Abstract
The Pacific thread herring, Opisthonema libertate, is a fishery resource in the eastern tropical Pacific, yet its population dynamics are poorly understood. The aims of this work were to document the metazoan parasite species in O. libertate from the Gulf of California in Mexico, determine latitudinal changes in their infection levels, and assess their potential as biological tags. Six parasitic species were identified: Myosaccium ecaude and Parahemiurus merus digeneans, Cribromazocraes cf. travassosi and Mazocraeoides georgei monogeneans, Pseudoterranova sp. nematodes, and Lepeophtheirus sp. copepods. The monogenean and copepod species are reported for the first time in O. libertate. Many fish were infected with digeneans, and there was a rare occurrence of other parasites. While the prevalence and median intensity of M. ecaude and P. merus significantly varied among sampling localities, a multivariate analysis revealed a distinct separation between some localities; these results suggest that individual fish form several discrete populations. However, the utility of these parasites as biological tags will be further probed because they cannot satisfy all requirements for good biological tags. The present results might be helpful in complementing other techniques to determine the movement and stock structure of O. libertate, albeit with certain limitations.
Key Contribution:
Spatial changes in infection levels of adult digeneans suggest that individuals of Pacific thread herring remain resident in certain areas. However, the reliability of these parasites as biological tags is questioned.
1. Introduction
The Pacific thread herring, Opisthonema libertate (Günther, 1867) (Clupeiformes, Dorosomatidae), is a small pelagic fish distributed from the Pacific coast of Baja California and the Gulf of California, in Mexico, to Peru [1]. Like other small pelagic fish species, O. libertate is ecologically essential due to its role in mediating energy transfer between trophic levels [2,3]. Additionally, this species substantially contributes to the small pelagic fishery in the Mexican Pacific, especially in the Gulf of California [4,5].
The Gulf of California is a marginal, semi-enclosed sea area exceeding 1100 km in length and is considered to be a tropical marine biodiversity hotspot that houses several small pelagic fish species hunted by commercial fishing fleets [6], all of which has resulted in higher fishing pressure on O. libertate during the past decade [7]. This situation requires the structural population information of various species in order to achieve sustainable management. This information still needs to be improved for O. libertate despite the ecological and commercial importance thereof. Limited evidence on the morphometric study of the fish body and otoliths suggests a structured population of this species in the Gulf of California [8], which needs further investigation using a broad spectrum of techniques covering the multiple characteristics of this fish species [9].
Parasites are essential ecological organisms in oceans and can provide valuable information that enables a better understanding of all aspects of fish biology [10]. They also function as biological tags and help locate fish movement to improve the probability of correctly assigning a fish to its original stock, which are fundamental requirements for effective management [11,12,13]. The selection of parasites as tags requires careful screening; however, because not all parasites can be used as tags, with long-living parasites being recommended the most for this [14,15], this highlights the importance of the necessity of a knowledge of fish parasites and the ability to determine the spatial variability thereof across broad geographical areas. Concerning the parasites of O. libertate, a previous study reported Kuhnia sp. and Polymicrocotyle manteri Lamothe-Argumedo, 1967 monogeneans, Myosaccium ecaude Montgomery 1957 and Parahemiurus merus (Linton 1910) digeneans, Proteocephalidea gen. sp. cestodes, and Pseudoterranova sp. nematodes from fish collected in a locality of the Mexican Central Pacific [16], while no information is yet available regarding their distribution in the Gulf of California.
As such, the aims of this study were to investigate the distribution of the metazoan parasite species in O. libertate from the Gulf of California, determine latitudinal changes in their infection levels, and assess their potential as biological tags.
2. Materials and Methods
Fish samples were collected from fishing hauls in six localities of the eastern Gulf of California in June and July 2022 (Figure 1 and Table 1) during a research cruise onboard the R/V “Dr. Jorge Carranza Fraser” of the Instituto Nacional de Pesca y Acuacultura (INAPESCA). The fishing hauls were made with a midwater net of four equal caps (i.e., top and bottom footrope length: 48.17 m). The trawls were towed at an average speed of 6.5 km h−1 for 45 min with an average depth of 25 m. The hauls were made by prior detection using echograms recorded 24 h d−1 with a Simrad EK60 scientific echo sounder (Simrad Kongsberg Maritime AS, Horten, Norway) equipped with five split-beam transducers (i.e., 18, 38, 70, 120, and 200 kHz). After the collected fish samples were sorted by hand, 257 fish specimens were isolated, identified to the genus level, and frozen at −4 °C pending further use.
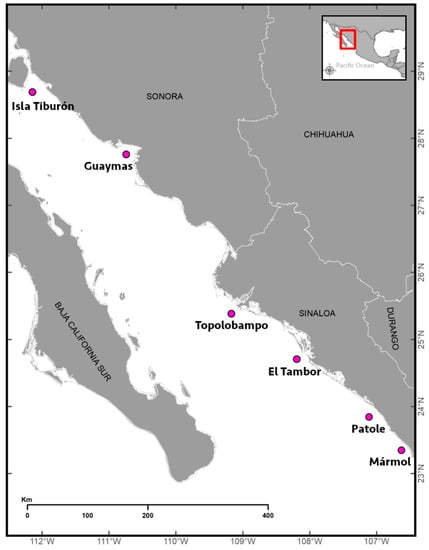
Figure 1.
Study area and sampling localities (pink dots) in the Gulf of California in Mexico.

Table 1.
Samples of Pacific thread herring, Opisthonema libertate, from six localities in the Gulf of California in Mexico.
At the laboratory, each frozen sample was thawed under ambient conditions and subjected to body weight (i.e., g), fork length (i.e., cm), and sex determination, and data were recorded. The fish were identified up to the species level using a previously published protocol [1]. The external surfaces, gills, cavities, internal organs, and musculature were examined for the presence of metazoan parasites using a stereomicroscope (Motic, Richmond, BC, Canada); all metazoan parasites were counted and preserved in 4% formalin. For morphological identification, platyhelminths were stained with Gomori’s trichrome reagent, dehydrated using graded ethanol series, cleared with methyl salicylate, and examined as permanent mounts in Canada balsam, while nematodes and crustaceans were cleared in lactic acid. All samples were examined using a compound microscope (Leica DMLB, Leica Microsystems, Wetzlar, Germany).
Fish length and weight were compared among the collected samples using one-way ANOVA. The prevalence and median intensity of parasitic infection was calculated for each parasite species [17,18], where the significant differences among the samples were determined by subjecting the prevalence values to Fisher’s exact test, and the intensity was compared with Mood’s median test (multiple comparisons) and bootstrap t-tests (pairwise comparisons), with 1000 replications using Qpweb software [18]. The mean intensity is commonly provided in parasitological studies; however, in the present study, we preferred to use the median intensity because it is a suitable metric to describe the typical level of infection in a sample [18]. To display the geographical location of the parasites that infect fish in each sampling locality, maps were created with the geographical co-ordinates of localities using the PBSmapping [19] and mapplots [20] packages in R software [21].
