The Danube Delta: The Achilles Heel of Danube River–Danube Delta–Black Sea Region Fish Diversity under a Black Sea Impact Scenario Due to Sea Level Rise—A Prospective Review
Abstract
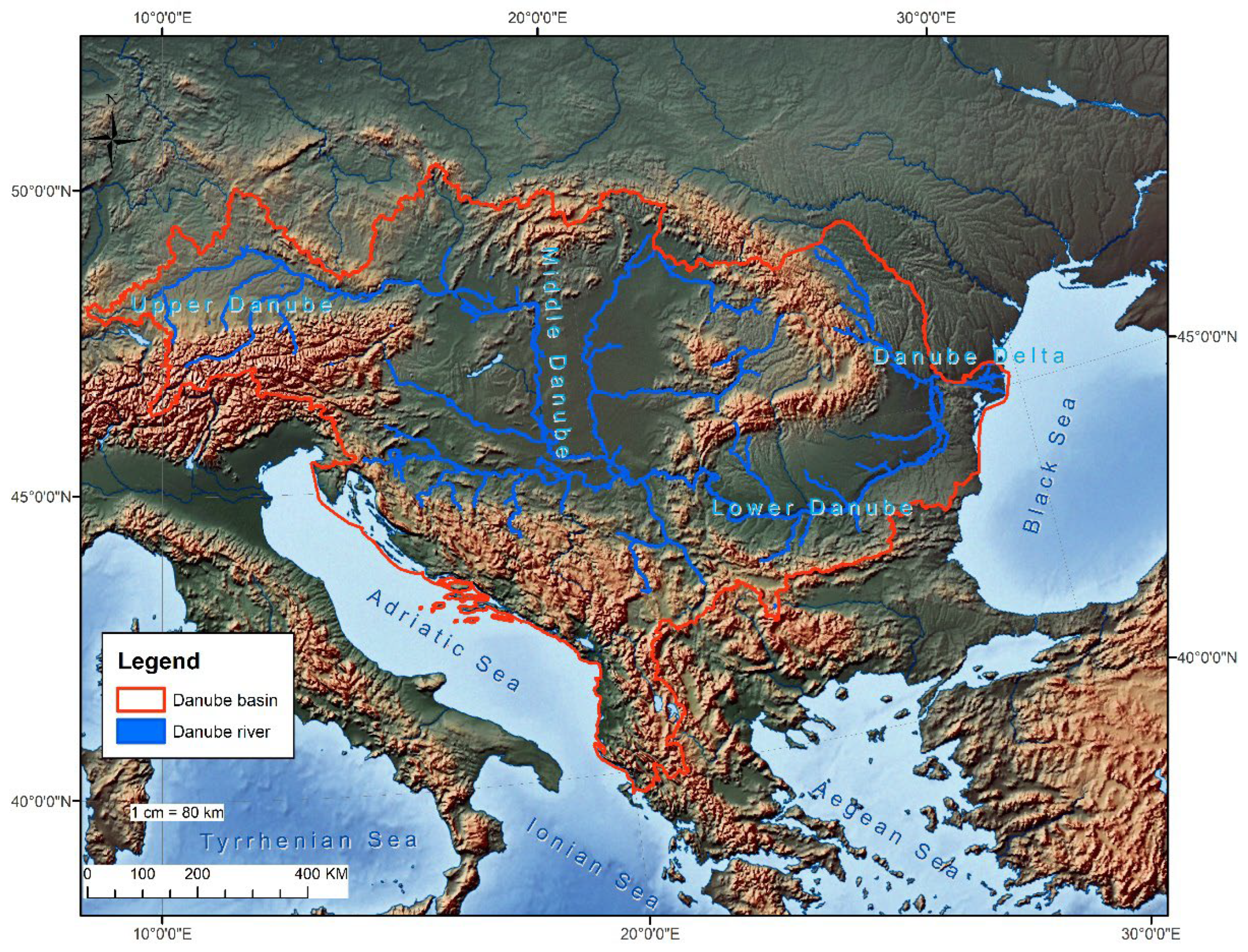
1. Introduction and Background
2. Results and Analysis
2.1. Global Sea Level Rise Elements: Short History, Present Situation, and Trends
2.2. Black Sea Level Fluctuation Elements: Dynamic History and Future Rise Potential Trends
2.3. Danube Delta Habitats’ Historical Exposure and Potential Trends and Risks to Potential Black Sea Level Rise
2.4. Potential General Consequences in Changing Danube Delta Microhabitats and Habitats Due to the Negative Potential Effects of Forecast Black Sea Level Rise
2.5. Potential Specific Negative Ecologic Consequences of Altering and Changing Danube Delta Habitats for Fish Fauna Species Presence/Absence, Distribution, and Status
2.5.1. Potential Specific Ecologic Negative Consequences of Altering and Changing Danube Delta Habitats for the First Category of Fish Species under Threat (Fish Species Presence/Absence, Distribution, and Status)
2.5.2. Potential Specific Ecologic Negative Consequences of Altering and Changing Danube Delta Habitats for the Second Category of Fish Species under Threat (Fish Species Presence/Absence, Distribution, and Status)
2.5.3. Potential Specific Ecologic Negative Consequences of Altering and Changing Danube Delta Habitats for the Third Category of Fish Species under Threat (Fish Species Presence/Absence, Distribution, and Status)
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prichard, S.B. Reconstructing the Rhone: The cultural politics of nature and nation in contemporary France, 1945–1997. Fr. Hist. Stud. 2004, 27, 765–799. [Google Scholar]
- Bănăduc, D.; Rey, S.; Trichkova, T.; Lenhardt, M.; Curtean-Bănăduc, A. The Lower Danube River–Danube Delta–North West Black Sea: A pivotal area of major interest for the past, present and future of its fish fauna—A short review. Sci. Total Environ. 2016, 545–546, 137–151, ISSN 0048-9607/1879-1026. [Google Scholar] [CrossRef]
- Meybeck, M. Global analysis of river systems: From Earth system controls to Anthropocene syndromes. Phil. Trans. R. Soc. Lond. B 2003, 358, 1935–1955. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Binder, E. The Habitats Along the Upper Danube in Germany and Changes to Them Induced by Human Impacts. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 27–48. [Google Scholar]
- Curtean-Bănăduc, A.; Bănăduc, D. Human impact effects on Târnava River basin aquatic Biodiversity (Transylvania, Romania). In Human Impact on Danube Watershed Biodiversity in the XXI Century, 1st ed.; Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Hardcover; Springer International Publishing: Cham, Switzerland, 2020; pp. 425–437. ISBN 978-3-030-37241-5/978-3-030-37242-2. [Google Scholar] [CrossRef]
- Burga, C.A.; Landolt, E. The Upper Engandine: Headwater Region of the River Inn. A Swiss Hot Spot of Plant Diversity and Premium Tourism Region. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 49–64. [Google Scholar]
- Hübl, E. Vegetation and Flora Near the Danube in Austria. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 65–86. [Google Scholar]
- Cianfaglione, K.; Pedrotti, F. Italy in the Danube Geography: Territory, Landscape, Environment, Vegetation, Fauna, Culture, Human Management and Outlooks for the Future. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 87–118. [Google Scholar]
- Curtean-Bănăduc, A.; Olosutean, H.; Bănăduc, D. Influence of Environmental Variables on the Structure and Diversity of Ephemeropteran Communities: A Case Study of the Timis River, Romania. Acta Zool. Bulg. 2016, 68, 215–224. [Google Scholar]
- Adámek, Z.; Jurajdová, Z.; Janáč, M.; Zahrádková, S.; Němejcová, D.; Jurajda, P. The Response of Fish Assemblages to Human Impacts Along the Lower Stretch of the Rivers Morava and Dyje (Danube River Basin, Czech Republic). In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 135–150. [Google Scholar]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuț, C.-M.; Bănăduc, D. The Benthic Trophic Corner Stone Compartment in POPs Transfer from Abiotic Environment to Higher Trophic Levels-Trichoptera and Ephemeroptera Pre-Alert Indicator Role. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Dekić, R.; Ivanc, A.; Ćetković, D.; Lolić, A. Anthropogenic Impact and Environmental Quality of Different Tributaries of the River Vrbas (Bosnia and Hertzegovina). In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 169–214. [Google Scholar]
- Bănăduc, D.; Maric, S.; Cianfaglione, K.; Afanasyev, S.; Somogy, D.; Nyeste, K.; Antal, L.; Kosko, J.; Caleta, M.; Wanzenbock, J.; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction? Sustainability 2022, 14, 13493. [Google Scholar] [CrossRef]
