Whiting (Merlangius merlangus) Grows Slower and Smaller in the Adriatic Sea: New Insights from a Comparison of Two Populations with a Time Interval of 30 Years
Abstract
1. Introduction
2. Materials and Methods
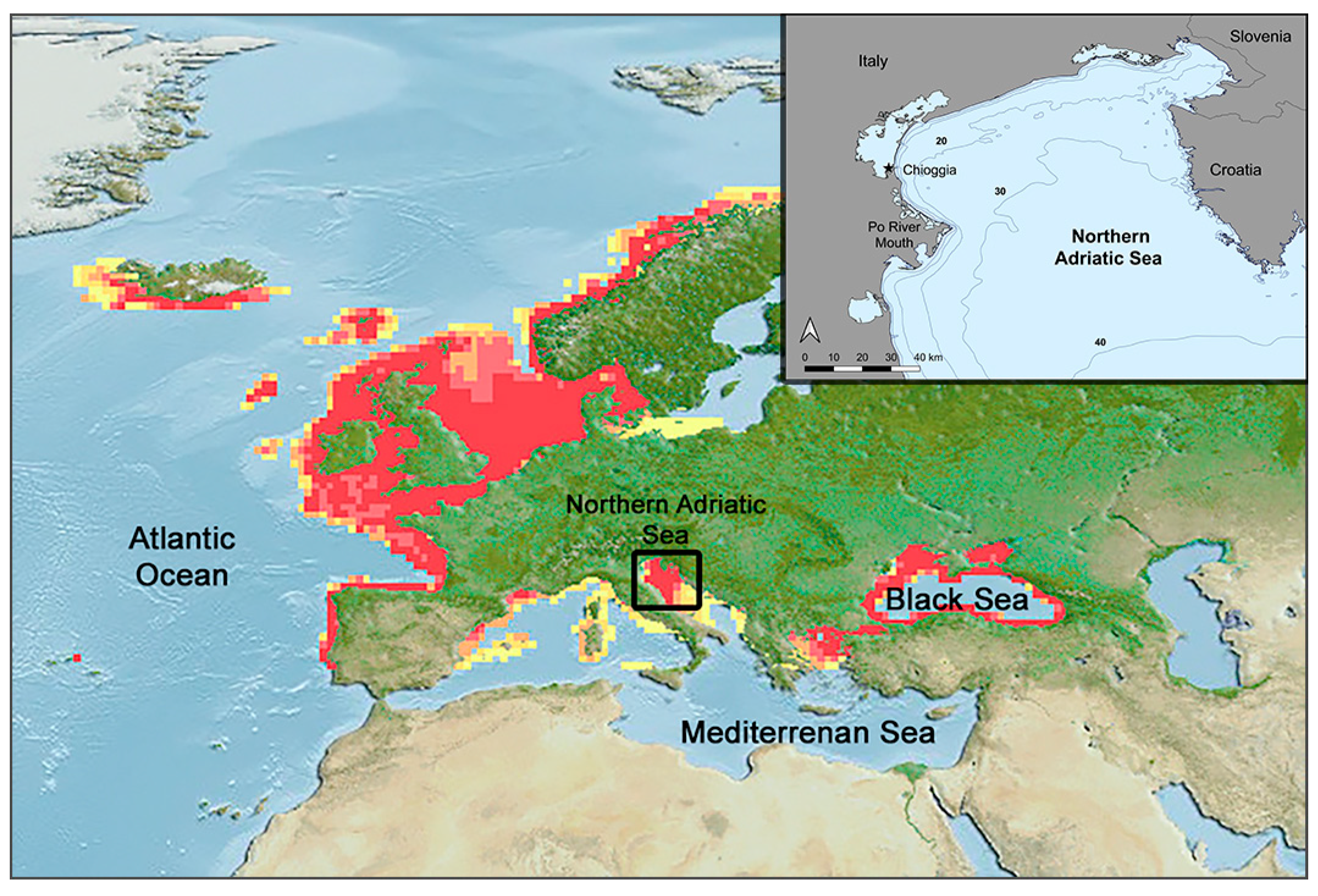
2.1. Study Area
2.2. Sampling Design and Population Parameters
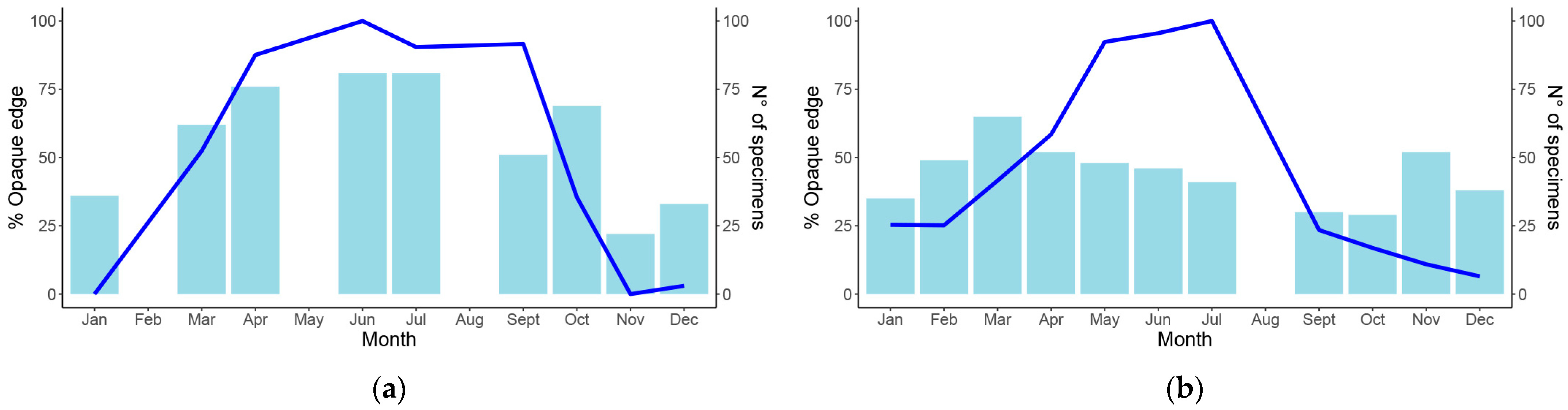
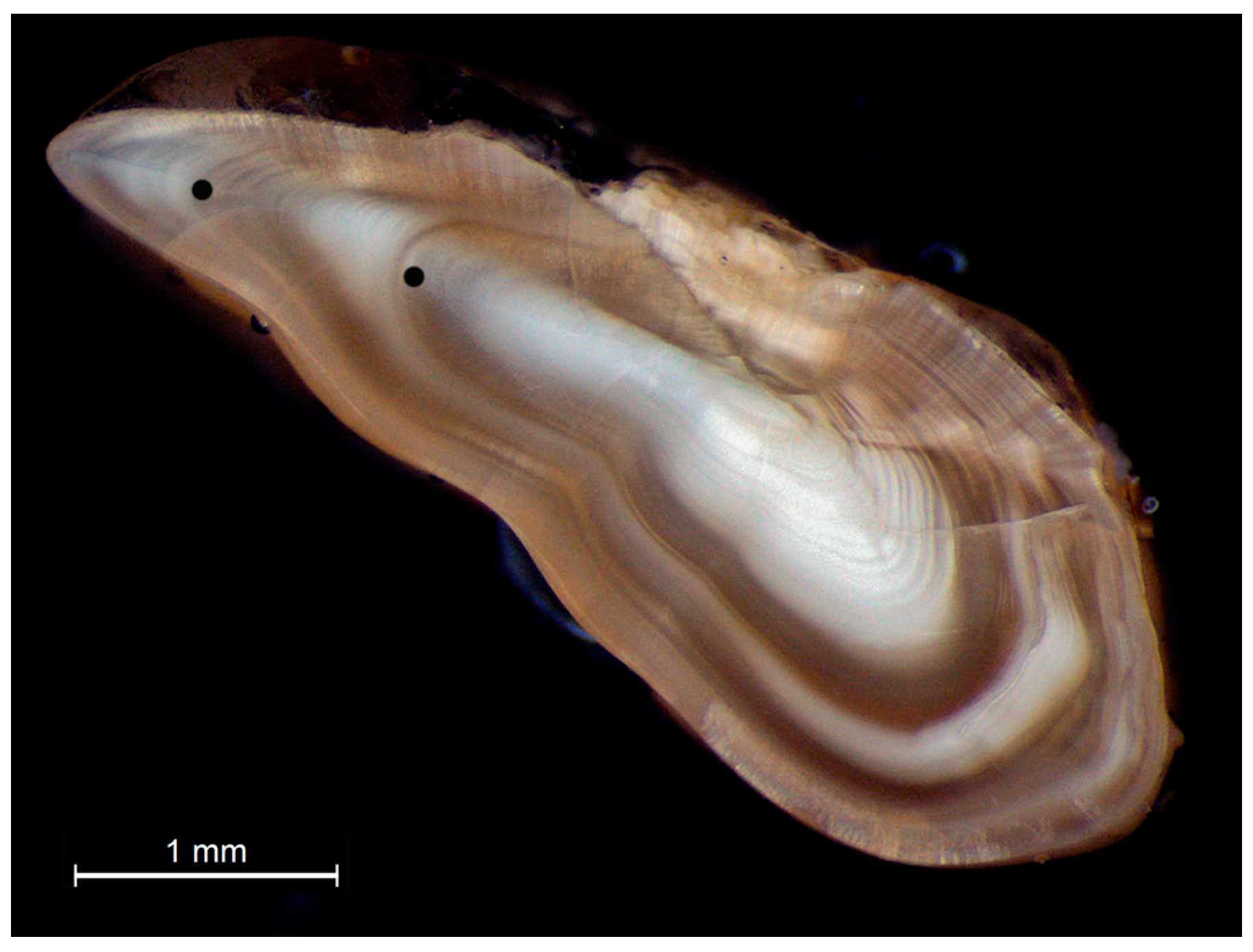
2.3. Age Estimation
2.4. Fecundity and Maturity Estimation
3. Results
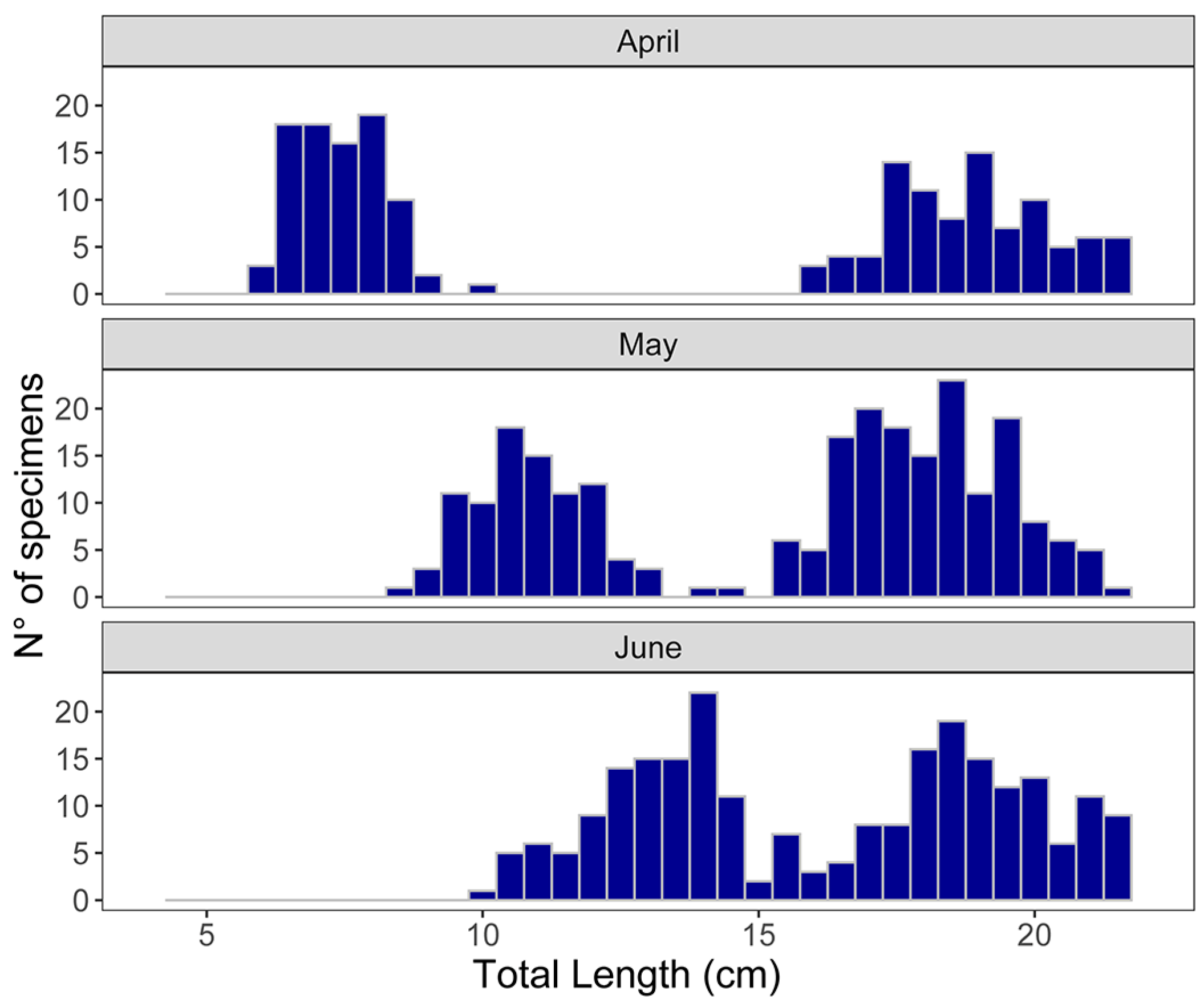
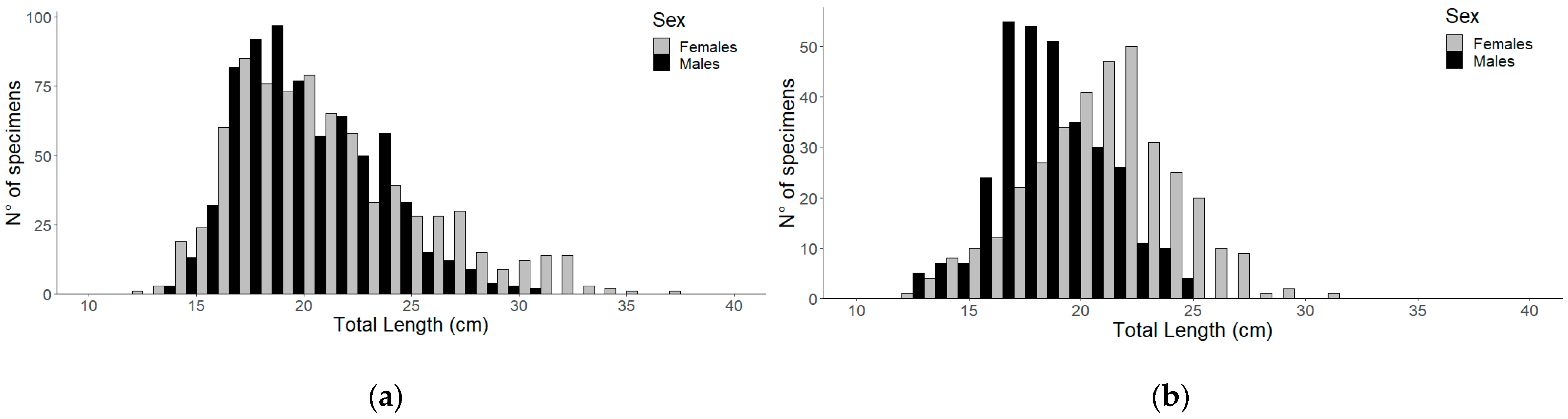
3.1. Population Structure
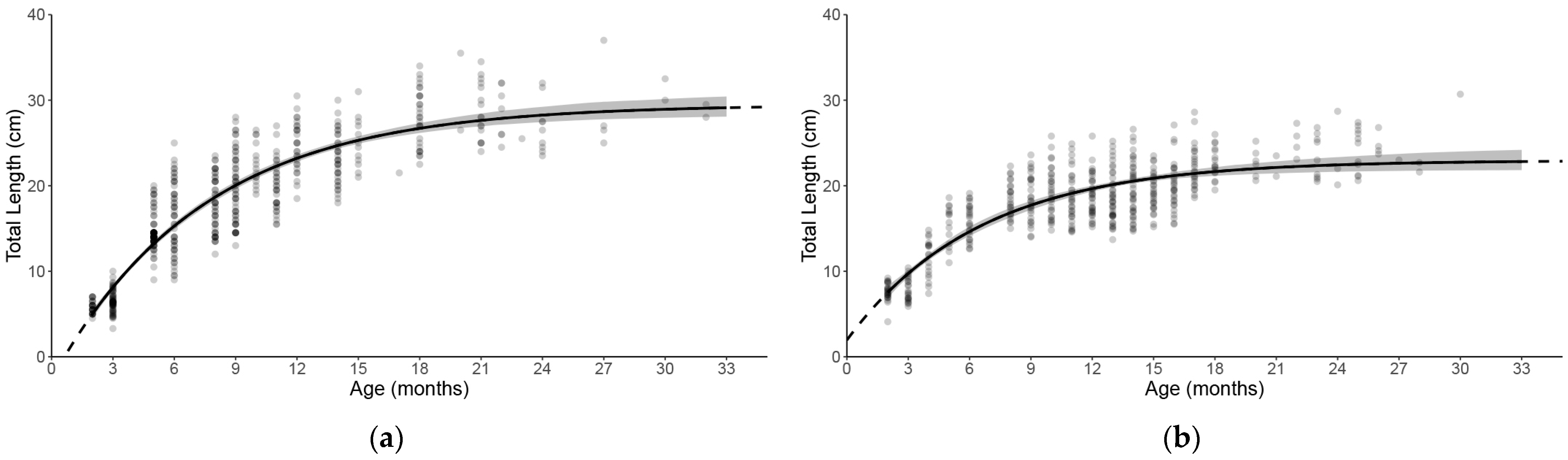
3.2. Growth
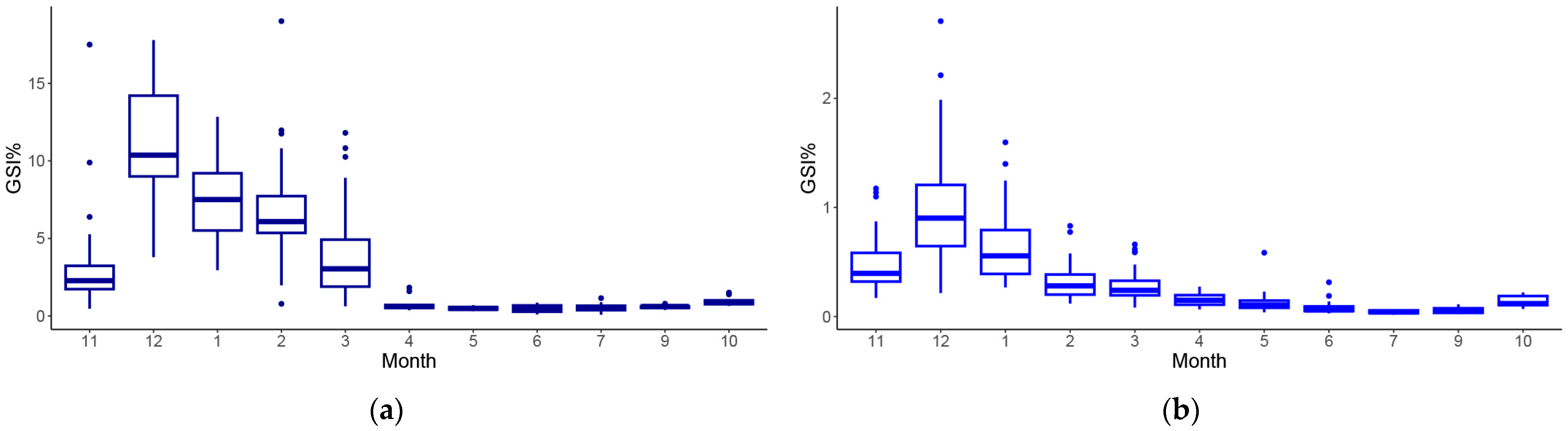
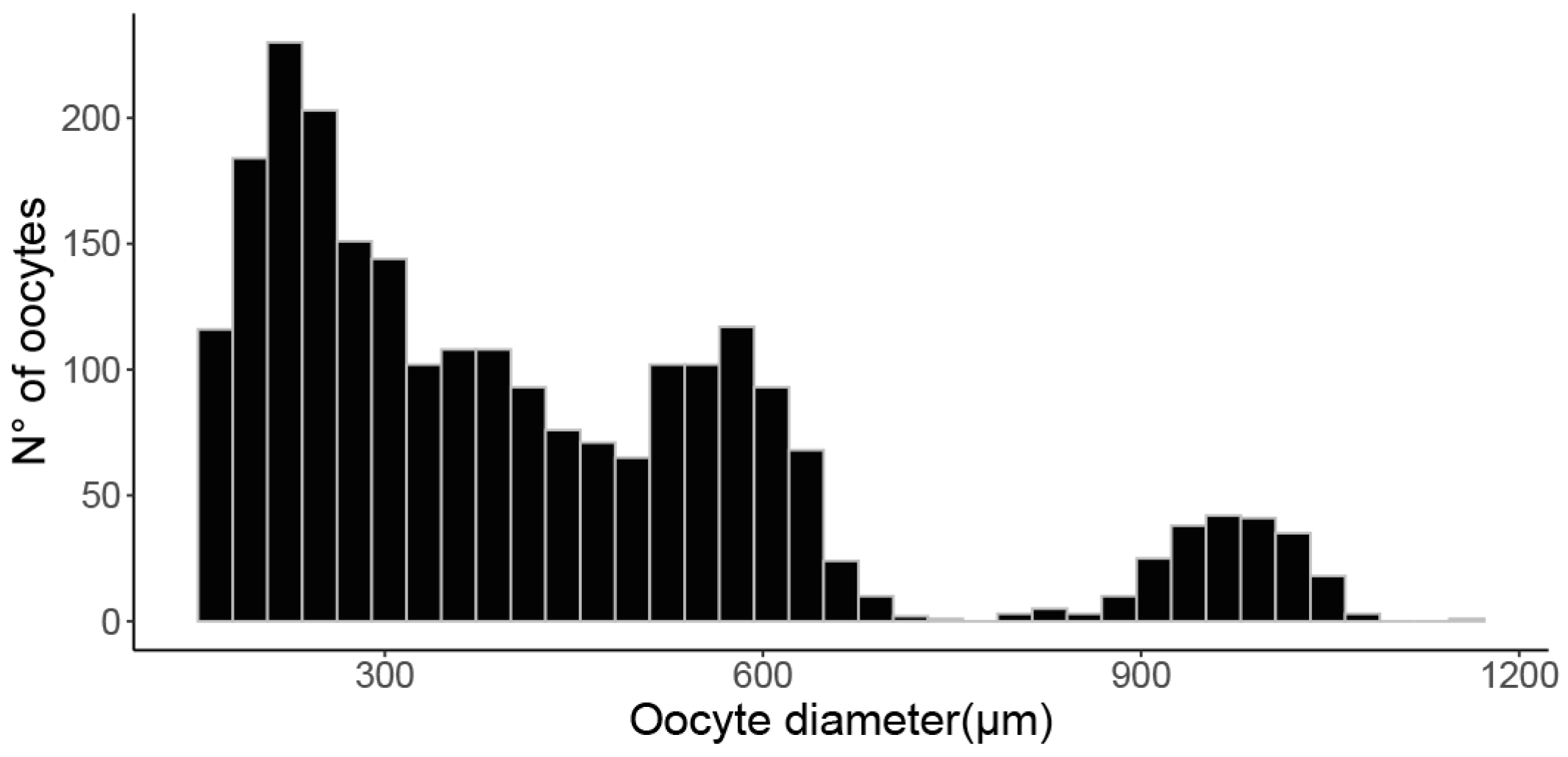
3.3. Maturity Estimation and Fecundity
4. Discussion
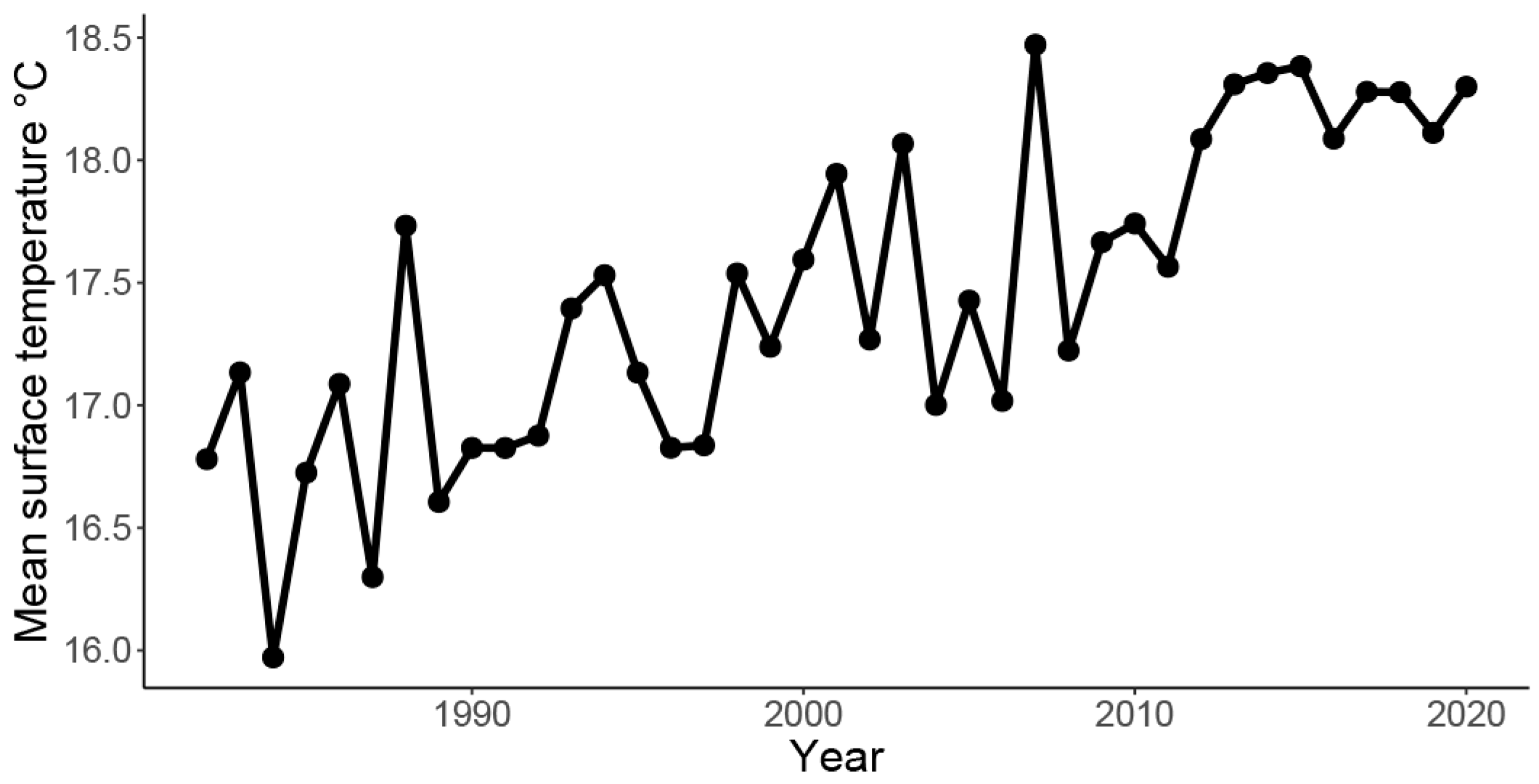
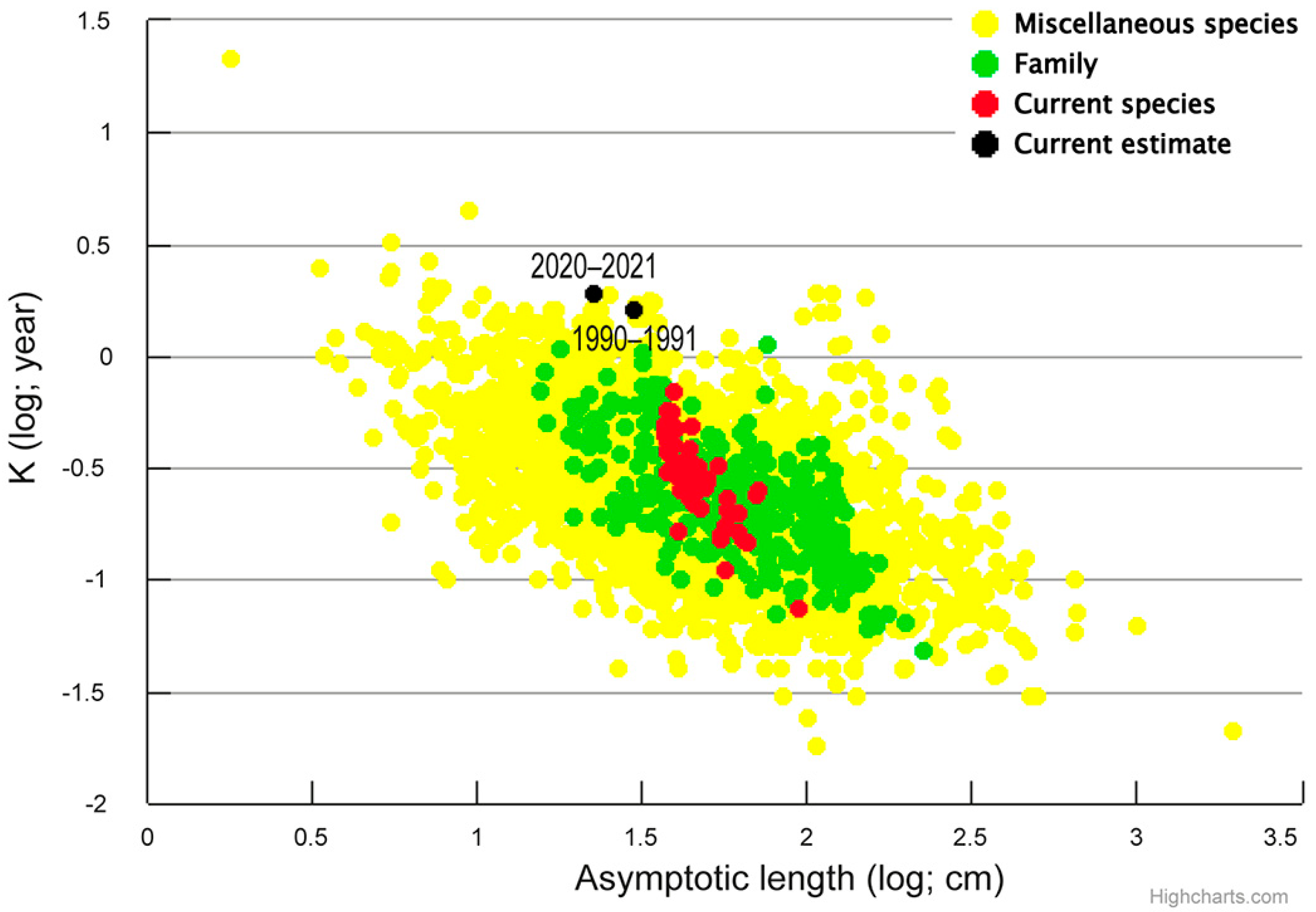
4.1. Historical Comparison
4.2. Population Biological Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Females | Males | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Quarters) | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | N | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | N |
| Total length (cm) | ||||||||||||||||||||||
| 12 | 1 | 1 | 2 | 0 | ||||||||||||||||||
| 13 | 6 | 2 | 8 | 5 | 5 | |||||||||||||||||
| 14 | 12 | 6 | 18 | 8 | 7 | 15 | ||||||||||||||||
| 15 | 2 | 4 | 6 | 4 | 4 | 2 | 10 | |||||||||||||||
| 16 | 3 | 4 | 7 | 2 | 4 | 2 | 8 | |||||||||||||||
| 17 | 3 | 4 | 2 | 9 | 4 | 4 | 2 | 10 | ||||||||||||||
| 18 | 3 | 4 | 2 | 9 | 4 | 4 | 3 | 2 | 13 | |||||||||||||
| 19 | 4 | 4 | 2 | 1 | 11 | 4 | 4 | 4 | 2 | 14 | ||||||||||||
| 20 | 3 | 4 | 2 | 2 | 11 | 2 | 4 | 6 | 2 | 14 | ||||||||||||
| 21 | 2 | 4 | 4 | 2 | 12 | 2 | 4 | 5 | 4 | 1 | 16 | |||||||||||
| 22 | 2 | 3 | 4 | 2 | 11 | 1 | 4 | 3 | 3 | 1 | 12 | |||||||||||
| 23 | 1 | 3 | 4 | 2 | 10 | 4 | 4 | 4 | 2 | 1 | 15 | |||||||||||
| 24 | 3 | 3 | 2 | 1 | 9 | 1 | 3 | 3 | 1 | 3 | 11 | |||||||||||
| 25 | 1 | 2 | 2 | 1 | 6 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 11 | |||||||||
| 26 | 3 | 7 | 3 | 13 | 1 | 1 | 2 | 2 | 3 | 1 | 10 | |||||||||||
| 27 | 1 | 3 | 5 | 2 | 1 | 12 | 1 | 2 | 1 | 2 | 1 | 7 | ||||||||||
| 28 | 1 | 2 | 2 | 1 | 1 | 7 | 2 | 1 | 1 | 4 | ||||||||||||
| 29 | 1 | 2 | 1 | 4 | 1 | 1 | 1 | 3 | ||||||||||||||
| 30 | 1 | 1 | 2 | 1 | 1 | 6 | 1 | 1 | 2 | |||||||||||||
| 31 | 1 | 2 | 1 | 1 | 5 | 0 | ||||||||||||||||
| 32 | 2 | 2 | 3 | 1 | 8 | 0 | ||||||||||||||||
| 33 | 1 | 1 | 2 | 0 | ||||||||||||||||||
| 34 | 1 | 1 | 2 | 0 | ||||||||||||||||||
| 35 | 1 | 1 | 0 | |||||||||||||||||||
| 37 | 1 | 1 | 0 | |||||||||||||||||||
| N | 42 | 52 | 39 | 25 | 14 | 10 | 6 | 1 | 1 | - | 190 | 37 | 45 | 35 | 23 | 15 | 8 | 11 | 3 | 1 | 2 | 180 |
| TL mean (cm) | 16.7 | 19.3 | 23.6 | 25.0 | 30.1 | 30.9 | 31.2 | 37.0 | 32.5 | - | 16.8 | 18.7 | 20.6 | 22.2 | 25.6 | 26.4 | 25.5 | 26.2 | 30.0 | 28.8 | ||
| TL SD | 3.0 | 4.1 | 3.4 | 3.1 | 2.7 | 3.4 | 1.2 | - | - | - | 3.0 | 3.2 | 2.9 | 2.3 | 2.7 | 1.8 | 1.3 | 1.0 | - | 1.1 | ||
| Unsexed juveniles | ||||||||||||||||||||||
| Age (quarters) | 3 | 6 | 9 | 12 | N | |||||||||||||||||
| Total length (cm) | ||||||||||||||||||||||
| 3 | 1 | 1 | ||||||||||||||||||||
| 4 | 7 | 7 | ||||||||||||||||||||
| 5 | 21 | 21 | ||||||||||||||||||||
| 6 | 35 | 35 | ||||||||||||||||||||
| 7 | 12 | 12 | ||||||||||||||||||||
| 8 | 8 | 8 | ||||||||||||||||||||
| 9 | 1 | 4 | 5 | |||||||||||||||||||
| 10 | 1 | 3 | 4 | |||||||||||||||||||
| 11 | 6 | 6 | ||||||||||||||||||||
| 12 | 7 | 7 | ||||||||||||||||||||
| 13 | 11 | 2 | 13 | |||||||||||||||||||
| 14 | 21 | 1 | 22 | |||||||||||||||||||
| N | 86 | 52 | 3 | - | 141 | |||||||||||||||||
| TL mean (cm) | 6.3 | 12.8 | 13.5 | - | ||||||||||||||||||
| TL SD | 1.2 | 1.6 | 0.5 | - | ||||||||||||||||||
| Females | Males | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Quarters) | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | N | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | N |
| Total length (cm) | ||||||||||||||||||||||||
