Abstract
Seasonal changes in feeding habits of hilsa (Tenualosa ilisha) were studied monthly in 2019 in the upper Meghna estuary, Bangladesh, through gut content analyses. Tychoplanktonic diatoms followed by copepods were abundant in all months and size groups of hilsa. The inverse relationship between niche breadth and relative length of the gut revealed hilsa consume a variety of food at their early stages while their feeding habit changes towards diatoms during the adult stage with the development of gill rakers. Young hilsas prefer mostly tychoplanktonic diatoms (Aulacoseira sp., Triceratium sp., Nitzschia sp., Synedra sp., and Coscinodiscus sp.) and copepods (Pseudodiaptomus sp.). However, adult hilsas rejected Aulacoseira sp. during their spawning season. Ingested sand grains correlated with tychoplankton, revealing their food value from epipelic microalgae and bacteria clinging to them and epibenthic foraging by young hilsas. Thus, this study revealed that young hilsas primarily feed on tychoplanktonic diatoms and copepods from water and near bottom of the upper Meghna estuary during January to June while later stages with the development of gill rakers allow hilsas to feed on planktonic diatoms. Epibenthic feeding needs to be considered more fully in evaluating the biology of hilsa and, perhaps, other clupeids, and in evaluating possible human impacts on this foraging habitat.
Keywords:
feeding modes; feeding plasticity; tychoplankton; epibenthic; zooplankton; hilsa shad; upper Meghna River Key Contribution:
Young hilsa primarily feed on tychoplanktonic diatoms and copepods from water and near bottom of the upper Meghna estuary during January to June while later stages with the development of gill rakers allow hilsa to feed on planktonic diatoms.
1. Introduction
Hilsa shad, mostly known as hilsa (Tenualosa ilisha, Hamilton 1822), is a clupeid species widely distributed in the northern Indian Ocean that migrates from saline to brackish and fresh waters for spawning or feeding purposes [1], especially through the Padma–Meghna River system in Bangladesh [2]. Hilsa catches from Bangladesh contributed the highest yield (50–60%) to the global harvest in 2019, followed by those from Myanmar (20–25%), India (15–20%), and other countries (5–10%), including Pakistan, Iran, Iraq, and Kuwait [2]. Hilsa is the most important fishery species in Bangladesh, with an annual production of 533,000 MT in 2018–2019, which contributed ~14% to the country’s total fishery production [3]. Hilsas are also of high nutritional value, as they contain significant levels of essential polyunsaturated fatty acids (PUFA), amino acids, minerals, and other lipids [4]. Among the 53 fin fish species reliant on the Meghna River, hilsa was not the major contributor [5]. However, the hilsa fishery plays a vital role in the employment of coastal fishers, earnings of foreign exchange, and poverty alleviation in Bangladesh. Moreover, human impacts on the sustainability of the hilsa fishery are not trivial [6].
Hilsa is an anadromous fish that spends its adult phase in marine habitats, reproductive phase in estuaries and rivers, and immature phase in major rivers and their tributaries. Therefore, feeding habits likely differ according to life phase, fish size, and food availability and in response to the season and other ecological factors [7]. Marine biologists and ecologists acknowledge that a thorough knowledge of the feeding habits of these anadromous fishes in different life phases is important for the sustainable management and protection of the hilsa fishery [8]. In this regard, ecosystem-based fishery management of hilsa fish, which depends on knowledge of the trophic dynamics and habitat–prey–predator interactions in the ecosystem, can be supported through gut content analysis [9].
Food availability is the most significant factor in determining the distribution of hilsa; low-salinity monsoon runoff, as large volumes of fresher, turbid and nutrient-rich water, supports breeding, migration, and the ecosystem’s productivity. Continual changes in food composition (phytoplankton, zooplankton, benthos, and fish) and feeding guilds (herbivorous, omnivorous, and carnivorous) may vary with variations in the physico-chemical factors of water, such as pH [10], dissolved oxygen [11], temperature [12], salinity [13], and inorganic nutrients [13,14,15].
The study of fish feeding habits through stomach content analysis has long been an important tool in fishery management [16]. Many methods (qualitative and quantitative) have been devised to assess fish dietary preferences through gut content analyses [17]. Nevertheless, most of the published research fails to provide comprehensive conclusions as to the season and the age-wise preferences of foods for hilsa. Studies on the seasonal changes of phytoplankton and zooplankton in the Meghna and Padma rivers in Bangladesh have been conducted [7,18]; however, long-term detailed studies, in particular, regarding feeding habits of size groups of hilsa in the upper Meghna Estuary have not yet been published. Therefore, the current study was performed to determine the variation of hilsa food preferences among different life stages in the upper Meghna Estuary.
2. Materials and Methods
2.1. Study Area and Period
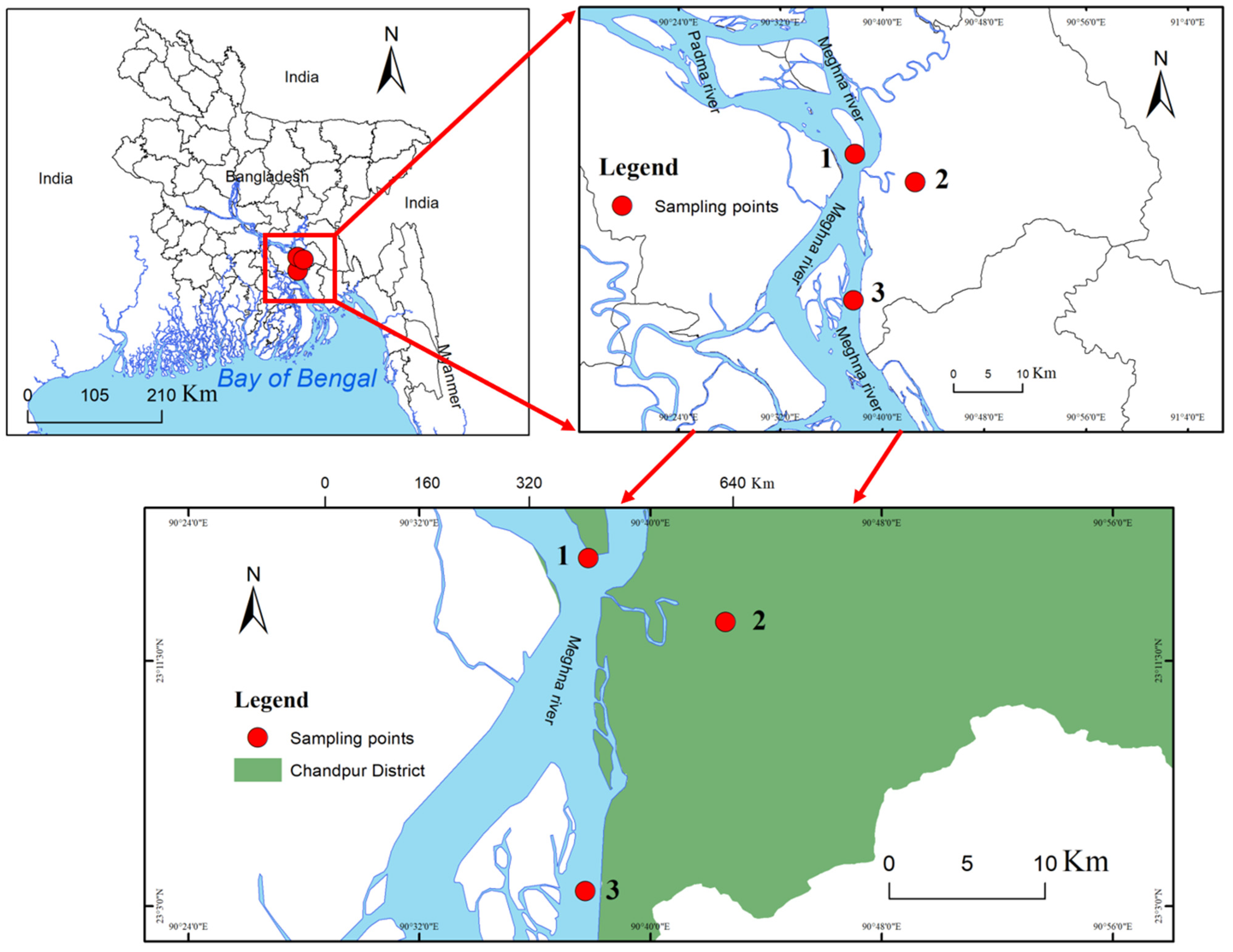