To study the effect of sampling locality differentiation based on parasite assemblages, non-metric multidimensional scaling (nMDS) was performed based on a Bray–Curtis similarity matrix of infection intensity data (i.e., excluding uninfected fish). The nMDS facilitates the creation of a points configuration in a specified number of dimensions in such a manner that the rank order agreement between the interpoint distances and the resemblance values is maximized. Distances to group centroids were visualized using bootstrap averaging (i.e., 50 iterations; correlation coefficient of rho = 0.99; m = 4 dimensions) [22]. A one-way permutational multivariate analysis of the variance (PERMANOVA; 1 × 6 factorial design, “sampling locality” as a fixed factor) was used to test the differentiation of group centroids; “host size” was introduced as a covariable (ANCOVA model); and the main effects were tested for after 9999 permutations and subsequent post-hoc pair-wise comparisons [23]. The Bray–Curtis similarity matrix was computed on square-root-transformed data to reduce the influence of extreme values when determining similarity in infections among individual fish. These analyses were performed with the use of PRIMER 7 with PERMANOVA+ Add On package.
3. Results
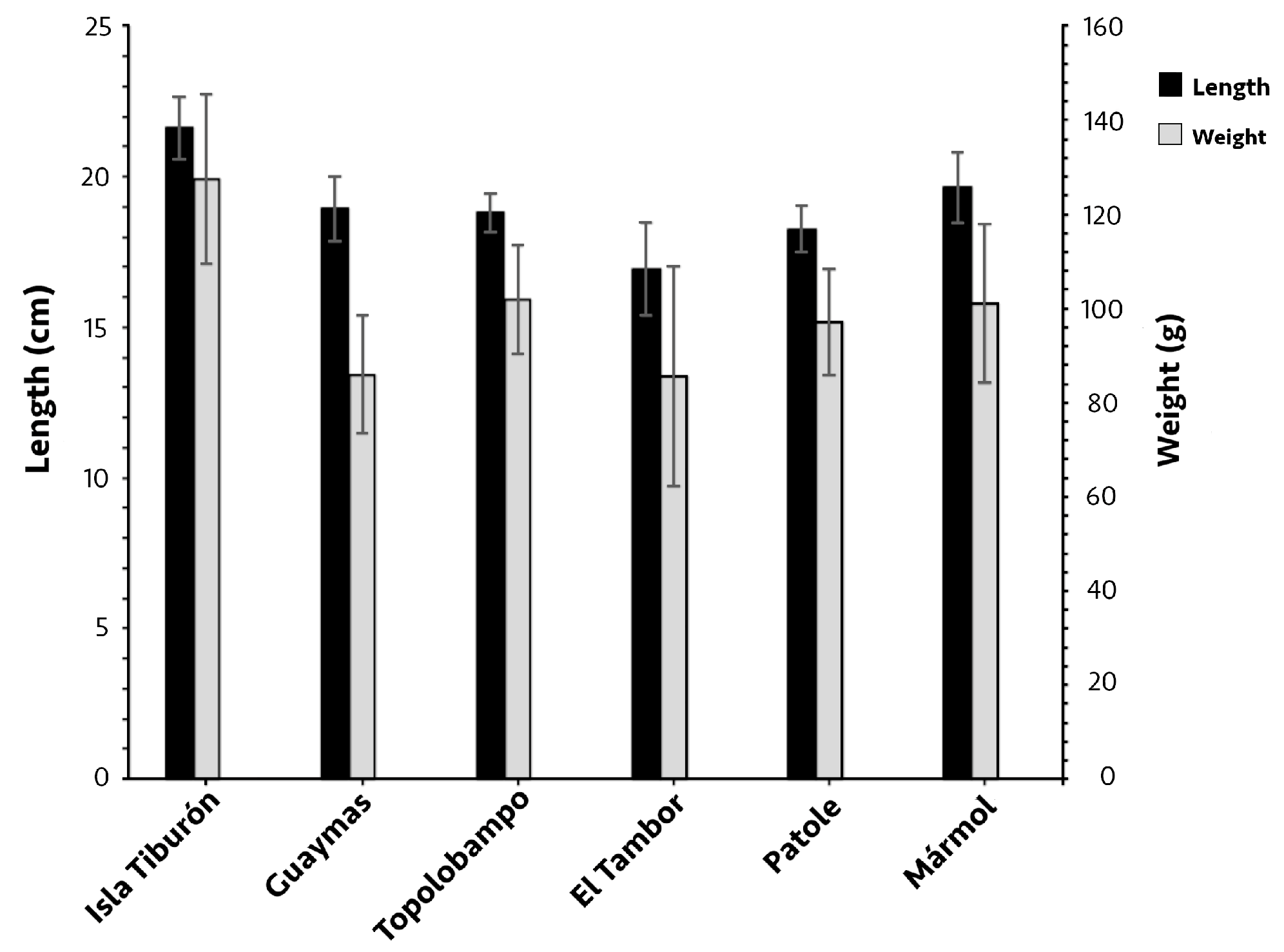
Of the 257 individuals of O. libertate analyzed, 166 were females, and 91 were males. The mean fork length and body weight significantly varied between some localities (p < 0.05; Figure 2), with both weight and length being notably higher at Isla Tiburón—21.6 ± 1 cm and 127 ± 18 g, respectively—compared to all other localities (p < 0.05), and the smallest fish were located in El Tambor; in this locality, while the fork length was significantly smaller (16.9 ± 1.5 cm) than in all the other localities, the body weight (85.5 ± 23.4 g) only differed from Isla Tiburón, Topolobampo, and Mármol. There was no significant difference in length between the females and males in each locality (p > 0.05); the weight of females in Topolobampo (104.2 g) was significantly higher than the males (89.4 g; p < 0.05).
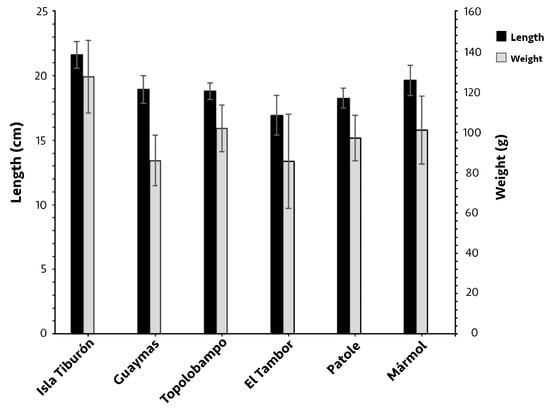
Figure 2.
Mean fork length and body weight of Pacific thread herring, Opisthonema libertate, from six sampling localities in the eastern Gulf of California; error bars show the standard deviation.
Among the six sampling localities, all collected specimens of O. libertate were infected with at least one species of parasite. The parasitofauna consisted of six species: two adult digeneans (i.e., M. ecaude and P. merus)- and one larval nematode (i.e., Pseudoterranova sp.)-infected stomach, and two adult monogeneans (i.e., Cribromazocraes cf. travassosi and Mazocraeoides georgei Price 1936)- and one juvenile copepod (i.e., Lepeophtheirus sp.)-infected gills (Figure 3). While the digeneans were highly prevalent at all sampling locations, the other species rarely appeared in some sampling localities with a prevalence and median intensity of ≤12% and ≤5 parasites/fish, respectively (Table 2). This was the only reason for applying comparative statistical tests to digenean prevalence and median intensity.
