Randall’s Threadfin Bream (Nemipterus randalli, Russell 1986) Poses a Potential Threat to the Northeastern Mediterranean Sea Food Web
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Stomach Content Analysis
2.4. Modelling Approach
3. Results
3.1. Stomach Content Analysis
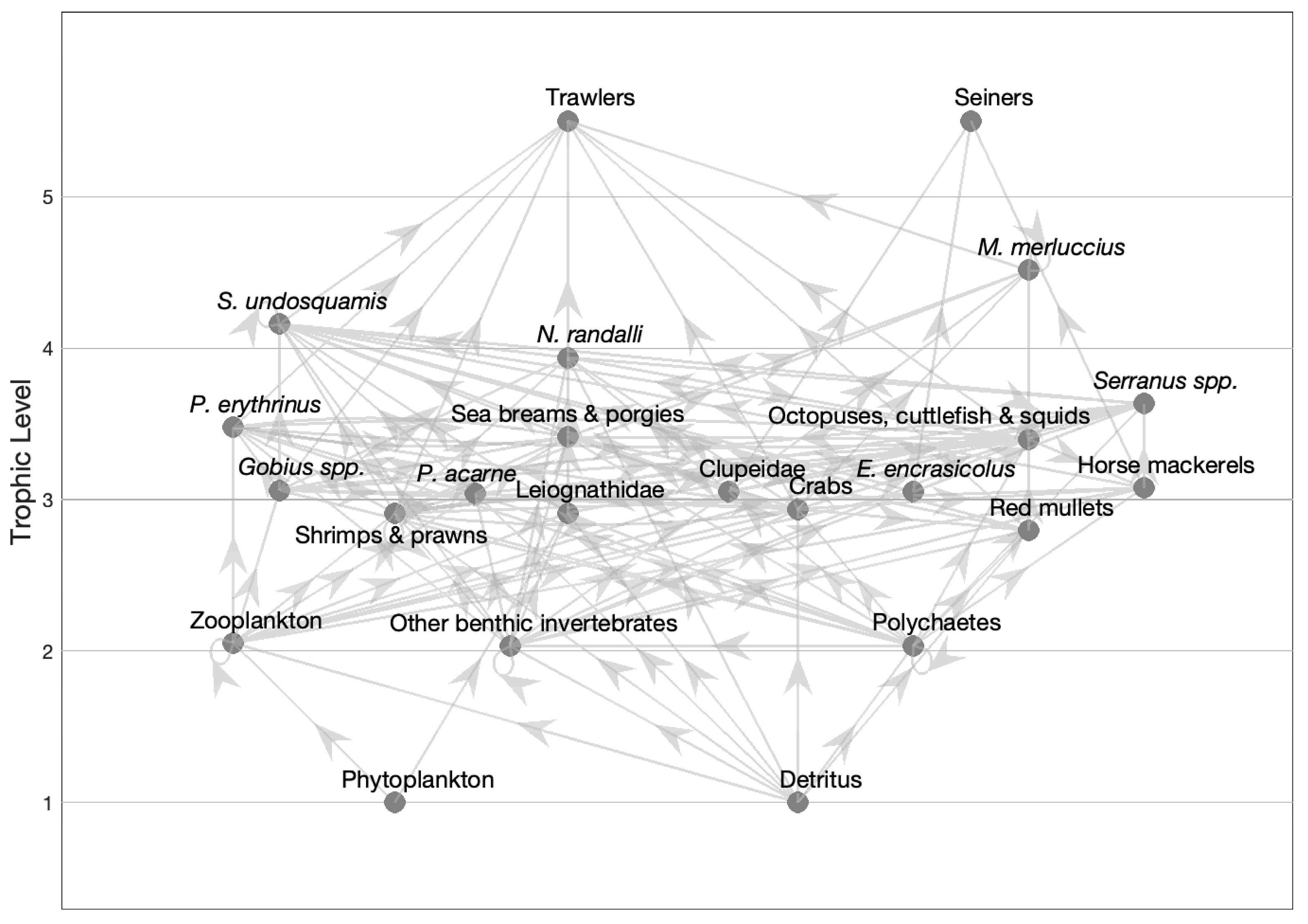
3.2. The Model
3.3. Model Data Quality
3.4. Model Summary Statistics
3.5. Mixed Trophic Impact Analysis
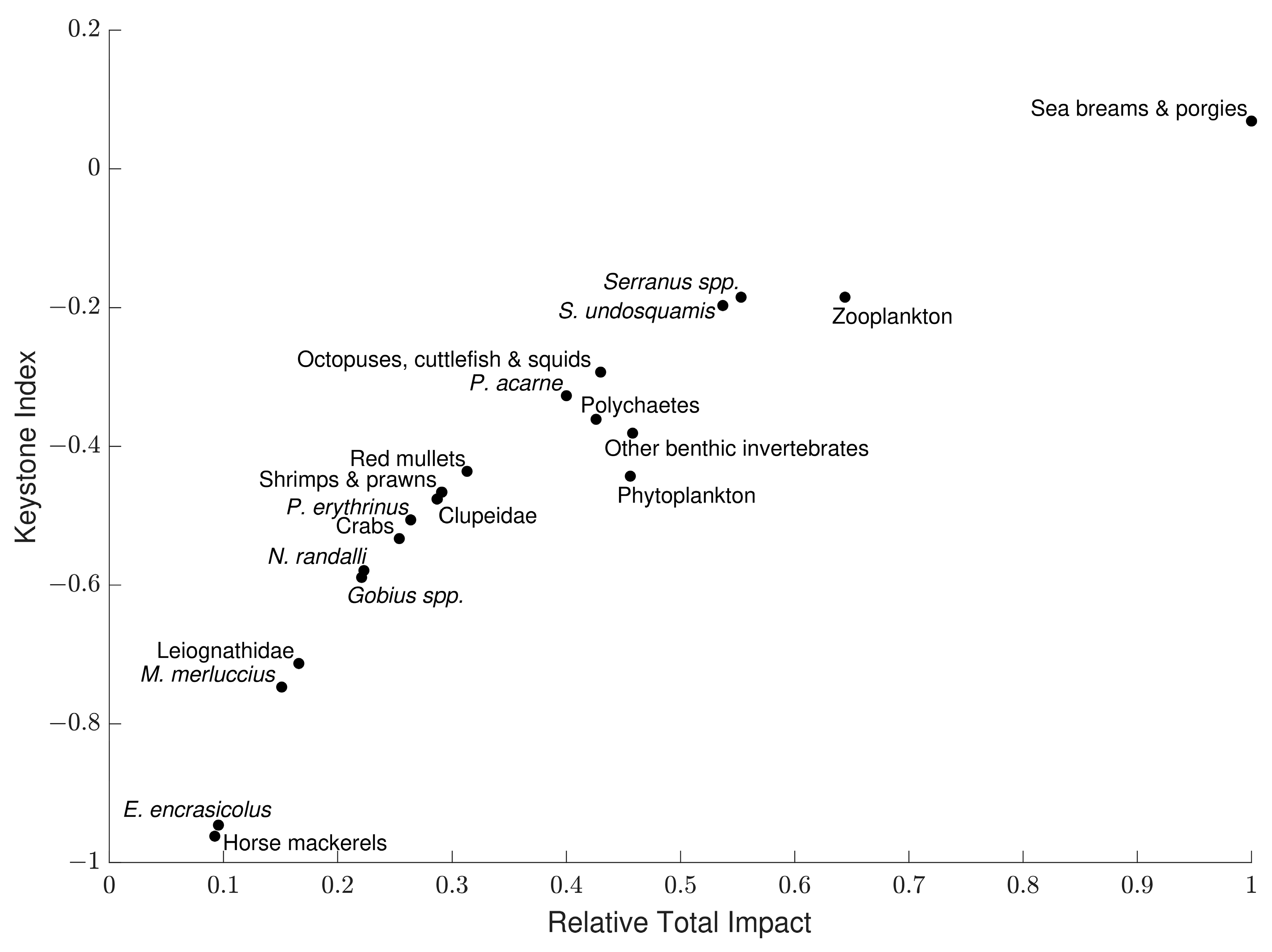
3.6. Keystoneness Analysis
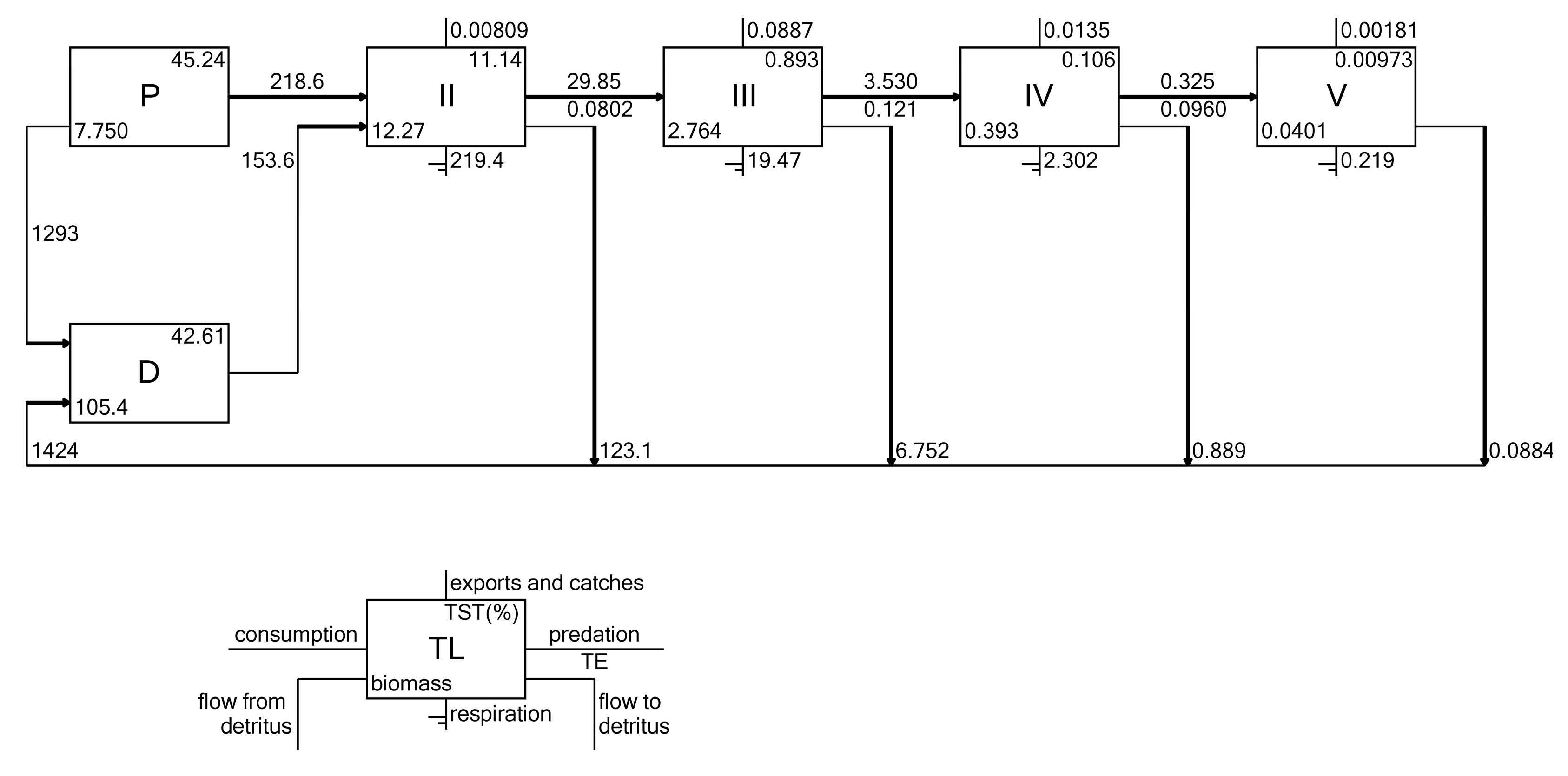
3.7. Energy Flows
4. Discussion
Limitations and Future Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Functional Groups | Original Value | Calibrated Value | Sources |
|---|---|---|---|
| Phytoplankton | |||
| Biomass | 7.75 | 7.75 | [68] |
| P/B | 195.1 | 195.1 | Calculated to match 151.2 gC/m2/y annual primary production as per [69] |
| Zooplankton | |||
| Biomass | 3.385 | 3.385 | [70] |
| P/B | 30.42 | 30.42 | [71] |
| Q/B | 92.18 | 92.18 | [18] |
| Diet | [18] | ||
| N. randalli | |||
| Biomass | 0.0374 | 0.0374 | Trawl survey |
| P/B | 0.936 | 0.936 | [72] |
| Q/B | 9.781 | 7.781 | Empirical equation by [35] using length–weight relationship and L∞ values from [73] and aspect ratio from trawl survey |
| Diet | Stomach content analysis, [28,53,74] | ||
| Other benthic invertebrates | |||
| Biomass | 0.0546 | 5.456 | Trawl survey |
| P/B | 1.15 | 1.15 | [10] |
| Q/B | 3.658 | 3.658 | [10] adjusted with Opitz’s correction factor [75] |
| Diet | Modified from [10] | ||
| Polychaetes | |||
| Biomass | 1.62 | 3.24 | [76] |
| P/B | 3.61 | 3.61 | [18] |
| Q/B | 16.93 | 16.93 | [10] adjusted with Opitz’s correction factor [75] |
| Diet | Modified from [10] | ||
| Crabs | |||
| Biomass | 0.0618 | 0.618 | Trawl survey |
| P/B | 2.42 | 2.42 | [10] |
| Q/B | 5.526 | 5.526 | [10] adjusted with Opitz’s correction factor [75] |
| Diet | Modified from [10] | ||
| Shrimps and prawns | |||
| Biomass | 0.251 | 0.251 | Trawl survey |
| P/B | 3.09 | 3.09 | [18] |
| Q/B | 12.27 | 11.27 | [18] |
| Diet | Modified from [10] | ||
| Octopuses, cuttlefish and squids | |||
| Biomass | 0.102 | 0.0612 | Trawl survey |
| P/B | 2.652 | 2.652 | [10] |
| Q/B | 14.22 | 14.22 | [10] adjusted with Opitz’s correction factor [75] |
| Diet | [77] | ||
| P. erythrinus | |||
| Biomass | 0.140 | 0.0837 | Trawl survey |
| P/B | 1.769 | 1.769 | [78] |
| Q/B | 8.432 | 8.432 | Empirical equation by [35] using length–weight relationship and L∞ (as (weighted average of max length divided by 0.95 [79]) values from [80] |
| Diet | [81] | ||
| P. acarne | |||
| Biomass | 0.230 | 0.161 | Trawl survey |
| P/B | 1.94 | 1.94 | [82] |
| Q/B | 10.69 | 10.69 | Empirical equation by [35] using length–weight relationship and L∞ values from [83] |
| Diet | [83] | ||
| Red mullets | |||
| Biomass | 0.45 | 0.315 | Trawl survey |
| P/B | 1.225 | 1.225 | [78] |
| Q/B | 9.894 | 9.894 | Empirical equation by [35] using length–weight relationship and L∞ values from [78] |
| Diet | [84] | ||
| M. merluccius | |||
| Biomass | 0.0209 | 0.0209 | Trawl survey |
| P/B | 2.41 | 2.41 | [85] |
| Q/B | 7.115 | 7.115 | Empirical equation by [35] using length–weight relationship and L∞ values from [85] |
| Diet | [86] | ||
| Gobius spp. | |||
| Biomass | 0.0018 | 0.360 | Trawl survey |
| P/B | 0.847 | 1.695 | Empirical equation by [34] using maximum age value from [87]. |
| Q/B | 11.07 | 11.07 | Empirical equation by [35] using length–weight relationship and L∞ values from [87] |
| Diet | [87] | ||
| S. undosquamis | |||
| Biomass | 0.0829 | 0.0829 | Trawl survey |
| P/B | 1.76 | 1.76 | [88] |
| Q/B | 8.285 | 8.285 | Empirical equation by [35] using length–weight relationship from [89] and L∞ from [88] |
| Diet | [90] | ||
| Sea breams and porgies | |||
| Biomass | 0.419 | 0.293 | Trawl survey |
| P/B | 0.415 | 0.415 | Empirical equation by [34] capitalising on weighted averages of calculated Z values using maximum age values from [36] |
| Q/B | 8.654 | 7.654 | Empirical equation by [35] using length–weight relationship from [91] and L∞ from [92] |
| Diet | [93] | ||
| Serranus spp. | |||
| Biomass | 0.0123 | 0.0864 | Trawl survey |
| P/B | 1.28 | 1.28 | [94] |
| Q/B | 12.215 | 10.215 | Empirical equation by [35] using W∞ from [95] |
| Diet | [96] | ||
| Leiognathidae | |||
| Biomass | 0.408 | 0.408 | Trawl survey |
| P/B | 0.961 | 0.961 | [97] |
| Q/B | 19.36 | 19.36 | Empirical equation by [35] using W∞ from [97] |
| Diet | [98] | ||
| Clupeidae | |||
| Biomass | 0.00224 | 0.447 | Trawl survey |
| P/B | 1.282 | 1.282 | [99] |
| Q/B | 14.21 | 14.21 | Empirical equation by [35] using length–weight relationship and L∞ from [99] |
| Diet | [100] | ||
| E. encrasicolus | |||
| Biomass | 0.00703 | 0.0703 | Trawl survey |
| P/B | 2.73 | 2.73 | [18] |
| Q/B | 12.23 | 12.23 | Empirical equation by [35] using length–weight relationship and L∞ from [101] |
| Diet | [102] | ||
| Horse mackerels | |||
