Abstract
This study aimed to evaluate the effects of dietary supplementation with nano-curcumin (NCur) and a nano-curcumin/chitosan blend (NCur/Ch) on growth performance, digestibility, immune response, antioxidant status, intestinal morphometric characters, and gene regulation in Nile tilapia. Fish (n = 180, initial body weight = 12.0 ± 0.53 g) received supplementary NCur at rates of 0 (control), 0.00625, and 0.0125, and NCur/Ch at rates of 0.00625 + 0.5 g/kg diet for 4 weeks. Growth performance parameters (final weight and length, body mass gain, specific growth, and length gain rates) were markedly increased, and the feed conversion ratio was significantly decreased in the NCur- and NCur/Ch-supplemented groups. Digestive enzyme (amylase), immune response markers (immunoglobulin M, nitrous oxide, and lysozyme activity), plasma albumin, and total protein were increased significantly, mainly with a diet supplemented with 0.00625 g NCur/kg. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose, and cortisol levels decreased in the supplemented groups compared to the control. Significantly increased glutathione peroxidase (GPx) and decreased malondialdehyde (MDA) levels were observed in the NCur/Ch group. Superoxide dismutase (SOD) activity was improved in the 0.0125 NCur group. Intestinal morphometric characters, including villus length, width, interspace, and goblet cell abundance, were increased to cope with improved growth performance and were associated with upregulation of insulin-like growth factor 1 (igf-1) and complement C-5 (cc5) compared to the control group. Therefore, NCur and an NCur/Ch blend could be supplemented in the Nile tilapia diets as a natural alternative to promote growth, digestion, immune status, liver function, antioxidant status, and related gene expression in O. niloticus.
Key Contribution:
1. The role of dietary supplementation with the nano-curcumin/chitosan blend on Nile tilapia performance, immune response, and antioxidant status was evaluated. 2. The nano-curcumin/chitosan blend revealed a synergistic effect in improving growth, feed utilization, and digestive processes, including digestive enzyme activity and gut histomorphology. 3. The immune response, antioxidant status, and some related gene expressions were enhanced by the nano-curcumin/chitosan blend supplementation.
1. Introduction
Dietary supplementation of nanoparticles in aquafeed is increasingly reported and widely applied due to its promising effects on fish overall performance and productivity [1]. The novel properties of nano-delivery come from their enhanced distribution, efficacy, and longer bioactive time over the traditional ones [2,3,4]. Nano-delivery also improves fish efficiency to absorb nutrients and drugs with poor absorption, improves the solubility of vitamins, protects the nutraceutical from pH, microorganisms, and enzymes before reaching the target, and improves vaccine activity [5,6]. Many feed supplements are natural nutraceuticals. Natural medicinal supplements and their active ingredients have been incorporated into aquaculture diets for health and performance improvement and for stress and disease resistance [7,8,9]. These compounds are reported as natural alternatives to chemicals possessing antibiotic, growth promoting, antioxidant, and immune-stimulating properties [10,11,12,13].
Curcumin (Cur) is a hydrophobic and polyphenolic natural-derived compound, representing 60–70% of the main active ingredient found in turmeric [14,15]. Dietary supplementation with Cur and Cur-nanomicelles improved growth performance, antioxidant status, body composition, and ameliorated the toxicity effects of silver nanoparticle in Cyprinus carpio [7]. Nile tilapia (Oreochromis niloticus) fed a Cur-supplemented diet up to 3% revealed improved growth performance, feed utilization, redox status, upregulated expression of heat shock protein gene, and increased low temperature tolerance [16]. In addition, dietary Cur in nano-form improved growth performance, digestive enzyme activities, antioxidant enzyme activities, and humoral immunity in Nile tilapia [17]. Therefore, Cur is proven to have growth-promoting, antioxidative, and immunostimulatory effects on different aquaculture species [15]. Nevertheless, Cur has many problematic delivery issues, including low bioavailability and poor absorption [14]. Moreover, the metabolism and elimination of Cur are rapid, owing to its unsteady structure, which leads to low levels in blood and animal tissues, and limits its persistence and usage [4,18]. Consequently, nano-delivery approaches have been utilized to improve Cur distribution, stability, and availability [19]. Nanoparticles secure better delivery and efficiency, thus reducing the amounts of curcumin required to be incorporated into diets, resulting in overall improved properties at a lower cost [7,20].
Chitosan (Ch) is a natural additives also used to enhance aquatic feeds. Ch is obtained through the deacetylation of crustacean chitin and is characterized by safety, compatibility, affordability, and biodegradability [21,22]. The inclusion of Ch in the diet of juvenile loach, Misgurnus anguillicaudatus, enhanced weight gain, lipid metabolism, and health status and modulated gut microbiota [23]. Ch improves the growth, immunity, and health status of C. carpio and O. niloticus [24,25]. Furthermore, the use of Ch nanoparticles is more efficient than the normal form due to their high surface area, higher bioavailability, easy absorption, and efficient delivery to the target site [22]. Dietary Ch nanoparticles significantly improved Nile tilapia growth performance and antioxidant status and stimulated immune responses [26]. Moreover, dietary Ch nanoparticles alleviated systemic inflammation induced by Yersinia ruckeri and stimulated the immune response of rainbow trout, Oncorhynchus mykiss [27].