The Eastern Ganges Delta is also known as the Meghna Estuary (Figure 1). It originates from the hilly regions of eastern India. After entering Bangladesh, confluent rivers form the Meghna River Estuary, which is hydrographically referred to as the ‘upper Meghna’, and which flows to the Bay of Bengal [19]. The Meghna River Estuary is the largest estuarine ecosystem of Bangladesh, therefore representing an important hilsa fishery habitat [7]. This study was conducted in the upper Meghna Estuary, which flows through Chandpur District (Figure 1), considered one of the most important sanctuaries of hilsa fish by the Bangladeshi government [7]. The Chandpur area of the Meghna River Estuary has a significant flow through the river system and is considered one of the most important breeding and nursery grounds of hilsa in Bangladesh. The monsoon rainy season in Bangladesh occurs from June through to mid-October; rainfall during this season accounts for 75–80% of the country’s annual rainfall. The tidal water level fluctuations in the study area are reported to be highest (4.2 m) during the spring tide in the monsoon [5]. Three stations in the upper Meghna estuary, Haimchor (S1), Katakhali (S2), and the Padma–Meghna confluence (S3), were selected for sampling (Figure 1).
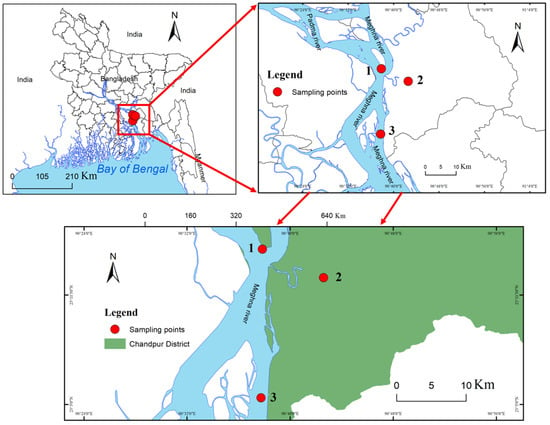
Figure 1.
The map shows the area of upper Meghna Estuary (Chandpur) and the red circles in the area show the location of the sampling stations.
2.2. Fish Collections
Monthly fish sampling in 2019 using gill nets with mesh sizes small enough to capture juvenile hilsa fish was performed (except for in July and August, owing to adverse monsoonal conditions). Hilsa specimens spanning different size ranges were collected by hilsa fishers. The freshly caught specimens were maintained in an insulated icebox and immediately brought to the laboratory, where they were immediately preserved in 10% buffered formalin. In total, 200 specimens (20 specimens per month) were collected (Table 1) and divided into seven different size groups (<10 g, 10–30 g, 30–50 g, 50–80 g, 100–200 g, 200–300 g, and 400–800 g in fresh weight).

Table 1.
Total number of sampled hilsa fish in different months from the upper Meghna River Estuary.
2.3. Water Collection for Plankton Analyses
Surface and bottom water samples (@ 250L) were collected at each station for the plankton study using a Kemmerer-type water sampler (Kemmerer, model 12.07, Netherlands) at each station. The samples were combined, then 250 L portions were filtered through plankton nets of a 200 µm mesh size for zooplankton analyses or 125 µm mesh size for phytoplankton analyses. Concentrated plankton samples were transferred to labeled plastic bottles and were preserved in 5% buffered formalin. Plankton specimens were identified to the genus level following Sahu et al. (2012), Hustedt (1985) and Yamaji (1966) [13,20,21]. The identification and counting of plankton in these samples were performed using a Sedgwick–Rafter counting cell chamber, following standard methods [22].
2.4. Gut Content Assessment
Sampled hilsa fishes were washed in distilled water and dried using tissue paper. The total length (mm) of each specimen was recorded, then digestive tracts were dissected from the esophagus to the anus and preserved in 10% buffered formalin. The region from the pyloric stomach to the gizzard was measured for length in mm, then immersed in distilled water and examined under a stereomicroscope for the presence of food items. This measured gut length was also used for calculating relative gut length later. Food organisms were identified to the genus level [20,21] using a Leica BMD epifluorescence microscope at the microscopy laboratory of the Department of Biology and Marine Biology, University of North Carolina Wilmington, USA, and other gut contents were noted, including unidentifiable or abiotic materials.
2.5. Gut Contents Analyses
Gut length measurements were conducted on 2–12 individuals in each size class. The relative length of the gut (RLG, total length of gut (mm)/total length of fish (mm) was applied to understand changes in fish feeding habits across size classes, as a longer relative gut length is thought to reflect a diet of less easily digestible foods [23].
Several standard metrics were utilized to describe and compare hilsa gut contents across size groups, sampling times and the availability of food types in the environment.
The frequency of occurrence (%F) method describes the proportions of the readily identifiable food items in the gut, although it gives little indication of the relative amount or mass of each food type present in the stomach. The frequency of occurrence of food items inside the gut was calculated using the following formula:
where %Fi = frequency of occurrence (%F) of the ith food type in the sample; ni = number of stomachs in which the ith food type was found in the gut; and N = total number of stomachs with food in the sample [16,24,25]. For example, in January, 2019 diatoms were present in all the 20 sampled fish guts, so %Fdiatoms = 100 × (20/20) = 100%.
%Fi = 100 × (ni/N)
The volumetric index (%V) method is useful particularly for herbivorous and detritivorous fishes, when numerical techniques are difficult to apply [25]. Each food type in the stomach was allotted a certain number of points based on its visually ranked proportion of gut volume. The diet component of the lowest volume was given 1 point. Then, 2, 4, 8, 16 and 32 points were allotted for each more voluminous food type, respectively. The sum of all the points for each food item was scaled down to percentages of the total, to calculate the percentage of volume (%V) of the food types (including detrital materials in each gut) for all the fish examined from each station and month. Percentage volumes within each subsample were calculated as %Vi = (number of points allocated against each food type (i) ÷ total points allocated to all groups of plankton) × 100.