| Biomass | 0.095 | 0.095 | Trawl survey |
| P/B | 1.66 | 1.6 | [101] |
| Q/B | 11.80 | 11.80 | [101] |
| Diet | [39,40] | ||
| Detritus | |||
| Biomass | 105.4 | 105.4 | Empirical equation by [103] using 151.2 gC/m2/y primary production and euphotic zone depth of 37 from [69] |
| # | Prey/Predator | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Phytoplankton | 0.7 | 0.03 | |||||||||||||||||
| 2 | Zooplankton | 0.05 | 0.02 | 0.11 | 0.1 | 0.338 | 0.11 | 0.284 | 0.15 | 0.725 | 0.97 | 1.0 | 0.975 | |||||||
| 3 | N. randalli | 0.01 | 0.01 | |||||||||||||||||
| 4 | Other benthic invertebrates | 0.009 | 0.005 | 0.41 | 0.4 | 0.54 | 0.331 | 0.086 | 0.209 | 0.001 | 0.307 | 0.183 | 0.042 | 0.02 | 0.003 | |||||
| 5 | Polychaetes | 0.003 | 0.027 | 0.034 | 0.42 | 0.22 | 0.09 | 0.107 | 0.106 | 0.235 | 0.863 | 0.037 | 0.029 | 0.102 | 0.01 | 0.001 | ||||
| 6 | Crabs | 0.227 | 0.02 | 0.025 | 0.175 | 0.076 | 0.039 | 0.174 | 0.001 | 0.027 | 0.04 | 0.2 | 0.003 | |||||||
| 7 | Shrimps and prawns | 0.329 | 0.01 | 0.055 | 0.075 | 0.226 | 0.016 | 0.114 | 0.005 | 0.02 | 0.134 | 0.003 | ||||||||
| 8 | Octopuses, cuttlefish and squids | 0.02 | 0.023 | 0.001 | 0.041 | 0.015 | ||||||||||||||
| 9 | P. erythrinus | 0.01 | 0.05 | |||||||||||||||||
| 10 | P. acarne | 0.01 | 0.1 | 0.1 | 0.05 | |||||||||||||||
| 11 | Red mullets | 0.005 | 0.134 | 0.035 | 0.126 | |||||||||||||||
| 12 | M. merluccius | 0.31 | ||||||||||||||||||
| 13 | Gobius spp. | 0.025 | 0.055 | 0.024 | 0.184 | 0.028 | 0.009 | 0.104 | ||||||||||||
| 14 | S. undosquamis | 0.008 | ||||||||||||||||||
| 15 | Sea breams and porgies | 0.016 | 0.013 | 0.164 | 0.08 | |||||||||||||||
| 16 | Serranus spp. | 0.062 | 0.128 | |||||||||||||||||
| 17 | Leiognathidae | 0.135 | 0.031 | 0.1 | ||||||||||||||||
| 18 | Clupeidae | 0.17 | 0.01 | 0.277 | 0.378 | 0.036 | 0.01 | |||||||||||||
| 19 | E. encrasicolus | 0.143 | 0.07 | |||||||||||||||||
| 20 | Horse mackerels | 0.051 | 0.02 | |||||||||||||||||
| 21 | Detritus | 0.25 | 0.026 | 0.968 | 0.966 | 0.12 | 0.19 | 0.232 | 0.382 | 0.101 | ||||||||||
| Import |
References
- Convention on Biological Diversity (CBD). Guiding Principles for the Prevention, Introduction and Mitigation of Impacts of Alien Invasive Species That Threaten Ecosystems, Habitats or Species; Convention on Biological Diversity (CBD): The Hague, The Netherlands, 2002. [Google Scholar]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Ben Rais Lasram, F.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity Patterns Shaped by Human Activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar]
- Öztürk, B. Status of Alien Species in the Black and Mediterranean Seas. Stud. Rev. Gen. Fish. Comm. Mediterr. 2010, 87, 103. [Google Scholar]
- Merkado, G.; Abramovich, S.; Abdu, U.; Almogi-Labin, A.; Pawlowski, J.; Holzmann, M. Breaking Biogeographic Barriers: Molecular and Morphological Evidences for the Lessepsian Invasion of Soritid Foraminifers to the Mediterranean Sea. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 22–27 April 2012; p. 560. [Google Scholar]
- Spanier, E.; Galil, B.S. Lessepsian Migration: A Continuous Biogeographical Process. Endeavour 1991, 15, 102–106. [Google Scholar] [CrossRef]
- Edelist, D.; Rilov, G.; Golani, D.; Carlton, J.T.; Spanier, E. Restructuring the Sea: Profound Shifts in the World’s Most Invaded Marine Ecosystem. Divers. Distrib. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Bilecenoğlu, M. Demersal Lessepsian Fish Assemblage Structure in the Northern Levant and Aegean Seas. J. Black Sea/Mediterr. Environ. 2016, 22, 46–59. [Google Scholar]
- Zakaria, H.Y. Article Review: Lessepsian Migration of Zooplankton through Suez Canal and Its Impact on Ecological System. Egypt. J. Aquat. Res. 2015, 41, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: A User’s Guide; Fisheries Centre, University of British Columbia: Vancouver, BC, Canada, 2005; Volume 154, p. 31. [Google Scholar]
- Tsagarakis, K.; Coll, M.; Giannoulaki, M.; Somarakis, S.; Papaconstantinou, C.; Machias, A. Food-Web Traits of the North Aegean Sea Ecosystem (Eastern Mediterranean) and Comparison with Other Mediterranean Ecosystems. Estuar. Coast. Shelf Sci. 2010, 88, 233–248. [Google Scholar] [CrossRef]
- Papapanagiotou, G.; Tsagarakis, K.; Koutsidi, M.; Tzanatos, E. Using Traits to Build and Explain an Ecosystem Model: Ecopath with Ecosim Modelling of the North Aegean Sea (Eastern Mediterranean). Estuar. Coast. Shelf Sci. 2020, 236, 106614. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Tsagarakis, K.; Sylaios, G.; Tsikliras, A.C. Ecosystem Trophic Structure and Fishing Effort Simulations of a Major Fishing Ground in the Northeastern Mediterranean Sea (Thermaikos Gulf). Estuar. Coast. Shelf Sci. 2022, 264, 107667. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Keramidas, I.; Tsagarakis, K.; Tsikliras, A.C. Ecosystem Models and Effort Simulations of an Untrawled Gulf in the Central Aegean Sea. Front. Mar. Sci. 2019, 6, 648. [Google Scholar]