- Đikanović, V.; Nikčević, M.; Mićković, B.; Hegediš, A.; Mrdak, D.; Pešić, V. Anthropogenic Pressures on Watercourses of the Danube River Basin in Montenegro, In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 241–256. [Google Scholar]
- Lenhardt, M.; Smederevac-Lalić, M.; Hegediš, A.; Skorić, S.; Cvijanović, G.; Višnjić-Jeftić, Ž.; Djikanović, V.; Jovičić, K.; Jaćimović, M.; Jarić, I. Human Impacts of Fish Fauna in the Danube River in Serbia: Current Status and Ecological Implicatins. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 257–280. [Google Scholar]
- Mišiková Elexová, E.; Makovinská, J. Assessment of the Aquatic Ecosystem in the Slovak Stretch of the Danube River. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 281–300. [Google Scholar]
- Maślanko, W.; Ferencz, B.; Dawidek, J. State and Changes of Natural Environment in Polish Part of the Danube River Basin Poland. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 301–326. [Google Scholar]
- Afanasyev, S.; Lyashenko, A.; Iarochevitch, A.; Lietytska, O.; Zorina-Sakharova, K.; Marushevska, O. Pressures and Impacts on Ecological Status of Surface Water Bodies in Ukrainian part of the Danube River Basin. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 327–359. [Google Scholar]
- Bakiu, R. Drina River (Sava’s Tributary of Danube River) and Human Impact in Albania. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 359–380. [Google Scholar]
- Kostov, V.; Slavevska-Stamenkovic, V.; Ristovska, M.; Stojov, V.; Marić, S. Characteristics of the Danube Drainage Area in the Republic of Macedonia. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 381–392. [Google Scholar]
- Kenderov, L.; Trichkova, T. Long-Term Changes in the Ecological Conditions of the Iskar River (Danube River Basin, Bulgaria). In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 393–424. [Google Scholar]
- Costea, G.; Push, M.T.; Bănăduc, D.; Cosmoiu, D.; Curtean-Bănăduc, A. A review of hydropower plants in Romania: Distribution, current knowledge, and their effects on fish in headwater streams. Renew. Sustain. Energy Rev. 2021, 54, 111003, ISSN 1364-0321. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Bănăduc, D.; Burcea, A.; Berg, V.; Lyche, J.L.; Gheorghe, L.M. Persistent organic pollutants in Mureş watershed. In The Impact of Persistent Organic Pollutants on Freshwater Ecosystems and Human Health; Curtean-Bănăduc, A., Ed.; Publisher Lucian Blaga, University of Sibiu: Sibiu, Romania, 2016; pp. 117–152. [Google Scholar]
- Curtean-Bănăduc, A.; Marić, S.; Gabor, G.; Didenko, A.; Rey Planelas, S.; Bănăduc, D. Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians? Conservation elements. Turk. J. Zool. 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Popa, G.-O.; Curtean-Bănăduc, A.; Bănăduc, D.; Florescu, I.E.; Burcea, A.; Dudu, A.; Georgescu, S.E.; Costache, M. Molecular markers reveal reduced genetic diversity in Romanian populations of Brown Trout, Salmo trutta L., 1758 (Salmonidae). Acta Zool. Bulg. 2016, 68, 399–406, ISSN 0324-0770. [Google Scholar]
- Beattie, A. The Danube: A Cultural History; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Almazov, A.A.; Bondar, C.; Diaconescu, C.; Ghederim, V.; Mihailov, A.N.; Mita, P.; Nichiforov, I.D.; Rai, I.A.; Rodionov, N.A.; Stănescu, S.; et al. Zona de vărsare a Dunării. Morfografie hidrologică; Tehnică: Bucureşti, Romania, 1963; 396p. [Google Scholar]
- Schiemer, F.; Gutti, G.; Keckeis, H.; Staraş, M. Ecological status and problems of the Danube River and its fish fauna: A review. In Proceedings of the Second Symposium on the Management of Large Rivers for Fishers; Welcomme, R., Petr, T., Eds.; RAP Publication 2004/16. FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2004; Volume I, pp. 273–279. [Google Scholar]
- Claudino-Sales, V. Danube Delta, Romania, Coastal World Heritage Sites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 93–97. Available online: https://link.springer.com/chapter/10.1007/978-94-024-1528-5_14 (accessed on 11 November 2022).
- Preoteasa, L.; Roberts, H.M.; Vespremeanu-Stroe, A.; Popa, I.; Duller, G.A.T. Records of Climate Change over Late-Holocene in the Danube Delta Coastal Dune System. Rev. Geomorfol. 2009, 11, 91–100. [Google Scholar]
- Drugescu, C. Zoogeografia României; Editura ALL: Bucureşti, Romania, 1994; p. 140. [Google Scholar]
- Morigi, C. A LGM and Pre-Boreal Danube paleo-Deltas evidenced on the Romanian Black Sea self. Geophys. Res. Abstr. 2007, 9, 00903, ISSN 1607-7962. [Google Scholar]
- Chirică, L. Modificările Climatice în Post-Glaciar şi Impactul lor Asupra Geosistemului Marii Negre. 2019, pp. 288–293. Available online: https://ibn.idsi.md/sites/default/files/imag_file/288-2934.pdf (accessed on 11 November 2022).