The index of relative importance (%IRI) is an integration of measurements of number, volume and frequency of occurrence to assist in evaluating the relationship of the various food items found in fish stomachs. %IRI was calculated by summing up the numerical and volumetric percentage values and multiplying it by the frequency of occurrence percentage value [26]:
where %Ni, %Vi and %Fi represent percentages of number, volume and frequency of occurrence of prey i, respectively. %Ni is the total number of dietary items (phytoplankton, zooplankton, or unidentified items) in each dietary group (i) divided by the total number of dietary items recorded per fish size class or per month multiplied by 100. %IRI values represent the ranking of food preference:
IRIi = (%Ni + %Vi) × %Fi
%IRI = (IRI of each group of plankton/total IRI of all groups of plankton) × 100
Niche breadth (NB) is ultimately dependent on variations in prey that can be ingested by the fish [27]. Levin’s measure of niche breadth was measured as NB = 1/(Σpi2) [28] where, pi = proportion of individual food item = number of each individual type of food item (or planktonic group) observed in each size class of hilsa gut ÷ total number of food items observed in guts of each size class of hilsa.
2.6. Standardized Niche Breadth
To standardize the measure of Levin’s niche breadth on a scale of 0 to 1, Levin’s measure of standardized niche breadth (Bs) was calculated from the following formula:
where n = total number of plankton recorded from every group of plankton in each size class of hilsa gut.
Bs = (NB − 1)/(n − 1)
2.7. Electivity Index (E)
We evaluated the diet types found in the gut vs. the food items found in the water column for analyzing Ivlev’s Electivity index (E) [29] from the following formula:
where Pg = percentage of a particular diet type in the gut; Pw = percentage of a particular diet type in the water. E values vary from −1 to +1. Positive values indicate selection for a certain food item while negative values indicate selection against that food type.
E = (Pg − Pw)/(Pg + Pw)
3. Results
3.1. Hilsa Gut Contents
We recorded 29 genera of microalgae in the analysis of hilsa guts (Table 2); among them, 11 genera are tychoplanktonic, 7 genera are planktonic and 1 genus is filamentous in nature. Other groups, including filamentous cyanobacteria, chlorophytes, filamentous charophytes, euglenas and polar, and centric diatoms were also recorded more rarely. Copepods (2 genera), rotifers (2 genera) and cladocerans (2 genera) were found as major groups of zooplankton in the guts of sampled hilsa. Guts of young hilsa fish contained a noticeable amount of sand particles along with detrital materials, but guts of adult hilsa fish did not contain any sand (Table 2).

Table 2.
Different types of planktonic genera observed from the hilsa gut contents.
3.2. Frequency of Occurrence (%F)
The dominant phytoplankton genera in hilsa guts with a high frequency of occurrence were the tychoplanktonic diatoms (95%) in January. The tychoplanktonic and planktonic algal frequencies of occurrence gradually decreased until December (Table 3). Some phytoplankton genera were found in specific months, such as the diatom Cyclotella sp. (%F = 90%) in January, the filamentous cyanobacterium Trichodesmium sp. (%F = 45%) in January, and the pennate diatom Nitzschia sp. (%F = 65%) and filamentous charophyte Mougeotia sp. (%F = 50%) in February (Table 3). Hilsa fry modify their feeding habits from tychoplanktonic to planktonic when they become larger (200–800 g) in size (Table 4, Figure 2). Tychoplanktonic species, notably Aulacoseira sp., most frequently (highest %F) occurred in hilsa fry (10–80 g), and Coscinodiscus sp. and Synedra sp. occurred frequently (highest %F) in the 10 g–800 g size classes of hilsa (Table 4, Figure 3). Hilsas in their early stages of life (10–30 g) prefer Copepoda and Cladocera (F% 58.08% and 48.81%, respectively) (Table 4). The detrital sand particles, %F, decreased with increased fish size and showed the highest value (15%) in the youngest sizes of hilsa fishes (below 10–50 g).

Table 3.
Feeding habits of Tenualosa ilisha by frequency of occurrence (%F), volumetric index (%V) and index of relative importance (%IRI) in months of January–December. N/A = not analyzed.

Table 4.
Frequency of occurrence (%F) of tychoplanktonic and holoplanktonic genera in gut contents of Tenualosa ilisha of different sizes (g) and in different months.
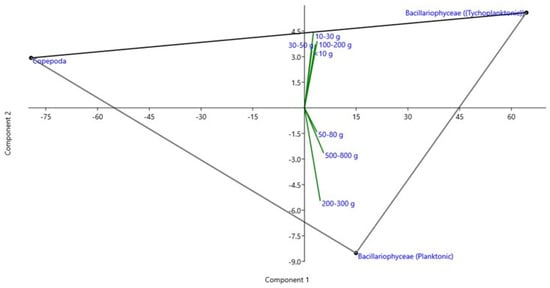
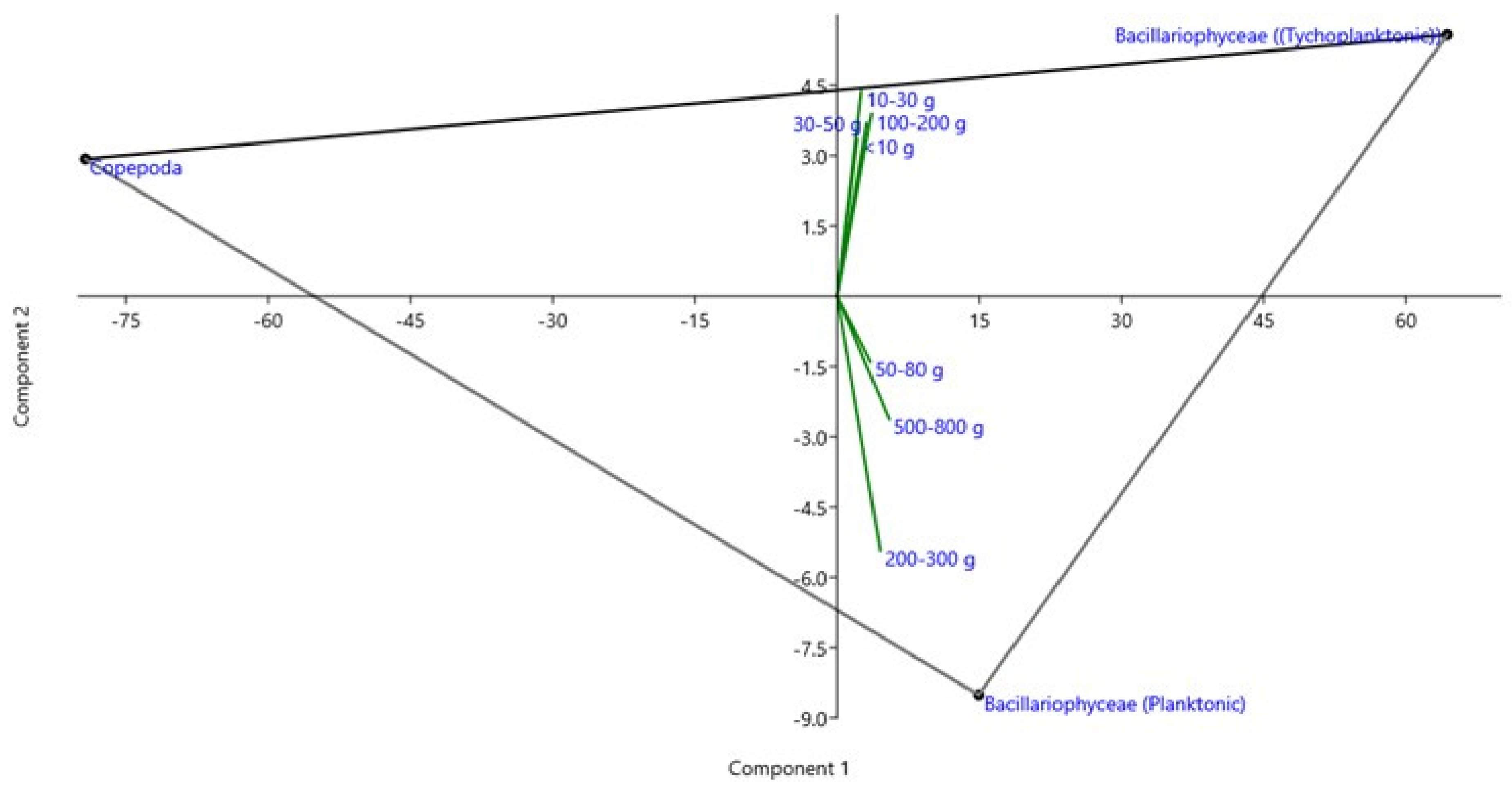
Figure 2.
