Abstract
Marine resources exploitation through bottom trawling affects marine ecosystems; thus, management should consider the presence of sensitive species as ecosystem health indicators. Epibenthic organisms such as sea pens are widely used to assess benthic conditions, as their populations are declining where trawling is intense. The Pomo/Jabuka Pits fishing ground in the Adriatic Sea, subject to various management measures over the years, is a nursery for European hake and hosts a small, but dense, population of Norway lobster and a remarkable abundance of pink shrimp. The sea pen Funiculina quadrangularis shares its habitat (sandy-muddy bottoms) with these crustaceans. Through UnderWater TeleVision surveys conducted from 2012 to 2019, F. quadrangularis abundance and distribution were quantified in relation to changes in the spatiotemporal distribution of fishing efforts. The average density (n/m2) of colonies was calculated for three periods: BEFORE implementation of measures (before 1 July 2015), during an INTERMEDIATE period in which limitations changed (2 July 2015 to 31 August 2017), and AFTER the implementation of a Fishery Restricted Area (from 1 September 2017). F. quadrangularis revealed an increase in density where fisheries were closed, even after a short period. This showed how management measures can positively influence epibenthic communities and that sea pens can be indicators of impact and/or recovery of habitats.
Keywords:
Funiculina quadrangularis; vulnerable marine ecosystem (VME) indicators; Jabuka/Pomo Pit; Fishery Restricted Area (FRA) Key Contribution:
Bottom trawling can impact marine ecosystems; benthic conditions can be evaluated by means of sensitive organisms such as sea pens. Through UnderWater TeleVision surveys conducted from 2012 to 2019, F. quadrangularis’ abundance and distribution were quantified in relation to changes in the spatiotemporal distribution of fishing efforts in the Pomo/Jabuka Pits area (Adriatic Sea). This showed how management measures can positively influence epibenthic communities.
1. Introduction
Overexploitation of marine resources, especially by means of bottom trawling, can change or degrade habitats and have negative impacts on marine ecosystems [1,2,3]. Bottom trawling, in fact, may lead to long-term changes in benthic communities and affect the trophic condition of benthic ecosystems [4,5,6,7]. In addition to the effects directly linked to the removal of organisms, a secondary cause can be, for example, the resuspension of sediment, which can have a suffocating effect on non-target benthic fauna and flora [8,9,10].
In order to maintain the sustainability of fish resources over time and the conservation of ecosystems, careful management should be based on a solid understanding of the structure and functioning of its components, including the effects of human and natural disturbances [11,12]. A variety of management techniques can be adopted that also take into account the presence of sensitive species as ecosystem health indicators useful to evaluate the effectiveness of measures implemented [12,13]. Depending on the biological traits of some species, the anthropogenic impact might result in fluctuations in abundance, which might be reflected also in changes in the ecosystem components and biodiversity [14,15]. The selection of an organism as an indicator species is crucial, as this should represent a link between the objectives of management measures and the actions [16].
Physical disturbance to the seabed as a result of fishing activities, including effects on benthic communities, is addressed by the criteria under Descriptor 6 (Sea-floor integrity) of the Marine Strategy Framework Directive (MSFD) of the European Union [17]. In particular, criterion D6C3 requires Member States to investigate the adverse effects of physical disturbance on each habitat type and derived changes in its biotic and abiotic structure and functions, for example, through the analysis of changes in species composition and their relative abundance, absence of particularly sensitive or fragile species or species providing a key function, and the size structure of species. Epibenthic organisms, such as sponges and sea pens, are among the most used species to assess benthic conditions [13,18]. Sea pens are colonial cnidarians belonging to the subclass Octocorallia, order Pennatulacea [19]. Sea pen forests may provide important three-dimensional habitats for fish and invertebrate species, thus contributing to the preservation of ecosystem functions in marine benthic ecosystems [20,21,22,23]. They can host eggs and larvae, and serve as safe habitat for young fish [24].
The European Commission has classified sea pen forests as vulnerable marine ecosystems (VMEs) and essential fish habitats (EFHs) because of their significant ecological roles and the sensitivity of pennatulaceans to human activities [25,26,27]. The presence of sea pens and possible changes in their distribution provide information in support of the evaluation of management strategies aimed at safeguarding vulnerable species sensitive to human impacts [20]. The General Assembly of the United Nations adopted resolution 61/105 to safeguard VMEs in consideration of the significance of their marine ecological services [27]. To successfully ensure the long-term conservation and sustainable use of marine resources, Resolution 70/75 emphasized the urgent need to also safeguard VMEs and mitigate the effects of bottom trawling on them [21].
Funiculina quadrangularis (Pallas, 1766) is a tall sea pen having polyps that develop from a square-sectioned calcareous axial rod with a peduncle at the base [26]. Individuals can reach 200 cm in length and have an axis that is up to 25% immersed in the sediment [26]. They are frequently characterized by a feather-like appearance [22,26]. Between 20 and 2000 m of depth, F. quadrangularis is adapted to muddy environments and frequently develops dense meadows [22]. F. quadrangularis was found in marine lakes and open waters and is globally distributed (in the Atlantic Ocean, especially in the North Sea, Mediterranean Sea and throughout the Pacific Ocean [23,24]). F. quadrangularis is not a target for fishing activities but may be a by-catch due to the fact that it shares the same habitat (on sandy-muddy sediments) as Nephrops norvegicus (Linnaeus, 1758) and Parapenaeus longirostris (Lucas, 1846) [26], two of the most important commercial crustaceans, especially in the Mediterranean Sea [28]. F. quadrangularis is considered a critically endangered species [29]; in fact, its populations are declining in areas where trawling activities targeting the aforementioned species are intense [25].
Mapping F. quadrangularis density over time and space might, thus, be useful not only to determine their distribution in the Mediterranean, but also to assess the conditions of the VMEs to which they belong [30,31]. Sea pens are commonly sampled by using trawl nets, grab samplers, or scuba divers directly cutting/removing the organism [24,32,33]. Less invasive methodologies based on the analysis of photo/video recorded through Remotely Operated Vehicles (ROVs) or towed camera systems, such as the UnderWater TeleVision (UWTV), are also used for the evaluation of the distribution of sea pens on the seabed [31,34,35,36]. In the Adriatic Sea, the UWTV methodology is used to derive N. norvegicus burrow densities in the Pomo/Jabuka Pits area (important for commercial fishing and subjected to various management strategies [37]) and was also trialled to assess the distribution in the area of other species of ecological interest, such as F. quadrangularis, and trawl marks [34]. The collected footage might indeed be helpful to conduct quantitative or semi-quantitative evaluation of species coexisting with N. norvegicus in its environment; OSPAR (the Oslo and Paris Convention for the Protection of the Marine Environment of the North-East Atlantic) underlined the possibility of using UWTV for the evaluation of sea pens [38].
The aim of this study is to assess changes over time in the abundance and distribution of F. quadrangularis in a particular region in which fishery restrictions changed in space and time, and therefore verify the actual potential of using this species as an indicator of human impact and/or the recovery of habitats after the implementation of management strategies.
2. Materials and Methods
2.1. Study Area
The Pomo/Jabuka Pits are three depressions delimited by the 200 m bathymetry located in the central Adriatic Sea ([39], Figure 1); this area is one of the main fishing grounds historically shared by the Italian and Croatian fleets [34,40]. The complex topography of the area and the Adriatic Sea oceanographic regimes make it a very particular environment in which the water exchange does not happen annually [41]. These conditions influence the nutrient cycle, with consequences on local biodiversity and on the trophic status of benthic communities [42]. Furthermore, alterations in species assemblages and possible consequences on trophic and ecosystem balances could probably be due to the synergistic action of fishing pressure and climate change [7,43,44,45,46,47].