- Chirică, L. Evoluţia Mării Negre şi a Zonei de Litoral în Pleistocenul Superior şi Holocen; CZU: 902/903 (92-96); Colegiul de Ecologie Chișinău: Chișinău, Moldova, 2020. [Google Scholar]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin—South-Eastern Carpathians—North-Western Black Sea coast area lakes, a broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E.; et al. Aquatic foods to nourish nations. Nature 2021, 598, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Perillo, G.M.E. (Eds.) . Deltas: Landforms, Ecosystems and Human Activities; IAHS Press: Wallingford, UK, 2013; 256p, ISBN 9781907161360. [Google Scholar]
- Bernhardt, J.R.; O’Connor, M.I. Aquatic biodiversity enhances multiple nutritional benefits to humans. Proc. Natl. Acad. Sci. USA 2021, 118, e1917487118. [Google Scholar] [CrossRef]
- World’s Leading Aquatic Scientific Societies Urgently Call for Cuts to Global Greenhouse Gas Emissions. 2020. Available online: https://www.sws.org/2020/09/14/worlds-leading-aquatic-scientific-societies-urgently-call-for-cuts-to-global-greenhouse-gas-emissions/ (accessed on 6 April 2022).
- Nelson, J.S. Fishes of the World, 3rd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Rodríguez-Rey, M.; Grenouillet, G. Disentangling the Drivers of the Sampling Bias of Freshwater Fish across Europe. Fishes 2022, 7, 383. [Google Scholar] [CrossRef]
- Giurescu, C.C. Istoria Pescuitului in România—Volume I; Editura Academiei Republicii Populare Române: Bucureşti, Romania, 1964; 389p. [Google Scholar]
- Petts, G.E.; Imhof, J.G.; Manny, B.A.; Maher, J.F.B.; Weisberg, S.B. Management of fish populations in large rivers: A review of tools and approaches. In Proceedings of the International, Large river symposium (LARS), Honey Harbour, ON, Canada, 14–21 September 1986; Dodge, D.P., Ed.; Canadian Special Publication of Fisheries and Aquatic Science: Ottawa, ON, Canada, 1989; pp. 578–588. [Google Scholar]
- Antipa, G. Fauna Ihtiologică a României; Inst. de Arte Grafice Carol Göbl.: București, Romania, 1909; p. 112. [Google Scholar]
- Bănărescu, P.M. Fauna Republicii Populare Române, Pisces-Osteichthyes, Volume XIII; Editura Academiei Republicii Populare Române: Bucureşti, Romania, 1964; p. 962. [Google Scholar]
- Bănărescu, P.M. Considerations on the threatened freshwater fishes of Europe. Ocrot. Nat. Med. Înconj. 1993, 37, 87–98. [Google Scholar]
- Oţel, V. Atlasul Peştilor din Rezervaţia Biosferei Delta Dunării; Centrul de Informare Tehnologică Delta Dunării: Tulcea, România, 2007; p. 480. [Google Scholar]
- Wolfgang, J.J.; Brown, M.; Campbell, I.C.; Finlayson, M.; Gopal, B.; Ramberg, L.; Warner, B.G. The comparative biodiversity of seven globally important wetlands: A synthesis. Aquat. Sci. 2006, 68, 400–414. [Google Scholar]
- Ramanathan, V. The greenhouse theory of climate change: A test by an inadvertent global experiment. Science 1988, 240, 293–299. [Google Scholar] [CrossRef]
- Helfman, G.S. Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources; Island Press: Washington, DC, USA, 2007. [Google Scholar]
- Laske, S. Surface Water Connectivity of Arctic Lakes Drives Paterns of Fish Species Richness and Composition, and Food Web Structure. Ph.D. Thesis, University of Alaska Fairbanks, Fairbanks, AK, USA, 2017. [Google Scholar]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple freshwater stressors-Key drivers for the future of freshwater environents. Front. Environ. Sci. 2023, 11, 1143706. [Google Scholar] [CrossRef]
- Năvodaru, I.; Buijse, A.D.; Staraş, M. Effects of Hydrology and Water Quality on the Fish Community in Danube Delta Lakes. Int. Rev. Hydrobiol. 2002, 87, 329–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, F.; Pan, M.; Van Niel, T.; Wegehenkel, M. Editorial hydrological processes in changing climate, land use, and cover change. Adv. Meteorol. 2016, 2016, 7273424. [Google Scholar] [CrossRef]
- Koenigstein, S.; Mark, F.C.; Goessling-Reisemann, S.; Reuter, H.; Pörtner, H.-O. Modeling climate change impacts on marine fish populations: Process based integration of ocean warming, acidification and other environmental drivers. Fish 2016, 17, 972–1004. [Google Scholar]
- NOAA. 2021. Available online: https://www.noaa.gov/media-release/new-online-tool-helps-communities-prepare-for-coastal-flooding (accessed on 13 November 2022).
- Syvitski, J.P.; Kettner, A.J.; Overeem, I.; Hutton, E.W.; Hannon, M.T.; Brakenridge, G.R.; Day, J.; Vörösmarty, C.; Saito, Y.; Giosan, L.; et al. Sinking deltas due to human activities. Nat. Geosci. 2009, 2, 681–686. [Google Scholar] [CrossRef]
- Bănăduc, D.; Bănăduc, A.; Lenhardt, M.; Gutti, G. “Porţile de Fier/Iron Gates” Gorges Area (Danube) Fish Fauna. Transylv. Rev. Syst. Ecol. Res. 2014, 16, 171–196. [Google Scholar] [CrossRef]
- Habersack, H. The (Dis)balance of Sediments in the Danube River Basin; ICPDR Secretariat: Vienna, Austria, 2018; Volume 3, Available online: https://www.icpdr.org/main/publications/danube-watch-3-2018-disbalance-sediments-danube-river-basin (accessed on 11 November 2022).
- Mierlă, M. Hydrological Risk Assessment for Danube Fluvial Delta. Ph.D. Thesis, Alexandru Ioan Cuza University, Iași, Romania, 2013. Available online: http://phdthesis.uaic.ro/PhDThesis/Mierl%C4%83,%20Marian,%20Hydrological%20risk%20assessment%20for%20Danube%20Fluvial%20Delta.pdf (accessed on 11 November 2022).