| 6 | 1 | 1 | 4 | 4 | ||||||||||||||||||||
| 7 | 1 | 1 | 4 | 1 | 5 | |||||||||||||||||||
| 8 | 3 | 2 | 5 | 3 | 3 | |||||||||||||||||||
| 9 | 1 | 1 | 2 | 2 | 2 | |||||||||||||||||||
| 10 | 2 | 2 | 2 | 2 | ||||||||||||||||||||
| 11 | 1 | 1 | 2 | 2 | ||||||||||||||||||||
| 12 | 0 | 6 | 6 | |||||||||||||||||||||
| 13 | 5 | 5 | 5 | 1 | 6 | |||||||||||||||||||
| 14 | 5 | 2 | 2 | 1 | 10 | 4 | 2 | 2 | 8 | |||||||||||||||
| 15 | 2 | 2 | 2 | 3 | 9 | 3 | 5 | 8 | 7 | 2 | 25 | |||||||||||||
| 16 | 4 | 2 | 4 | 4 | 14 | 3 | 4 | 6 | 8 | 2 | 23 | |||||||||||||
| 17 | 4 | 4 | 5 | 4 | 1 | 18 | 4 | 4 | 7 | 7 | 2 | 24 | ||||||||||||
| 18 | 2 | 4 | 6 | 7 | 2 | 21 | 2 | 3 | 5 | 8 | 4 | 22 | ||||||||||||
| 19 | 1 | 3 | 5 | 5 | 2 | 16 | 2 | 7 | 5 | 5 | 19 | |||||||||||||
| 20 | 4 | 6 | 6 | 4 | 20 | 4 | 3 | 4 | 6 | 1 | 3 | 1 | 22 | |||||||||||
| 21 | 3 | 6 | 7 | 5 | 1 | 22 | 2 | 3 | 4 | 1 | 3 | 2 | 1 | 16 | ||||||||||
| 22 | 4 | 5 | 7 | 7 | 23 | 3 | 2 | 2 | 2 | 1 | 10 | |||||||||||||
| 23 | 1 | 4 | 6 | 4 | 1 | 1 | 17 | 1 | 1 | 4 | 1 | 7 | ||||||||||||
| 24 | 3 | 3 | 4 | 1 | 1 | 12 | 1 | 1 | 1 | 3 | ||||||||||||||
| 25 | 2 | 3 | 6 | 1 | 3 | 2 | 17 | 0 | ||||||||||||||||
| 26 | 1 | 1 | 2 | 3 | 1 | 8 | 0 | |||||||||||||||||
| 27 | 2 | 5 | 2 | 9 | 0 | |||||||||||||||||||
| 28 | 1 | 1 | 2 | 0 | ||||||||||||||||||||
| 29 | 1 | 1 | 0 | |||||||||||||||||||||
| 30 | 1 | 1 | 1 | 3 | 0 | |||||||||||||||||||
| 31 | 1 | 1 | 0 | |||||||||||||||||||||
| 32 | 0 | 0 | ||||||||||||||||||||||
| 33 | 0 | 0 | ||||||||||||||||||||||
| 34 | 0 | 0 | ||||||||||||||||||||||
| 35 | 0 | 0 | ||||||||||||||||||||||
| 36 | 0 | 0 | ||||||||||||||||||||||
| 37 | 0 | 0 | ||||||||||||||||||||||
| N | 6 | 29 | 29 | 50 | 57 | 40 | 3 | 15 | 9 | 2 | - | 240 | 15 | 30 | 22 | 40 | 46 | 29 | 5 | 13 | 7 | 2 | - | 209 |
| TL mean (cm) | 8.1 | 14.5 | 19.1 | 20.0 | 20.5 | 22.9 | 23.3 | 28.0 | 25.8 | 28.8 | - | 8.1 | 14.4 | 17.7 | 17.6 | 17.7 | 19.7 | 22.2 | 22.1 | 22.2 | 22.2 | - | ||
| TL SD | 1.2 | 3.1 | 2.6 | 2.9 | 2.9 | 2.8 | 2.1 | 2.4 | 1.2 | 2.8 | - | 1.3 | 2.5 | 1.9 | 1.9 | 2.1 | 2.2 | 1.3 | 1.4 | 1.3 | 0.8 | - | ||
| Unsexed juveniles | ||||||||||||||||||||||||
| Age (quarters) | 3 | 6 | 9 | 12 | N | |||||||||||||||||||
| Total length (cm) | ||||||||||||||||||||||||
| 4 | 1 | 1 | ||||||||||||||||||||||
| 6 | 8 | 8 | ||||||||||||||||||||||
| 7 | 10 | 10 | ||||||||||||||||||||||
| 8 | 5 | 5 | ||||||||||||||||||||||
| 9 | 2 | 1 | 3 | |||||||||||||||||||||
| N | 26 | 1 | - | - | 27 | |||||||||||||||||||
| TL mean (cm) | 7.4 | 9.3 | - | - | ||||||||||||||||||||
| TL SD | 1.1 | - | - | - | ||||||||||||||||||||
References
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS ONE 2013, 8, e79889. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Watson, R.; Pauly, D. Signature of Ocean Warming in Global Fisheries Catch. Nature 2013, 497, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Ben Souissi, J.; Busoni, G.; Coco, S.; Chryssanthi, A.; Fanelli, E.; et al. Climate Change, Biological Invasions, and the Shifting Distribution of Mediterranean Fishes: A Large-Scale Survey Based on Local Ecological Knowledge. Glob. Chang. Biol. 2019, 25, 2779–2792. [Google Scholar] [CrossRef]
- Casini, M.; Bartolino, V.; Molinero, J.C.; Kornilovs, G. Linking Fisheries, Trophic Interactions and Climate: Threshold Dynamics Drive Herring Clupea harengus Growth in the Central Baltic Sea. Mar. Ecol. Prog. Ser. 2010, 413, 241–252. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F. Fishing down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Barausse, A.; Michieli, A.; Riginella, E.; Palmeri, L.; Mazzoldi, C. Long-Term Changes in Community Composition and Life-History Traits in a Highly Exploited Basin (Northern Adriatic Sea): The Role of Environment and Anthropogenic Pressures. J. Fish Biol. 2011, 79, 1453–1486. [Google Scholar] [CrossRef]
- Kuparinen, A.; Hutchings, J.A. Consequences of Fisheries-Induced Evolution for Population Productivity and Recovery Potential. Proc. R. Soc. B Biol. Sci. 2012, 279, 2571–2579. [Google Scholar] [CrossRef]