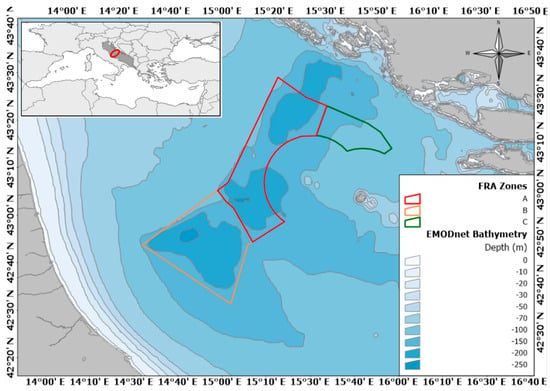
Figure 1.
The location of the study area within the Mediterranean basin is indicated by a red circle in the top-left rectangle; the main map shows the bathymetry of the Central Adriatic Sea (source: [39]) and the Jabuka/Pomo Pits Fishery Restricted Area zones “A”, “B”, and “C”, according to Recommendation GFCM/41/2017/3 [48].
This area is the main nursery for the European hake, Merluccius merluccius (Linnaeus, 1758), in the Adriatic, and the presence of muddy bottoms and other exogenous factors make it the habitat of a population of small-but-dense Norway lobster, N. norvegicus, individuals [49,50,51]. Among the other crustacean species occurring in the area, a commercial and ecological relevance is attributable to the pink shrimp, P. longirostris, which showed periodic fluctuations in the area, probably linked to environmental parameter changes, and an abundance peak in 2017 [52,53]. Also possibly linked to climate change, a crustacean species shift also occurred in the area: the squat lobster Munida intermedia (Milne-Edwards and Bouvier, 1899), was replaced by Iridonida speciosa (von Martens, 1878) (previously known as Munida speciosa), first observed in 2003 [54]. Another gadoid species dwelling in the area, the blue whiting Micromesistius poutassou (Risso, 1827), was reported to experience fluctuations in abundance over time, probably as a result of environmental variations and fishing exploitation [55]. Therefore, the Pomo/Jabuka Pits represent a VME and an EFH worthy of implementation of appropriate management measures aimed at protecting the demersal fish stocks, enhancing the densities of organisms in terms of biomass and abundance, and protecting possible VMEs while ensuring the sustainable exploitation of the main resources [37,56]. For this reason, a series of management measures were implemented in the area over time [55,57], which led to the establishment, in 2017, of a Fishery Restricted Area (FRA) by the General Fisheries Commission for the Mediterranean (GFCM, [48]). The FRA is divided into three zones: “A”, which is closed to all fishing activity, “B,” where bottom trawling is regulated with licenses and a number of fishing days (two days allowed per licensed vessel per week, fishing vessels using twin bottom otter trawls can fish one day per week), and fishery is banned from 1 September to 31 October, and zone “C,” where trawling is permitted through licenses on Saturdays and Sundays (from 5.00 am to 10.00 pm), whereas vessels fishing with bottom-set nets, set longlines and traps can fish from Monday 05.00 am till Thursday 22.00 pm, and fishing is also banned from 1 September to 31 October (fishing activity with purse seiners and pelagic trawlers targeting anchovy or sardine is prohibited in the FRA; Figure 1). The European Parliament and Council ratified the FRA with Regulation 2019/982 [58]. The Pomo/Jabuka Pits FRA became permanent with GFCM Recommendation 44/2021 [59]. Therefore, the intensity and distribution of fishing activity in the region changed over time as a consequence of the aforementioned changes in management measures. In fact, before the implementation of the management measures, the fishing pressure in the area was quite high and was exerted by a significant number of vessels [12,37]; since the FRA was established, in addition to the spatial and temporal limitations, the number of authorized vessels is regulated by the competent authorities and the annual list of authorized vessels is reported in the GFCM portal (https://www.fao.org/gfcm/data/fleet/fras/en/, accessed on 16 June 2023).
2.2. Sample Collection and Video Analysis
From 2009 to 2019 (except for 2011 and 2018), the Ancona section of the Institute of Marine Biological Resources and Biotechnologies of the Italian National Research Council (CNR-IRBIM) and the Institute of Oceanography and Fisheries (IOF) of Split (Croatia) partnered on an annual UWTV survey covering the entire area of the three meso-Adriatic depressions, conducted on board the research vessel Dallaporta (LOA 35.30 m, 258 GT, 1100 HP) [34,37,57,60]. The surveys were carried out under the auspices of the FAO–ADRIAMED project [60]; in 2013, thanks to the Italian National Flagship Program RITMARE, the UWTV equipment owned by CNR was completely renewed [61], and from 2015 to 2019 it was also supported (for the complementary experimental trawl fishery component) by the Italian Ministry of Agricultural, Food, and Forestry Policies (MIPAAF; [62]).
The 60 fixed stations firstly defined in the area according to a random stratified sampling design [34] were maintained over time and are still consistent with the stratification currently in use based on the FRA zones, as they mainly overlap the original strata [37]; not all stations were studied in all the surveys due to ship time and weather condition limitations [60]. During each survey, a Kongsberg Simrad colour camera mounted on a sledge was towed on the sea floor at about a 1-knot speed. The camera’s field of view was set at a constant width of 80 cm. A custom data logger, synchronized with the video deck unit and with a CTD (Conductivity, Temperature, Depth) probe recording environmental parameters [63], acquired the vessel GPS (Global Positioning System) position (as a proxy of the sledge position) once per minute to allow the quantification of the surface viewed. After each survey, trained readers reviewed the collected footage following a specific protocol to derive estimates of the target features (e.g., burrows) and/or organisms [64,65]. Each station considered valid for N. norvegicus assessment purposes consisted of a minimum of 7 good minutes (normally out of about 10–20 min recorded per station) complying with the speed and visibility criteria set for the Adriatic in [60]. Recently, the surface viewed in all stations of the time series was recalculated to take into account the difference in the bottom surface covered by the sledge and the vessel route [37].
UWTV is used to evaluate N. norvegicus following specific standards in several European countries [66]. However, the fact that it uses a fixed field of view makes it suitable for collecting other ecological data potentially useful in the context of an ecosystem approach to fisheries management [34]. Figure 2 is composed of frames extrapolated from UWTV footage collected in the Adriatic Sea showing the environment and some recorded organisms. Recently, the UWTV footage collected in the Pomo Pits area was in fact further analysed with the aim to derive information on the epibenthic communities subjected to physical perturbations and the objects of interest in the context of Descriptor 6 of the MSFD, among which in particular is F. quadrangularis [67].
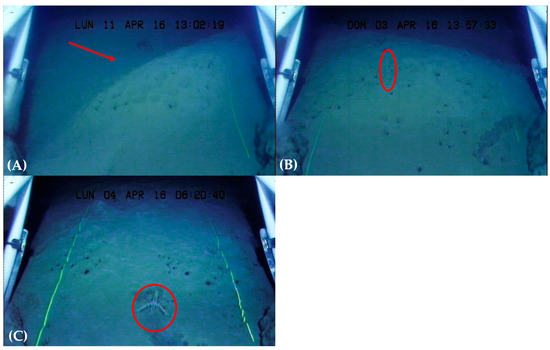
Figure 2.
Images obtained by means of UWTV: (A) trawl track created by the passage of the otter door of a bottom trawler; (B) inside the red circle is a specimen of F. quadrangularis; (C) the red circle shows a specimen of N. norvegicus, one of the species with whom sea pens share the habitat; in all frames are visible burrows made by various organisms.