- Năstase, G. Vaile submarine ale Dunarii, Cogâlnicului, Nistrului şi Niprului. Bul. Soc. Geogr. 1972, 10, 1–10. [Google Scholar]
- Panin, N.; Panin, S.; Herz, N.; Noakes, J.E. Radiocarbon dating of Danube Delta deposits. Quat. Res. 1983, 19, 249–255. [Google Scholar] [CrossRef]
- Goodell, J. The Water Will Come; Little, Brown and Company: New York, NY, USA; Boston, MA, USA; London, UK, 2017; ISBN 978-316-26024-4. [Google Scholar]
- Mccall, G.; Akpan, A.; Bănăduc, D.; Figureroa, F.D.; Fontoura, N.; Hitchcock, R.K.; Kar, D. The Estuarine Ecological Knowledge Network Makes Progress: International Project Sites and Potential Ways Forward. Mar. Technol. Soc. J. 2022, 56, 116–117. [Google Scholar] [CrossRef]
- Messner, F.; Meyer, V. Flood damage, vulnerability and risk perception—Challenges for flood damage research. In Flood Risk Management: Hazards, Vulnerability and Mitigation Measures; Schanze, J., Zeman, E., Marsalek, J., Eds.; Springer: New York, NY, USA, 2006; pp. 149–168. [Google Scholar]
- Raaijmakers, R.; Krywkow, J.; van der Veen, A. Flood risk perceptions and spatial multi-criteria analysis: An exploratory research for hazard mitigation. Nat. Hazards 2008, 46, 307–322. [Google Scholar] [CrossRef]
- Ayres, P.G. Shaping Ecology: The Life of Arthur Tansley; John Wiley & Sons: Chichester, UK; Malden, MA, USA, 2012. [Google Scholar]
- Armaş, I.; Avram, E. Perception of flood risk in Danube Delta, Romania. Nat. Hazards 2009, 50, 269–287. [Google Scholar] [CrossRef]
- Holmes, R. Environmental Ethics: Duties to and Values in the Natural World; Temple University Press: Philadelphia, PA, USA, 1989. [Google Scholar]
- Nuttle, W.K.; Brinson, M.; Cajon, D.; Callaway, J.C.; Christian, R.R.; Chmura, G.L.; Conner; William, H.; Day, R.H.; Ford, M.; et al. Conserving Coastal Wetlands Despite Sea Level Rise. Environ. Sci. Fac. Staff. Publ. 1997, 38, 257–264. [Google Scholar]
- Church, J.A.; Gregory, J.M. Encyclopedia of Ocean Sciences; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Day, J.W.; Rybczyk, J.M. Human-Induced Problems (uses and abuses). In Treatise on Estuarine and Coastal Science; Academic Press: London, UK, 2011. [Google Scholar]
- Woodworth, P.L.; Menéndez, M.; Gehrels, R.W. Evidence for Century-Timescale Acceleration in Mean Sea Levels and for Recent Changes in Extreme Sea Levels. Surv. Geophys. 2011, 32, 603–618. [Google Scholar] [CrossRef]
- Rignot, E.; Box, J.E.; Burgess, E.; Hanna, E. Mass balance of the Greenland ice sheet from 1958 to 2007. Geophys. Res. Lett. 2008, 35, L20502. [Google Scholar] [CrossRef]
- Kaitlin, A.; Naughten De Rydt, J.; Rsier, S.H.R.; Jenkins, A.; Holland, P.R.; Ridley, J.K. Two-timescale response of a large Antarctic ice shelf to climate change. Nat. Commun. 2021, 12, 1991. [Google Scholar]
- Corell, R.W. Challenges of Climate Change: An Arctic Perspective. AMBIO J. Hum. Environ. 2006, 35, 148–152. [Google Scholar] [CrossRef]
- Gehrels, R. Rising Sea Levels as an indicator of Global Change. In Climate Change; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Lalljee, B.; Awnindra, K. Singh. In Biodiversity and Climate Change Adaptation in Tropical Islands; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Haeberli, W.; Whiteman, C. Snow and Ice-Related Hazards, Risks and Disasters; Elsevier: Amsterdam, The Netherlandsm, 2015. [Google Scholar]
- Nicholls, R.J. Resilience; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Primavera, J.H.; Lee, S.Y. World Seas: And Environmental Evaluation, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Miller, T. Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kirezci, E.; Young, I.R.; Ranasinghe, R.; Muis, S.; Nicholls, R.J.; Lincke, D.; Hinkel, J. Projections of global-scale extreme sea levels and resulting episodic coastal flooding over the 21st Century. Sci. Rep. 2020, 10, 11629. [Google Scholar] [CrossRef]
- Tsimplis, M.N.; Baker, T.F. Sea level drop in the Mediterranean Sea: An indicator of deep water salinity and temperature changes? Geophys. Res. Lett. 2000, 27, 1731–1734. [Google Scholar] [CrossRef]
- Bindoff, N.L.; Willebrand, J.; Artale, A.; Cazenave, J.; Gregory, S.; Gulev, K.; Hanawa, C.; Le Quéré, S.; Levitus, Y.; Nojiri, C.K.; et al. Observations: Oceanic Climate Change and Sea Level. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Timmermann, A.; McGregor, S.; Jin, F.-F. Wind effects on past and future regional sea level trends in the southern Indo-Pacidic. J. Clim. 2010, 23, 4429–4437. [Google Scholar] [CrossRef]
- Xie, S.-P.; Deser, C.G.; Vecchi, J.; Ma, H.; Teng, H.; Wittenberg, A. Global warming pattern formation: Sea surface temperature and rainfall. J. Clim. 2010, 23, 966–986. [Google Scholar] [CrossRef]
- Dronkers, J.; Gilbert, J.T.E.; Butler, L.W.; Carey, J.J.; Campbell, J.; James, E.; McKenzie, C.; Misdorp, R.; Quin, N.; Ries, K.L.; et al. Strategies for Adaptation to Sea Level Rise; Report of the IPCC Coastal Zone Management Subgroup; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 1990. [Google Scholar]
- Bijlsma, L.; Ehler, C.N.; Klein, R.J.T.; Kulshrestha, S.M.; McLean, R.F.; Mimura, N.; Nicholls, R.J.; Nurse, L.A.; Perez Nieto, H.; Stakhiv, E.Z.; et al. Coastal zones and small islands. In Impacts, Adaptations and Mitigation of Climate Change: Scientific-Technical Analyses. Contribution of Working Group II to the Second Assessment Report of the Intergovernmental Panel on Climate Change; Watson, R.T., Zinyowera, M.C., Moss, R.H., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 289–324. [Google Scholar]