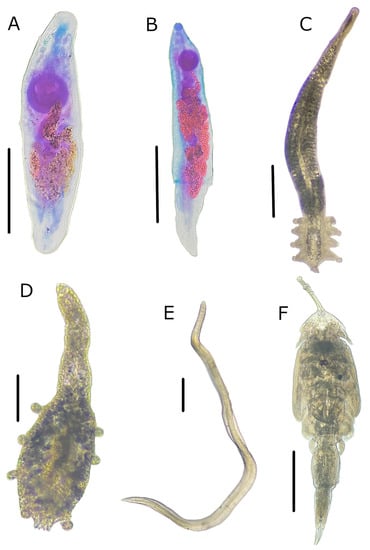
Figure 3.
Parasite species found in Pacific thread herring, Opisthonema libertate (Günther, 1867), from the eastern Gulf of California in Mexico. (A) Myosaccium ecaude Montgomery 1957; (B) Parahemiurus merus (Linton 1910); (C) Cribromazocraes cf. travassosi; (D) Mazocraeoides georgei Price 1936; (E) Pseudoterranova sp.; (F) Lepeophtheirus sp. Scale bars: 250 µm for (A); 500 µm for (B,C,E); 100 µm for (D), and 1000 µm for (F).

Table 2.
Parameters of parasite infection in Pacific thread herring, Opisthonema libertate, by six species of metazoan parasites in six localities in the Gulf of California in Mexico.
The prevalence and intensity of infection of M. ecaude and P. merus did not differ significantly between the female and male fish (p > 0.05).
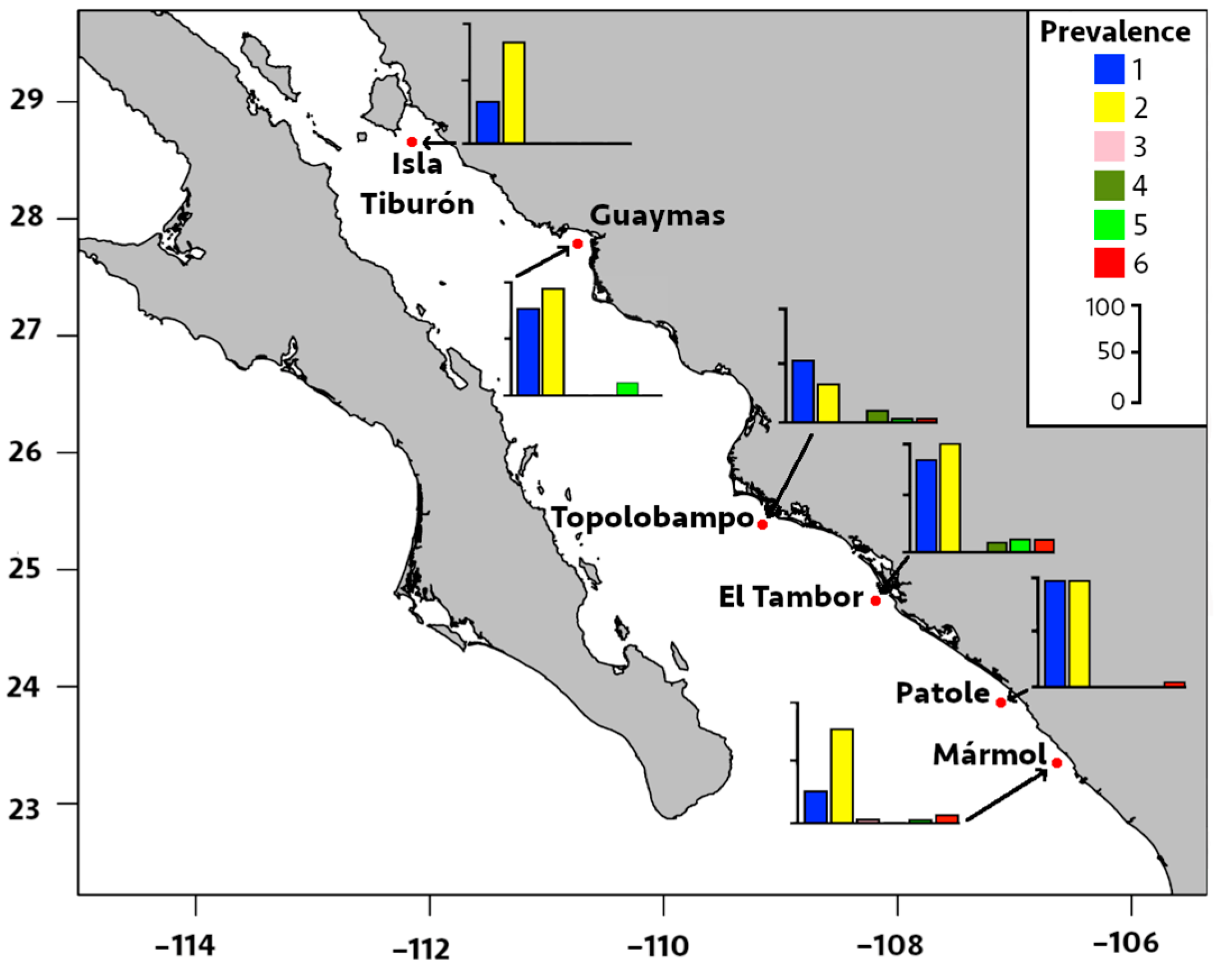
Regarding locality distribution, the significant prevalence and median intensity of digeneans were recorded in El Tambor and Patole (Table 2 and Figure 4 and Figure 5). The prevalence of P. merus was 100% in El Tambor, which was significantly higher (p < 0.05) than in Isla Tiburón, Topolobampo, and Mármol. This was followed by P. merus with 96% prevalence in Patole, which was significantly higher (p < 0.05) than Topolobampo and Mármol. In contrast, the prevalence of P. merus was significantly lower (33%, p < 0.05) in Topolobampo compared to all other localities. Moreover, the median intensity of P. merus was also significantly higher in Patole (20 parasites/fish, p < 0.05) than in all other localities, except for El Tambor, while significantly lower in Topolobampo (3 parasites/fish, p < 0.05) than all other localities, except for Isla Tiburón.
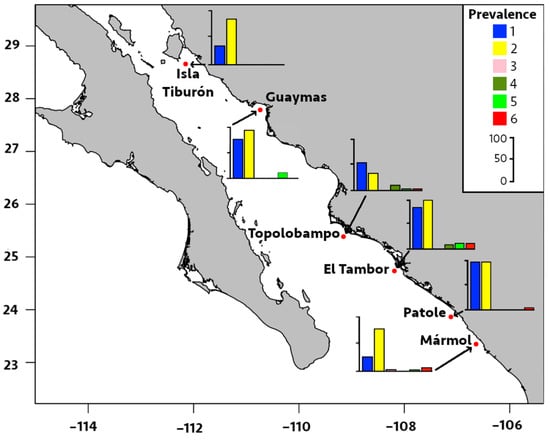
Figure 4.