2.3. Data Analysis
All data acquired during the UWTV surveys are stored in a database created using the Geographic Information System (GIS) Manifold® System Release 8 (http://www.georeference.org/doc/manifold.htm, accessed on 16 April 2023), which allows for the validation and processing of GPS data to determine the swept area for each minute, mapping, and various types of analyses [37,60,62]. The analyses carried out within this study took into account the densities of F. quadrangularis colonies (n/m2) calculated for each of the recorded minutes considered valid during the footage readings carried out to determine the densities of N. norvegicus burrows. Because F. quadrangularis is distributed in patches and the density values per station obtained could be very low, in order to obtain a finer spatial distribution, in this work, estimates were calculated at the level of one minute. The density (n/m2) of colonies per minute was calculated using the number of individuals of F. quadrangularis counted divided by the swept area. In order to assess the F. quadrangularis density variation as a potential effect of the management measures implemented over time and space, an approach was adopted that is very similar to that used in Chiarini et al. [55]; in the latter study, in fact, a short-term evaluation of changes in fisheries management measures occurring in the Pomo/Jabuka Pits Area was carried out through a Before–Intermediate–After Multiple Sites (BIAMS) approach (a variant of the classic “before-after-control-impact” model design also adopted in an attempt to compare contiguous strata with different characteristics overcoming the unavailability of an adequate independent control site). To facilitate a possible comparison with the results obtained in the area for other species, within this study, only UWTV data collected from 2012 onward were considered; therefore, 8 UWTV surveys conducted in the period 2012–2019 (except for 2018) were taken into account. Overall, 3244 min of video were analysed, for a total of about 85,541 m2 of the seabed. The same three time steps defined in Chiarini et al. [55] were also adopted to allow possible comparisons of the species response: a period “BEFORE” the implementation of the first management measures (from 1 January 2012 to 1 July 2015), the “INTERMEDIATE” stage (from 2 July 2015 to 31 August 2017), in which management measures have changed over time following national regulations [68,69], and “AFTER” the adoption of Italian and Croatian regulations analogous to the subsequent FRA application regulation [70] (from 1 September 2017 to 1 January 2020). The average density of colonies over the considered periods (i.e., “BEFORE”, “INTERMEDIATE”, and “AFTER”) in each of the three zones (“A”, “B”, and “C”) was analysed to assess any variations in the F. quadrangularis population. Average densities are not theoretically influenced by differences in surface; furthermore, by taking into consideration periods rather than single survey years, the limitation caused by the difference in the number (and location) of minutes recorded in each zone every year should be reduced. The standard deviation was also calculated to account for variability. In addition, the homogeneity of variance was assessed by a Levene test and, to address homoscedasticity, a one-way ANOVA (type II) was performed on the dataset and the Tukey test was used as a post hoc analysis. Regarding heteroscedasticity, a non-parametric ANOVA (Kruskal–Wallis test) and Games–Howell post hoc test were adopted. Each of the aforementioned periods were considered as the level of a temporal factor accounting for the fisheries management measures adopted in the study area from 2012 to 2019. To determine significance, the reference p-value was set at 0.05. These statistical analyses, and the production of related graphics, were performed using R software and the rstatix, car, dplyr and ggplot2 packages [71,72,73,74].
Furthermore, a spatial grid of 2 × 2 nautical mile cells (surface corresponding to 13.72 km2 each) was built for the Pomo/Jabuka Pits area by means of the GIS. Figure 3 highlights the cells in the entire study area which were effectively monitored throughout the time frame under examination. Mean density (n/m2) of colonies per cell was calculated using all data from 2012 to 2019 and also for the three considered periods (i.e., “BEFORE”, “INTERMDIATE”, and ”AFTER”) in order to obtain density maps.
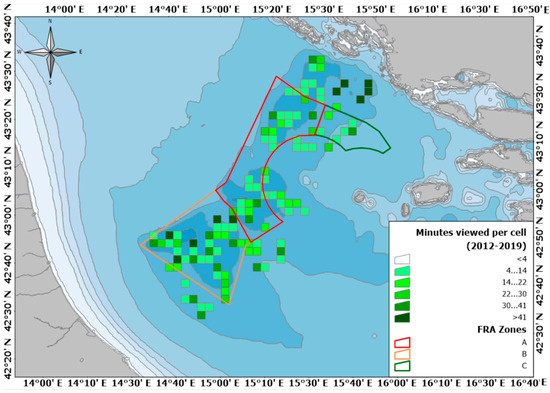
Figure 3.
Map of the study area: the polygons indicate the three zones of the Jabuka/Pomo Pits FRA, cells of 2 × 2 nautical miles show the area covered by the UWTV surveys, while their green palette indicates the number of minutes recorded within each.
Persistence per cell from 2012 to 2019 was also calculated using an adaptation of the Getis G statistic adopted to identify spatial hotspots [75,76]. The persistence estimates were obtained by dividing the number of surveys in which the species was found in a particular cell by the total number of surveys in which that cell was visited. Only cells visited more than 1 time (i.e., in at least 2 surveys) and in which F. quadrangularis was recorded at least once were considered to calculate this index. Persistence was also calculated over the single period “BEFORE” in order to create a baseline showing the initial situation before the implementation of the management measures, to be used for comparison in future analyses of medium- to long-term effects.
3. Results
3.1. Spatial Distribution of Colonies and Density by Period for Each Zone (“A”,“B”, and “C”)
In the entire study area, in total, 138 colonies of F. quadrangularis were found, and the resulting average density per minute was 0.004 n/m2 (±0.002). The spatial distribution of the average density of colonies per cell over the three considered periods and over the entire time frame of this study is shown in Figure 4. Figure 5, instead, shows the average densities of F. quadrangularis colonies calculated for each considered zone and period.
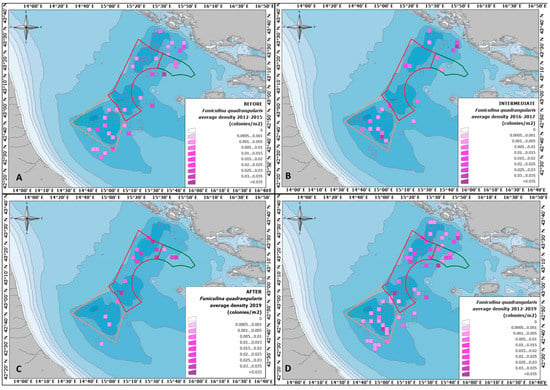
Figure 4.
Map of the study area: the polygons indicate the three zones of the FRA, the 2 × 2 nautical mile cells coloured with a purple palette indicate the average density of F. quadrangularis colonies (no colonies were ever recorded in the empty cells); panels (A–C) refer respectively to periods “BEFORE”, ”INTERMEDIATE”, and ”AFTER”, while panel (D) shows values calculated per each cell over the entire time frame of the study (2012–2019).
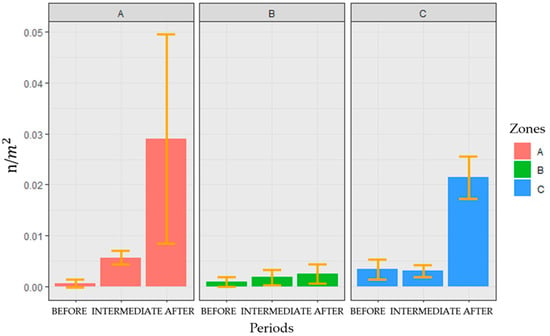
Figure 5.
Average densities (n/m2) of F. quadrangularis colonies recorded in the three FRA zones (“A”, “B”, “C”) for the three considered periods (“BEFORE”, “INTERMEDIATE”, “AFTER”); yellow bars indicate the standard deviation.
3.1.1. Area “A” across the Periods
In total, 75 colonies of F. quadrangularis were counted in zone ”A”, and the average density value calculated over the entire time frame of the study was 0.004 n/m2 (±0.01). The highest value of density was found in the period “AFTER” (maximum density = 0.176 n/m2), whereas the lowest was in the period “BEFORE” (minimum density other than 0 values = 0.031 n/m2). Given the heterogeneity of the variance, the Kruskal–Wallis test was performed (p-value < 0.001). The Games–Howell post hoc test proved that the period “AFTER” is significantly different from the other periods, showing an increase in density over time (p-value = 0.006 obtained for the comparison of “BEFORE” and ”AFTER” and p-value = 0.014 for that between “INTERMEDIATE” and ”AFTER”; Figure 5).