- van Alphen, J.; Haasnoot, M.; Diermanse, F. Uncertain Accelerated Sea-Level Rise, Potential Consequences, and Adaptive Strategies in The Netherlands. Water 2022, 14, 1527. [Google Scholar] [CrossRef]
- Emery, K.O.; Aubrey, D.G. Sea Levels, Land Levels, and Tide Gauges; Springer: New York, NY, USA, 1991; Price DM 118.00 (hard covers); ISBN 3-540-97449-0. [Google Scholar]
- Müller, G.I. Marea Neagră prezentare generală 1-29. In Diversitatea Lumii Vii—Determinatorul Ilustrat al Flrei şi Faunei României 1-383; Godeanu, S.P., Müller, D.I., Eds.; Editura Bucura Mond: Bucureşti, Romania, 1995. [Google Scholar]
- Ujvari, I. Geografia Apelor României; Edit. Științifică: București, Romania, 1972; 591p. [Google Scholar]
- Sorokin, I. The Black Sea; The Black Sea. Ed. Nauka Acad. Sc. USSR: Moscow, Russia, 1982; 216p. [Google Scholar]
- Bondar, C. Trends in the evolution of the mean Black sea level. Meteorol. Hydrol. 1989, 19, 23–28. [Google Scholar]
- Şelariu, O. Asupra Oscilaţiilor de Nivel ale Mării Negre la Constanţa; Studii și Cercetări Geografice asupra Dobrogei, Societatea de Știinșe Geografice, Filiala: Constanţa, Romania, 1972. [Google Scholar]
- Panin, N. Impacts of expected climate changes and sea-level rise on Romanian Black Sea shore, especially on the Danube Delta area. In Implication of Climate Change in the Black Sea Region; Gerges, M., Ed.; WG. 19/contribution 8, 1-11; UNEP (Oceans and Coastal areas Programme Activity Centre): Istambul, Turkey, 1992. [Google Scholar]
- Ascherson, N. Black Sea (Vintage 1996); Bennett Books Ltd.: Los Angeles, CA, USA, 1996; ISBN 0-09-959371-8. [Google Scholar]
- King, C. The Black Sea: A History; Oxford University Press: Oxford, UK, 2004; ISBN 0-19-924161-9. [Google Scholar]
- Ghervas, S. The Black Sea in Armitage; Armitage, D., Bashford, A., Sivasundaram, S., Eds.; Oceanic Histories Cambridge; Cambridge University Press: Cambridge, UK, 2017; pp. 234–266. ISBN 978-1-1083-0071-2. [Google Scholar] [CrossRef]
- Marea Neagră în Zona Litoralului Românesc: Monografie Hidrologică; Serviciul Studii-Documentare si Publicatii Tehnice al Institutului de meteorologie si hidrologie: Bucharest, Romania, 1973; IMH 516p.
- Antipa, G. Problemele Stiintifice si Economice ale Deltei Dunarii; Analele Inst. Geol. Român: Bucharest, Romania, 1917. [Google Scholar]
- Polishchuk, V.V. Biogeographical Aspects of Studying the Reservoirs of the Danube Basin within the USSR; Polishchuk, V.V., Garasevich, I.G., Eds.; Nauk. Dumka: Bucharest, Rumania, 1986; 212p. [Google Scholar]
- Polishchuk, V.V. Biogeography of continental water bodies of Ukraine and adjacent territories. Holocene complexes. Gidrobiol. J. 1996, 32, 3–25. [Google Scholar]
- Polishchuk, V.V. On the boreal elements of the fauna of the Black Sea basin. Gidrobiol. J. 1978, 14, 17–24. [Google Scholar]
- Pons, L.J. Chapter I Morphology, climate, geology and soils. In Environmental Status Reports Vol. 4, Conservation status of the Danube Delta; International Union for Conservation of Nature: Gland, Switzerland, 1992; pp. 1–22. [Google Scholar]
- Panin, N. Global Changes, Sea Level Rising and the Danube Delta: Risks and responses. In Global Changes, Sea Level Rising and the Danube Delta, Proceedings of the International Workshop on Modern and Ancient Sedimentary Environments and Processes, Moeciu, Romania, 8–15 October 1998; Geo-Eco-Marina, 4/1999; Panin, N., Ed.; National Institute of Marine Geology and Geo-Ecology: Bucharest, Romania, 1999. [Google Scholar]
- Panin, N. Danube Delta: Geology, Sedimentology, Evolution; Français, S., Ed.; ASF: Fontaibleau, France, 1998. [Google Scholar]
- Bondar, C.; Panin, N. The Danube Delta Hydrologic DataBase and Modelling. In Final Report EC: Fluxes of Greenhouse Gases in the North-Western Region of the Black Sea Coastal Zone: Influence of the Danube River System; Geo-Eco-Marina: Bucharest, Romania, 2000; 35p. [Google Scholar]
- Hanganu, J.; Doroftei, M.; Sârbu, I.; Ştefan, N. Physical Landscape—Chapter 1. In The Bio-politics of the Danube Delta, Nature, History, Policies; Iordache, C., Ed.; Lexington Books: Blue Ridge Summit, PA, USA, 2015; pp. 1–452. [Google Scholar]
- Ciocârlan, V. Flora Deltei Dunarii; Edit. CERES: Bucharest, Romania, 1994. [Google Scholar]
- Martin-Ortega, J.; Ferrier, R.C.; Gordon, I.J.; Khan, S. Water Ecosystem Services: A Global Perspective; Cambridge University Press: Cambridge, UK, 2015; ISBN 978-1-107-10037-4. [Google Scholar]
- Tsybulskiy, A.I.; Afanasyev, S.A.; Gukov, Y.A. On the Hydrobiological Regime of the Lena River Delta. Hydrobiol. J. 2002, 38, 3–10. [Google Scholar] [CrossRef]
- Popescu-Gorj, A.; Scobiola-Palade, X. L’entomofaune de l’ile de Letea (Delta du Danube). Introduction, généralités. Trav. Muséum Natl. D’histoire Nat. Grigore Antipa 1968, 12, 107–116. [Google Scholar]
- Panin, N. Some aspects of fluvial and marine processes in the Danube Delta. An. Inst. Geol. Geophys. Géogr. Ser. Géographie 1976, 33, 25–36. [Google Scholar]
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a sustainable resource and generator of secondary resources in the 21st century: Stressors, threats, risks, management and protection strategies, and conservation approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef] [PubMed]
- Bondar, C. Secular evolution of some components of the hydrological Danube regime and of the mean level of the Black sea. Proc. World Coast Conf. 1993, 17, 891–893. [Google Scholar]
- Lepsi, I. Materiale Pentru Studiul Deltel Dundrii. I; Buletinul Muzeului Regional Bassarabia: Chișinău, Moldova, 1942; Volume 10, pp. 94–325. [Google Scholar]
