967, 22, 907–922. [Google Scholar] [CrossRef]
- Henderson, R.J.; Tocher, D.R. The lipid composition and biochemistry of freshwater fish. Prog. Lipid Res. 1987, 26, 281–347. [Google Scholar] [PubMed]
- Sargent, J.R.; Henderson, R.J.; Tocher, D.R. The Lipids. In Fish Nutrition, 2nd ed.; Halver, J.E., Ed.; Academic Press: San Diego, CA, USA, 1989; pp. 154–219. [Google Scholar]
- Fink, P. Invasion of quality: High amounts of essential fatty acids in the invasive Ponto-Caspian mysid Limnomysis benedeni. J. Plankton Res. 2013, 35, 907–913. [Google Scholar] [CrossRef]
- Kattner, G.; Fricke, H.S.G. Simple gas-liquid chromatography method for simultaneous determination of fatty acids and alcohols in wax esters of marine organisms. J. Chromatogr. 1986, 361, 263–268. [Google Scholar] [CrossRef]
- Colchen, T.; Gisbert, E.; Kraus, D.; Pasquet, A.; Fontaine, P. Improving pikeperch larviculture by combining environmental, feeding and population factors. Aquac. Rep. 2020, 17, 100337. [Google Scholar] [CrossRef]
- Péter, G.; Lukic, J.; Alvestad, R.; Horváth, Z.; Nagy, Z.; Rónyai, A.; Bársony, P.; Ljubobratovic, U. Nursing of Pike-Perch (Sander lucioperca) in Recirculating Aquaculture System (RAS) Provides Growth Advantage in Juvenile Growth Phase. Animals 2023, 13, 347. [Google Scholar] [CrossRef]
- Ballesteros-Redondo, L.; Palm, H.W.; Bährs, H.; Wacker, A.; Bischoff, A.A. Pikeperch larviculture (Sander lucioperca [L., 1758]) with Brachionus plicatilis (Mueller, 1786) (Rotifera) and Apocyclops panamensis (Marsh, 1913) (Copepoda). J. World Aquac. Soc. 2023, 54, 1026–1039. [Google Scholar] [CrossRef]
- Bischoff, A.A.; Kubitz, M.; Wranik, C.M.; Ballesteros-Redondo, L.; Fink, P.; Palm, H.W. The Effect of Brachionus calyciflorus (Rotifera) on Larviculture and Fatty Acid Composition of Pikeperch (Sander lucioperca (L.)) Cultured under Pseudo-GreenWater Conditions. Sustainability 2022, 14, 6607. [Google Scholar] [CrossRef]
- Bischoff, A.A.; Kubitz, M.; Wranik, C.M.; Pfefferkorn, H.; Augustin, C.B.; Hagen, W.; Palm, H.W. Fatty acid utilization of pikeperch (Sander lucioperca (Linnaeus, 1758)) larvae under starvation conditions during early development. Bull. Fish Biol. 2018, 17, 59–73. [Google Scholar]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The Lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Ed.; Academic Press: Amsterdam, The Netherlands, 2002; pp. 181–257. [Google Scholar]
- Henrotte, E.; Overton, J.L.; Kestemont, P. Effects of dietary n-3 and n-6 fatty acid levels on egg and larval quality of Eurasian perch. Cybium 2008, 32, 271–272. [Google Scholar]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Sargent, J.R.; Bell, J.G.; Bell, M.V.; Henderson, R.J.; Tocher, D.R. Requirement criteria for essential fatty acids. J. Appl. Ichthyol. 1995, 11, 183–198. [Google Scholar] [CrossRef]
- Mourente, G. Accumulation of DHA (docosahexaenoic acid; 22:6n-3) in larval and juvenile fish brain. In The Big Fish Bang; Institute of Marine Research: Bergen, Norway, 2003; pp. 239–248. [Google Scholar]
- Henrotte, E.; Mandiki, R.S.N.M.; Prudencio, A.T.; Vandecan, M.; Mélard, C.; Kestemont, P. Egg and larval quality, and egg fatty acid composition of Eurasian perch breeders (Perca fluviatilis) fed different dietary DHA/EPA/AA ratios. Aquac. Res. 2010, 41, 53–61. [Google Scholar] [CrossRef]
- Schulz, C.; Knaus, U.; Wirth, M.; Rennert, B. Effects of varying dietary fatty acid profile on growth performance, fatty acid, body and tissue composition of juvenile pike perch (Sander lucioperca). Aquac. Nutr. 2005, 11, 403–413. [Google Scholar] [CrossRef]
- Imentai, A.; Raskovic, B.; Steinbach, C.; Rahimnejad, S.; Yanes-Roca, C.; Policar, T. Effects of first feeding regime on growth performance, survival rate and development of digestive system in pikeperch (Sander lucioperca) larvae. Aquaculture 2020, 529, 735636. [Google Scholar] [CrossRef]
- Imentai, A.; Gilannejad, N.; Martinez-Rodriguez, G.; Lopez, F.J.M.; Martinez, F.P.; Penka, T.; Dzyuba, V.; Dadras, H.; Policar, T. Effects of first feeding regime on gene expression and enzyme activity in pikeperch (Sander lucioperca) larvae. Front. Mar. Sci. 2022, 9, 864536. [Google Scholar] [CrossRef]
- Hamza, N.; Ostaszewska, T.; Kestemont, P. Development and Functionality of the Digestive System in Percid Fishes Early Life Stages. In Biology and Culture of Percid Fishes: Principles and Practices, 1st ed.; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 239–264. [Google Scholar]
- Dhert, P.; Lavens, P.; Duray, M.; Sorgeloos, P. Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using ω3-HUFA-enriched live food. Aquaculture 1990, 90, 63–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).