3.1.2. Area “B” across the Periods
In zone “B”, 37 colonies were found, and the average density value calculated over the entire time frame of the study was 0.003 n/m2 (±0.003). Both the highest and the lowest density values were found in the period “BEFORE” (maximum density = 0.095 n/m2, minimum density other than 0 values = 0.032 n/m2). Given the homogeneity of the variance, a one-way ANOVA was performed, but no significant differences among the periods were found, even if Figure 5 shows a slight increase in density over time.
3.1.3. Area “C” across the Periods
A total of 26 colonies were found in zone “C”, and the average density value calculated over the entire time frame of the study was 0.003 n/m2 (±0.001). The highest density was found in the period “AFTER” (maximum density = 0.091 n/m2), whereas the lowest was found in the period “BEFORE” (minimum density other than 0 values = 0.029 n/m2). Given the heterogeneity of the variance, the Kruskal–Wallis test was performed (p-value = 0.002). The Games–Howell post hoc test proved that the period “AFTER” is significantly different from the others, showing an increase in density over time (p-value = 0.004 obtained for the comparison of “BEFORE” and ”AFTER” and p-value = 0.005 for that between “INTERMEDIATE” and ”AFTER”; Figure 5).
3.2. Persistence per Cell
The persistence index, calculated for each cell within the entire study area across the entire time frame of the study (Figure 6A), showed the highest values (>0.9) in zones “A” and “C”. The minimum value of persistence was recorded in Croatian territorial waters (=0.166), whereas the maximum value was found in zone “A” (=1). The persistence index calculated in each cell for the “BEFORE” period is shown in Figure 6B. The lowest recorded value (=0.333) in this case was in zone “B”; the highest was in Croatian territorial waters (=1).
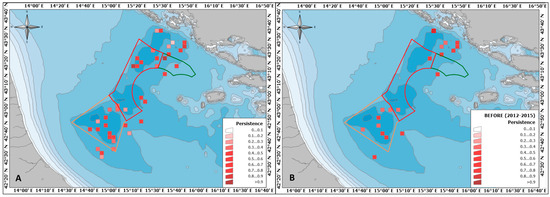
Figure 6.
Map of F. quadrangularis colonies’ persistence in the Pomo/Jabuka Pits area calculated across the whole considered UWTV time series (2012–2019; panel (A)) and only for the “BEFORE” period (panel (B)); the 2 × 2 nautical mile cells indicate portions of seabed visually inspected at least one time and in which colonies were seen at least once; the red palette indicates values calculated for each cell (from 0 to 1); the polygons indicate the three zones of the FRA.
4. Discussion
This study advances the understanding of F. quadrangularis’ geographic range in the Adriatic Sea. Twelve species of sea pens are actually present in the Mediterranean Sea, of which six are distributed in the Adriatic Sea, with a southward decline in abundance [30]. The presence of F. quadrangularis in the Off Ancona area, in the Pomo/Jabuka Pits, and off the Montenegrin coast is worthy of note [30,31]. Sessile organisms are damaged by severe bottom trawling, endangering the benthic environment and its related fish resources [26,77]. The abundance and location of sea pens in the Adriatic basin may have been influenced by the high level of fishing activity [30,78]. Historical information on the abundance and distribution of Pennatulacea in the Adriatic Sea is lacking, and it is challenging to identify an original, undisturbed state. The quantity and geographic distribution of sea pens may have been reduced in the Mediterranean basin, especially in the Adriatic Sea, due to intense trawling targeting commercial species [30]. The use of Sentinels of Seabed (SoS) indicators could help to map VMEs in the Mediterranean and evaluate anthropogenic impacts, as described by Serrano et al. [13]. The historical analysis of the distribution of F. quadrangularis in the Pomo/Jabuka Pits area is therefore noteworthy; being nowadays a FRA, it can be used to test the recovery times of species of high ecological importance.
4.1. Effects of Different Protection Levels on Funiculina quadrangularis
The development of F. quadrangularis distribution maps could provide indications on the state of the benthic component impacted by bottom trawling [13,23,31,34]. The slow growth rate, late sexual maturity and lack of withdrawl capacity make this species sensitive to trawl impact [24,26,79]. In fact, Downie et al. [33] reported F. quadrangularis to be less resilient to trawling in the Greater North Sea and Celtic Seas than other sea pens such as Pennatula phosphorea (Linnaeus, 1758) and Virgularia mirabilis (Müller, 1776). However, according to Pierdomenico et al. [80], the relationship between trawling intensity and the abundance of this species is not straightforward in the southern Tyrrhenian Sea (i.e., the lowest abundances were recorded in areas with strong fishing pressure, but relatively high values were observed in areas subject to intermediate effort), as it is for another VME indicator such as the bamboo coral Isidella elongata (Esper, 1788). While performing ROV video survey transects in a no-take reserve and some control areas in the northwestern Mediterranean Sea, Vigo et al. [36] found a higher abundance of Norway lobster and other fish species within the no-take reserve than in the control area, but only a slightly higher, but not significant, abundance of F. quadrangularis in the no-take area after 2.5 years since the implementation. The study area considered in the present work was instead subjected to various management measures, and consequent levels of fishing pressure, which varied over time and space. This condition allowed to evaluate the effects of different levels of trawl impact on sea pen colonies. The average density of F. quadrangularis colonies showed variations over the entire study period (2012–2019), particularly in zones “A” and “C”, which appeared to be positively influenced by the management measures. In fact, the average F. quadrangularis density in these two zones consistently increased, especially in the “AFTER” period following the establishment of the FRA. Although some environmental differences between the two zones, such as the difference in bathymetry and circulation of the Adriatic Sea, do not allow to directly compare them, it is evident that despite a theoretically similar level of protection in the latter period, zones “B” and “C” showed different results. The modest increase in zone “B” is likely the result of a fishing pressure still strong enough to condition the colonies’ presence and growth compared to the other zones. Therefore, the results of this study suggest that under effective trawling management and, likely, favourable environmental conditions, F. quadrangularis can increase in density within a few years. These findings are quite in agreement with those resulting for other benthic species (e.g., N. norvegicus) in Chiarini et al. [55,57].
4.2. Ecological Aspects and Colonies’ Persistence
Although it turns out to be a species sensitive to trawling, the unbranched form and elasticity of F. quadrangularis allow the persistence of this species even in heavily trawled regions, but at a lower density than in well-preserved populations [80,81]. In this study, an index was tested to determine which regions within the Pomo/Jabuka Pits had more persistent F. quadrangularis patches. A possible limitation of this index is that it does not account for the time sequence of the records within each cell over the different years, so it probably should not be applied in its current form to long time series when two records could paradoxically be at the beginning and end. However, in shorter (and more homogeneous) time series, as in the case of the “BEFORE” period, it can provide useful information to compare with subsequent time steps. In fact, when considering the entire time frame of the study, zones “A” and “C” recorded the highest persistence values. This might be correlated with management measures, but a further comparison with persistence calculated for the “AFTER” period, considering more than one survey/year, would be interesting to confirm this.
It would be interesting to also include in future medium- to long-term evaluations the distribution and displacement of fishing efforts directly estimated by means of Vessel Monitoring Systems (VMSs) and/or Automatic Identification Systems (AISs) [82,83,84,85]. To allow a better understanding of the response of the abundance and distribution of sea pen colonies to fishing pressure, more complex analyses could also be performed on the acquired datasets, including the main physical characteristics of the study area (e.g., bathymetry) and possible local variations over time of environmental parameters influencing benthic communities (e.g., temperature, salinity, oxygen, etc. [57,63]).