- Gâştescu, P.; Oltean, M.; Nichersu, I.; Constantinescu, A. Ecosystems of the Romanian Danube Delta Biosphere Reserve, Explanation to a Map 1:175.000; RIZA Werkdocument 99.032x; Institutul de Cercetare şi Proiectare Delta Dunarii: Tulcea, Romania; Institutul de Geografie: Bucharest, Romania; Institutul de Biologie: Bucharest, Romania; Institute of Inland Water Management and Waste Water Treatment RIZA: Lelystad, The Netherlands, 1998. [Google Scholar]
- Schneider, E. Chapter 2, The impact of the hydrological regime on the diversity of natural habitats in the Danube Delta. In The Bio-politics of the Danube Delta, Nature, History, Policies; Iordache, C., Ed.; Lexington Books: Blue Ridge Summit, PA, USA, 2015. [Google Scholar]
- Hanganu, J.; Gridin, M.; Drost, H.J.; Chifu, J.; Stefan, N.; Sârbu, I. Explanation to the Vegetation Map of the Romanian Danube Delta Biosphere Reserve 1:150000; Flevobericht No. 356. Rijkswaterstaat Directorate Flevoland, Lelystad (Map sc. 1: 100.000 and Text-Book; Danube Delta Institute: Tulcea, Romania; Institute for Biology Iasi: Iași, Romania; Directorate General for Water Management, Transport and Public Works: The Hague, The Netherlands, 1994. [Google Scholar]
- Dister, E. The Function, Evaluation and Relicts of Near-Natural Flood-plains, Limnologie aktuel 2, Kinzelbach R ed Biologie der Donau; Gustav Fisher Verlag: Stuttgart, Germany; Jena, Germany; New York, NY, USA, 1994; pp. 317–329. [Google Scholar]
- Cioacă, E.; Dimache, D.; Schneider, E. Hydrological regime within the Danube Delta Biosphere Reserve-Letea forest ecosystem case study. Sci. Ann. Danub. Delta Inst. 2005, 11, 142–151. [Google Scholar]
- Marin, G.; Schneider, E. (Eds.) Ecological Restoration in the Danube Delta Biosphere Reserve/Romania, Babina and Cernovca Islands; Danube Delta National Institute and WWF: Rastatt, Germany, 1997; p. 121. [Google Scholar]
- Schneider, E.; Tudor, M. Chapter 11.2 Flora in Gastescu P and Stiuca R ed. Delta Dunarii; Editura CD Press: Bucharest, Romania, 2008; pp. 138–144. [Google Scholar]
- Bănăduc, D. Important area for fish—Natura 2000 (SCI) for Gobio albipinnatus Lukasch, 1933 species in the Danube Delta (Romania). Acta Oecologica 2007, 14, 127–135. [Google Scholar]
- Hanganu, J.; Dubyna, D.; Zhmud, E.; Grigoraş, I.; Menke, U.; Drost, H.; Stefan, N.; Sârbu, I. Vegetation of the Biosphere Reserve Danube Delta—With Transboundary Vegetation Map on a 1:150.000 Scale; RIZA Rapport 2002.049; Danube Delta National Institute: Tulcea, Romania; Kholodny MG—Institute of Botany and Danube Delta Biosphere Reserve, Ukraine and RIZA: Lelystad, The Netherlands, 2002. [Google Scholar]
- Antipa, G. Die Störe und ihre Wanderungen in den europäischen Gewässer, mit besonderer Berücksichtigung der Störe der Donau und des Schwarzen Meeres. In Bericht an den Internationalen Fischerei—Kongreß in Wien; Österreichs Fischerei: Mondsee, Austria, 1905; 22p. [Google Scholar]
- Bănărescu, P.M. Peşti rari şi cu areal restrans din fauna ţării noastre şi problema ocrotirii lor. Ocrot. Nat. 1965, 9, 5–21. [Google Scholar]
- Antipa, G. Regiunea inundabilă a Dunării; Strada Doamnei: Bucharest, Romania, 1910; 318p. [Google Scholar]
- Antipa, G. Wissenschaftliche Und Wirtschaftliche Probleme Des Donaudeltas; Göbl: Bukarest, Romania, 1915. [Google Scholar]
- Antipa, G. Dunărea şi Problemele ei Ştiinţifice, Economice şi Politice; Cartea Românească: Bucharest, Romania, 1921; 191p. [Google Scholar]
- Antipa, G. Die biologischen Grundlagen und der mechanismus der Fischproduktion in den Gewässern der unteren Donau. Bull. Sect. Sci. Acad. Roum. 1928, 11, 21–40. [Google Scholar]
- Antipa, G. Pescăriile şi Regiunea inundabilă a Dunării în Cadrul Economiei Naţionale şi Mondiale; Monitorul Oficial şi Imprimeriile Statului, Imprimeria Centrală: Bucharest, Romania, 1933. [Google Scholar]
- Antipa, G. Elemente nouă în fauna apelor dulci din România. In Grigore Antipa: Hommage À Son Oeuvre; Imprimeria Natzionalǎ: București, Romania, 1938; pp. 85–91. [Google Scholar]
- Antipa, G. Die Donau. Ihre Politische Und Wirtschaftliche Bedeutung Im Leben Des Rumänischen Vlkes; Die Dacia-bücher: Bukarest, Romania, 1941. [Google Scholar]
- Bănărescu, P.M. Cyclostomata si Chondrichthyes; Fauna, R.S.R., Ed.; Edit. Academiei: Bucharest, Romania, 1969; Volume 12. [Google Scholar]
- Năvodaru, I.; Năstase, A. What fish and how many there are in Danube delta? Sci. Ann. Danub. Delta Inst. 2011, 17, 71–82. [Google Scholar]
- Tockner, K. Freshwaters: Global Distribution, Biodiversity, Ecosystem Services, and Human Pressures. In Handbook of Water Resources Management: Discourses, Concepts and Examples; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gough, P.; Philipsen, P.; Schollema, P.; Wanningen, H. From Sea to source. In International Guidance for the Restoration of Fish Migration Highways; Regional Water Authority Hunze en Aa᾿s Pstbus 195, 9640 AD: Veendam, The Netherlands, 2012; pp. 1–300. [Google Scholar]
- Năvodaru, I.; Staraş, M.; Banks, R. Management of sturgeon stocks of the lower Danube River system. In Proceedings of the Delta’s: State-of Art Protection and Management, Conference Proceedings, Tulcea, Romania, 26–31 July 1999; pp. 229–237. [Google Scholar]
- Khelifi, N.; Boualleg, C.; Sahtout, F.; Kaouachi, N.; Mouaissia, W.; Morad Bensouillah, M. Feeding habits of Carassius carassius (Cyprinidae) in Beni Haroun Dam (north-east of Algeria). AACL Bioflux 2017, 10, 1596–1609. [Google Scholar]
- Copp, G.H.; Sayer, C.D. Demonstrating the practical impact of publications in aquatic conservation-the case of crucian carp Carassius carassius in the east of England. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1753–1757. [Google Scholar] [CrossRef]