Prevalence of infection by six metazoan parasites in Pacific thread herring, Opisthonema libertate, from six localities in the eastern Gulf of California in Mexico. The bar chart represents the prevalence of infection in each locality; each bar corresponds to a parasite species, and the parasites are represented by the following numbers: 1 = M. ecaude; 2 = P. merus; 3 = Pseudoterranova sp.; 4 = Lepeophtheirus sp.; 5 = Cribromazocraes cf. travassosi; 6 = M. georgei.
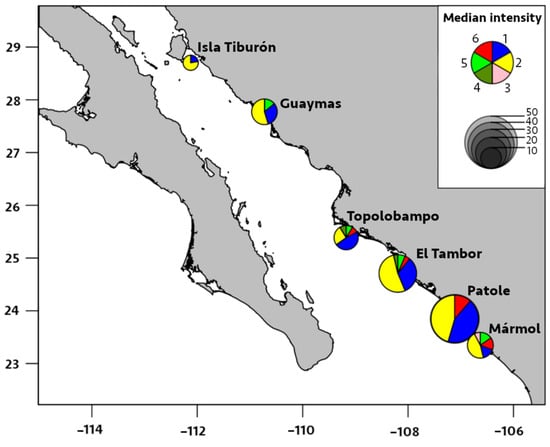
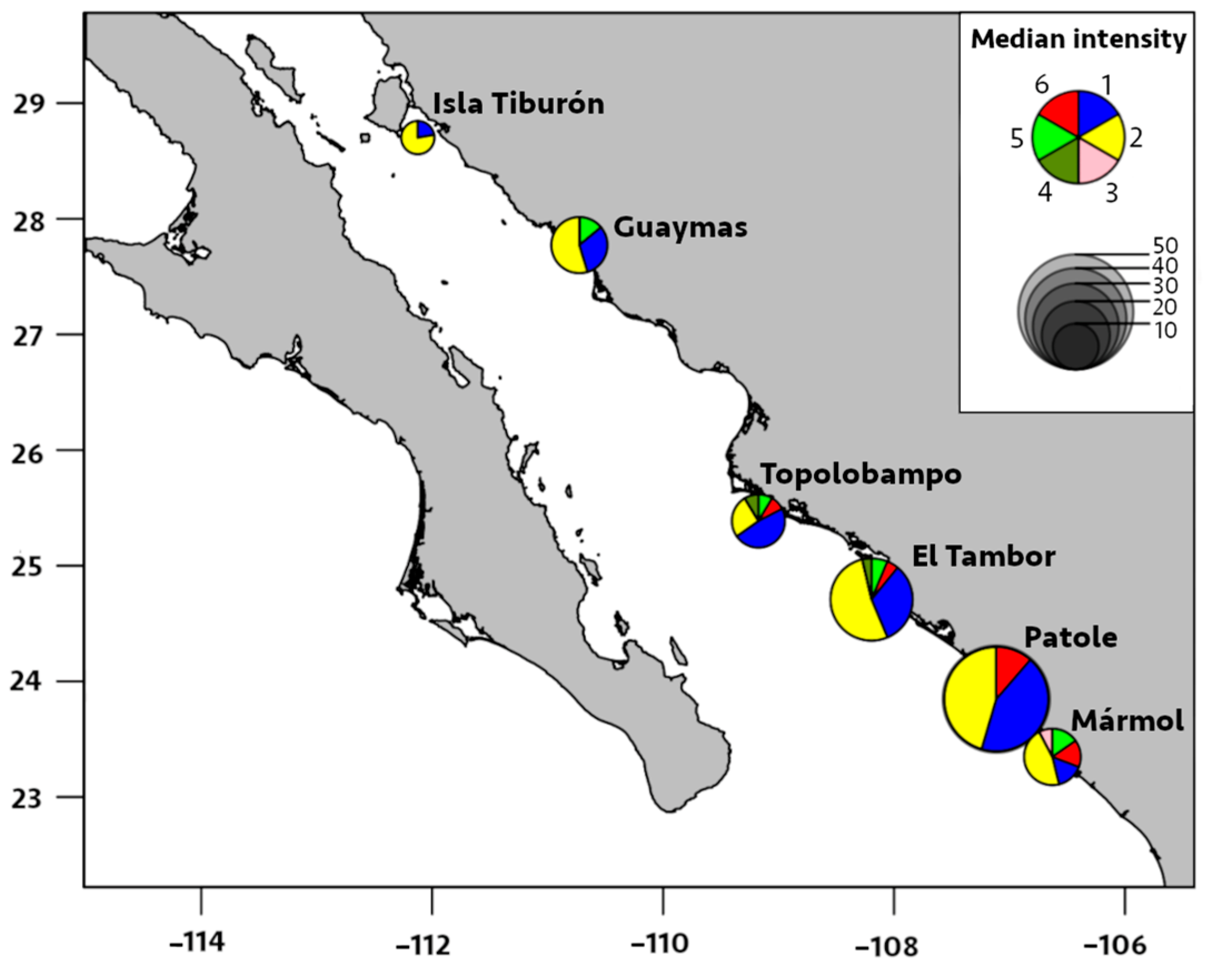
Figure 5.
Median intensity of infection by six species of metazoan parasites in Pacific thread herring, Opisthonema libertate, from six localities in the eastern Gulf of California in Mexico. The radius of each pie chart represents the total intensity of parasites of all species found in a locality; each slice corresponds to a parasite species, and the parasites are represented by the following numbers: 1 = M. ecaude; 2 = P. merus; 3 = Pseudoterranova sp.; 4 = Lepeophtheirus sp.; 5 = Cribromazocraes cf. travassosi; 6 = M. georgei.
The prevalence of M. ecaude reached 96% in Patole and was significantly higher (p < 0.05) than all other localities, except for El Tambor (85%), with the median intensity reaching 19 parasites/fish in Patole, which was significantly higher than all other areas, except for Mármol (p < 0.05). This was followed by M. ecaude with the second highest median intensity of 10 parasites/fish in El Tambor, being only significantly higher than in Isla Tiburón and Guaymas (p < 0.05).