F. quadrangularis is a significant habitat-forming species that helps create three-dimensional bathyal environments, improves ecological functionality, and provides essential habitat for fish and invertebrates [30,77,86,87]. Therefore, the assessment of its persistence can offer important information for the correct management of the study area; in fact, the possibility of geographically locating the most persistent patches, associated with the measurement of density, can indicate which are the zones most in need of protection.
The use of non-invasive methodologies such as ROVs or UWTV for the collection of information on benthic indicator species is well established and, for the evaluation of the long-term effects of the management measures in place in the study area, it would be desirable to maintain the UWTV time series in the future, or at least repeat video surveys from time to time [26,36,56].
5. Conclusions
The Adriatic Sea geographic range of F. quadrangularis has been improved by this work, which also supports the mapping of VMEs and EFHs. This study demonstrates that effective management measures can have a positive influence on epibenthic communities and highlights the potential of sea pens as indicators of impact on and/or recovery of exploited habitats. These findings might be used in the planning and monitoring of sensitive marine areas. Furthermore, these results could also be used in the context of the Blue Economy, allowing for the exploitation of the benefits offered to commercial fishing due to the maintenance of intact benthic habitats. However, more studies, using video surveys, are required to assess the long-term effects of the management strategies applied in the Pomo/Jabuka Pits area on the sea pen community. It would be relevant to include, in future, analyses of the abundance and distribution of commercially and ecologically important species, and more variables, such as environmental parameters or VMS and AIS data for authorized fleets operating in zones “B”, “C”, and surrounding areas, which are also useful to monitor the displacement of fishing efforts.
Author Contributions
Conceptualization, M.M.; Data curation, M.M. and L.Z.; Formal analysis, M.M. and L.Z.; Funding acquisition, M.M.; Investigation, F.D., A.B., L.Z., P.S., P.P., D.M., I.I. and M.M.; Methodology, M.M. and L.Z.; Project administration, M.M. and N.V.; Supervision, M.M., I.I. and N.V.; Writing—original draft, M.M. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided for the collection and processing of the data used in this study by the FAO AdriaMed regional project, the RITMARE Flagship Project of the Italian Ministry of University and Research, the Direzione Generale della Pesca Marittima e dell’Acquacoltura of the Italian Ministry of Agricultural, Food, and Forestry Policies (through a series of agreements with CNR-IRBIM; CUP J52I15003990001, J53C17000540001), and by the Italian Institute for Environmental Protection and Research (through an agreement with CNR-IRBIM for the study of epimegabenthic communities subjected to physical perturbations carried out in the context of Descriptor 6 of the European Marine Strategy Framework Directive).
Institutional Review Board Statement
This study did not involve the removal of animals from the environment; the institutional review board statement is not applicable.
Data Availability Statement
All data are stored at CNR IRBIM Ancona and can be shared upon the reasonable request of qualified researchers. A portion of the dataset was also collected with permits of the Croatian Institutions. The authors received formal authorization to use the data for scientific purposes by the competent authorities, but no special access privileges were granted.
Acknowledgments
The authors want to thank for their precious contributions Giordano Giuliani, Giovanni Canduci, Camilla Croci, and the crew of RV Dallaporta during the scientific surveys.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript, or in the decision to publish the results.
References
- Clark, M.R.; Althaus, F.; Schlacher, T.A.; Williams, A.; Bowden, D.A.; Rowden, A.A. The Impacts of Deep-Sea Fisheries on Benthic Communities: A Review. ICES J. Mar. Sci. 2016, 73, i51–i69. [Google Scholar] [CrossRef]
- Farella, G.; Tassetti, A.N.; Menegon, S.; Bocci, M.; Ferrà, C.; Grati, F.; Fadini, A.; Giovanardi, O.; Fabi, G.; Raicevich, S.; et al. Ecosystem-Based MSP for Enhanced Fisheries Sustainability: An Example from the Northern Adriatic (Chioggia-Venice and Rovigo, Italy). Sustainability 2021, 13, 1211. [Google Scholar] [CrossRef]
- Van Denderen, P.D.; Hintzen, N.T.; Van Kooten, T.; Rijnsdorp, A.D. Temporal Aggregation of Bottom Trawling and Its Implication for the Impact on the Benthic Ecosystem. ICES J. Mar. Sci. 2015, 72, 952–961. [Google Scholar] [CrossRef]
- Pusceddu, A.; Bianchelli, S.; Martín, J.; Puig, P.; Palanques, A.; Masqué, P.; Danovaro, R. Chronic and Intensive Bottom Trawling Impairs Deep-Sea Biodiversity and Ecosystem Functioning. Proc. Natl. Acad. Sci. USA 2014, 111, 8861–8866. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, A.; Bianchelli, S.; Danovaro, R. Quantity and Biochemical Composition of Particulate Organic Matter in a Highly Trawled Area (Thermaikos Gulf, Eastern Mediterranean Sea). Adv. Oceanogr. Limnol. 2015, 6, 21–32. [Google Scholar] [CrossRef]
- Pommer, C.D.; Olesen, M.; Jhansen, Ø.L.S. Impact and Distribution of Bottom Trawl Fishing on Mud-Bottom Communities in the Kattegat. Mar. Ecol. Prog. Ser. 2016, 548, 47–60. [Google Scholar] [CrossRef]
- Jennings, S.; Pinnegar, J.K.; Polunin, N.V.C.; Warr, K.J. Impacts of Trawling Disturbance on the Trophic Structure of Benthic Invertebrate Communities. Mar. Ecol. Prog. Ser. 2001, 213, 127–142. [Google Scholar] [CrossRef]
- Hutchings, P. Review of the Effects of Trawling on Macrobenthic Epifaunal Communities. Mar. Freshw. Res. 1990, 41, 111–120. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Collie, J.S.; Hall, S.J.; Jennings, S.; Poiner, I.R. Modification of Marine Habitats by Trawling Activities: Prognosis and Solutions. Fish Fish. 2002, 3, 114–136. [Google Scholar] [CrossRef]
- Lauria, V.; Garofalo, G.; Fiorentino, F.; Massi, D.; Milisenda, G.; Piraino, S.; Russo, T.; Gristina, M. Species Distribution Models of Two Critically Endangered Deep-Sea Octocorals Reveal Fishing Impacts on Vulnerable Marine Ecosystems in Central Mediterranean Sea. Sci. Rep. 2017, 7, 8049. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, D.; Rodríguez, J.; Abdul Malak, D. Development and Testing of a New Framework for Rapidly Assessing Legal and Managerial Protection Afforded by Marine Protected Areas: Mediterranean Sea Case Study. J. Environ. Manage. 2016, 167, 29–37. [Google Scholar] [CrossRef]
- Bastardie, F.; Angelini, S.; Bolognini, L.; Fuga, F.; Manfredi, C.; Martinelli, M.; Nielsen, J.R.; Santojanni, A.; Scarcella, G.; Grati, F. Spatial Planning for Fisheries in the Northern Adriatic: Working toward Viable and Sustainable Fishing. Ecosphere 2017, 8, e01696. [Google Scholar] [CrossRef]
- Serrano, A.; de la Torriente, A.; Punzón, A.; Blanco, M.; Bellas, J.; Durán-Muñoz, P.; Murillo, F.J.; Sacau, M.; García-Alegre, A.; Antolínez, A.; et al. Sentinels of Seabed (SoS) Indicator: Assessing Benthic Habitats Condition Using Typical and Sensitive Species. Ecol. Indic. 2022, 140, 108979. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Papadopoulou, N.; Sevastou, K.; Franco, A.; Teixeira, H.; Piroddi, C.; Katsanevakis, S.; Fürhaupter, K.; Beauchard, O.; Cochrane, S.; et al. Report on Identification of Keystone Species and Processes across Regional Seas. 2014, pp. 1–103. Available online: https://hal.science/hal-01790558 (accessed on 27 April 2023).