- Papantoniou, G.; Giannoulaki, M.; Stoumboudi, M.T.; Lefkaditou, E.; Tsagarakis, K. Food Web Interactions in a Human Dominated Mediterranean Coastal Ecosystem. Mar. Environ. Res. 2021, 172, 105507. [Google Scholar] [CrossRef]
- Keramidas, I.; Dimarchopoulou, D.; Tsikliras, A.C. Modelling and Assessing the Ecosystem of the Aegean Sea, a Major Hub of the Eastern Mediterranean at the Intersection of Europe and Asia. Reg. Stud. Mar. Sci. 2022, 56, 102704. [Google Scholar] [CrossRef]
- Michailidis, N.; Corrales, X.; Karachle, P.K.; Chartosia, N.; Katsanevakis, S.; Sfenthourakis, S. Modelling the Role of Alien Species and Fisheries in an Eastern Mediterranean Insular Shelf Ecosystem. Ocean Coast. Manag. 2019, 175, 152–171. [Google Scholar] [CrossRef]
- Corrales, X.; Ofir, E.; Coll, M.; Goren, M.; Edelist, D.; Heymans, J.J.; Gal, G. Modeling the Role and Impact of Alien Species and Fisheries on the Israeli Marine Continental Shelf Ecosystem. J. Mar. Syst. 2017, 170, 88–102. [Google Scholar] [CrossRef]
- Saygu, İ.; Heymans, J.J.; Fox, C.J.; Özbilgin, H.; Eryaşar, A.R.; Gökçe, G. The Importance of Alien Species to the Food Web and Bottom Trawl Fisheries of the Northeastern Mediterranean, a Modelling Approach. J. Mar. Syst. 2020, 202, 103253. [Google Scholar] [CrossRef]
- Rice, M.A. Intensive Fishing Effort and Market Controls as Management Tools for Invasive Aquatic Species: A Review. Asian Fish. Sci. 2021, 34, 383–392. [Google Scholar]
- Yemisken, E.; Dalyan, C.; Eryilmaz, L. Catch and Discardfish Species of Trawl Fisheries in the Iskenderun Bay (Northeastern Mediterranean) with Emphasis on Lessepsian and Chondricthyan Species. Mediterr. Mar. Sci. 2014, 15, 380–389. [Google Scholar] [CrossRef] [Green Version]
- Golani, D.; Sonin, O. The Japanese Threadfin Bream Nemipterus Japonicus, a New Indo-Pacific Fish in the Mediterranean Sea. J. Fish Biol. 2006, 68, 940–943. [Google Scholar] [CrossRef]
- Lelli, S.; Colloca, F.; Carpentieri, P.; Russell, B.C. The Threadfin Bream Nemipterus randalli (Perciformes: Nemipteridae) in the Eastern Mediterranean Sea. J. Fish Biol. 2008, 73, 740–745. [Google Scholar] [CrossRef]
- Bilecenoglu, M.; Russell, B. Record of Nemipterus randalli Russell, 1986 (Nemipteridae) from Iskenderun Bay, Turkey. Cybium Int. J. Ichthyol. 2008, 32, 279–280. [Google Scholar]
- Gülşahin, A.; Kara, A. Record of Nemipterus randalli Russell, 1986 from the Southern Aegean Sea (Gökova Bay, Turkey). J. Appl. Ichthyol. 2013, 29, 933–934. [Google Scholar] [CrossRef]
- Aydin, İ.; Akyol, O. Occurrence of Nemipterus randalli Russell, 1986 (Nemipteridae) off Izmir Bay, Turkey. J. Appl. Ichthyol. 2017, 33, 533–534. [Google Scholar] [CrossRef]
- Uyan, U.; Filiz, H.; Top, N.; Tarkan, A.S. Assessment of Invasiveness Potential of Nemipterus randalli in Mediterranean Sea by Aquatic Species Invasiveness Screening Kit (AS-ISK). In Proceedings of the Workshop on Risk Assessment Tools in Aquatic Species, Proceedings Book, Duzce, Turkey, 28–29 April 2016; pp. 62–63. [Google Scholar]
- Bilge, G.; Filiz, H.; Yapici, S.; Tarkan, A.S.; Vilizzi, L. A Risk Screening Study on the Potential Invasiveness of Lessepsian Fishes in the South-Western Coasts of Anatolia. Acta Ichthyol. Piscat. 2019, 49, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Yapici, S.; Fïlïz, H. Biological Aspects of Two Coexisting Native and Non-Native Fish Species in the Aegean Sea: Pagellus erythrinus vs. Nemipterus randalli. Mediterr. Mar. Sci. 2019, 20, 594–602. [Google Scholar] [CrossRef]
- Uyan, U.; Filiz, H.; Tarkan, A.S.; Çelik, M.; Top, N. Age and Growth of Nemipterus randalli in the Southern Aegean Sea, Turkey. J. Black Sea/Mediterr. Environ. 2019, 25, 140–149. [Google Scholar]
- Akgün, Y. The Trophic and Fishery Impact of Invasive Nemipterus randalli (Russell, 1986) in the Northeastern Mediterranean Sea. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2022. [Google Scholar]
- Dunn, A.; Harley, S.J.; Doonan, I.J.; Bull, B. Calculation and Interpretation of Catch-per-Uniteffort (CPUE) Indices. N. Z. Fish. Assess. Rep. 2000, 1, 44. [Google Scholar]
- Gulland, J.A. Report of the FAO/IOP Workshop on the Fishery Resources of the Western Indian Ocean South of the Equator Mah? Food and Agriculture Organization of the United Nations: Rome, Italy, 1979. [Google Scholar]
- Allen, K.R. Relation between Production and Biomass. J. Fish. Board Can. 1971, 28, 1573–1581. [Google Scholar] [CrossRef]
- Hoenig, J.M. Empirical Use of Longevity Data to Estimate Mortality Rates. Fish. Bull. 1983, 81, 898–903. [Google Scholar]
- Palomares, M.L.D.; Pauly, D. Predicting Food Consumption of Fish Populations as Functions of Mortality, Food Type, Morphometrics, Temperature and Salinity. Mar. Freshw. Res. 1998, 49, 447–453. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. 2023. Available online: www.fishbase.org (accessed on 28 April 2023).