Loading plot of rotated principal component analysis (PCA) of the different groups of microalgal preference observed by the gut content of seven sizes of hilsa fish based on the frequency of occurrence (%F) in the study area. Young and adult sizes of hilsa showed tychoplanktonic and planktonic algal preference, respectively.
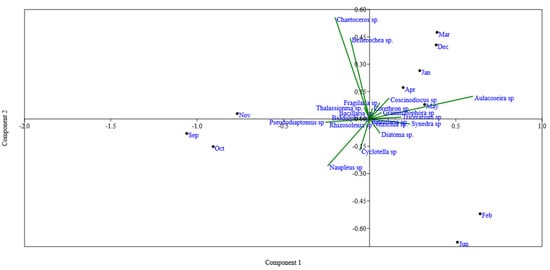
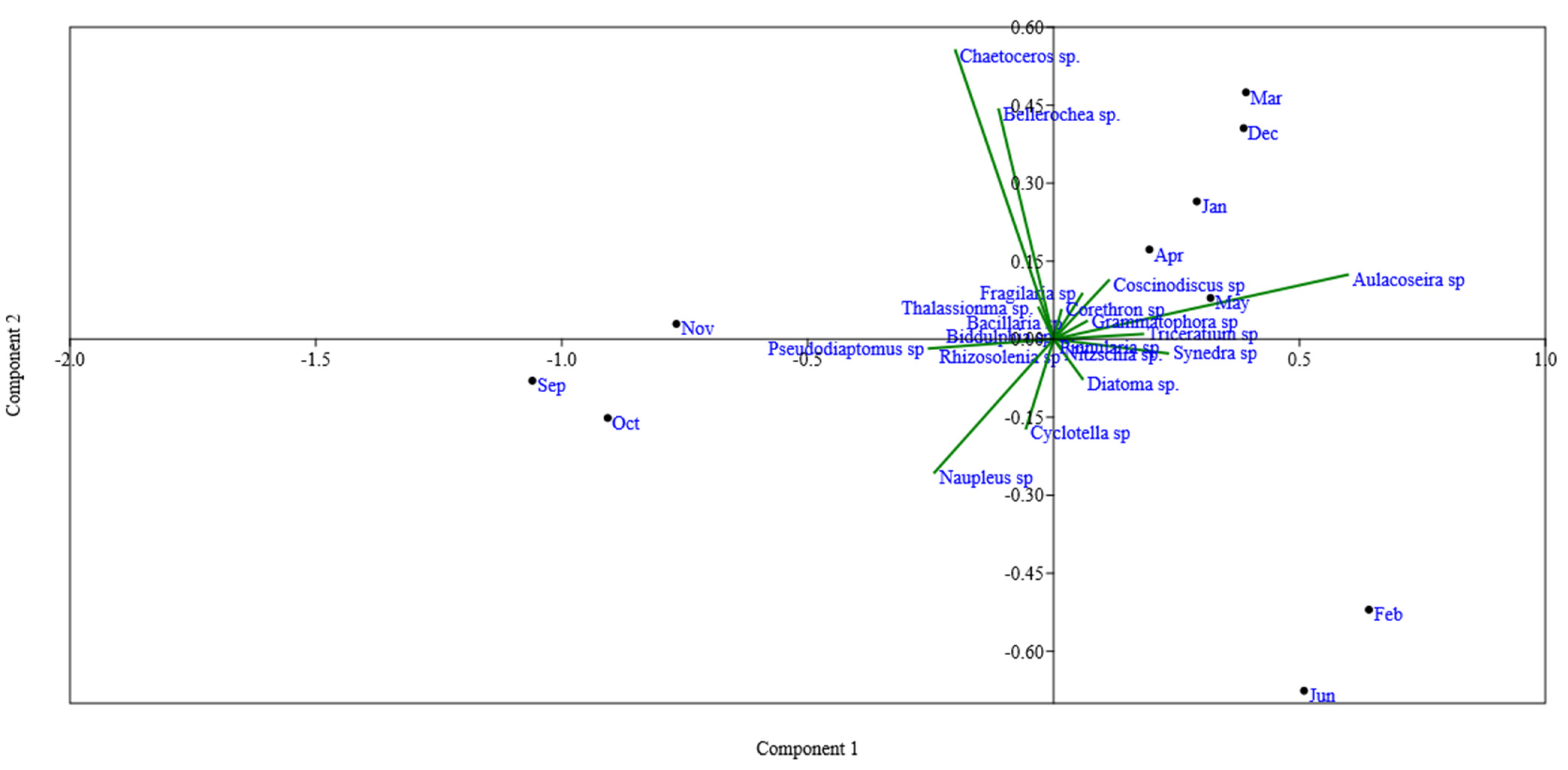
Figure 3.
Biplot of principal component analysis (PCA) showed the seasonal changes in the epibenthic feeding habits of hilsa in the study area. The epibenthic microalgae Aulacoseira sp. showed the highest feeding preference (January–June) followed by Coscinodiscus sp. while those species were completely avoided during spawning season (September–November).