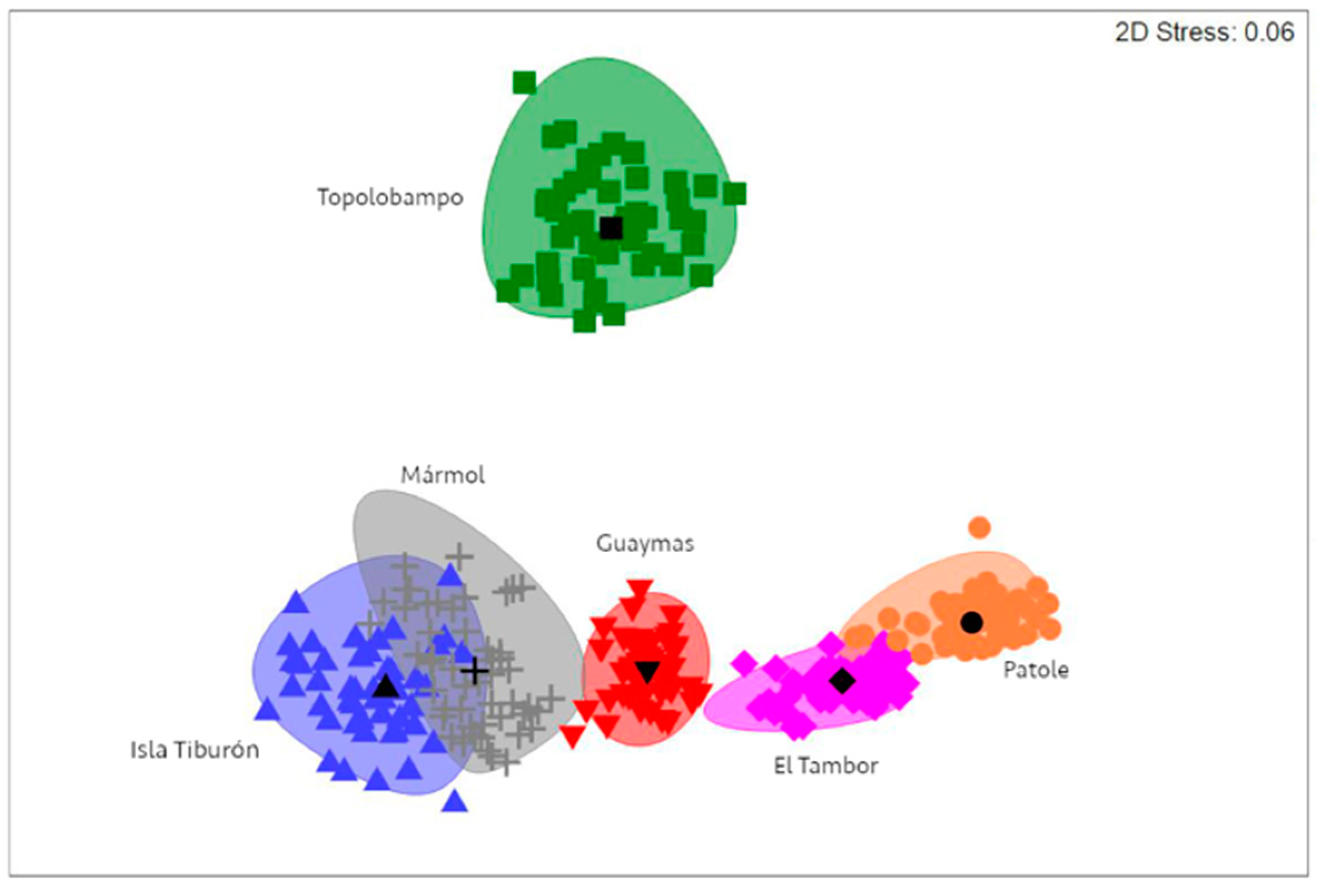
A Bray–Curtis similarity matrix was computed for the intensity of P. merus and M. ecaude infection, followed by bootstrap-average-based nMDS ordination generation, which showed an apparent separation between some sampling localities, with clear separation observed for Topolobampo, in addition to El Tambor and Patole separated from Isla Tiburón, Guaymas, and Mármol (Figure 6). According to the PERMANOVA, there was significant variability in the intensity of P. merus and M. ecaude infection between localities (Table 3). Furthermore, pairwise tests indicated significant differences in all cases (Pperm < 0.01), except between Isla Tiburón and Mármol and between El Tambor and Patole (Pperm > 0.05).
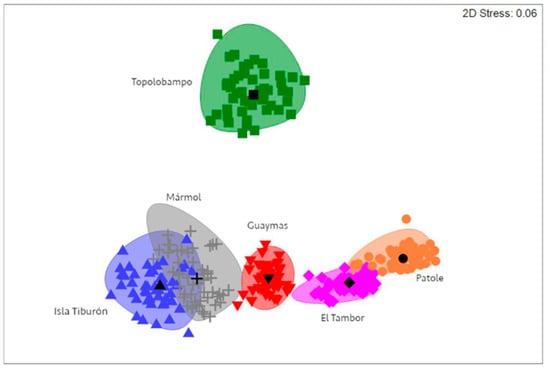
Figure 6.
Non-metric multidimensional scaling representation showing separation of sampling localities based on infection intensity of P. merus and M. ecaude in Pacific thread herring, Opisthonema libertate, from the eastern Gulf of California using a Bray–Curtis similarity index.

Table 3.
PERMANOVA results of comparisons of infection intensity of Parahemiurus merus and Myosaccium ecaude in Pacific thread herring, Opisthonema libertate, across six localities in the Gulf of California.
4. Discussion
4.1. Parasite Species
The novelty of this study lies in reporting the parasitofauna in O. libertate from the Gulf of California in Mexico. While six fish parasitic species—including P. merus, M. ecaude, and Pseudoterranova sp.—were previously documented for O. libertate from Chamela Bay on the central Pacific coast of Mexico [16]; Cribromazocraes cf. travassosi, M. georgei, and Lepeophtheirus sp. have not been previously recorded in this fish species.
Cribromazocraes cf. travassosi reported herein morphologically resembles Cribromazocraes travassosi, which was originally described by Santos and Kohn [24]; however, our collected specimens were insufficient for correct identification. Moreover, C. travassosi has only been found on clupeiform fishes from Brazil, specifically on the Sardinella brasiliensis, Opisthonema oglinum, Cetengraulis edentulus, and Harengula clupeola [25,26,27,28]. Similarly, M. georgei has been reported in at least seven clupeiform species from the western Atlantic [25,28,29,30,31,32]. It should be noted that some of these records referred to either Mazocraeoides opisthonema, Mazocraeoides hargisi, or Mazocraeoides olentangiensis, all of which are synonymously used for M. georgei [31,33]. According to Sailaja et al., only 13 of the 25 species described under the Mazocraeoides genus were valid [33]. Moreover, M. georgei has only been reported in Opisthonema oglinum in the Atlantic but not in any Pacific species of Opisthonema [29]. The Lepeophtheirus copepods were also never identified at the species level, as they were found at the juvenile (i.e., chalimus) stage. In this study, the copepods were assigned to Lepeophtheirus based on body shape, appendage structure, and the absence of lunules in the frontal plate, and no literature is yet available on Lepeophtheirus in any Opisthonema species. The findings of C. cf. travassosi, M. georgei, and Lepeophtheirus sp., therefore, constitute new host and geographical records.