- Turkstat Turkish Statistical Institute. 2023. Available online: https://biruni.tuik.gov.tr/medas/?locale=tr (accessed on 21 June 2023).
- Sea Around Us Concepts, Design and Data. 2020. Available online: Seaaroundus.Org (accessed on 21 June 2023).
- Piroddi, C.; Coll, M.; Steenbeek, J.; Moy, D.M.; Christensen, V. Modelling the Mediterranean Marine Ecosystem as a Whole: Addressing the Challenge of Complexity. Mar. Ecol. Prog. Ser. 2015, 533, 47–65. [Google Scholar]
- Heymans, J.J.; Coll, M.; Link, J.S.; Mackinson, S.; Steenbeek, J.; Walters, C.; Christensen, V. Best Practice in Ecopath with Ecosim Food-Web Models for Ecosystem-Based Management. Ecol. Model. 2016, 331, 173–184. [Google Scholar] [CrossRef]
- Link, J.S. Adding Rigor to Ecological Network Models by Evaluating a Set of Pre-Balance Diagnostics: A Plea for PREBAL. Ecol. Model. 2010, 221, 1580–1591. [Google Scholar] [CrossRef]
- Ulanowicz, R.E. Information Theory in Ecology. Comput. Chem. 2001, 25, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ulanowicz, R.E. Ascendancy: A Measure of Ecosystem Performance. In Handbook of Ecosystem Theories and Management; CRC Press Inc.: Boca Raton, FL, USA, 2000; p. 303. [Google Scholar]
- Costanza, R.; Mageau, M. What Is a Healthy Ecosystem? Aquat. Ecol. 1999, 33, 105–115. [Google Scholar] [CrossRef]
- Odum, E.P. The Strategy of Ecosystem Development: An Understanding of Ecological Succession Provides a Basis for Resolving Man’s Conflict with Nature. Science 1969, 164, 262–270. [Google Scholar]
- Finn, J.T. Measures of Ecosystem Structure and Function Derived from Analysis of Flows. J. Theor. Biol. 1976, 56, 363–380. [Google Scholar] [CrossRef]
- Christensen, V.; Pauly, D. Flow Characteristics of Aquatic Ecosystems. In Trophic Models of Aquatic Ecosystems; Christensen, V., Pauly, D., Eds.; ICLARM: Manila, Philippines, 1993; pp. 338–352. [Google Scholar]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres Jr, F. Fishing down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V. Primary Production Required to Sustain Global Fisheries. Nature 1995, 374, 255–257. [Google Scholar] [CrossRef]
- Lindeman, R. The Trophic-Dynamic Aspect of Ecology. Ecology 1942, 23, 399–418. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Puccia, C.J. Mixed trophic impacts in ecosystems. In Coenoses; Springer: Berlin/Heidelberg, Germany, 1990; pp. 7–16. [Google Scholar]
- Libralato, S.; Christensen, V.; Pauly, D. A Method for Identifying Keystone Species in Food Web Models. Ecol. Model. 2006, 195, 153–171. [Google Scholar] [CrossRef]
- Gurlek, M.; Erguden, S.; Yaglioglu, D.; Turan, F.; Demirhan, S.; Gurlek, M.; Gungor, M.; Ozbalcilar, B.; Ozcan, T.; Univerity, M.K. Feeding Habits of Indo-Pacific Species Nemipterus randalli Russel, 1986 (Nemipteridae) in Iskenderun Bay, Eastern Mediterranean Sea. Rapp. Comm. Int. Médit. 2010, 39, 539. [Google Scholar]
- Morissette, L. Complexity, Cost and Quality of Ecosystem Models and Their Impact on Resilience: A Comparative Analysis, with Emphasis on Marine Mammals and the Gulf of St. Lawrence. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2007. [Google Scholar]
- Coll, M.; Palomera, I.; Tudela, S.; Sardà, F. Trophic Flows, Ecosystem Structure and Fishing Impacts in the South Catalan Sea, Northwestern Mediterranean. J. Mar. Syst. 2006, 59, 63–96. [Google Scholar]
- Coll, M.; Santojanni, A.; Palomera, I.; Tudela, S.; Arneri, E. An Ecological Model of the Northern and Central Adriatic Sea: Analysis of Ecosystem Structure and Fishing Impacts. J. Mar. Syst. 2007, 67, 119–154. [Google Scholar] [CrossRef]
- Ortiz, M.; Rodriguez-Zaragoza, F.; Hermosillo-Nuñez, B.; Jordán, F. Control Strategy Scenarios for the Alien Lionfish Pterois Volitans in Chinchorro Bank (Mexican Caribbean): Based on Semi-Quantitative Loop Analysis. PLoS ONE 2015, 10, e0130261. [Google Scholar] [CrossRef]
- Ünal, V.; Tıraşın, E.M.; Tosunoğlu, Z. An Ecosystem Approach to Fisheries Management for Small-Scale Fisheries in Gökova Marine Protected Area, Turkey: Challenges Encountered during the Transition Process. In Transition towards an Ecosystem Approach to Fisheries in the Mediterranean Sea–Lessons Learned through Selected Case Studies; Food and Agriculture Organization: Rome, Italy, 2022. [Google Scholar]
- Giorgi, F. Climate Change Hot-Spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Shaltout, M. Recent Sea Surface Temperature Trends and Future Scenarios for the Red Sea. Oceanologia 2019, 61, 484–504. [Google Scholar]
- García-Monteiro, S.; Sobrino, J.A.; Julien, Y.; Sòria, G.; Skokovic, D. Surface Temperature Trends in the Mediterranean Sea from MODIS Data during Years 2003–2019. Reg. Stud. Mar. Sci. 2022, 49, 102086. [Google Scholar]