- Degnbol, P. Indicators as a Means of Communicating Knowledge. ICES J. Mar. Sci. 2005, 62, 606–611. [Google Scholar] [CrossRef]
- European Commission. Commission Decision (EU) 2017/848 of 17 May 2017-Laying down Criteria and Methodological Standards on Good Environmental Status of Marine Waters and Specifications and Standardized Methods for Monitoring and Assessment; European Commission: Brussels, Belgium, 2017; Official Journal L 125; pp. 43–74. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017D0848 (accessed on 27 April 2023).
- Burgos, J.M.; Buhl-Mortensen, L.; Buhl-Mortensen, P.; Ólafsdóttir, S.H.; Steingrund, P.; Ragnarsson, S.; Skagseth, Ø. Predicting the Distribution of Indicator Taxa of Vulnerable Marine Ecosystems in the Arctic and Sub-Arctic Waters of the Nordic Seas. Front. Mar. Sci. 2020, 7, 131. [Google Scholar] [CrossRef]
- Williams, G.C. The Global Diversity of Sea Pens (Cnidaria: Octocorallia: Pennatulacea). PLoS ONE 2011, 6, e22747. [Google Scholar] [CrossRef]
- Cogswell, A.; Kenchington, E.; Lirette, C.; Murillo, F.J.; Campanis, G.; Campbell, N.; Ollerhead, N. Layers Utilized by an ArcGIS Model to Approximate Commercial Coral and Sponge By-Catch in the NAFO Regulatory Area. 2011. Available online: https://archive.nafo.int/open/sc/2011/scr11-072.pdf (accessed on 26 April 2023).
- United Nations. Resolution 70/75: Sustainable Fisheries, Including through the 1995 Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 Relating to the Conservation and Management of Straddling Fiss; United Nations: New York, NY, USA, 2016; p. 35. Available online: https://sdgs.un.org/documents/ares7075-sustainable-fisheries-including-thr-23183 (accessed on 28 April 2023).
- Hughes, D.J. Sea Pens and Burrowing Megafauna: An Overview of Dynamics and Sensitivity Characteristics for Conservation Management of Marine SACs. Science 1998, 3, 1–105. [Google Scholar]
- Felder, D.L.; Camp, D.K. Gulf of Mexico: Origin, Waters, and Biota. 2009, Volume 1. Available online: https://decapoda.nhm.org/pdfs/31409/31409.pdf (accessed on 25 April 2023).
- Wright, E.P.; Kemp, K.; Rogers, A.D.; Yesson, C. Genetic Structure of the Tall Sea Pen Funiculina quadrangularis in NW Scottish Sea Lochs. Mar. Ecol. 2015, 36, 659–667. [Google Scholar] [CrossRef]
- Ardizzone, G.D. An Introduction to Sensitive and Essential Fish Habitats Identification and Protection in the Mediterranean Sea; STECF: Rome, Italy, 2006; p. 63. [Google Scholar]
- Greathead, C.F.; Donnan, D.W.; Mair, J.M.; Saunders, G.R. The Sea Pens Virgularia mirabilis, Pennatula phosphorea and Funiculina quadrangularis: Distribution and Conservation Issues in Scottish Waters. J. Mar. Biol. Assoc. UK 2007, 87, 1095–1103. [Google Scholar] [CrossRef]
- Rogers, A.D.; Gianni, M. The Implementation of UNGA Resolutions 61/105 and 64/72 in the Management of Deep-Sea Fisheries on the High Seas. 2010. Available online: http://www.stateoftheocean.org/pdfs/61105-Implemention-finalreport.pdf (accessed on 18 April 2023).
- FAO; GFCM. Fishery and Aquaculture Statistics. GFCM Capture Production 1970-2020 (FishStatJ); FAO Fisheries and Aquaculture Division: Rome, Italy, 2022; Available online: https://www.fao.org/fishery/en/topic/166235?lang=en (accessed on 10 April 2023).
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species; IUCN Version 2022-2; IUCN: Gland, Switzerland, 2022; Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/2022-2_RL_Stats_Table_1a.pdf (accessed on 25 April 2023).
- Bastari, A.; Pica, D.; Ferretti, F.; Micheli, F.; Cerrano, C. Sea Pens in the Mediterranean Sea: Habitat Suitability and Opportunities for Ecosystem Recovery. ICES J. Mar. Sci. 2018, 75, 1722–1732. [Google Scholar] [CrossRef]
- Mačić, V.; Đorđević, N.; Đurović, M.; Petović, S.; Russo, T. Improving Knowledge of Funiculina quadrangularis and Vulnerable Marine Ecosystems in the South Adriatic. Mediterr. Mar. Sci. 2022, 23, 805–816. [Google Scholar] [CrossRef]
- Edwards, D.C.B.; Moore, C.G. Reproduction in the Sea Pen Funiculina quadrangularis (Anthozoa: Pennatulacea) from the West Coast of Scotland. Estuar. Coast. Shelf Sci. 2009, 82, 161–168. [Google Scholar] [CrossRef]
- Downie, A.L.; Noble-James, T.; Chaverra, A.; Howell, K.L. Predicting Sea Pen (Pennatulacea) Distribution on the UK Continental Shelf: Evidence of Range Modification by Benthic Trawling. Mar. Ecol. Prog. Ser. 2021, 670, 75–91. [Google Scholar] [CrossRef]
- Martinelli, M.; Morello, E.B.; Isajlović, I.; Belardinelli, A.; Lucchetti, A.; Santojanni, A.; Atkinson, R.J.A.; Vrgoč, N.; Arneri, E. Towed Underwater Television towards the Quantification of Norway Lobster, Squat Lobsters and Sea Pens in the Adriatic Sea. Acta Adriat. 2013, 54, 3–12. [Google Scholar]
- Miatta, M.; Snelgrove, P.V.R. Sea Pens as Indicators of Macrofaunal Communities in Deep-Sea Sediments: Evidence from the Laurentian Channel Marine Protected Area. Deep. Res. Part I Oceanogr. Res. Pap. 2022, 182, 103702. [Google Scholar] [CrossRef]
- Vigo, M.; Navarro, J.; Aguzzi, J.; Bahamon, N.; García, J.A.; Rotllant, G.; Recasens, L.; Company, J.B. ROV-Based Monitoring of Passive Ecological Recovery in a Deep-Sea No-Take Fishery Reserve. Sci. Total Environ. 2023, 883, 163339. [Google Scholar] [CrossRef]
- ICES. Working Group on Nephrops Surveys (WGNEPS; Outputs from 2021). ICES Sci. Rep. 2022, 4, 183. [Google Scholar] [CrossRef]
- OSPAR. Meeting of the Intersessional Correspondence Group on the Implementation Follow up of Measures for the Protection and Conservation of Species and Habitats (ICG-POSH), Paris, France, 9–11 October 2018. Roadmap for the Implementation of Collective Actions W. 2018, pp. 1–8. Available online: https://www.ospar.org/site/assets/files/38906/0205a5_actionsheet13_mpashabitats.pdf (accessed on 2 May 2023).