4.2. Infection-Level Changes
Changes in the prevalence and intensity of infection with P. merus and M. ecaude, being significantly lower in Topolobampo than in El Tambor and Patole, as well as the separation of the localities indicated by the nMDS and PERMANOVA, suggest a discontinuous distribution of both digenean species; this is possibly due to the latitudinal variability in oceanographic conditions (e.g., water temperature) and the availability of intermediate hosts. Topolobampo is located on the border of the central and southern regions of the gulf, where the abundance of zooplankton is known to be lower than the southern area comprised of El Tambor and Patole [34]. The zooplankton communities are largely formed by copepods [34], which typically act as second intermediate hosts of hemiurid digeneans [35]. The copepod community structure between the southern and central regions differs in response to contrasting environmental conditions [34,36]. The southern region (i.e., the entrance of the gulf) covers the California Current, the Mexican Coastal Current, and the Gulf of California waters and results in particular oceanographic conditions, such as relatively higher seawater temperatures and species assemblages, which facilitate tropical species domination [36,37,38,39,40]. Some studies have reported that increased temperature positively affects the transmission of digeneans, which could be the reason for higher infection levels [41,42]. As such, the relatively higher abundance of intermediary hosts and higher seawater temperature could have caused the highest parasite infection levels in O. libertate caught in El Tambor and Patole to some extent.
Based on the above findings, it is envisaged that the parasite-infection levels in Mármol are comparable to El Tambor and Patole; these levels were lower in Mármol, however, and were highly similar to Isla Tiburón and to Guaymas to a limited extent. While increased temperature positively affects digenean transmission, other local factors may be more critical, so latitudinal gradients in infection levels may not be observed [43]. The eastern coast of the Gulf of California has spatially distinct environmental conditions influenced by agriculture, mariculture, upwellings, seasonal currents, river discharges, and hypoxia [44,45]; these environmental factors along the shallow coastal waters might influence host–parasite interactions differently, thereby making noticeable spatial patterns that are challenging to observe.
4.3. Potential Biological Tags
Parasites are generally reliable biological tags that can improve the understanding of many marine fish stocks [12,14]. The principle underlying this approach is that fish harboring a given parasite species come from geographical areas where this parasite is endemic [12,14]. Parasites can also be used as a tag within their endemic areas, where differences in the behavior and feeding habits of different host populations or the abundance of intermediate hosts can translate into significantly different infection levels in different geographical areas [46]. One prerequisite selection criterion is based on significant levels of parasitic infections in different parts of the study area [14], making P. merus and M. ecaude digeneans well-suited candidates. Weston et al. employed this criterion to identify different stocks of South African sardine, Sardinops sagax (Clupeiformes, Alosidae), using a larval-stage (metacercariae) digenean [47]. Unlike metacercariae, adult digeneans are thought to have short life spans, which hinders their use as biological tags [14,15]; according to Hemmingsen and MacKenzie, however, some adult digeneans might serve as potential indicators for fish movements [48]. Moreover, Jacobson et al. clarified Pacific sardine migration using M. ecaude abundance data, pointing out that this parasite has a sufficiently long life span to evaluate the movement of its fish host [13].
Based on the observed differences in P. merus and M. ecaude infection, it is possible to hypothesize that the individuals of O. libertate could have limited movement between certain geographical areas within the Gulf of California. The collected fish specimens in this study were mostly < 20 cm in length, and they could be regarded as non-migrant based on their size. While the clupeiforms’ movements throughout the life cycle are not well understood [49]; according to Lo et al., fish < 20 cm in length appear to be unable to migrate due to insufficient energy resources to swim long distances [50].
The overlap between El Tambor and Patole shown by nMDS suggests that fish were moving between these locations or that the environmental and ecological conditions affecting parasite infection were similar, owing to the contiguous nature of the localities [11]; in contrast, the overlap between Isla Tiburón and Mármol may be confusing, since these localities represented the northern and southern extremes of the studied area and might be due to the similarity of infection with P. merus and M. ecaude. It is worth noting, however, that fish in Isla Tiburón were not infected with the other parasite species found in O. libertate from other localities. Nematodes and monogeneans were found in Mármol fish but not in Isla Tiburón; as such, from an ecological perspective, these could be considered different populations based on parasite assemblage differences [12]. A possible structured population of O. libertate was also described by Pérez-Quiñonez et al. based on fish morphometrics [8]; they determined that specimens from the central region of the gulf (i.e., Guaymas) represent a different morphotype than specimens from the southern region (i.e., Mazatlán). Similarly, Jacob–Cervantes et al. differentiated southern and northern stocks based on body size [51]. Thus, integrating parasites using other techniques might bring better support when delineating movement patterns and identifying stocks of thread herrings in the future, which might then provide helpful information to improve the management and sustainable use of marine resources in the context of fisheries.
4.4. Limitations
The limitations of the study might be the differences in host sizes among fishing areas because this can influence parasite burdens. As suggested by Braicovich et al., however, fish length was incorporated as a covariate in the PERMANOVA to “correct” the effect of this variable [52]. The parasitic burden of fish typically increases with age or size, possibly due to large hosts having more surface area for parasite attachment and consuming more food, thereby resulting in higher exposure to infective stages as they grow and leading to cumulative patterns of parasite abundance [53]. Interestingly, in the present study, the smallest fish caught in El Tambor presented a high parasite burden.
5. Conclusions
In this study, the observed changes in parasite infection in O. libertate suggest a discontinuous distribution of P. merus and M. ecaude among localities in the Gulf of California in Mexico. While these changes may indicate that fish—particularly those measuring < 20 cm in length—form discrete populations across the gulf, this is not a solid conclusion because these parasites satisfy some—but not all—of the selection criteria to be used as good biological tags [14]. In the future, it is necessary to continue parasite surveys of O. libertate in more locations across the Gulf of California while considering possible drivers such as seasonality and host traits (e.g., diet and ontogeny).
Author Contributions
F.N.M.-S. conceived the study, attained funding, performed data analysis, and wrote the manuscript; D.G.L.-M. performed laboratory work, conducted the data analysis, and wrote the manuscript; J.R.F.V.-Z. performed fieldwork and drafted the article; E.M.-E. and F.A. drafted and critically revised the article for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) of the Universidad Nacional Autónoma de México (UNAM) through Project IA200523. The Instituto de Ciencias del Mar y Limnología, UNAM, financed the article processing charge. The National Fisheries and Aquaculture Institute of Mexico (INAPESCA) funded the research surveys. The National Council of Science and Technology of Mexico (CONACYT) provided postgraduate student scholarships to D.L.-M and J.R.F.V.-Z.
Institutional Review Board Statement
This study included no endangered or protected species. No live animals were caught specifically for this project. Fish examined for parasites were obtained from fishing hauls during a research cruise focused on fisheries stock assessments. The National Commission for Fisheries and Aquaculture issued the fishing permit (PPF/DGOPA-004/22).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon reasonable request.
Acknowledgments
Special thanks to Instituto Nacional de Pesca y Acuacultura (INAPESCA) for the facilities provided for collecting samples and to the crew of the ship Jorge Carranza for their support on the cruise. We would also like to thank Ireri Zacilha Renteria Caballero, Luis Eduardo Cabanillas Tapia, Félix Rodríguez López, and Roberto Cruz García for their help during our field and laboratory work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or data interpretation; in the writing of the manuscript; or in the decision to publish the results.