- Turan, C.; Gürlek, M. Climate Change and Biodiversity Effects in Turkish Seas. Nat. Eng. Sci. 2016, 1, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Por, F.D. Climate Optimum Rejuvenates the Mediterranean Marine World. Integr. Zool. 2010, 5, 112–121. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global Climate Change Amplifies the Entry of Tropical Species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Gücü, A.C. Impact of Depth and Season on the Demersal Trawl Discard. Turk. J. Fish. Aquat. Sci. 2012, 12, 817–830. [Google Scholar]
- Giakoumi, S.; Pey, A.; Di Franco, A.; Francour, P.; Kizilkaya, Z.; Arda, Y.; Raybaud, V.; Guidetti, P. Exploring the Relationships between Marine Protected Areas and Invasive Fish in the World’s Most Invaded Sea. Ecol. Appl. 2019, 29, e01809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C. Management Priorities for Marine Invasive Species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef]
- El Hourany, R.; Fadel, A.; Gemayel, E.; Abboud-Abi Saab, M.; Faour, G. Spatio-Temporal Variability of the Phytoplankton Biomass in the Levantine Basin between 2002 and 2015 Using MODIS Products. Oceanologia 2017, 59, 153–165. [Google Scholar] [CrossRef]
- Yucel, N. Spatio-Temporal Variability of the Size-Fractionated Primary Production and Chlorophyll in the Levantine Basin (Northeastern Mediterranean). Oceanologia 2018, 60, 288–304. [Google Scholar] [CrossRef]
- Yılmaz, A.Z.; Besiktepe, S. Annual Variations in Biochemical Composition of Size Fractionated Particulate Matter and Zooplankton Abundance and Biomass in Mersin Bay, NE Mediterranean Sea. J. Mar. Syst. 2010, 81, 260–271. [Google Scholar] [CrossRef]
- Zervoudaki, S.; Christou, E.D.; Nielsen, T.G.; Siokou-Frangou, I.; Assimakopoulou, G.; Giannakourou, A.; Maar, M.; Pagou, K.; Krasakopoulou, E.; Christaki, U.; et al. The Importance of Small-Sized Copepods in a Frontal Area of the Aegean Sea. J. Plankton Res. 2007, 29, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Tartar, Ü.; Yeldan, H. Some Population Dynamic Parameters of Northeastern Mediterranean (Iskenderun Bay) Threadfin Bream, Nemipterus randalli Russell, 1986. Acta Biol. Turc. 2022, 35, 3–7. [Google Scholar]
- Erguden, D.; Turan, C.; Gurlek, M.; Yaglioglu, D.; Gungor, M. Age and Growth of the Randall’s Threadfin Bream Nemipterus randalli (Russell, 1986), a Recent Lessepsian Migrant in Iskenderun Bay, Northeastern Mediterranean. J. Appl. Ichthyol. 2010, 26, 441–444. [Google Scholar] [CrossRef]
- Gilaad, R.-L.; Galil, B.S.; Diamant, A.; Goren, M. The Diet of Native and Invasive Fish Species along the Eastern Mediterranean Coast (Osteichthyes). Zool. Middle East 2017, 63, 325–335. [Google Scholar] [CrossRef]
- Opitz, S. Trophic Interactions in Caribbean Coral Reefs; WorldFish Center: Penang, Malaysia, 1996; Volume 1085. [Google Scholar]
- Ergev, M.B. A Baseline Study for Determination of Macrobenthic Epi/ Infaunal Species in the Northeastern Mediterranean. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2002; p. 364. [Google Scholar]
- Martínez-Baena, F.; Navarro, J.; Albo-Puigserver, M.; Palomera, I.; Rosas-Luis, R. Feeding Habits of the Short-Finned Squid Illex Coindetii in the Western Mediterranean Sea Using Combined Stomach Content and Isotopic Analysis. J. Mar. Biol. Assoc. UK 2016, 96, 1235–1242. [Google Scholar] [CrossRef]
- Bingel, F. Erdemli-İçel Bölgesi Balıkçılığı Geliştirme Projesi Kesin Raporu (Final Report on the Improvement of the Fishery in Erdemli-İcel Region); Proje No 80070010; Institute of Marine Sciences-Middle East Technical Univiversitesi: Erdemli-Mersin, Turkey, 1981; Volume 154. [Google Scholar]
- Pauly, D. Fish Population Dynamics in Tropical Waters: A Manual for Use with Programmable Calculators; WorldFish Center: Penang, Malaysia, 1984; Volume 8. [Google Scholar]
- Lteif, M.; Jemaa, S.; Mouawad, R.; Khalaf, G.; Lelli, S.; Fakhri, M. Population Biology of the Common Pandora, Pagellus erythrinus (Linnaeus, 1758) along the Lebanese Coast, Eastern Mediterranean. Egypt. J. Aquat. Res. 2020, 46, 57–62. [Google Scholar] [CrossRef]
- Šantić, M.; Paladin, A.; Rađa, B. Feeding Habits of Common Pandora Pagellus erythrinus (Sparidae) from Eastern Central Adriatic Sea. Cybium 2011, 35, 83–90. [Google Scholar] [CrossRef]
- Gurbet, R.; Akyol, O.; Yalcin, E. Catch per Unit Effort and Mortality Rates of Two Sparid Species, Pagellus Acarne and Pagellus erythrinus from Bottom Trawl Fishery in Izmir Bay, Aegean Sea. J. Anim. Vet. Adv. 2012, 11, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, D. Age, Growth, and Diet of Axillary Seabream, Pagellus Acarne (Actinopterygii: Perciformes: Sparidae), in the Central Aegean Sea. Acta Ichthyol. Piscat. 2018, 48, 329–339. [Google Scholar]
- Chérif, M.; Amor, M.B.; Selmi, S.; Gharbi, H.; Missaoui, H.; Capapé, C. Food and Feeding Habits of the Red Mullet, Mullus Barbatus (Actinopterygii: Perciformes: Mullidae), off the Northern Tunisian Coast (Central Mediterranean). Acta Ichthyol. Piscat. 2011, 41, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Çiçek, E.; Avşar, D. Population Parameters, Mortality and Exploitation Rates of European Hake, Merluccius Merluccius (Linnaeus, 1758) in Iskenderun Bay (Off Karatas Coasts, Adana). Ecol. Life Sci. 2010, 5, 146–154. [Google Scholar]