3.3. Volumetric Index (%V)
The percentage of volume (%V) of the stomach (including detrital material) contents of hilsa revealed (Table 5, Figure 3) results similar to those in Table 4, Figure 2; frequency of occurrence (F%) measures showed little difference in values. Tychoplanktonic genera ranged from 47.08 to 66.75%; planktonic genera ranged from 16.68 to 25.02%; filamentous forms ranged from 1.47 to 12.51%; Phormidiaceae ranged from 0.78 to 6.25%; and Dinophyceae ranged from 0.18 to 3.12% among the microalgae. Copepoda ranged from 1.56 to 12.50% among zooplankton (Table 5) in all hilsa size groups. Copepoda also occupied the highest (6.21–12.50) %V in early stages (<10–50 g) and the lowest %V of the guts in adult stages (100 g–800 g) of hilsa, respectively. Hilsa mostly consume tychoplanktonic algae in their early stages of life in addition to copepods (Table 5, Figure 2). The highest amount (5.05%) of detrital sand particles was noticed in the early stages of hilsa (<10 g) and the content decreased (Table 5) with the increasing size of the hilsa (up to 80 g). Ingested sand materials were positively correlated (p < 0.05) with tychoplanktonic, planktonic, and filamentous groups of phytoplankton (except Chlorophyceae), and negatively correlated with zooplankton groups (Table 6). A strong positive correlation (r = 0.992, p < 0.05) of sand particles and unidentified materials might be noted with organic debris.

Table 5.
Feeding habits of Tenualosa ilisha by frequency of occurrence (%F) and volumetric index (%V) by size class.

Table 6.
Correlations between the volumes of ingested sand grains and the volumes of different food items estimated from the guts of young hilsa in the study. Bold items denote the significant relations (r, p < 0.05).** indicates highly significant values.
3.4. Index of Relative Importance (IRI%)
Tychoplanktonic Bacillariophyceae with an IRI% of 23.63 to 79.57% constituted the bulk of food items in the gut contents of hilsa, and Copepoda ranked second (1.87 to 43.54%) in all studied months (Table 3). Tychoplanktonic microalgae among other Bacillariophyceae constituted the bulk of food items (highest IRI%) throughout the year, indicating the epibenthic feeding habits of hilsa (Table 3). However, sand particles might also have microalgae (tychoplanktonic, planktonic and filamentous) or bacteria clinging on them that added food value although they drive no separate value in IRI% (Table 3). Therefore, the ranking of feeding habits of hilsa based on IRI% was tychoplanktonic > planktonic > filamentous > Cyanophyceae > Dinophyceae > Euglenophyceae > Chlorophyceae; that of zooplankton was Copepoda > Rotifera > Cladocera (Table 3).
3.5. Niche Breadth (NB) and Relative Length of Gut (RLG)
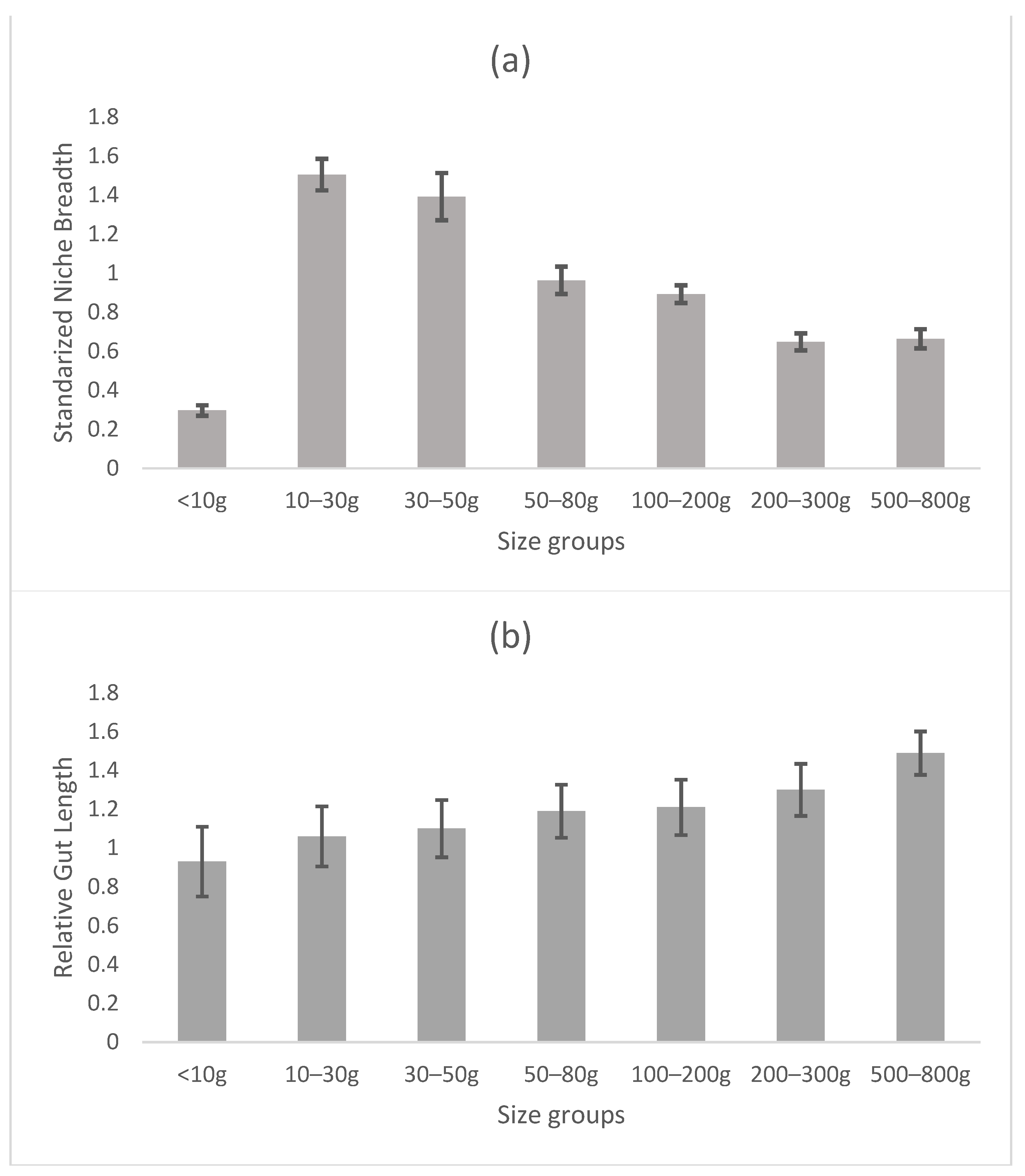
NB was calculated to understand the natural resource partitioning status of hilsa of different sizes. The RLG of hilsa was calculated to verify the changes in feeding habits in the different size groups. The standardized NB and RLG are summarized in Figure 4. The present study recorded a gradual decrease in the standardized NB of hilsa from fry (0.19) to adult (0.07), indicating greater diet specialization with age. The RLG revealed that the relative gut length increased with hilsa sizes from fry (RLG: 0.88) to adult (RLG: 1.49) (Figure 4) suggesting greater consumption of phytoplankton foods as fish age.
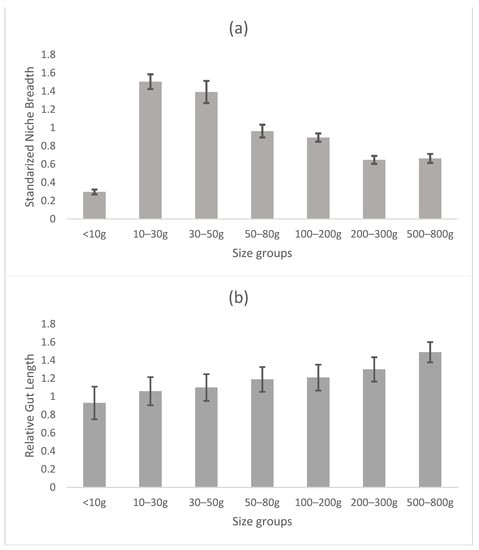
Figure 4.