- Fortibuoni, T.; Giovanardi, O.; Pranovi, F.; Raicevich, S.; Solidoro, C.; Libralato, S. Analysis of Long-Term Changes in a Mediterranean Marine Ecosystem Based on Fishery Landings. Front. Mar. Sci. 2017, 4, 33. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards Sustainability in World Fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; Molinos, J.G.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of Marine Organisms to Climate Change across Oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Pauly, D.; Cheung, W.W.L. Sound Physiological Knowledge and Principles in Modeling Shrinking of Fishes under Climate Change. Glob. Chang. Biol. 2018, 24, e15–e26. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Olsen, E.M.; Ohlberger, J.; Saborido-Rey, F.; Murua, H.; Piñeiro, C.; Stenseth, N.C. Contrasting Evolutionary Demography Induced by Fishing: The Role of Adaptive Phenotypic Plasticity. Ecol. Appl. 2014, 24, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Free, C.M.; Thorson, J.T.; Pinsky, M.L.; Oken, K.L.; Wiedenmann, J.; Jensen, O.P. Impacts of Historical Warming on Marine Fisheries Production. Science 2019, 365, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Moullec, F.; Barrier, N.; Drira, S.; Guilhaumon, F.; Marsaleix, P.; Somot, S.; Ulses, C.; Velez, L.; Shin, Y.J. An End-to-End Model Reveals Losers and Winners in a Warming Mediterranean Sea. Front. Mar. Sci. 2019, 6, 345. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Pauly, D. Impacts and Effects of Ocean Warming on Marine Fishes. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; Laffoley, D., Baxter, J.M., Eds.; IUCN: Gland, Switzerland, 2016; pp. 239–253. [Google Scholar]
- Genner, M.J.; Sims, D.W.; Southward, A.J.; Budd, G.C.; Masterson, P.; Mchugh, M.; Rendle, P.; Southall, E.J.; Wearmouth, V.J.; Hawkins, S.J. Body Size-Dependent Responses of a Marine Fish Assemblage to Climate Change and Fishing over a Century-Long Scale. Glob. Chang. Biol. 2010, 16, 517–527. [Google Scholar] [CrossRef]
- Hidalgo, M.; Rouyer, T.; Bartolino, V.; Cerviño, S.; Ciannelli, L.; Massutí, E.; Jadaud, A.; Saborido-Rey, F.; Durant, J.M.; Santurtún, M.; et al. Context-Dependent Interplays between Truncated Demographies and Climate Variation Shape the Population Growth Rate of a Harvested Species. Ecography 2012, 35, 637–649. [Google Scholar] [CrossRef]
- Engelhard, G.H.; Righton, D.A.; Pinnegar, J.K. Climate Change and Fishing: A Century of Shifting Distribution in North Sea Cod. Glob. Chang. Biol. 2014, 20, 2473–2483. [Google Scholar] [CrossRef]
- O’Connor, M.I.; Holding, J.M.; Kappel, C.V.; Duarte, C.M.; Brander, K.; Brown, C.J.; Bruno, J.F.; Buckley, L.; Burrows, M.T.; Halpern, B.S.; et al. Strengthening Confidence in Climate Change Impact Science. Glob. Ecol. Biogeogr. 2015, 24, 64–76. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; Ben Rais Lasram, F.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under Siege: Spatial Overlap between Marine Biodiversity, Cumulative Threats and Marine Reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Colloca, F.; Scarcella, G.; Libralato, S. Recent Trends and Impacts of Fisheries Exploitation on Mediterranean Stocks and Ecosystems. Front. Mar. Sci. 2017, 4, 244. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Response to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien Species in a Warmer World: Risks and Opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Bazairi, H.; Ben Haj, S.; Boero, F.; Cebrian, D.; De Juan, S.; Limam, A.; Lleonart, J.; Torchia, G.; Rais, C. The Mediterranean Sea Biodiversity: State of the Ecosystems, Pressures, Impacts; Unep-Map-Rac/Spa: Tunis, Tunisia, 2010; pp. 1–100. [Google Scholar]
- Cardinale, M.; Scarcella, G. Mediterranean Sea: A Failure of the European Fisheries Management System. Front. Mar. Sci. 2017, 4, 72. [Google Scholar] [CrossRef]