References
- Berry, D.H.; Barret, I. Gillraker analysis and speciation in the thread herring genus Opisthonema. Bull. Inter-Amer. Trop. Tuna Comm. 1963, 7, 137–190. [Google Scholar]
- Cury, P.; Bakun, A.; Crawford, R.J.; Jarre, A.; Quinones, R.A.; Shannon, L.J.; Verheye, H.M. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Hernández-Padilla, J.C.; Ruíz-Barreiro, T.M.; Salcedo-Bojórquez, S.; Espinosa-Romero, M.J.; Zetina-Rejón, M.J.; Arreguín-Sánchez, F. The ecological role of Opisthonema libertate and Cetengraulis mysticetus on ecosystem order in the southeastern Gulf of California, Mexico. Turk. J. Fish. Aquat. Sci. 2017, 17, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Lyle, F. Periodic fluctuations capture thread herring (Opisthonema spp.) in the Gulf of California, 1972–1990. CalCOFI Rep. 1992, 33, 124–129. [Google Scholar]
- Ruiz-Domínguez, M.; Quiñonez-Velázquez, C. Age, growth, and mortality of Opisthonema libertate on the coasts of northwestern Mexico. Cien. Mar. 2018, 44, 235–250. [Google Scholar] [CrossRef]
- Lanz, E.; Nevárez-Martínez, M.; López-Martínez, J.; Dworak, J.A. Small pelagic fish catches in the Gulf of California associated with sea surface temperature and chlorophyll. CalCOFI 2009, 50, 134–146. [Google Scholar]
- Ruiz-Domínguez, M.; Quiñonez-Velázquez, C.; Arizmendi-Rodríguez, D.I.; Gómez-Muñoz, V.M.; Nevárez-Martínez, M.O. Assessment of the exploitable biomass of thread herring (Opisthonema spp.) in northwestern Mexico. Acta Oceanol. Sin. 2021, 40, 53–65. [Google Scholar] [CrossRef]
- Pérez-Quiñonez, C.I.; Quiñonez-Velázquez, C.; García-Rodríguez, F.J. Detecting Opisthonema libertate (Günther, 1867) phenotypic stocks in northwestern coast of Mexico using geometric morphometrics based on body and otolith shape. Lat. Am. J. Aquat. Res. 2018, 46, 779–790. [Google Scholar] [CrossRef]
- Begg, G.A.; Waldman, J.R. An holistic approach to fish stock identification. Fish. Res. 1999, 43, 35–44. [Google Scholar] [CrossRef]
- Timi, J.T.; Poulin, R. Why ignoring parasites in fish ecology is a mistake. Int. J. Parasitol. 2020, 50, 755–761. [Google Scholar] [CrossRef]
- Moore, B.R.; Welch, D.J.; Newman, S.J.; Lester, R.J.G. Parasites as indicators of movement and population connectivity of a non-diadromous, tropical estuarine teleost: King threadfin Polydactylus macrochir. J. Fish Biol. 2012, 81, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Kamiya, T. Parasites as biological tags of fish stocks: A meta-analysis of their discriminatory power. Parasitology 2015, 142, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Baldwin, R.; Banks, M.; Emmett, R. Use of parasites to clarify residency and migration patterns of Pacific sardine (Sardinops sagax) in the California Current. Fish. Bull. 2019, 117, 196–211. [Google Scholar] [CrossRef]
- MacKenzie, K.; Abaunza, P. Parasites as biological tags for stock discrimination of marine fish: A guide to procedures and methods. Fish. Res. 1998, 38, 45–56. [Google Scholar] [CrossRef]
- Cantatore, D.M.P.; Timi, J.T. Marine parasites as biological tags in South American Atlantic waters, current status and perspectives. Parasitology 2015, 142, 5–24. [Google Scholar] [CrossRef]
- Pérez-Ponce de León, G.; García Prieto, L.; Rosas Villa, C. Helmintofauna de Opisthonema libertate y Harengula thrissina (Osteichthyes: Clupeidae) de la bahía de Chamela, Jalisco, México. Rev. Biol. Trop. 2000, 48, 759–763. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Schnute, J.T.; Boers, N.; Haigh, R.; Grandin, C.; Johnson, G.; Wessel, P.; Antonio, F. Mapping Fisheries Data and Spatial Analysis Tools. R Package Version 2.66.53. 2013. Available online: http://CRAN.R-project.org/package=PBSmapping (accessed on 2 May 2023).
- Gerritsen, H. Mapplots: Data Visualisation on Maps. R Package Version 1.4. 2013. Available online: http://CRAN.R-project.org/package=mapplots (accessed on 2 May 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 2 May 2023).
- Clarke, K.R.; Gorley, R. PRIMER v7: User Manual/Tutorial; Primer-E Limited: Plymouth, UK, 2015. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E Limited: Plymouth, UK, 2008. [Google Scholar]
- Santos, C.P.; Kohn, A. Description of Cribomazocraes travassosi n. sp. (Monogenea: Mazocraeidae), a fish parasite from the Atlantic Ocean. Mem. Inst. Oswaldo Cruz 1992, 87, 247–250. [Google Scholar] [CrossRef]
- Luque, J.L.; Vinas, R.A.; Paraguassú, A.R.; Alves, D.R. Metazoários Parasitos das sardinhas Sardinella brasiliensis e Harengula clupeola (Osteichthyes, Clupeidae) do litoral do Estado do Rio de Janeiro, Brasil. Rev. Uni. Rural. Ser. Cien. Vida 2000, 22, 71–76. [Google Scholar]
- Moreira, J.; Paschoal, F.; Cezar, A.D.; Luque, J.L. Community ecology of the metazoan parasites of Brazilian sardinella, Sardinella brasiliensis (Steindachner, 1879) (Actinopterygii: Clupeidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Braz. J. Biol. 2015, 75, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.; Paschoal, F. Community ecology of the metazoan parasites of the Atlantic thread herring, Opisthonema oglinum (Lesueur, 1818) (Actinopterygii: Clupeidae) from the Sepetiba Bay, Rio de Janeiro, Brazil. Braz. J. Biol. 2020, 81, 418–423. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.D.; Benicio, L.; Moreira, J.; Paschoal, F.; Pereira, F.B. Parasite communities and their ecological implications: Comparative approach on three sympatric clupeiform fish populations (Actinopterygii: Clupeiformes), off Rio de Janeiro, Brazil. Parasitol. Res. 2022, 121, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Hargis, W.J. Monogenetic trematodes of Gulf of Mexico fishes. part VII: The superfamily Diclidophoroidea Price, 1936 (Continued). Quart. J. Florida Acad. Sci. 1955, 18, 113–119. [Google Scholar]