- EMODnet; EMODnet Bathymetry Consortium. EMODnet Digital Bathymetry (DTM), Ifremer; EMODnet: Oostende, Belgium, 2016. [Google Scholar] [CrossRef]
- Russo, T.; Morello, E.B.; Parisi, A.; Scarcella, G.; Angelini, S.; Labanchi, L.; Martinelli, M.; D’Andrea, L.; Santojanni, A.; Arneri, E.; et al. A Model Combining Landings and VMS Data to Estimate Landings by Fishing Ground and Harbor. Fish. Res. 2018, 199, 218–230. [Google Scholar] [CrossRef]
- Marini, M.; Maselli, V.; Campanelli, A.; Foglini, F.; Grilli, F. Role of the Mid-Adriatic Deep in Dense Water Interception and Modification. Mar. Geol. 2016, 375, 5–14. [Google Scholar] [CrossRef]
- Taviani, M.; Angeletti, L.; Beuck, L.; Campiani, E.; Canese, S.; Foglini, F.; Freiwald, A.; Montagna, P.; Trincardi, F. Reprint of “On and off the Beaten Track: Megafaunal Sessile Life and Adriatic Cascading Processes”. Mar. Geol. 2016, 375, 146–160. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The Effects of Fishing on Marine Ecosystems. Adv. Mar. Biol. 1998, 34, 201–212. [Google Scholar] [CrossRef]
- Lucey, S.M.; Nye, J.A. Shifting Species Assemblages in the Northeast US Continental Shelf Large Marine Ecosystem. Mar. Ecol. Prog. Ser. 2010, 415, 23–33. [Google Scholar] [CrossRef]
- Albouy, C.; Guilhaumon, F.; Araújo, M.B.; Mouillot, D.; Leprieur, F. Combining Projected Changes in Species Richness and Composition Reveals Climate Change Impacts on Coastal Mediterranean Fish Assemblages. Glob. Chang. Biol. 2012, 18, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Soininen, J.; Snoeijs, P. Warming Leads to Higher Species Turnover in a Coastal Ecosystem. Glob. Chang. Biol. 2010, 16, 1181–1193. [Google Scholar] [CrossRef]
- Lavergne, S.; Mouquet, N.; Thuiller, W.; Ronce, O. Biodiversity and Climate Change: Integrating Evolutionary and Ecological Responses of Species and Communities. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 321–350. [Google Scholar] [CrossRef]
- GFCM. Recommendation GFCM/41/2017/3 on the Establishment of a Fisheries Restricted Area in the Jabuka/Pomo Pit in the Adriatic Sea; GFCM: Rome, Italy, 2017. [Google Scholar]
- Zupanovic, S.; Jardas, I. A Contribution to the Study of Biology and Population Dynamics of the Adriatic Hake, Merluccius merluccius (L.). Acta Adriat. 1986, 27, 97–146. [Google Scholar]
- Angelini, S. Towards an Ecosystem Approach to Fisheries for Nephrops norvegicus and Merluccius merluccius Inhabiting the Central Adriatic Sea. 2016. Available online: http://amsdottorato.unibo.it/7380/1/Angelini_Silvia_tesi.pdf (accessed on 3 May 2023).
- Angelini, S.; Martinelli, M.; Santojanni, A.; Colella, S. Biological Evidence of the Presence of Different Subpopulations of Norway Lobster (Nephrops norvegicus) in the Adriatic Sea (Central Mediterranean Sea). Fish. Res. 2020, 221, 105365. [Google Scholar] [CrossRef]
- Colloca, F.; Mastrantonio, G.; Lasinio, G.J.; Ligas, A.; Sartor, P. Parapenaeus longirostris (Lucas, 1846) an Early Warning Indicator Species of Global Warming in the Central Mediterranean Sea. J. Mar. Syst. 2014, 138, 29–39. [Google Scholar] [CrossRef]
- Martinelli, M.; Angelini, S.; Belardinelli, A.; Caccamo, G.; Cacciamani, R.; Calì, F.; Canduci, G.; Chiarini, M.; Croci, C.; Domenichetti, F.; et al. Accordo Tra MIPAAF e CNR-IRBIM Ancona in Merito Alla Proposta Progettuale Relativa Alle Attività Di Monitoraggio Periodico Delle Fosse Di Pomo e All’attuazione Di Misure Che, Nel Rispetto Dei Piani Di Gestione, Comportino Il Mantenimento Delle Condizioni Ambientali Idonee Alla vita e All’accrescimento dei Molluschi Bivalvi, Ponendo in Essere Misure Supplementari Tese a Proteggere le Diverse Fasi del Ciclo Biologico Delle Specie Interessate (CUP J41F19000080001)-Parte Monitoraggio Fosse di Pomo periodo 2019-2020; Secondo interim report-Luglio 2020; CNR-IRBIM: Ancona, Italy, 2020. [Google Scholar]
- Froglia, C.; Gramitto, M.; Martinelli, M.; Betulla, M. Long Term Changes in the Decapod Crustaceans Assemblage in the Western Meso-Adriatic Depression (Pomo Pit). Crustac. Soc. Mid-Year 2017, 1, 19–22. [Google Scholar]
- Chiarini, M.; Guicciardi, S.; Zacchetti, L.; Domenichetti, F.; Canduci, G.; Angelini, S.; Belardinelli, A.; Croci, C.; Giuliani, G.; Scarpini, P.; et al. Looking for a Simple Assessment Tool for a Complex Task: Short-Term Evaluation of Changes in Fisheries Management Measures in the Pomo/Jabuka Pits Area (Central Adriatic Sea). Sustainability 2022, 14, 7742. [Google Scholar] [CrossRef]
- GFCM. GFCM-SAC Report of the Working Group on Vulnerable Marine Ecosystems and Essential Fish Habitats (WGVME-EFH); FAO: Rome, Italy, 2023; p. 41. [Google Scholar]
- Chiarini, M.; Guicciardi, S.; Angelini, S.; Tuck, I.D.; Grilli, F.; Penna, P.; Domenichetti, F.; Canduci, G.; Belardinelli, A.; Santojanni, A.; et al. Accounting for Environmental and Fishery Management Factors When Standardizing CPUE Data from a Scientific Survey: A Case Study for Nephrops norvegicus in the Pomo Pits Area (Central Adriatic Sea). PLoS ONE 2022, 17, e0270703. [Google Scholar] [CrossRef] [PubMed]
- EU. Regulation (EU) 2019/982 of the European Parliament and of the Council of 5 June 2019 Amending Regulation (EU) No 1343/2011 on Certain Provisions for Fishing in the GFCM (General Fisheries Commission for the Mediterranean) Agreement Area; 2019, Official Journal L 164, pp. 1–22. Available online: http://data.europa.eu/eli/reg/2019/982/oj (accessed on 5 May 2023).