- García-Monteiro, S.; Sobrino, J.A.; Julien, Y.; Sòria, G.; Skokovic, D. Surface Temperature Trends in the Mediterranean Sea from MODIS Data during Years 2003–2019. Reg. Stud. Mar. Sci. 2022, 49, 102086. [Google Scholar] [CrossRef]
- Tortonese, E. The Main Biogeographical Features and Problems of the Mediterranean Fish Fauna. Copeia 1964, 1964, 98–107. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Guilhaumon, F.; Albouy, C.; Somot, S.; Thuiller, W.; Mouillot, D. The Mediterranean Sea as a “cul-de-Sac” for Endemic Fishes Facing Climate Change. Glob. Chang. Biol. 2010, 16, 3233–3245. [Google Scholar] [CrossRef]
- Vallisneri, M.; Scapolatempo, M.; Tommasini, S. Reproductive Biology of Merlangius merlangus, L. (Osteichthyes, Gadidae) in the Northern Adriatic Sea. Acta Adriat. 2006, 47, 159–165. [Google Scholar]
- Vallisneri, M.; Vecchi, A.; Manfredi, C. The Biological Cycle of Merlangius merlangus (Linnaeus, 1758) (Osteichthyes, Gadidae) in the Northern and Middle Adriatic Sea. Biol. Mar. Medit. 2004, 11, 652–656. [Google Scholar]
- Reynolds, J.D.; Dulvy, N.K.; Goodwin, N.B.; Hutchings, J.A. Biology of Extinction Risk in Marine Fishes. Proc. R. Soc. B Biol. Sci. 2005, 272, 2337–2344. [Google Scholar] [CrossRef]
- Russo, A.; Artegiani, A. Adriatic Sea Hydrography. Sci. Mar. 1996, 60, 33–43. [Google Scholar]
- Hopkins, T.S. The Structure of Ionian and Levantine Seas. Rep. Meteorol. Oceanogr. 1992, 41, 35–36. [Google Scholar]
- Ludwig, W.; Dumont, E.; Meybeck, M.; Heussner, S. River Discharges of Water and Nutrients to the Mediterranean and Black Sea: Major Drivers for Ecosystem Changes during Past and Future Decades? Prog. Oceanogr. 2009, 80, 199–217. [Google Scholar] [CrossRef]
- Fonda Umani, S. Pelagic Production and Biomass in the Adriatic Sea. Sci. Mar. 1996, 60, 65–77. [Google Scholar]
- Russo, A.; Carniel, S.; Sclavo, M.; Krzelj, M. Climatology of the Northern-Central Adriatic Sea. In Modern Climatology; Simon Wang, S.Y., Ed.; InTech: Rijeka, Croatia, 2012; pp. 177–212. [Google Scholar] [CrossRef]
- Supić, N.; Grbec, B.; Vilibić, I.; Ivančić, I. Long-Term Changes in Hydrographic Conditions in Northern Adriatic and Its Relationship to Hydrological and Atmospheric Processes. Ann. Geophys. 2004, 22, 733–745. [Google Scholar] [CrossRef]
- Kaschner, K.; Rius-Barile, J.; Kesner-Reyes, K.; Garilao, C.; Kullander, S.O.; Rees, T.; Froese, R. AquaMaps: Predicted Range Maps for Aquatic Species. Available online: www.aquamaps.org (accessed on 10 September 2022).
- Bolger, T.; Connolly, P.L. The Selection of Suitable Indices for the Measurement and Analysis of Fish Condition. J. Fish Biol. 1989, 34, 171–182. [Google Scholar] [CrossRef]
- Vitale, F.; Worsøe Clausen, L.; Nì Chonchuir, G. Handbook of Fish Age Estimation Protocols and Validation Methods. In ICES Cooperative Research Report; ICES: Copenhagen, Denmark, 2019; Volume 346. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, Precision and Quality Control in Age Determination, Including a Review of the Use and Abuse of Age Validation Methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Beamish, R.J.; Fournier, D.A. A Method for Comparing the Precision of a Set of Age Determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Chang, W.Y.B. A Statistical Method for Evaluating the Reproducibility of Age Determination. Can. J. Fish. Aquat. Sci. 1982, 39, 1208–1210. [Google Scholar] [CrossRef]
- Kimura, D.K. Likelihood Methods for the von Bertalanffy Growth Curve. Fish. Bull. 1980, 77, 765–776. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: https://fishbase.mnhn.fr/popdyn/PopGrowthList.php?ID=29&Genus Name=Merlangius&SpeciesName=merlangus&fc=183 (accessed on 10 December 2022).
- ICES CM 2007/ACFM:33; Report of the Workshop on Sexual Maturity Staging of Cod, Whiting, Haddock and Saithe (WKMSCWHS). ICES: Copenhagen, Denmark, 2008. [CrossRef]
- Murua, H.; Kraus, G.; Saborido-Rey, F.; Witthames, P.R.; Thorsen, A.; Junquera, S. Procedures to Estimate Fecundity of Marine Fish Species in Relation to Their Reproductive Strategy. J. Northwest Atl. Fish. Sci. 2003, 33, 33–54. [Google Scholar] [CrossRef]