- Lyach, R. In situ management options to improve crucian carp (Carassius carassius, L.) and brown trout (Salmo trutta, L.) populations status in Central Europe: A case study from the Czech Republic. Ecol. Evol. 2022, 12, 7. [Google Scholar] [CrossRef]
- Copp, G.H. The habitat diversity and fish reproductive function of floodplain ecosystems. Environ. Biol. Fishes 1989, 26, 1–26. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. Comprehensive analysis of >30 years of data on stream fish population trends and conservation status in Bavaria, Germany. Biol. Conserv. 2018, 226, 311–320. [Google Scholar] [CrossRef]
- Hohausová, E.; Jurajda, P. Restoration of a river backwater and its influence on fish assemblages. Czech J. Anim. Sci. 2005, 50, 473–482. [Google Scholar] [CrossRef]
- Copp, G.H.; Warrington, S.; Wesley, K.J. Management of an ornamental pond as a conservation site for a threatened native fish species, crucian carp Carassius carassius. In Pond Conservation in Europe; Springer: Dordrecht, The Netherlands, 2007; pp. 149–155. [Google Scholar]
- Wheeler, A.C. Ponds and fishes in Epping Forest. Essex Lond. Nat. 1998, 77, 107–146. [Google Scholar]
- Allardi, J.; Keith, P. Atlas Préliminaire des Poisons d’eau Douce de France; Coll. Patrimoines Naturels; Secrétariat Faune Flore, Muséum National d’Histoire Naturelle: Paris, France, 1991; 234p. [Google Scholar]
- Bănărescu, P.M. Pisces (Pești) 215–260. In Cartea Roșie a Vertebratelor din România; Botnariuc, N., Tatole, V., Eds.; Academia Româna: Bucharest, Romania, 2005; Volume 4. [Google Scholar]
- Tkachenko, V.; Andriycthenko, K.; Volkova, O.; Belyaev, V.; Kuzmenko, M.; Shirokaya, Z. Ichthyofauna state and fish productivity of lower reaches of Danube in Ukraine. Sci. Bull. Uzhhorod Univ. Ser. Biol. 2004, 15, 129–133. [Google Scholar]
- Copp, G.H.; Tarkan, A.S.; Godard, M.J.; Edmonds, N.J.; Wesley, K.J. Preliminary assessment of feral goldfish impacts on ponds, with particular reference to native crucian carp. Aquat. Invasions 2010, 5, 413–422. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, J.T.; Kuang, Y.Y.; Xu, R.; Zhao, Z.X.; Hou, G.Y.; Liang, H.W.; Sun, X.W. The transcriptiomes of the crucian carp complex (Carassius auratus) provide insights into the distinction between unisexual triploids and sexual diploids. Int. J. Mol. Sci. 2014, 15, 9386–9406. [Google Scholar] [CrossRef]
- Holopainen, I.J.; Tonn, W.M.; Paszkowski, C.A.; Pitkänen, A.K. Habitat use, diel activity, and growth of crucian carp in a manipulated pond. Int. Ver. Für Theor. Und Angew. Limnol. Verhandlungen 1988, 23, 1743–1750. [Google Scholar] [CrossRef]
- Pettersson, L.B.; Brönmark, C. Trading off safety against food: State dependent habitat choice and foraging in crucian carp. Oecologia 1993, 95, 353–357. [Google Scholar] [CrossRef]
- Sayer, C.D.; Copp, G.H.; Emson, D.; Godard, M.J.; Zieba, G.; Wesley, K.J. Towards conservation of crucian carp Carassius carassius: Understanding the extent and causes of decline within part of its native English range. J. Fish Biol. 2011, 79, 1608–1624. [Google Scholar] [CrossRef]
- Năstase, A.; Năvodaru, I. Researches of fish communities from Roşu-Puiu and Matiţa-Merhei lake-cmplexes in 2008. Sci. Ann. Danub. Delta Inst. 2010, 16, 23–32. [Google Scholar]
- Sac, G.; Özuluğ, M. Effects of environmental variables on the distribution of fish assemblages in an endorheic stream (Istambul, Turkey). Fresenius Environ. Bull. 2017, 26, 7150–7159. [Google Scholar]
- Kottelat, M. European freshwater fishes. An heuristic checklist of the freshwater fishes of Europe (exclusive former USSR), with an introduction for non-systematists and coments on nomenctature and conservation. Biologia 1997, 52 (Suppl. 5), 1–271. [Google Scholar]
- Wheeler, A. Freshwater fishes of Britain and Europe; Rainbow Books, Elsley House: London, UK, 1992; 124p. [Google Scholar]
- Leonte, V.; Ruga, C. Asupra prezenţei lui Rutilus (Pararutilus) frisii (Nordman) în fauna piscicolă a complexului Razelm. Bul. I.C.P. 1956, 15, 105–106. [Google Scholar]
- Năstase, A.; Năvodaru, I.; Cernișencu, I. The fish communities of lake-complexes from Danube Delta Biosphere Reserve (DDBR) in Spring-Summer and Autumn of 2016. Sci. Ann. Danub. Delta Inst. 2019, 24, 63–76, ISSN 1824-614X/2247-9902. [Google Scholar] [CrossRef]
- Nalbant, T. Some problems in the systematic of the genus Cobitis and its relatives (Pisces, Ostariophysi, Cobitidae). Rev. Roum. Biol. 1993, 38, 101–110. [Google Scholar]
- Riede, K. Global Register of Migratory Species—From Global to Regional Scales; Final Report of the R&D-Projekt 808 05 081; Federal Agency for Nature Conservation: Bonn, Germany, 2004; 329p.
- Nalbant, T. Studies on loaches (Pisces: Ostariophysi: Cobitidae). I, An Evaluation of the Valid Genera of Cobitinae. Trav. Du Muséum D’histoire Nat. ‘Grigore Antipa’ 1994, 34, 375–380. [Google Scholar]
- Fricke, R.; Bilecenoglu, M.; Sari, H.M. Annotated checklist of fish and lamprey species (Gnathostoma and Petromyzontomorphi) of Turkey, including a Red List of threatened and declining species. Stuttg. Beitr. Naturk. Sea A 2007, 706, 1–172. [Google Scholar]
- Miller, P.J. Gobiidae. In Checklist of the Fishes of the North-Eastern Atlantic and of the Mediterranean (CLOFNAM); Hureau, J.C., Monod, T., Eds.; UNESCO: Paris, France, 1979; Volume 1, pp. 483–515. [Google Scholar]
- Romero, P. An Etymological Dictionary of Taxonomy. Madrid, Unpublished, 2002. Available online: https://fishbase.mnhn.fr/references/fbrefsummary.php?ID=45335 (accessed on 2 February 2023).