Relationship between standardized niche breadth (a) and relative length of gut of Tenualosa ilisha of different size groups (b). Bars indicate standard deviation.
3.6. Electivity Index (EI)
The electivity index (EI) revealed a seasonal change in the phytoplankton and zooplankton species preferences of hilsa. Aulacoseira sp., Rhizosolenia sp., Synedra sp. and Coscinodiscus sp. were always favored from January to June (Figure 3). During spawning season (September and November), hilsa fish entirely avoided ingesting Aulacoseira sp. while Copepoda (Pseudodiaptomus sp) was found to most commonly consume zooplankton species throughout the study period and highly preferred this in the spawning season (0.35–0.64) (Figure 4).
4. Discussion
4.1. Hilsa Gut Contents
The number of phytoplankton (29) and zooplankton genera (6) recorded from the hilsa guts in this report did not show as diverse an assemblage of planktonic organisms as that found in the entire Meghna River Estuary [7], demonstrating patterns of selectivity in feeding. The shorter digestive tracts in smaller fish are thought to decrease the volume of food they can ingest at a time [30]. The substantial presence of Bacillariophyceae as the main component of the hilsa diet recorded in this study, however, corroborates the results of hilsa fish gut contents for the entire Meghna River Estuary [7] and Hoogly River, India [23].
4.2. Frequency of Occurrence (%F)
Hilsa fed primarily on tychoplanktonic diatoms and copepods, which is supported by other studies [7,23], although they did not distinguish tychopelagic from holoplanktonic forms or report sand grains in gut contents. The frequencies (%F) of sand particles we observed to be decreased with increased fish sizes, suggesting feeding by young hilsa in the nearshore, in epibenthic habitats. The presence of sand particles in hilsa guts, however, was also reported by [31]. Hora and Nair (1940) [32] reported sand grains in hilsa guts from the West Bengal waters, India, and they suggested that hilsa fish feed at the bottom, but no other studies considered the presence of sand in hilsa guts as indicators of feeding habitat in Bangladeshi riverine areas. Therefore, young hilsa feeding in the upper Meghna Estuary appear to be occur in nearshore bottom areas, as evidenced by the abundance of soft sediment benthic and tychopelagic diatoms, e.g., Biddulphia sp., Pinnularia sp., Synedra sp., Cyclotella sp., Nitzschia sp., Fragillaria sp. and Grammatophora sp. microalgae in their guts. We also found hilsa preferences for tychoplankton, mainly tychoplanktonic diatoms, in most life stages, but the fish also consume zooplankton in the early stages.
4.3. Volumetric Index (%V)
%V analyses yielded almost the same high values throughout the year for diatoms followed by copepods; however, the gut volumes copepods were higher in winter (November–February) than in summer (May–June). Similarly to the frequency of occurrence, volumetric analysis revealed the tychoplanktonic diatoms as important food items (47.08–66.75%) during the fry to adult stages of hilsa, corroborating the results of the study “Clupeids in the Estuary of the River Pendas, Malaysia” [33], but the percentages in the present study were higher, suggesting longer gut passage times are required to digest foods of plant origin such as microalgae during adult stages. Although there is no report on the seasonal variation in the food and feeding habits of hilsa from the upper Meghna Estuary, Hasan et al. (2016) [7] also made the generalization that hilsa mainly feed on phytoplankton with a small quantity of zooplankton. Most clupeids are capable of particulate and filter feeding of a wide range of particle sizes. Their gill rakers are coarser than those of true filter feeders [34], but they do allow the entrapment of microzooplankton and larger phytoplankton [35]. Like Hilsa, juvenile herring and the American shad [36] consume zooplankton. Our finding of greenish sand particles in young hilsa is the first report of the epibenthic feeding behaviors of hilsa in the upper Meghna Estuary. Lower riverine and nearshore areas in the study areas have been considered the most suitable habitats for hilsa fry [5]. The absence of teeth in the mouth and masticatory apparatus in the pharynx of hilsa is compensated for by the presence of a highly developed muscular gizzard-like pyloric stomach, gill rakers and moderately long intestines, which indicates microphagous feeding ability [37]. Gut analysis of different life stages of hilsa reveals that young hilsas are tychoplanktonic, benthic and copepod feeders while the adult hilsa mainly feeds on planktonic microalgae and copepods [32]. Adult hilsa also feed consistently on minute organisms such as diatoms, rotifers, green algae and protozoans [37]. The results of this study showing the consumption of copepods and the remarkable presence of sand grains and tychopelagic microalgae in young hilsa indicate that young hilsas aggregate near the bottom, feed epibenthicly and consume copepods aggregating near the bottom during day time. Sand grains in young hilsa guts strongly indicate epibenthic feeding and the incidental ingestion or actual deliberate ingestion of sand grains with attached microflora.
4.4. Index of Relative Importance (%IRI)
%IRI is usually estimated to rank the food items consumed by fishes. As with the %F and %V, %IRI also showed the dominance of Bacillariophyceae followed by copepods in hilsa gut contents. %IRI ranked tychoplanktonic diatoms and planktonic diatoms as first and second most important, respectively. The zooplankton ranking was Copepoda > Rotifera > Cladocera. %IRI cannot account for sand grains as the plankton samples were not evaluated for sand grain contents [38] and really could not be, as it was impossible to sample sand grains in the water column the way the fish themselves might.
4.5. Electivity Index (EI)
Diatoms and copepods, the most preferred food items for young hilsa in the Meghna River Estuary could be consistent with prior studies [7,39] conducted in the lower Meghna Estuary. Five genera of tychoplanktonic diatoms (Aulacoseira sp., Tricratium sp., Nitzschia sp., Synedra sp., and Coscinodiscus sp.) and one genus of each rotifer (Brachionus sp.) and copepod (Pseudodiaptomus sp.) were the most favored food items of young hilsas as those sizes (<10 g–80 g size) of hilsa were sampled during the monsoon (January to June) only. Adult hilsas during their spawning season (September to November) completely avoided Aulacoseira sp. Although there is no report to date on the age-wise species/genus selectivity of hilsa, Hasan et al. (2016) [7] concluded the group-wise preference (Bacillariophyceae and copepoda) of young hilsa which simply corroborated the results of our study but the negative electivity for zooplankton (−EI value) among all the sizes of hilsa could indicate greater difficulty in catching zooplankton.
4.6. Niche Breadth (NB) and Relative Length of Gut (RLG)
The niche breadth (NB) of hilsa decreased from 0.35 during the fry stage to 0.09 during the adult stage in the present study, which revealed hilsas as more generalized feeders during early stages (<10−80 g) and more specialized feeders during adult stages (100–800 g). The gradual increase in the RLG observed from fry to adult also revealed changes in feeding habits with the increase in hilsa size [40]. Again, this may reflect the development of gill rakers as filters in later stages of hilsa growth, coupled with less near-bottom foraging behavior evidenced by the lack of sand grains in the gut contents in adult-stage fish. We interpret this to mean that younger fish need to eat whatever they can handle in a food-dense near-bottom environment, but that bigger fish become more selective as their abilities improve.