- Sroufe Jr, S.A. Mazocraeoides olentangiensis, n. sp., a monogenetic trematode parasitic on the gills of the gizzard shad, Dorosoma cepedianum (Le Sueur). J. Parasitol. 1958, 44, 643–646. [Google Scholar] [CrossRef]
- Kohn, A.; Santos, C.P. First report of Mazocroeoides georgei price, 1936 and Mazocraeoides opisthonema Hargis, 1955 in Brazil with new synonysms (Monogenea, Mazocraeidae). Mem. Inst. Oswaldo Cruz 1988, 83, 437–440. [Google Scholar] [CrossRef]
- Alarcos, A.J.; Etchegoin, J.A. Parasite assemblages of estuarine-dependent marine fishes from Mar Chiquita coastal lagoon (Buenos Aires Province, Argentina). Parasitol. Res. 2010, 107, 1083–1091. [Google Scholar] [CrossRef]
- Sailaja, B.; Shameem, U.; Madhavi, R. Four species of Mazocraeoides Price, 1936 (Monogenea: Mazocraeidae), including two new species from clupeiform fishes off Visakhapatnam coast, Bay of Bengal. Zootaxa 2019, 4608, 233. [Google Scholar] [CrossRef]
- Quiroz-Martínez, B.; Salas-de-León, D.A.; Gil-Zurita, A.; Monreal-Gómez, M.A.; Coria-Monter, E.; Durán-Campos, E. Latitudinal and archipelago effect on the composition, distribution, and abundance of zooplanktonic organisms in the Gulf of California. Oceanologia 2023, 65, 371–385. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The role of zooplankton in the transmission of helminth parasites to fish. Rev. Fish Biol. Fish. 1995, 5, 336–371. [Google Scholar] [CrossRef]
- Jiménez-Pérez, L.C.; Lara-Lara, J.R. Zooplankton biomass and copepod community structure in the Gulf of California during the 1982–1983 El Niño event. CalCOFI Rep. 1988, 29, 122–128. [Google Scholar]
- Soto-Mardones, L.; Marinone, S.G.; Parés-Sierra, A. Time and spatial variability of sea surface temperature in the Gulf of California. Cien. Mar. 1999, 25, 1–30. [Google Scholar] [CrossRef]
- Aceves-Medina, G.; Jiménez-Rosenberg, S.P.A.; Hinojosa-Medina, A.; Funes-Rodríguez, R.; Saldierna-Martínez, R.J.; Smith, P.E. Fish larvae assemblages in the Gulf of California. J. Fish Biol. 2004, 65, 832–847. [Google Scholar] [CrossRef]
- Portela, E.; Beier, E.; Barton, E.D.; Castro, R.; Godínez, V.; Palacios-Hernández, E.; Fiedler, P.C.; Sánchez-Velasco, L.; Trasviña, A. Water masses and circulation in the tropical Pacific off central Mexico and surrounding areas. J. Phys. Oceanogr. 2016, 46, 3069–3081. [Google Scholar] [CrossRef]
- Gutiérrez-Bravo, J.G.; Tenorio-Fernandez, L.; Jiménez-Rosenberg, S.P.; Sánchez-Velasco, L. Three-dimensional distribution of larval fish habitats at the entrance of the Gulf of California in the tropical-subtropical convergence region off Mexico (April 2012). J. Plankton Res. 2022, 44, 130–144. [Google Scholar] [CrossRef]
- Studer, A.; Poulin, R. Seasonal dynamics in an intertidal mudflat: The case of a complex trematode life cycle. Mari. Ecol. Prog. Ser. 2012, 455, 79–93. [Google Scholar] [CrossRef][Green Version]
- Selbach, C.; Poulin, R. Some like it hotter: Trematode transmission under changing temperature conditions. Oecologia 2020, 194, 745–755. [Google Scholar] [CrossRef]
- Studer, A.; Widmann, M.; Poulin, R.; Krkošek, M. Large scale patterns of trematode parasitism in a bivalve host: No evidence for a latitudinal gradient in infection levels. Mar. Ecol. Prog. Ser. 2013, 491, 125–135. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Álvarez-Borrego, S.; Ruiz-Fernández, A.C.; García-Hernández, J.; Jara-Marini, M.E.; Bergés-Tiznado, M.E.; Piñón-Gimate, A.; Alonso-Rodríguez, R.; Soto-Jiménez, M.F.; Frías-Espiricueta, M.G.; et al. Environmental status of the Gulf of California: A pollution review. Earth-Sci. Rev. 2017, 166, 181–205. [Google Scholar] [CrossRef]
- Sánchez, A.; Aguíñiga-García, S.; Rey-Villiers, N. Evidence of hypoxia in the eastern coast of the Gulf of California as induced by stable nitrogen isotopes in surface sediments. Cont. Shelf Res. 2022, 239, 104716. [Google Scholar] [CrossRef]
- MacKenzie, K.; Campbell, N.; Mattiucci, S.; Ramos, P.; Pinto, A.L.; Abaunza, P. Parasites as biological tags for stock identification of Atlantic horse mackerel Trachurus trachurus L. Fish. Res. 2008, 89, 136–145. [Google Scholar] [CrossRef]
- Weston, L.F.; Reed, C.C.; Hendricks, M.; Winker, H.; van der Lingen, C.D. Stock discrimination of South African sardine (Sardinops sagax) using a digenean parasite biological tag. Fish. Res. 2015, 164, 120–129. [Google Scholar] [CrossRef]
- Hemmingsen, W.; MacKenzie, K. Latitudinal variations in the occurrence of some cod parasites along the west coast of Norway. Mar. Biol. Res. 2013, 9, 431–436. [Google Scholar] [CrossRef]
- Hunnam, K. The biology and ecology of tropical marine sardines and herrings in Indo-West Pacific fisheries: A review. Rev. Fish. Biol. Fisheries 2021, 31, 449–484. [Google Scholar] [CrossRef]
- Lo, N.C.H.; Macewicz, B.J.; Griffith, D.A. Migration of Pacific sardine (Sardinops sagax) off the west coast of United States in 2003–2005. Bull. Mar. Sci. 2011, 87, 395–412. [Google Scholar] [CrossRef]
- Jacob-Cervantes, M.L.; Vallarta-Zárate, J.R.F.; Becerra-Arroyo, D.; Rendón-Martínez, J.R. Indicadores de la existencia de dos unidades biológicas de sardina crinuda Opisthonema libertate en la región norte y sur del golfo de California. Memorias del XXIII Taller del Comité Técnico de Pelágicos Menores, La Paz, Mexico, 10–12 June 2015; Centro Interdiciplinacio de Ciencias Marinas, IPN: La Paz, Mexico, 2015. [Google Scholar]
- Braicovich, P.E.; Ieno, E.N.; Sáez, M.; Despos, J.; Timi, J.T. Assessing the role of host traits as drivers of the abundance of long-lived parasites in fish-stock assessment studies. J. Fish Biol. 2016, 89, 2419–2433. [Google Scholar] [CrossRef]
- Poulin, R. Variation in the intraspecific relationship between fish length and intensity of parasitic infection: Biological and statistical causes. J. Fish Biol. 2000, 56, 123–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).