- Baensch, H.A.; Riehl, R. Aquarien Atlas. Band 4. Mergus Verlag GmbH; Verlag für Natur-und Heimtierkunde: Melle, Germany, 1995; 864p. [Google Scholar]
- Nalbant, T.; Oţel, V. Knipwitschia Cameliae (Pisces: Gobiidae) a New Species of Dwarf Gobies from the Panizzae Group. Some Problems of Its Protection; Ocrotirea Naturii şi Mediului Inconjurător 39, 1–2; Editura Academiei: Bucharest, Romania, 1995; pp. 51–57. [Google Scholar]
- Scott, W.B.; Crossman, E.J. Freshwater fishes of Canada; Bulletin No. 184; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1973. [Google Scholar]
- Birstein, V.J. Sturgeons and paddlefishes: Threatened fishes in need of conservation. Conserv. Biol. 1993, 7, 773–787. [Google Scholar] [CrossRef]
- Năvodaru, I.; Staraş, M.; Buijse, A.D.; De Leew, J.J. Changes in fish population in Danube delta lakes: Effects of hydrology and water quality change. Review of results and potential for rehabilitation. Ecohydrol. Hydrobiol. 2005, 5, 245–256. [Google Scholar]
- Konecny, R.; Tollner, P.; Krejči, V.; Nemetz, S.; Wolf-Ott, F.; Chovanec, A.; Naderer, A.; Becker, B.; Steiner, F.; Winkler, B.; et al. Polder Soutok—Integrative flood protection and river restoration at the confluence of Morava and Dyje. In European River Restoration Conference, Vienna, Austria, 11–13 September 2013, 5th ed.; European Union: Brussels, Belgium, 2013. [Google Scholar]
- Naseka, A.M.; Freyhof, J. Romanogobio parvus, a new gudgeon from River Kuban, southern Russia (Cyprinidae, Gobioninae). Ichthyol. Explor. Freshwat. 2004, 15, 17–23. [Google Scholar]
- Wheeler, A. Key to the Fishes of Northern Europe; Frederick Warne: London, UK, 1978. [Google Scholar]
- Cocan, D.; Mireşan, V. Ihtiologie. In Sistematica şi Morfologia Peştilor; Colorama Publishing House: Cluj-Napoca, Romania, 2018; Volume 1, pp. 1–559. [Google Scholar]
- McDowall, R.M. The evolution of diadromy in fishes (revisited) and its place in phylogenetic analysis. Rev. Fish Biol. Fish. 1997, 7, 443–462. [Google Scholar] [CrossRef]
- Miller, P.J. Gobiidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1019–1085. [Google Scholar]
- Chao, L.N.; Trewavas, E. Sciaenidae. In Check-List of the Fishes the Eastern Tropical Atlantic (CLOFETA); Quero, J.C., Hureau, J.C., Karrer, C., Post, A., Saldanha, L., Eds.; JNICT: Lisbon, Portugal; SEI: Paris, France; UNESCO: Paris, France, 1990; Volume 2, pp. 813–826. [Google Scholar]
- Maugé, L.A. Atherinidae. In Check-List of the Fishes of the Eastern Tropical Atlantic (CLOFETA); Quero, J.C., Hureau, J.C., Karrer, C., Post, A., Saldanha, L., Eds.; JNICT: Lisbon, Portugal; SEI: Paris, France; UNESCO: Paris, France, 1990; Volume 2, pp. 604–605. [Google Scholar]
- Zander, C.D. Blenniidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1096–1112. [Google Scholar]
- Thomson, J.M. Mugilidae. In Check-List of the Freshwater Fishes of Africa (CLOFFA); Daget, J., Gosse, J.-P., Thys van den Audenaerde, D.F.E., Eds.; ISNB: Brussels, Belgium ; MRAC: Tervuren, Belgium ; ORSTOM: Paris, France, 1986; Volume 2, pp. 344–349. [Google Scholar]
- Eschmeyer, W.N.; Herald, E.S.; Hammann, H. A Field Guide to Pacific Coast Fishes of North America; Houghton Mifflin Company: Boston, MA, USA, 1983; 336p. [Google Scholar]
- Harrison, I.J. Mugilidae. Lisas. In Guia FAO para Identification de Especies para lo Fines de la Pesca, Pacifico Centro-Oriental; Fischer, W., Krupp, F., Schneider, W., Sommer, C., Carpenter, K.E., Niem, V., Eds.; FAO: Rome, Italy, 1995; Volume 3, pp. 1293–1298. [Google Scholar]
- Dooley, J.K. Pomatomidae. In Check-List of the Fishes of the Eastern Tropical Atlantic (CLOFETA); Quero, J.C., Hureau, J.C., Karrer, C., Post, A., Saldanha, L., Eds.; JNICT: Lisbon, Portugal; SEI: Paris, France; UNESCO: Paris, France, 1990; Volume 2, pp. 721–722. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bănăduc, D.; Afanasyev, S.; Akeroyd, J.R.; Năstase, A.; Năvodaru, I.; Tofan, L.; Curtean-Bănăduc, A. The Danube Delta: The Achilles Heel of Danube River–Danube Delta–Black Sea Region Fish Diversity under a Black Sea Impact Scenario Due to Sea Level Rise—A Prospective Review. Fishes 2023, 8, 355. https://doi.org/10.3390/fishes8070355
Bănăduc D, Afanasyev S, Akeroyd JR, Năstase A, Năvodaru I, Tofan L, Curtean-Bănăduc A. The Danube Delta: The Achilles Heel of Danube River–Danube Delta–Black Sea Region Fish Diversity under a Black Sea Impact Scenario Due to Sea Level Rise—A Prospective Review. Fishes. 2023; 8(7):355. https://doi.org/10.3390/fishes8070355
Chicago/Turabian StyleBănăduc, Doru, Sergey Afanasyev, John Robert Akeroyd, Aurel Năstase, Ion Năvodaru, Lucica Tofan, and Angela Curtean-Bănăduc. 2023. "The Danube Delta: The Achilles Heel of Danube River–Danube Delta–Black Sea Region Fish Diversity under a Black Sea Impact Scenario Due to Sea Level Rise—A Prospective Review" Fishes 8, no. 7: 355. https://doi.org/10.3390/fishes8070355
APA StyleBănăduc, D., Afanasyev, S., Akeroyd, J. R., Năstase, A., Năvodaru, I., Tofan, L., & Curtean-Bănăduc, A. (2023). The Danube Delta: The Achilles Heel of Danube River–Danube Delta–Black Sea Region Fish Diversity under a Black Sea Impact Scenario Due to Sea Level Rise—A Prospective Review. Fishes, 8(7), 355. https://doi.org/10.3390/fishes8070355