5. Conclusions
This study examined the seasonal and size variation of feeding preferences of hilsa fishes from the upper Meghna Estuary, Bangladesh. A total of twenty nine microalgae and six zooplankton genera were recorded from the gut contents of hilsa. Microalgae, primarily tychoplanktonic diatoms, were most important in terms of percent frequency (%F) and percent volume (%V) in the stomachs across all hilsa size groups, whereas zooplankton occurred more frequently in the smaller sizes of hilsa. Tychoplanktonic diatoms ranked first, and copepods ranked second in importance as dietary items based on the index of relative importance (IRI%). Electivity (EI) positively responded to tychoplanktonic diatoms and zooplankton during January to June. Five genera of tychoplanktonic diatoms (Aulacoseira sp., Tricratium sp., Nitzschia sp., Synedra sp., and Coscinodiscus sp.) and 1 genus of rotifer (Brachionus sp.) and copepod (Pseudodiaptomus sp.) were the most favored food items of young hilsa while the tychoplanktonic diatom, Aulacoseira sp., was avoided during the spawning season by adult hilsas. The dietary selectivity of hilsas was higher in the monsoon season than in winter, reflecting more abundant food supplies. Ingested sand grains were positively correlated with microalgae identified from the guts of young hilsas (%V), demonstrating epibenthic foraging by young hilsa. This study also revealed an inverse relationship between niche breadth (NB) and relative length of gut (RLG), correlating with the observations that young hilsas feed broadly on tychoplanktonic diatoms and copepods from the near-bottom of the upper Meghna Estuary during January to June, while later stages with the development of gill rakers allow hilsas to feed more exclusively on planktonic diatoms. Epibenthic feeding needs to be considered more fully in evaluating the biology of hilsa and, perhaps, other clupeids, and in evaluating possible human impacts on this foraging habitat.
6. Patents
There are patents resulting from the work reported in this manuscript.
Author Contributions
Conceptualization, M.J.S., L.B.C. and P.K.S.; methodology, M.J.S., L.B.C. and A.K.D.; software, M.J.S.; validation, M.J.S., L.B.C., P.K.S. and M.A.B.; formal analysis, M.J.S., L.B.C. and M.M.H.; investigation, A.K.D.; resources, M.J.S.; data curation, A.K.D.; writing—original draft preparation, M.J.S.; writing—review and editing, L.B.C., P.K.S. and M.J.S.; visualization, M.M.S.; supervision, M.J.S.; project administration, Y.M.; funding acquisition, M.J.S. and M.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Noakhali Science and Technology University Ethical Committee (NSTUEC) (protocol code: NSTU/SCI/EC/2023/149, date of approval: 18 May 2023).
Informed Consent Statement
Not Applicable.
Data Availability Statement
All authors ensure that our manuscript is ethically sound and meets industry-recognized standards.
Acknowledgments
We are deeply indebted to Alison Taylor, Director of the Richard M. Dillaman Microscopy Facility, Department of Biology and Marine Biology, University of North Carolina, Wilmington, USA, for her permission to use a modern microscope.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wahab, M.A.; Beveridge, M.C.M.; Phillips, M.J. Hilsa: Status of fishery and potential for aquaculture. Penang Malays. World Fish. Proc. 2019, 16, 196. [Google Scholar]
- Mahmud, Y. (Ed.) Hilsa Fisheries Research and Development in Bangladesh; Bangladesh Fisheries Research Institute: Mymensingh, Bangladesh, 2020; 309p. [Google Scholar]
- Ahmed, M.; Mitu, S.J.; Schneider, P.; Alam, M.; Mozumder, M.M.H.; Shamsuzzaman, M.M. Socio-Economic Conditions of Small-Scale Hilsa Fishers in the Meghna River Estuary of Chandpur, Bangladesh. Sustainability 2021, 13, 12470. [Google Scholar] [CrossRef]
- Alam, A.K.M.; Mohanty, B.P.; Hoq, M.E.; Thilsted, S. Nutritional values, consumption and utilization of Hilsa Tenualosa ilisha (Hamilton 1822). In Proceedings of the Regional Workshop on Hilsa: Potential for Aquaculture, Dhaka, Bangladesh, 16–17 September 2012; Wahab, M.A., Beveridge, M.C.M., Phillips, M.J., Eds.; WorldFish: Penang, Malaysia, 2019; pp. 159–187. [Google Scholar]
- Hossain, M.S.; Das, N.G.; Sarker, S.; Rahaman, M.Z. Fish diversity and habitat relationship with environmental variables at Meghna river estuary, Bangladesh. Egypt. J. Aquat. Res. 2012, 38, 213–226. [Google Scholar] [CrossRef]
- Shaha, D.C.; Hasan, J.; Kundu, S.R.; Yusoff, F.M.; Salam, M.A.; Khan, M.; Haque, F.; Ahmed, M.; Rahman, M.J.; Wahab, M.A. Dominant phytoplankton groups as the major source of polyunsaturated fatty acids for hilsa (Tenualosa ilisha) in the Meghna estuary Bangladesh. Sci. Rep. 2022, 12, 20980. [Google Scholar] [CrossRef]
- Hasan, K.M.M.; Ahmed, Z.F.; Wahab, M.A.; Mohammed, E.Y. Food and Feeding Ecology of Hilsa (Tenualosa ilisha) in Bangladesh’s Meghna River Basin; International Institute for Environment and Development: London, UK, 2016. [Google Scholar]
- Khan, A.A.; Fatima, M. Feeding ecology of the Grey mullet, Rhinomugil corsula (Hamilton) from the river Yamuna, North India. Asian Fish. Sci. 1994, 7, 256–266. [Google Scholar] [CrossRef]
- Dutta, S.; Maity, S.; Bhattacharyya, S.B.; Sundaray, J.K.; Hazra, S. Diet composition and intensity of feeding of Tenualosa ilisha (Hamilton, 1822) occurring in the northern Bay of Bengal, India. Proc. Zool. Soc. 2014, 67, 33–37. [Google Scholar]
- Schmidt, L.E.; Hansen, P.J. Allelopathy in the prymnesiophyte Chrysochromulina polylepis: Effect of cell concentration, growth phase and pH. Mar. Ecol. Prog. Ser. 2001, 216, 67–81. [Google Scholar] [CrossRef]
- Weiss, R.F. The solubility of nitrogen, oxygen and argon in water and seawater. In Deep Sea Research and Oceanographic Abstracts; Elsevier: Amsterdam, The Netherlands, 1970; Volume 17, pp. 721–735. [Google Scholar] [CrossRef]
- Gogoi, P.; Sinha, A.; Das Sarkar, S.; Chanu, T.N.; Yadav, A.K.; Koushlesh, S.K.; Borah, S.; Das, S.K.; Das, B.K. Seasonal influence of physicochemical parameters on phytoplankton diversity and assemblage pattern in Kailash Khal, a tropical wetland, Sundarbans, India. Appl. Water Sci. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Sahu, G.; Satpathy, K.K.; Mohanty, A.K.; Sarkar, S.K. Variations in Community Structure of Phytoplankton in Relation to Physicochemical Properties of Coastal Waters, Southeast Coast of India. Indian J. Geo-Mar. Sci. 2012, 41, 223–241. Available online: https://www.researchgate.net/publication/233967860_Variations_in_community_structure_of_phytoplankton_in_relation_to_physicochemical_properties_of_coastal_waters_southeast_coast_of_India (accessed on 14 June 2023).