- Choy, S.C. A Rapid Method for Removing and Counting Eggs from Fresh and Preserved Decapod Crustaceans. Aquaculture 1985, 48, 369–372. [Google Scholar] [CrossRef]
- Pearson, A.G.E. Histochemistry: Theoretical and Applied. Vol. 2: Analytical Technology; Churchill Livingstone: Edinburgh, UK, 1985. [Google Scholar] [CrossRef]
- Saber, S.; Macías, D.; Ortiz de Urbina, J.; Kjesbu, O.S. Stereological Comparison of Oocyte Recruitment and Batch Fecundity Estimates from Paraffin and Resin Sections Using Spawning Albacore (Thunnus alalunga) Ovaries as a Case Study. J. Sea Res. 2015, 95, 226–238. [Google Scholar] [CrossRef]
- Murua, H.; Saborido-Rey, F. Female Reproductive Strategies of Marine Fish Species of the North Atlantic. J. Northwest Atl. Fish. Sci. 2003, 33, 23–31. [Google Scholar] [CrossRef]
- Hunter, J.R.; Macewicz, B.J.; Chyan-Huei Lo, N.; Kimbrell, C.A. Fecundity, Spawning, and Maturity of Female Dover Sole Microstomus Pacificus, with an Evaluation of Assumptions and Precision. Fish. Bull. 1992, 90, 101–128. [Google Scholar]
- Fisher, J.A.D.; Frank, K.T.; Leggett, W.C. Breaking Bergmann’s Rule: Truncation of Northwest Atlantic Marine Fish Body Sizes. Ecology 2010, 91, 2499–2505. [Google Scholar] [CrossRef]
- Bianchi, G.; Gislason, H.; Graham, K.; Hill, L.; Jin, X.; Koranteng, K.; Manickchand-Heileman, S.; Payá, I.; Sainsbury, K.; Sanchez, F.; et al. Impact of Fishing on Size Composition and Diversity of Demersal Fish Communities. ICES J. Mar. Sci. 2000, 57, 558–571. [Google Scholar] [CrossRef]
- Shin, Y.J.; Rochet, M.J.; Jennings, S.; Field, J.G.; Gislason, H. Using Size-Based Indicators to Evaluate the Ecosystem Effects of Fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef]
- Yemane, D.; Field, J.G.; Leslie, R.W. Indicators of Change in the Size Structure of Fish Communities: A Case Study from the South Coast of South Africa. Fish. Res. 2008, 93, 163–172. [Google Scholar] [CrossRef]
- Edwards, C.T.T.; Plagányi, É.E. Protecting Old Fish through Spatial Management: Is There a Benefit for Sustainable Exploitation? J. Appl. Ecol. 2011, 48, 853–863. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Knust, R. Climate Change Affects Marine Fishes Through the Oxygen Limitation of Thermal Tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef]
- Atkinson, D.; Sibly, R.M. Why Are Organisms Usually Bigger in Colder Environments? Making Sense of a Life History Puzzle. Trends Ecol. Evol. 1997, 12, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Tropical Fishes: Patterns and Propensities. J. Fish Biol. 1998, 53, 1–17. [Google Scholar] [CrossRef]
- Lindmark, M.; Ohlberger, J.; Gårdmark, A. Optimum Growth Temperature Declines with Body Size within Fish Species. Glob. Chang. Biol. 2022, 28, 2259–2271. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Marine Biodiversity of the Mediterranean Sea: Situation, Problems and Prospects for Future Research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Bonacci, O.; Vrsalović, A. Differences in Air and Sea Surface Temperatures in the Northern and Southern Part of the Adriatic Sea. Atmosphere 2022, 13, 1158. [Google Scholar] [CrossRef]
- Cohen, D.M.; Inada, T.; Iwamoto, T.; Scialabba, N. FAO Species Catalogue. Vol. 10. Gadiform Fishes of the World (Order Gadiformes). An Annotated and Illustrated Catalogue of Cods, Hakes, Grenadiers and Other Gadiform Fishes Known to Date. FAO Fish. Syn. 1990, 125, 319–327. [Google Scholar]
- Forster, J.; Hirst, A.G.; Atkinson, D. Warming-Induced Reductions in Body Size Are Greater in Aquatic than Terrestrial Species. Proc. Natl. Acad. Sci. USA 2012, 109, 19310–19314. [Google Scholar] [CrossRef]
- Casini, M.; Käll, F.; Hansson, M.; Plikshs, M.; Baranova, T.; Karlsson, O.; Lundström, K.; Neuenfeldt, S.; Gårdmark, A.; Hjelm, J. Hypoxic Areas, Density-Dependence and Food Limitation Drive the Body Condition of a Heavily Exploited Marine Fish Predator. R. Soc. Open Sci. 2016, 3, 160416. [Google Scholar] [CrossRef]