- GFCM. Recommendation GFCM/44/2021/2 on the Establishment of a Fisheries Restricted Area in the Jabuka/Pomo Pit in the Adriatic Sea (Geographical Subarea 17), Amending Recommendation; GFCM: Rome, Italy, 2021. [Google Scholar]
- Martinelli, M.; Belardinelli, A.; Guicciardi, S.; Penna, P.; Domenichetti, F.; Croci, C.; Angelini, S.; Medvesek, D.; Froglia, C.; Scarpini, P. Support the Monitoring of Fisheries and Fisheries Resources in the Adriatic Sea” Report of Task 2 “To Perform the Appraisal of Nephrops Norvegicus in the Central Adriatic Sea (GFCM GSA 17) through Underwater Television Surveys; ISMAR: Ancona, Italy, 2017. [Google Scholar]
- Martinelli, M.; Belardinelli, A.; Guicciardi, S.; Penna, P.; Domenichetti, F.; Croci, C.; Angelini, S.; Medvesek, D.; Scarpini, P.; Micucci, D.; et al. SP2_LI1_WP1_UO05_D01-Rapporto Della Campagna 2015 (Ex SP2_WP1_AZ3_UO05_D03-Report 3° UWTV Survey–RITMARE)-RITMARE La Ricerca ITaliana per Il MARE; ISMAR: Ancona, Italy, 2016. [Google Scholar]
- Martinelli, M.; Angelini, S.; Belardinelli, A.; Chiarini, M.; Croci, C.; Domenichetti, F.; Guicciardi, S.; Scarpini, P.; Santojanni, A.; Lorenzo, Z. Final report Modulo 6. Monitoraggio Fosse Di Pomo Periodo 2017-2018 (Esteso Primavera 2019) Convenzione Tra MIPAAFT e CNR-ISMAR Ancona per Uno Studio Propedeutico Al Rinnovo Dell’affidamento Della Gestione Della Pesca Dei Molluschi Bivalvi Ai Consorzi Di Gestione–CUP J53C17000540001; IRBIM-CNR: Ancona, Italy, 2019. [Google Scholar]
- Penna, P.; Grilli, F.; Belardinelli, A.; Domenichetti, F.; Scarpini, P.; Martinelli, M. Pomo Pits Pressure/Temperature/Salinity/Oxygen Profiles Spring Dataset 2012–2021. Seanoe 2022. [Google Scholar] [CrossRef]
- ICES. Working Group on Nephrops Surveys (WGNEPS; Outputs from 2019). ICES Sci. Rep. 2020, 2, 85. [Google Scholar]
- Aguzzi, J.; Bahamon, N.; Doyle, J.; Lordan, C.; Tuck, I.D.; Chiarini, M.; Martinelli, M.; Company, J.B. Burrow Emergence Rhythms of Nephrops norvegicus by UWTV and Surveying Biases. Sci. Rep. 2021, 11, 5797. [Google Scholar] [CrossRef]
- Aguzzi, J.; Chatzievangelou, D.; Robinson, N.J.; Bahamon, N.; Berry, A.; Carreras, M.; Company, J.B.; Costa, C.; del Rio Fernandez, J.; Falahzadeh, A.; et al. Advancing Fishery-Independent Stock Assessments for the Norway Lobster (Nephrops norvegicus) with New Monitoring Technologies. Front. Mar. Sci. 2022, 9, 969071. [Google Scholar] [CrossRef]
- Martinelli, M.; Scarcella, G.; Sabatini, L.; Zacchetti, L.; Luzi, F.; Domenichetti, F. Convenzione tra ISPRA e CNR-IRBIM per la realizzazione di attività condivise, finalizzate a dare attuazione alle previsioni del d. lgs 13 ottobre 2010 n. 190, nell’ambito della Strategia Marina nel triennio 2021–2023. Modulo comunità epimegabentoniche–Mar Adriatico (GSA 17), Final report; IRBIM-CNR: Ancona, Italy, 2023. [Google Scholar]
- MIPAAF. Modalità Attuative per La Pesca Nella Fossa Di Pomo; Official Gazette of the Italian Republic No 2 of 03/01/2017; Ministero Delle Politiche Agricole Alimentari e Forestali: Rome, Italy, 2017. [Google Scholar]
- MIPAAF. Arresto Temporaneo Obbligatorio Delle Unità Autorizzate All’esercizio Della Pesca Con Il Sistema Strascico-Annualità 2015; Official Gazette of the Italian Republic No 162 of 15/07/2015; Ministero Delle Politiche Agricole Alimentari e Forestali: Rome, Italy, 2015. [Google Scholar]
- MIPAAF. Misure per La Pesca Nella Fossa Di Pomo; Ministero Delle Politiche Agricole Alimentari e Forestali: Rome, Italy, 2017; Available online: https://www.politicheagricole.it/flex/cm/pages/ServeAttachment.php/L/IT/D/9%252F0%252F4%252FD.583cb74efe7278af0dc3/P/BLOB%3AID%3D11345/E/pdf?mode=download (accessed on 12 May 2023).
- Wickham, H. ggplot2 Elegant Graphics for Data Analysis, 3rd. ed; Springer: New York, NY, USA, 2016; pp. 3–10. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. A Grammar of Data Manipulation [R Package Dplyr Version 1.0.0]. Media. 2020. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 10 April 2023).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Kassambara, A. Rstatix:Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.1. 2022. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 12 April 2023).
- Colloca, F.; Garofalo, G.; Bitetto, I.; Facchini, M.T.; Grati, F.; Martiradonna, A.; Mastrantonio, G.; Nikolioudakis, N.; Ordinas, F.; Scarcella, G.; et al. The Seascape of Demersal Fish Nursery Areas in the North Mediterranean Sea, a First Step towards the Implementation of Spatial Planning for Trawl Fisheries. PLoS ONE 2015, 10, e0119590. [Google Scholar] [CrossRef] [PubMed]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Mastrototaro, F.; Chimienti, G.; Acosta, J.; Blanco, J.; Garcia, S.; Rivera, J.; Aguilar, R. Isidella elongata (Cnidaria: Alcyonacea) Facies in the Western Mediterranean Sea: Visual Surveys and Descriptions of Its Ecological Role. Eur. Zool. J. 2017, 84, 209–225. [Google Scholar] [CrossRef]
- Lotze, H.K.; Coll, M.; Dunne, J.A. Historical Changes in Marine Resources, Food-Web Structure and Ecosystem Functioning in the Adriatic Sea, Mediterranean. Ecosystems 2011, 14, 198–222. [Google Scholar] [CrossRef]
- Eno, N.C.; MacDonald, D.S.; Kinnear, J.A.M.; Amos, S.C.; Chapman, C.J.; Clark, R.A.; Bunker, F.S.P.D.; Munro, C. Effects of Crustacean Traps on Benthic Fauna. ICES J. Mar. Sci. 2001, 58, 11–20. [Google Scholar] [CrossRef]
- Pierdomenico, M.; Russo, T.; Ambroso, S.; Gori, A.; Martorelli, E.; D’Andrea, L.; Gili, J.M.; Chiocci, F.L. Effects of Trawling Activity on the Bamboo-Coral Isidella elongata and the Sea Pen Funiculina quadrangularis along the Gioia Canyon (Western Mediterranean, Southern Tyrrhenian Sea). Prog. Oceanogr. 2018, 169, 214–226. [Google Scholar] [CrossRef]
- Bo, M.; Canese, S.; Spaggiari, C.; Pusceddu, A.; Bertolino, M.; Angiolillo, M.; Giusti, M.; Loreto, M.F.; Salvati, E.; Greco, S.; et al. Deep Coral Oases in the South Tyrrhenian Sea. PLoS ONE 2012, 7, e49870. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; D’Andrea, L.; Parisi, A.; Cataudella, S. VMSbase: An R-Package for VMS and Logbook Data Management and Analysis in Fisheries Ecology. PLoS ONE 2014, 9, e100195. [Google Scholar] [CrossRef]
- Russo, T.; D’Andrea, L.; Parisi, A.; Martinelli, M.; Belardinelli, A.; Boccoli, F.; Cignini, I.; Tordoni, M.; Cataudella, S. Assessing the Fishing Footprint Using Data Integrated from Different Tracking Devices: Issues and Opportunities. Ecol. Indic. 2016, 69, 818–827. [Google Scholar] [CrossRef]
- Ferrà Vega, C.; Tassetti, A.N.; Grati, F.; Scarcella, S.; Fabi, G. AIS as a Useful System to Support the Identification of Fisheries Restricted Areas. Licence: CC-NC-SA 3.0 IGO. FAO Fish Forum, B. Abstr.. 2018, p. 338. Available online: https://www.fao.org/3/cb7622en/cb7622en.pdf (accessed on 10 May 2023).
- Tassetti, A.N.; Ferrà, C.; Fabi, G. Rating the Effectiveness of Fishery-Regulated Areas with AIS Data. Ocean Coast. Manag. 2019, 175, 90–97. [Google Scholar] [CrossRef]
- De Clippele, L.H.; Buhl-Mortensen, P.; Buhl-Mortensen, L. Fauna Associated with Cold Water Gorgonians and Sea Pens. Cont. Shelf Res. 2015, 105, 67–78. [Google Scholar] [CrossRef]
- Grinyó, J.; Francescangeli, M.; Santín, A.; Ercilla, G.; Estrada, F.; Mecho, A.; Fanelli, E.; Costa, C.; Danovaro, R.; Company, J.B.; et al. Megafaunal assemblages in deep-sea ecosystems of the Gulf of Cadiz, northeast Atlantic ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2022, 183, 103738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).