- Cartes, J.E.; Rey, J.; Lloris, D.; De Sola, L.G. Influence of Environmental Variables on the Feeding and Diet of European Hake (Merluccius Merluccius) on the Mediterranean Iberian Coasts. J. Mar. Biol. Assoc. UK 2004, 84, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Halit, F.; Toğulga, M. Age and Growth, Reproduction and Diet of the Black Goby, (Gobius Niger) from Aegean Sea, Turkey. JFS.com 2009, 3, 245–265. [Google Scholar] [CrossRef]
- Gökçe, G.; Sangün, L.; Özbilgin, H.; Bilecenoglu, M. Growth and Mortality of the Brushtooth Lizardfish (Saurida Undosquamis) in Iskenderun Bay (Eastern Mediterranean Sea) Using Length Frequency Analysis. J. Appl. Ichthyol. 2007, 23, 697–699. [Google Scholar] [CrossRef]
- El-Etreby, S.; Saber, M.; El-Ganainy, A.; El-bokhty, A.-A. Age, Growth and Reproduction of the Lizard Fish Saurida Undosquamis from the Gulf of Suez, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2013, 17, 25–34. [Google Scholar]
- Özyurt, C.E.; Yeşilçimen, H.Ö.; Mavruk, S.; Kiyağa, V.B.; Perker, M. Assessment of Some of the Feeding Aspects and Reproduction of S. undosquamis Distributed in the İskenderun Bay. Turk. J. Fish. Aquat. Sci. 2017, 17, 51–60. [Google Scholar]
- Cicek, E.; Avsar, D.; Yeldan, H.; Ozutok, M. Length–Weight Relationships for 31 Teleost Fishes Caught by Bottom Trawl Net in the Babadillimani Bight (Northeastern Mediterranean). J. Appl. Ichthyol. 2006, 22, 290–292. [Google Scholar] [CrossRef]
- Apostolidis, C.; Stergiou, K.I. Estimation of Growth Parameters from Published Data for Several M Editerranean Fishes. J. Appl. Ichthyol. 2014, 30, 189–194. [Google Scholar] [CrossRef]
- Taieb, A.H.; Sley, A.; Ghorbel, M.; Jarboui, O. Feeding Habits of Sparus Aurata (Sparidae) from the Gulf of Gabes (Central Mediterranean). Cah. Biol. Mar. 2013, 54, 263–270. [Google Scholar]
- Tserpes, G.; Tsimenides, N. Age, Growth and Mortality of Serranus cabrilla (Linnaeus, 1758) on the Cretan Shelf. Fish. Res. 2001, 51, 27–34. [Google Scholar] [CrossRef]
- Soykan, O.; İLKYAZ, A.T.; METİN, G.; Kinacigil, H.T. Growth and Reproduction of Brown Comber (Serranus Hepatus Linnaeus, 1758) in the Central Aegean Sea, Turkey. Turk. J. Zool. 2013, 37, 211–217. [Google Scholar] [CrossRef]
- Bilecenoglu, M. Growth and Feeding Habits of the Brown Comber, Serranus Hepatus (Linnaeus, 1758) in Izmir Bay, Aegean Sea. Acta Adriat. 2009, 50, 105–110. [Google Scholar]
- Özütok, M.; Avşar, D. Preliminary Estimation of Growth, Mortality and the Exploitation Rates of the Silverbelly (Leiognathus Klunzingeri Steindachner, 1898) Population from the Yumurtalık Bight, Northeastern Mediterranean Coast of Turkey. Turk. J. Fish. Aquat. Sci. 2004, 4, 59–64. [Google Scholar]
- Jayabalan, N. Food and Feeding Habits of the “ponyfish Leiognathus Splenden~” (f).Sces, Leiognathidae) from Porto Novo Coast. Indian J. Mar. Sci. 1991, 20, 157–160. [Google Scholar]
- Tsikliras, A.C.; Koutrakis, E.T. Growth and Reproduction of European Sardine, Sardina Pilchardus (Pisces: Clupeidae), in Northeastern Mediterranean. Cah. Biol. Mar. 2013, 54, 365–374. [Google Scholar]
- Sever, T.M.; Bayhan, B.; Taskavak, E. A Preliminary Study on the Feeding Regime of European Pilchard (Sardina Pilchardus Walbaum 1792) in Izmir Bay, Turkey, Eastern Aegean Sea. Naga 2005, 28, 41–48. [Google Scholar]
- Gücü, A.C.; Sakınan, S.; Karataş, E.; Ok, M.; Tüer, M.; Yalçın, E.; Örek, Y.A.; Bingel, F. Kuzeydoğu Akdeniz Küçük Pelajik Balık Stoklarındaki Değişimlerin Araştırılması; 108O566 Project Report; The Scientific and Technological Research Council of Turkey: Ankara, Turkey, 2011. [Google Scholar]
- Karachle, P.K.; Stergiou, K.I. Feeding and Ecomorphology of Three Clupeoids in the N Aegean Sea. Mediterr. Mar. Sci. 2014, 15, 9–26. [Google Scholar] [CrossRef]
- Pauly, D.; Soriano-Bartz, M.L.; Palomares, M.L.D. Improved Construction, Parametrization and Interpretation of Steady-State Ecosystem Models. In Trophic Models of Aquatic Ecosystems; ICLARM Conference Proceedings, 26, Christensen, V., Pauly, D., Eds.; ICLARM, WorldFish: Penang, Malaysia, 1993; pp. 1–13. [Google Scholar]


| Functional Group | Species and Taxa Included |
|---|---|
| Detritus | Sediment and water-column detritus |
| Phytoplankton | Planktonic algae |
| Zooplankton | Fodder micro- and mesozooplankton |
| Nemipterus randalli | N. randalli |
| Other benthic invertebrates | Philine spp., Anseropoda placenta (Pennant, 1777); Echinaster (Echinaster) sepositus (Retzius, 1783); Pennatula phosphorea Linnaeus, 1758; Pennatula rubra (Ellis, 1764); Antedon spp.; Coscinasterias tenuispina (Lamarck, 1816) Gastropoda Bivalvia |
| Polychaetes | All taxa |
| Crabs | Pagurus prideaux Leach, 1815; Medorippe lanata (Linnaeus, 1767); Charybdis (Archias) longicollis Leene, 1938 |