- Kolding, J.; Haug, L.; Stefansson, S. Effect of Ambient Oxygen on Growth and Reproduction in Nile Tilapia (Oreochromis niloticus). Can. J. Fish. Aquat. Sci. 2008, 65, 1413–1424. [Google Scholar] [CrossRef]
- Panfili, J.; de Pontual, H.; Troadec, H.; Wright, P.J. Manual of Fish Sclerochronology; Ifremer-lRD Coedition: Brest, France, 2002. [Google Scholar]
- Choat, J.H.; Axe, L.M. Growth and Longevity in Acanthurid Fishes; An Analysis of Otolith Increments. Mar. Ecol. Prog. Ser. 1996, 134, 15–26. [Google Scholar] [CrossRef]
- Fablet, R.; Pecquerie, L.; de Pontual, H.; Høie, H.; Millner, R.; Mosegaard, H.; Kooijman, S.A.L.M. Shedding Light on Fish Otolith Biomineralization Using a Bioenergetic Approach. PLoS ONE 2011, 6, e27055. [Google Scholar] [CrossRef]
- Piroddi, C.; Coll, M.; Liquete, C.; Macias, D.; Greer, K.; Buszowski, J.; Steenbeek, J.; Danovaro, R.; Christensen, V. Historical Changes of the Mediterranean Sea Ecosystem: Modelling the Role and Impact of Primary Productivity and Fisheries Changes over Time. Sci. Rep. 2017, 7, 44491. [Google Scholar] [CrossRef]
- Santojanni, A.; Arneri, E.; Bernardini, V.; Cingolani, N.; Di Marco, M.; Russo, A. Effects of Environmental Variables on Recruitment of Anchovy in the Adriatic Sea. Clim. Res. 2006, 31, 181–193. [Google Scholar] [CrossRef]
- Macias, D.; Garcia-Gorriz, E.; Piroddi, C.; Stips, A. Biogeochemical Control of Marine Productivity in the Mediterranean Sea during the Last 50 Years. Glob. Biogeochem. Cycles 2014, 28, 897–907. [Google Scholar] [CrossRef]
- Hussy, K.; Coad, J.O.; Farrell, E.D.; Clausen, L.W.; Clarke, M.W. Sexual Dimorphism in Size, Age, Maturation, and Growth Characteristics of Boarfish (Capros aper) in the Northeast Atlantic. ICES J. Mar. Sci. 2012, 69, 1729–1735. [Google Scholar] [CrossRef]
- Lauerburg, R.A.M.; Keyl, F.; Kotterba, P.; Floeter, J.; Temming, A. Sex-Specific Food Intake in Whiting Merlangius merlangus. J. Fish Biol. 2015, 86, 1729–1753. [Google Scholar] [CrossRef]
- Yildiz, T.; Saadet Karakulak, F. Age, Growth and Mortality of Whiting (Merlangius merlangus, Linnaeus, 1758) from the Western Black Sea, Turkey. Turkish J. Fish. Aquat. Sci. 2019, 19, 793–804. [Google Scholar] [CrossRef]
- Hayward, A.; Gillooly, J.F. The Cost of Sex: Quantifying Energetic Investment in Gamete Production by Males and Females. PLoS ONE 2011, 6, e16557. [Google Scholar] [CrossRef] [PubMed]
- Barneche, D.R.; White, C.R.; Marshall, D.J. Fish Reproductive-energy Output Increases Disproportionately With Body Size. Science 2018, 645, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Giovanardi, O.; Rizzoli, M. Biological Data, Collected during the Pipeta Expeditions, on the Whiting, Merlangius merlangus (L.) in the Adriatic Sea. FAO Fish. Rep. 1984, 290, 149–153. [Google Scholar]
- Ottersen, G.; Hjermann, D.O.; Stenseth, N.C. Changes in Spawning Stock Structure Strengthen the Link between Climate and Recruitment in a Heavily Fished Cod (Gadus morhua) Stock. Fish. Oceanogr. 2006, 15, 230–243. [Google Scholar] [CrossRef]
- Mazlum, R.E.; Bilgin, S. Age, Growth, Reproduction and Diet of the Whiting, Merlangius merlangus euxinus (Nordmann, 1840), in the Southeastern Black Sea. Cah. Biol. Mar. 2014, 3, 463–474. [Google Scholar]
- Bilgin, S.; Bal, H.; Taşçı, B. Length Based Growth Estimates and Reproduction Biology of Whiting, Merlangius merlangus euxinus (Nordman, 1840) in the Southeast Black Sea. Turkish J. Fish. Aquat. Sci. 2012, 12, 871–881. [Google Scholar] [CrossRef]
- Mir-Arguimbau, J.; Balcells, M.; Raventós, N.; Martín, P.; Sabatés, A. Growth, Reproduction and Their Interplay in Blue Whiting (Micromesistius poutassou, Risso, 1827) from the NW Mediterranean. Fish. Res. 2020, 227, 105540. [Google Scholar] [CrossRef]
- Gerritsen, H.D.; Armstrong, M.J.; Allen, M.; McCurdy, W.J.; Peel, J.A.D. Variability in Maturity and Growth in a Heavily Exploited Stock: Whiting (Merlangius merlangus, L.) in the Irish Sea. J. Sea Res. 49 2003, 49, 69–82. [Google Scholar] [CrossRef]