- Cahoon, L. Tychoplankton. In Encyclopedia of Estuaries; Kennish, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2016; p. 721. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Wang, Y.S.; Cheng, H.; Zhang, J.D.; Fei, J. Spatial variation of phytoplankton community structure in Daya Bay, China. Ecotoxicology 2015, 24, 1450–1458. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach content analysis: A review of methods and their applications. J. Fish Biol. Southampt. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Manoharan, J.; Gopalakrishnan, A.; Varadharajan, D.; Thilagavathi, B.; Priyadharsini, S. Stomach content analysis of Terapon jarbua (Forsskal) from Parangipettai coast, South East Coast of India. Adv. Appl. Sci. Res. 2012, 3, 2605–2621. [Google Scholar]
- Ahsan, D.A.; Kabir, A.N.; Rahman, M.M.; Mahabub, S.; Yesmin, R.; Faruque, M.H.; Naser, M.N. Plankton composition, abundance and diversity in hilsa (Tenualosa ilisha) migratory rivers of Bangladesh during spawning season. Dhaka Univ. J. Biol. Sci. 2012, 21, 177–189. [Google Scholar] [CrossRef]
- Rashid, S.M.A. Coastal Biodiversity—A Review. Report Prepared for Long Term Monitoring Research and Analysis of Bangladesh Coastal Zone. 2019. Available online: https://www.researchgate.net/profile/Rashid-Sma/publication/338855465_Coastal_Biodiversity_of_Bangladesh_-_A_Review/links/5e300a2e299bf10a659925cf/Coastal-Biodiversity-of-Bangladesh-A-Review.pdf (accessed on 14 June 2023).
- Hustedt, F. The Pennate Diatoms. A Translation of Hustedt’s ‘Die Kieselalgen Deutschlands, Österreichs und der Schweiz unter Berücksichtigung der übrigen Länder Europas Sowie der Angrenzenden Meeresgebiete. 2. Teil (1959)’ with Supplement from Jensen NG. 1985. Available online: https://www.worldcat.org/title/pennate-diatoms-a-translation-of-hustedts-die-kieselalgen-2-teil/oclc/12960768 (accessed on 14 June 2023).
- Yamaji, I. Illustrations of the Marine Plankton of Japan; Hoikusha: Osaka, Japan, 1966; p. 372. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992; Volume 6, Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1818549 (accessed on 14 June 2023).
- De, D.; Anand, P.S.; Sinha, S.; Suresh, V.R. Study on Preferred Food Items of Hilsa (Tenualosa isilha). Available online: https://www.ripublication.com/ijafst_spl/ijafstv4n7spl_02.pdf (accessed on 14 June 2023).
- Bowen, S.H. Quantitative description of the diet. In Fisheries Techniques, 2nd ed.; American Fisheries Society: Bethesda, Maryland, 1996; pp. 513–532. [Google Scholar]
- Hynes, H.B.N. The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J. Anim. Ecol. 1950, 19, 36–58. [Google Scholar] [CrossRef]
- Pinkas, L. Food habits of albacore, bluefin tuna and bonito in California waters. Calif. Dept. Fish Game Fish Bull. 1971, 152, 1–139. [Google Scholar]
- Landaeta, M.F.; Suárez-Donoso, N.; Bustos, C.A.; Balbontín, F. Feeding habits of larval Maurolicus parvipinnis (Pisces: Sternoptychidae) in Patagonian fjords. J. Plankton Res. 2011, 33, 1813–1824. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments; Princeton Monographs Series. No. 2; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar] [CrossRef]
- Ivlev, V.S. Experimental Ecology of the Feeding of Fishes; Yale University Press: New Haven, CT, USA, 1961; 302p, Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1538235 (accessed on 14 June 2023).
- Ribble, D.O.; Smith, M.H. Relative intestine length and feeding ecology of freshwater fishes. Growth 1983, 47, 292. [Google Scholar]
- Karna, S.K.; Guru, B.C.; Panda, S. Food and feeding habits of Tenualosa ilisha (Hamilton, 1822) from India’s largest brackish water lagoon. Fisheries 2014, 3, 123–125. [Google Scholar]
- Hora, S.L.; Nair, K.K. Further observations on the bionomics and fishery of the Indian shad, Hilsa ilisha (Hamilton), in Bengal waters. Rec. Zool. Surv. India 1940, 42, 35–50. [Google Scholar] [CrossRef]
- Ara, R.; Arshad, A.; Musa, L.; Amin, S.M.N.; Kuppan, P. Feeding habits of larval fishes of the family Clupeidae (Actinopterygii: Clupeiformes) in the estuary of River Pendas, Johor, Malaysia. J. Fish. Aquat. Sci. 2011, 6, 816. [Google Scholar] [CrossRef]
- Durbin, A.G.; Durbin, E.G. Grazing rates of the Atlantic menhaden Brevoortia tyrannus as a function of particle size and concentration. Mar. Biol. 1975, 33, 265–277. [Google Scholar] [CrossRef]
- James, A.G. Are clupeid microphagists herbivorous or omnivorous? A review of the diets of some commercially important clupeids. S. Afr. J. Mar. Sci. 1988, 7, 161–177. [Google Scholar] [CrossRef]
- Collette, B.B.; Klein-MacPhee, G. Bigelow and Schroeder’s Fishes of the Gulf of Maine, 3rd ed.; Smithsonian Institution Press: Washington, DC, USA; London, UK, 2002; Volume 4, p. 372. [Google Scholar] [CrossRef]
- Hossain, M.A.; Das, I.; Genevier, L.; Hazra, S.; Rahman, M.; Barange, M.; Fernandes, J.A. Biology and fisheries of Hilsa shad in Bay of Bengal. Sci. Total Environ. 2019, 651, 1720–1734. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.K.; Calver, M.C.; Dickman, C.R. The index of relative importance: An alternative approach to reducing bias in descriptive studies of animal diets. Wildl. Res. 2002, 29, 415–421. [Google Scholar] [CrossRef]
- Akter, A.; Rahman, M.A.; Isaac, S.; Sarker, M.J. Zooplankton in the Gut Content of Indian Shad (Tenualosa ilisha): Case Study at the Meghna River Estuary, Bangladesh 2016. Available online: https://www.rroij.com/open-access/zooplankton-in-the-gut-content-of-indian-shad-tenualosa-ilishacase-study-at-the-meghna-river-estuary-bangladesh-.pdf (accessed on 14 June 2023).
- De, D.K.; Datta, N.C. Studies on Certain Aspects of the Morpho-Histology of Indian Shad Hilsa, Tenualosa ilisha (Hamilton) in Relation to Food and Feeding Habits. 2011. Available online: https://agris.fao.org/agris-search/search.do?recordID=IN2022026203 (accessed on 14 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).