| Shrimps and prawns | Penaeus japonicus Spence Bate, 1888; Penaeus kerathurus (Forskål, 1775); Parapenaeus longirostris (Lucas, 1846); Squilla mantis (Linnaeus, 1758); Erugosquilla massavensis (Kossmann, 1880) |
| Octopuses, cuttlefish and squids | Eledone moschata (Lamarck, 1798); Octopus vulgaris Cuvier, 1797; Sepia officinalis Linnaeus, 1758; Illex coindetii (Vérany, 1839); Loligo vulgaris Lamarck, 1798; Rhombosepion elegans (Blainville, 1827); Rhombosepion orbignyanum (Férussac, 1826); Sepietta oweniana (d’Orbigny, 1841) |
| Pagellus erythrinus (Linnaeus, 1758) | P. erythrinus |
| Pagellus acarne (Risso, 1827) | P. acarne |
| Red mullets | Mullus barbatus Linnaeus, 1758 and Mullus surmuletus Linnaeus, 1758 |
| Merluccius merluccius (Linnaeus, 1758) | M. merluccius |
| Gobius spp. | Gobius bucchichi Steindachner, 1870; Gobius niger Linnaeus, 1758; Vanderhorstia mertensi Klausewitz, 1974 |
| Saurida undosquamis (Richardson, 1848) | S. undosquamis |
| Sea breams and porgies | Boops boops (Linnaeus, 1758); Dentex macrophthalmus (Bloch, 1971); Diplodus annularis (Linnaeus, 1758); Diplodus sargus (Linnaeus, 1758); Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817); Lithognathus mormyrus (Linnaeus, 1758); Evynnis ehrenbergii (Valenciennes, 1830); Pagrus pagrus (Linnaeus, 1758); Sparus aurata Linnaeus, 1758; Spicara flexuosum Rafinesque, 1810; Spicara smaris (Linnaeus, 1758) |
| Serranus spp. | Serranus hepatus (Linnaeus, 1758); Serranus cabrilla (Linnaeus, 1758) |
| Leiognathidae | Equulites elongatus (Günther, 1874); Equulites klunzingeri (Steindachner, 1898) |
| Clupeidae | Dussumieria elopsoides Bleeker, 1849; Sardina pilchardus (Walbaum, 1792); Sardinella aurita Valenciennes, 1847; Sardinella maderensis (Lowe, 1838) |
| Engraulis encrasicolus (Linnaeus, 1758) | E. encrasicolus |
| Horse mackerels | Trachurus mediterraneus (Steindachner, 1868) and Trachurus trachurus (Linnaeus, 1758) |
| Group | Diet Item | Weight (%) |
|---|---|---|
| Crustaceans | Squilla spp. | 26.99 |
| Charybdis longicollis | 10.95 | |
| Unidentified crabs | 8.53 | |
| Stamatopoda | 6.11 | |
| Penaeus japonicus | 4.26 | |
| Penaeus kerathurus | 2.95 | |
| Unidentified shrimps | 1.28 | |
| Unidentified crustaceans | 1.2 | |
| Macropthalmus spp. | 0.94 | |
| Penaeus spp. | 0.53 | |
| Alpheidae | 0.12 | |
| Other Decapoda | 0.09 | |
| Fish | Clupea spp. | 12.32 |
| Unidentified teleost fish | 8.93 | |
| Serranus hepatus | 4.49 | |
| Equulites elongatus | 2.53 | |
| Vanderhorstia mertensi | 1.78 | |
| Saurida undosquamis | 1.44 | |
| Sparidae | 1.12 | |
| Echinoderms | Ophiaderma longicaudum (Bruzelius, 1805) | 0.75 |
| Anseropoda placenta | 0.11 | |
| Other Echinodermata | 0.02 | |
| Other | Lophotrochozoa | 1.88 |
| Digested organic material | 0.39 | |
| Endoparasites | 0.29 |
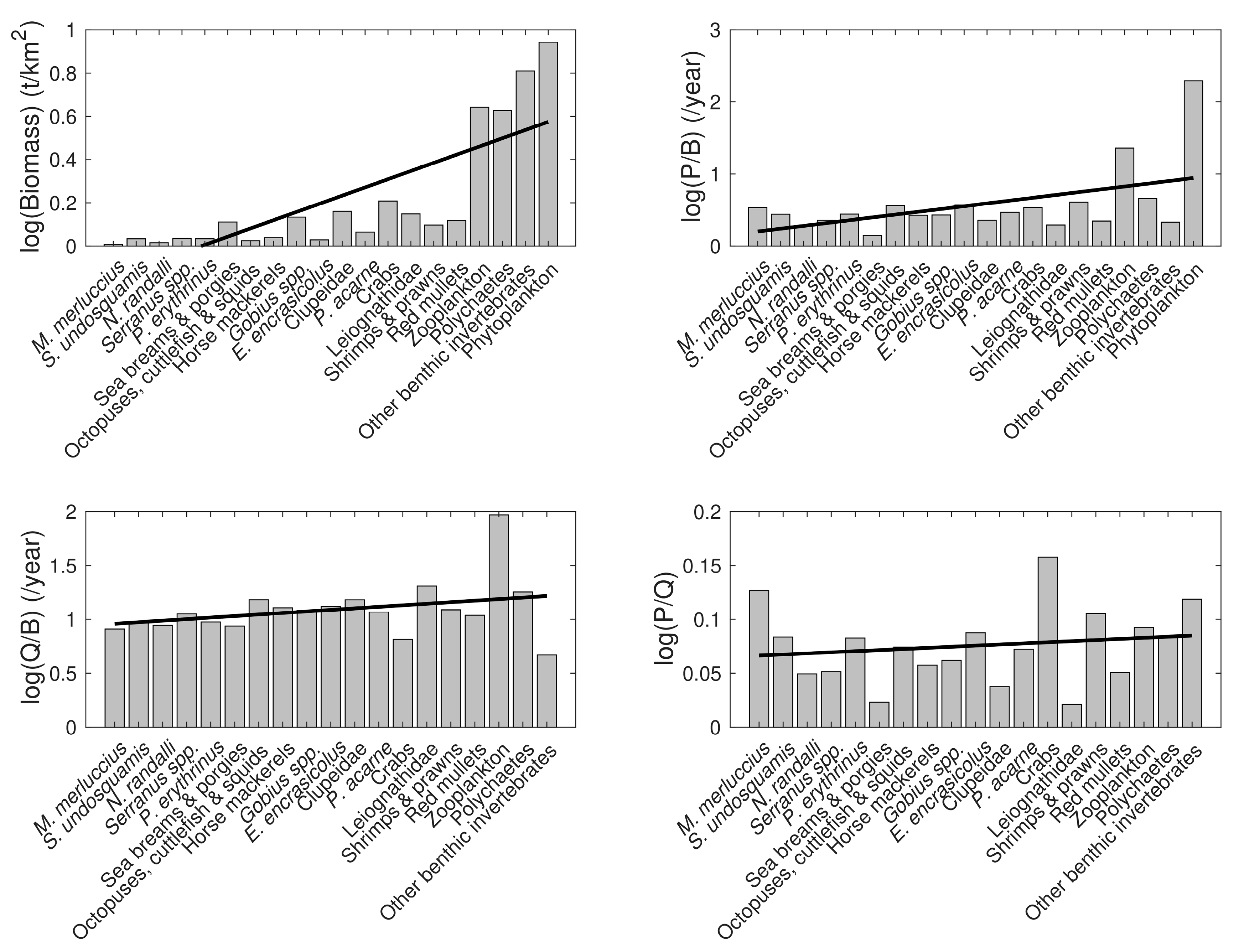
| Group/Species | Trophic Level (TL) | Biomass (t km−2) | P/B (y−1) | Q/B (y−1) | EE | P/Q | Landings (Tonnes km−2 y−1) | |
|---|---|---|---|---|---|---|---|---|
| By Seiners | By Trawlers | |||||||
| Phytoplankton | 1 | 7.75 | 195.1 | 0.14 | ||||
| Zooplankton | 2.05 | 3.385 | 21.9 | 92.18 | 0.43 | 0.24 | ||
| N. randalli | 3.94 | 0.037 | 0.94 | 7.78 | 0.45 | 0.12 | ||
| Other benthic invertebrates | 2.03 | 5.456 | 1.15 | 3.66 | 0.87 | 0.31 | ||
| Polychaetes | 2.04 | 3.24 | 3.61 | 16.93 | 0.85 | 0.21 | ||
| Crabs | 2.94 | 0.618 | 2.42 | 5.53 | 0.93 | 0.44 | 6.51 × 10−5 | |
| Shrimp and prawns | 2.91 | 0.251 | 3.09 | 11.27 | 0.95 | 0.27 | 0.016 | |
| Octopuses, cuttlefish and squids | 3.40 | 0.061 | 2.65 | 14.22 | 0.93 | 0.19 | 0.008 | |
| P. erythrinus | 3.48 | 0.084 | 1.77 | 8.43 | 0.88 | 0.21 | 0.009 | |
| P. acarne | 3.04 | 0.161 | 1.94 | 10.69 | 0.83 | 0.18 | ||
| Red mullets | 2.80 | 0.315 | 1.23 | 9.89 | 0.89 | 0.12 | 0.013 | |
| M. merluccius | 4.51 | 0.021 | 2.41 | 7.11 | 0.93 | 0.34 | 0.001 | |
| Gobius spp. | 3.06 | 0.36 | 1.69 | 11.07 | 0.85 | 0.15 | 0.001 | |
| S. undosquamis | 4.16 | 0.083 | 1.76 | 8.29 | 0.04 | 0.21 | 0.001 | |
| Sea breams and porgies | 3.41 | 0.293 | 0.41 | 7.65 | 0.91 | 0.05 | 0.018 | |
| Serranus spp. | 3.63 | 0.086 | 1.28 | 10.21 | 0.96 | 0.13 | ||
| Leiognathidae | 2.91 | 0.408 | 0.96 | 19.36 | 0.38 | 0.05 | ||
| Clupeidae | 3.05 | 0.447 | 1.28 | 14.21 | 0.86 | 0.09 | 0.042 | |
| E. encrasicolus | 3.05 | 0.07 | 2.73 | 12.23 | 0.84 | 0.22 | 0.001 | |
| Horse mackerels | 3.08 | 0.095 | 1.67 | 11.8 | 0.36 | 0.14 | 0.003 | |
| Detritus | 1 | 105.35 | 0.11 | |||||
| Parameter | Value | Unit |
|---|---|---|
| Sum of all consumption | 423.93 | t/km2/year |
| Sum of all exports | 1270.62 | t/km2/year |
| Sum of all respiratory flows | 241.38 | t/km2/year |
| Sum of all flows to detritus | 1424.15 | t/km2/year |
| Total system throughput | 3360.08 | t/km2/year |
| Sum of all production | 1609.76 | t/km2/year |
| Total net primary production | 1512 | t/km2/year |
| Net system production | 1270.62 | t/km2/year |
| Total biomass (excluding detritus) | 23.22 | t/km2 |
| Total biomass/total throughput (B/T) | 0.007 | year |