- Timmerman, C.A.; Marchal, P.; Denamiel, M.; Couvreur, C.; Cresson, P. Seasonal and Ontogenetic Variation of Whiting Diet in the Eastern English Channel and the Southern North Sea. PLoS ONE 2020, 15, e0239436. [Google Scholar] [CrossRef] [PubMed]
- ICES CM 1998/G:14; Report of the Workshop on Otolith Ageing of North Sea Whiting. ICES: Copenhagen, Denmark, 1998. [CrossRef]
- Ross, S.D.; Hüssy, K. A Reliable Method for Ageing of Whiting (Merlangius merlangus) for Use in Stock Assessment and Management. J. Appl. Ichthyol. 2013, 29, 825–832. [Google Scholar] [CrossRef]
- Polat, N.; Gümücs, A. Ageing of Whiting (Merlangius merlangus euxinus, Nord., 1840) Based on Broken and Burnt Otolith. Fish. Res. 1996, 28, 231–236. [Google Scholar] [CrossRef]
- Yildiz, T.; Uzer, U.; Yemişken, E.; Karakulak, F.S.; Kahraman, A.E.; Çanak, Ö. Conserve Immatures and Rebound the Potential: Stock Status and Reproduction of Whiting (Merlangius merlangus [Linnaeus, 1758]) in the Western Black Sea. Mar. Biol. Res. 2021, 17, 815–827. [Google Scholar] [CrossRef]
- Pauly, D. Why Do Fish Reach First Maturity When They Do? J. Fish Biol. 2022, 101, 333–341. [Google Scholar] [CrossRef]
- Ismen, A. Fecundity of Whiting, Merlangius merlangus euxinus (L.) on the Turkish Black Sea Coast. Fish. Res. 1995, 22, 309–318. [Google Scholar] [CrossRef]
- Tortonese, E. Fauna d’Italia; Edizioni Calderini: Bologna, Italy, 1970. [Google Scholar]
- Ungaro, N.; Marano, G.; Piccinetti, C. Adriatic, Black Sea: The Whiting Doubt. Cybium 1995, 19, 311–315. [Google Scholar]
- Şalcıoğlu, A.; Gubili, C.; Krey, G.; Sönmez, A.Y.; Bilgin, R. Phylogeography and Population Dynamics of the Eastern Mediterranean Whiting (Merlangius merlangus) from the Black Sea, the Turkish Straits System, and the North Aegean Sea. Fish. Res. 2020, 229, 105614. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Sarmiento, J.L.; Dunne, J.; Frölicher, T.L.; Lam, V.W.Y.; Palomares, M.L.D.; Watson, R.; Pauly, D. Shrinking of Fishes Exacerbates Impacts of Global Ocean Changes on Marine Ecosystems. Nat. Clim. Chang. 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Lloret, J.; Sabatés, A.; Muñoz, M.; Demestre, M.; Solé, I.; Font, T.; Casadevall, M.; Martín, P.; Gómez, S. How a Multidisciplinary Approach Involving Ethnoecology, Biology and Fisheries Can Help Explain the Spatio-Temporal Changes in Marine Fish Abundance Resulting from Climate Change. Glob. Ecol. Biogeogr. 2015, 24, 448–461. [Google Scholar] [CrossRef]
- Lloret, J.; Serrat, A.; Thordarson, G.; Helle, K.; Jadaud, A.; Bruno, I.; Ordines, F.; Sartor, P.; Carbonara, P.; Rätz, H.J. The Poor Health of Deep-Water Species in the Context of Fishing Activity and a Warming Climate: Will Populations of Molva Species Rebuild or Collapse? J. Fish Biol. 2021, 98, 1572–1584. [Google Scholar] [CrossRef] [PubMed]

| Age (Quarters) | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Length (cm) | ||||||||||||
| 3 | 1 | 1 | ||||||||||
| 4 | 7 | 7 | ||||||||||
| 5 | 21 | 21 | ||||||||||
| 6 | 35 | 35 | ||||||||||
| 7 | 12 | 12 | ||||||||||
| 8 | 8 | 8 | ||||||||||
| 9 | 1 | 4 | 5 | |||||||||
| 10 | 1 | 3 | 4 | |||||||||
| 11 | 6 | 6 | ||||||||||
| 12 | 8 | 1 | 9 | |||||||||
| 13 | 22 | 4 | 26 | |||||||||
| 14 | 41 | 14 | 55 | |||||||||
| 15 | 6 | 8 | 2 | 16 | ||||||||
| 16 | 5 | 8 | 2 | 15 | ||||||||
| 17 | 7 | 8 | 4 | 19 | ||||||||
| 18 | 7 | 8 | 5 | 2 | 22 | |||||||
| 19 | 8 | 8 | 6 | 3 | 25 | |||||||
| 20 | 5 | 8 | 8 | 4 | 25 | |||||||
| 21 | 4 | 8 | 9 | 6 | 1 | 28 | ||||||
| 22 | 3 | 7 | 7 | 5 | 1 | 23 | ||||||
| 23 | 1 | 7 | 8 | 6 | 2 | 1 | 25 | |||||
| 24 | 4 | 3 | 5 | 4 | 1 | 3 | 20 | |||||
| 25 | 1 | 1 | 4 | 3 | 2 | 3 | 2 | 1 | 17 | |||
| 26 | 4 | 8 | 5 | 2 | 3 | 1 | 23 | |||||
| 27 | 1 | 4 | 5 | 4 | 2 | 2 | 1 | 19 | ||||
| 28 | 1 | 2 | 2 | 3 | 2 | 1 | 11 | |||||
| 29 | 1 | 3 | 1 | 1 | 1 | 7 | ||||||
| 30 | 1 | 1 | 3 | 1 | 1 | 1 | 8 | |||||
| 31 | 1 | 2 | 1 | 1 | 5 | |||||||
| 32 | 2 | 2 | 3 | 1 | 8 | |||||||
| 33 | 1 | 1 | 2 | |||||||||
| 34 | 1 | 1 | 2 | |||||||||
| 35 | 1 | 1 | ||||||||||
| 37 | 1 | 1 | ||||||||||
| N | 86 | 131 | 100 | 74 | 48 | 29 | 18 | 17 | 4 | 2 | 2 | 511 |
| TL mean (cm) | 6.3 | 15.2 | 18.9 | 22.2 | 23.6 | 27.8 | 28.9 | 27.5 | 28.9 | 31.3 | 28.8 | |
| TL SD | 1.2 | 3.2 | 3.8 | 3.5 | 3.1 | 3.5 | 3.5 | 3.1 | 5.5 | 1.8 | 1.1 |
| Age (Quarters) | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Length (cm) | ||||||||||||
| 3 | 0 | |||||||||||
| 4 | 1 | 1 | ||||||||||
| 5 | 0 | |||||||||||
| 6 | 13 | 13 | ||||||||||
| 7 | 15 | 1 | 16 | |||||||||
| 8 | 11 | 2 | 13 | |||||||||
| 9 | 5 | 2 | 7 | |||||||||
| 10 | 2 | 2 | 4 | |||||||||
| 11 | 3 | 3 | ||||||||||
| 12 | 6 | 6 | ||||||||||
| 13 | 10 | 1 | 11 | |||||||||
| 14 | 9 | 2 | 4 | 3 | 18 | |||||||
| 15 | 5 | 7 | 10 | 10 | 2 | 34 | ||||||
| 16 | 7 | 6 | 10 | 12 | 2 | 37 | ||||||
| 17 | 8 | 8 | 12 | 11 | 3 | 42 | ||||||
| 18 | 4 | 7 | 11 | 15 | 6 | 43 | ||||||
| 19 | 1 | 5 | 12 | 10 | 7 | 35 | ||||||
| 20 | 8 | 9 | 10 | 10 | 1 | 3 | 1 | 42 | ||||
| 21 | 3 | 8 | 10 | 9 | 2 | 3 | 2 | 1 | 38 | |||
| 22 | 4 | 5 | 7 | 10 | 2 | 2 | 2 | 1 | 33 | |||
| 23 | 1 | 4 | 7 | 4 | 2 | 4 | 2 | 24 | ||||
| 24 | 3 | 3 | 5 | 2 | 2 | 15 | ||||||
| 25 | 2 | 3 | 6 | 1 | 3 | 2 | 17 | |||||
| 26 | 1 | 1 | 2 | 3 | 1 | 8 | ||||||
| 27 | 2 | 5 | 2 | 9 | ||||||||
| 28 | 1 | 1 | 2 | |||||||||
| 29 | 1 | 1 | ||||||||||
| 30 | 1 | 1 | 1 | 3 | ||||||||
| 31 | 1 | 1 | ||||||||||
| 32 | 0 | |||||||||||
| 33 | 0 | |||||||||||
| 34 | 0 | |||||||||||
| 35 | 0 | |||||||||||
| 37 | 0 | |||||||||||
| N | 47 | 60 | 51 | 90 | 103 | 69 | 8 | 28 | 16 | 4 | - | 476 |
| TL mean (cm) | 7.7 | 14.4 | 18.5 | 19 | 19.2 | 21.6 | 22.6 | 25.5 | 24.2 | 27.9 | - | |
| TL SD | 1.2 | 2.8 | 2.4 | 2.8 | 2.9 | 3 | 1.6 | 3.6 | 2.2 | 5 | - |
| L∞ | k | t0 | φ | |
|---|---|---|---|---|
| 1990–1991 combined sexes | 29.47 | 1.633 | 0.052 | 3.15 |
| 2020–2021 combined sexes | 22.84 | 1.889 | −0.043 | 2.99 |
| 1990–1991 females | 35.14 | 1.219 | 0.045 | 3.17 |
| 2020–2021 females | 25.11 | 1.663 | −0.04 | 3.02 |
| 1990–1991 males | 26.7 | 1.831 | 0.059 | 3.11 |
| 2020–2021 males | 20.52 | 2.236 | −0.02 | 2.97 |
| χ2 | p-Value | ||
|---|---|---|---|
| 1990–1991 vs. 2020–2021 | L∞ | 33.25 | <0.01 |
| k | 1.1 | 0.29 | |
| Whole model | 255.78 | <0.01 | |
| Females vs. males 2020–2021 | L∞ | 19.23 | <0.01 |
| k | 2.5 | 0.11 | |
| Whole model | 94.62 | <0.01 | |
| Females vs. males 1990–1991 | L∞ | 42.39 | <0.01 |
| k | 13.21 | <0.01 | |
| Whole model | 80.07 | <0.01 | |
| Females 2020–2021 vs. 1990–1991 | L∞ | 28.64 | <0.01 |
| k | 3.75 | 0.05 | |
| Whole model | 175.78 | <0.01 | |
| Males 2020–2021 vs. 1990–1991 | L∞ | 29.16 | <0.01 |
| k | 1.45 | 0.23 | |
| Whole model | 177.69 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calì, F.; Stranci, F.; La Mesa, M.; Mazzoldi, C.; Arneri, E.; Santojanni, A. Whiting (Merlangius merlangus) Grows Slower and Smaller in the Adriatic Sea: New Insights from a Comparison of Two Populations with a Time Interval of 30 Years. Fishes 2023, 8, 341. https://doi.org/10.3390/fishes8070341
Calì F, Stranci F, La Mesa M, Mazzoldi C, Arneri E, Santojanni A. Whiting (Merlangius merlangus) Grows Slower and Smaller in the Adriatic Sea: New Insights from a Comparison of Two Populations with a Time Interval of 30 Years. Fishes. 2023; 8(7):341. https://doi.org/10.3390/fishes8070341
Chicago/Turabian StyleCalì, Federico, Federica Stranci, Mario La Mesa, Carlotta Mazzoldi, Enrico Arneri, and Alberto Santojanni. 2023. "Whiting (Merlangius merlangus) Grows Slower and Smaller in the Adriatic Sea: New Insights from a Comparison of Two Populations with a Time Interval of 30 Years" Fishes 8, no. 7: 341. https://doi.org/10.3390/fishes8070341
APA StyleCalì, F., Stranci, F., La Mesa, M., Mazzoldi, C., Arneri, E., & Santojanni, A. (2023). Whiting (Merlangius merlangus) Grows Slower and Smaller in the Adriatic Sea: New Insights from a Comparison of Two Populations with a Time Interval of 30 Years. Fishes, 8(7), 341. https://doi.org/10.3390/fishes8070341