| Total primary production/total respiration (Pp/R) | 6.26 | - |
| Total primary production/total biomass (Pp/B) | 65.11 | /year |
| Transfer efficiency from primary producers | 5.77 | % |
| Transfer efficiency from detritus | 12.56 | % |
| Mean transfer efficiency (TE) | 9.77 | % |
| Connectance index | 0.30 | - |
| System omnivory index (SOI) | 0.13 | - |
| Shannon diversity index | 1.90 | - |
| Total catch | 0.11 | t/km2/year |
| Mean trophic level of catch (mTLc) | 3.14 | - |
| Gross efficiency (catch/net primary production) | 0.0001 | - |
| Mean trophic level of community (≥3.25) | 3.61 | - |
| Primary production required to sustain catches (PPRc) | 0.68 | % |
| Predatory cycling index (PCI) | 3.49 | % |
| Finn’s cycling index (FCI) | 2.24 | % |
| Finn’s mean path length (PL) | 2.22 | - |
| Ascendancy | 48.3 | % |
| Overhead | 51.7 | % |
| Capacity | 7954 | flowbits |
| Ecopath pedigree index | 0.63 | - |
| Indicators | This Study | Israeli Coast [17] | Cyprus Coast [16] | North Aegean Sea [10] | Pagasitikos Gulf, Aegean Sea [13] | Thermaikos Gulf, Aegean Sea [12] | Saronikos Gulf, Aegean Sea [14] | North Aegean Sea [11] | North and Central Adriatic Sea [55] | South Catalan Sea [56] | Mersin Bay, Levant Sea [18] | Mediterranean Sea [39] | Unit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2019 | 2008–2012 | 2015.2017 | 2003–2006 | 2008 | 1998–2000 | 1998–2000 | 1993 | 1990s | 1994 | 2009–2013 | 2000s | |
| Sum of all respiratory flows | 241.38 | - | - | 269.48 | 486 | 417 | 571 | 271.68 | 421.09 | 327.16 | 254.63 | 290 | t/km2/year |
| Sum of all flows to detritus | 1424.15 | - | - | 562.53 | 761 | 868 | 1297 | 566.46 | 1387.46 | 416.91 | 292.12 | 1467 | t/km2/year |
| Total system throughput | 3360.08 | 631.89 | 841 | 1976 | 2951 | 3185 | 3925 | 1984.75 | 3844 | 1657 | 1149.53 | 4000 | t/km2/year |
| Total net primary production | 1512 | - | - | 535.48 | 712 | 923 | 1243 | 535.47 | 1149.85 | 386.68 | 368.65 | 1610 | t/km2/year |
| Net system production | 1270.62 | - | - | 265.99 | 227 | 506 | 672 | 263.80 | 728.76 | 59.52 | 114.2 | 1320 | t/km2/year |
| Total biomass (excluding detritus) | 23.22 | 8.69 | 18.77 | 33.04 | 78 | 40 | 38.94 | 33.98 | 130.3 | 59.99 | 23.49 | 42.74 | t/km2 |
| Total biomass/total throughput | 0.007 | - | - | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0.02 | - | year |
| Total primary production/total respiration | 6.26 | 4.26 | 2.04 | 1.99 | 1.47 | 2.21 | 2.17 | 1.97 | 2.73 | 1.18 | 1.45 | 5.55 | - |
| Mean transfer efficiency | 9.77 | 19 | 16.93 | 17.4 | - | - | 14.77 | - | 10 | 12.6 | 9.37 | 9.2 | % |
| Connectance index | 0.30 | - | - | - | - | - | 0.332 | 0.28 | - | 0.20 | 0.27 | 0.1 | - |
| System omnivory index | 0.13 | 0.19 | 0.23 | 0.18 | 0.25 | 0.2 | 0.23 | 0.24 | 0.19 | 0.19 | 0.16 | 0.27 | - |
| Total catch | 0.11 | 0.93 | 0.65 | 2.35 | - | - | 2.75 | 2.93 | 2.44 | 5.36 | 0.42 | - | t/km2/year |
| Mean trophic level of catch | 3.14 | 3.37 | 3.25 | 3.47 | - | - | 3.36 | 3.47 | 3.07 | 3.12 | 3.29 | 3.08 | - |
| Gross efficiency (catch/net primary production) | 0.0001 | - | - | 0.004 | - | - | 0.002 | 0.01 | 0.002 | 0.0014 | 0.001 | 0.00026 | - |
| Primary production required to sustain catches | 0.68 | 11.34 | 7.07 | 3.45 | - | - | 4.66 | - | 6.59 | 9.45 | 6.79 | 1.46 | % |
| Predatory cycling index | 3.49 | - | - | - | - | - | 14.77 | - | 3.97 | 3.33 | 3.67 | 10.96 | % |
| Finn’s cycling index | 2.24 | 5.78 | 9.3 | 14.6 | - | - | 12.53 | - | 14.7 | 25.19 | 10.09 | 4.98 | % |
| Finn’s mean path length | 2.22 | 2.63 | 3.21 | - | - | - | 3.121 | - | 5.41 | 4.27 | - | - | - |
| Ascendancy | 48.3 | - | - | 21.6 | - | - | 24.5 | - | 27 | 25.5 | 24.32 | 42.9 | % |
| Overhead | 51.7 | - | - | 78.4 | - | - | 75.5 | - | 73 | 74.5 | 75.66 | 57.1 | % |
| Capacity | 7954 | - | - | 9162.5 | - | - | 15,785 | - | 15,406.7 | 7119.3 | 4773.98 | - | flowbits |
| Ecopath pedigree index | 0.63 | 0.54 | 0.62 | 0.61 | 0.53 | 0.53 | 0.65 | - | 0.66 | 0.67 | 0.63 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgun, Y.; Akoglu, E. Randall’s Threadfin Bream (Nemipterus randalli, Russell 1986) Poses a Potential Threat to the Northeastern Mediterranean Sea Food Web. Fishes 2023, 8, 402. https://doi.org/10.3390/fishes8080402
Akgun Y, Akoglu E. Randall’s Threadfin Bream (Nemipterus randalli, Russell 1986) Poses a Potential Threat to the Northeastern Mediterranean Sea Food Web. Fishes. 2023; 8(8):402. https://doi.org/10.3390/fishes8080402
Chicago/Turabian StyleAkgun, Yagmur, and Ekin Akoglu. 2023. "Randall’s Threadfin Bream (Nemipterus randalli, Russell 1986) Poses a Potential Threat to the Northeastern Mediterranean Sea Food Web" Fishes 8, no. 8: 402. https://doi.org/10.3390/fishes8080402
APA StyleAkgun, Y., & Akoglu, E. (2023). Randall’s Threadfin Bream (Nemipterus randalli, Russell 1986) Poses a Potential Threat to the Northeastern Mediterranean Sea Food Web. Fishes, 8(8), 402. https://doi.org/10.3390/fishes8080402