The use of Ch in drug or active ingredient delivery has increased due to several properties of Ch including bio-adhesion, biodegradability, biocompatibility, and low toxicity. Furthermore, Ch improved the solubility and stability of coated materials, and enhanced delivery to the target site and cell uptake [28]. Hence, it is assumed that the integration between nano-curcumin (NCur) and a chitosan (NCur/Ch) blend could better improve overall fish performance. Thus, with limited literature addressing this integration, the current work aim to assess the effect of dietary NCur and an NCur/Ch blend on growth performance, digestion, immune and antioxidant status, intestinal function, and some gene expression in Nile tilapia, which is among the highest farmed and most profitable fish species worldwide due to its nutrition value and availability.
2. Materials and Methods
2.1. Preparation of Curcumin Nanoparticles
2.1.1. Synthesis of Curcumin Nanoparticles
Curcumin nanoparticles were synthesized by a sonochemical method in which ultrasonic waves led to the formation and growth of microbubbles. These microbubbles had extreme temperatures and pressure in the inner and outer sides of the bubbles. After the collapsed curcumin (Sigma, Chennai, India) molecules were subjected to these extreme conditions, which induced the nucleation of nanoparticles, they were exposed to rapid cooling, leading to the synthesis of curcumin nanoparticles. Olive oil (Khaled Khoshala Co., Cairo, Egypt) acted as a stabilizer, coating curcumin nanoparticle to prevent aggregation. An amount of 0.01 g of curcumin was added to 10 mL of olive oil and stirred for 1 h at 600× g, and then the solution was subjected to sonication for 2 h at the conditions of plus time of 4 s, rest time of 1 s, temperature maintained below 80 °C, total time of sonication of 1.5 h, and 75% amplitude.
2.1.2. Synthesis of Nano-curcumin and Chitosan Capsule (NCur/Ch)
Chitosan (0.5 g) was dissolved in 100 mL of 2% acetic acid in a 250 mL beaker and heated until boiling; then, 25 mL of the prepared curcumin nanoparticles was added. An amount of 1 g of sodium tripolyphosphate (STPP) dissolved in 50 mL of double-deionized water was added dropwise to the boiling dissolved NCur/Ch mixture until a yellow fiber-like appearance was obtained. The mixture was transferred to a 2 L beaker of double-distilled water and incubated until the NCur/Ch capsule precipitated. Then, the secondary products dissolved in the top beaker solution were removed. The final step was repeated twice; then, the precipitate was dried at room temperature.
2.1.3. Characterization of Curcumin Nanoparticles
Characterization included shape, size, and identification steps. Identification was carried out to confirm that the synthesis occurred without any contamination, change in chemistry, or crystallography of curcumin nanoparticles, using an X-ray diffraction (XRD) instrument (EQUINOX 1000 X-ray Diffractometer, Thermo Scientific Company, Waltham, MA, USA). The shape characterization was performed to illustrate the morphology and shape of curcumin nanoparticles. Size analysis was carried out by dynamic light scattering (DLS) Nano Sight NS500, Malvern Instruments Ltd. (Malvern, UK). Shape analysis was carried out using a scanning electron microscope (SEM; Prisma E, Thermo Scientific Company, Waltham, MA, USA) and transmission electron microscope (TEM; EM-2100, high-resolution magnification).
2.2. Diets and Experimental Conditions
In total, 4 experimental diets were prepared by adding NCur at rates of 0 (control), 0.00625, and 0.0125 and Ncur/Ch at rates of 0.00625 + 0.5 g/kg commercial basal diet (Aller® Aqua, 6th of October city, Egypt; 30% protein, Table 1) [26]. The ingredients were mixed, extruded to obtain uniform pellets, dried at room temperature, and then stored at 4 °C until use.

Table 1.
Composition and approximate analysis of basal diet.
One hundred and eighty Nile tilapia (O. niloticus) were obtained from a private fish farm at Elqnater Elkhairia, Qalubia governorate, Egypt. Fish were transported to the wet aquatic laboratory at Benha University, Egypt, to perform the current study. Fish (initial body weight = 12.0 ± 0.53 g and initial length = 8 ± 0.42 cm) were kept in a 1000 L fiberglass tank and fed a control basal diet for 12 days as acclimatization period. Fish were distributed at a density of 15 fish per 90 L rectangular tanks divided into triplicates with free water flow. Fish were fed to satiation twice daily (at 09:00 a.m. and 17:00 p.m.) for 4 weeks. Water quality was routinely checked and kept at 25.0 ± 0.6 °C temperature, 5.8 ± 0.32 mg/L dissolved oxygen, 0.25–0.31 mg/L ammonia (NH3), and 7 ± 0.2 pH.
2.3. Growth Performance
The weight and length of all fish were recorded at the start and end of the experiment as follows:
Body mass gain (BMG; %) = 100 × [(final body mass − initial body mass)/(initial body] mass).
Specific growth rate (SGR; % day−1) = 100 × [(ln final body mass g − ln initial body mass g)/trial days]
Feed conversion ratio (FCR) = consumed feed/weight gain
Length gain rate % = 100 × [(final body length cm − initial body length cm)/initial body length cm].
2.4. Digestive Enzymes
At the end of the experiment, 9 fish from each group (3 fish/replicate) were used for intestinal sample collection in phosphate-buffered saline of pH 7.4. After homogenization, the samples were centrifuged for 15 min at 6000× g at 2–8 °C to obtain the supernatant. Digestive enzymes’ (amylase, lipase, and protease) activities in homogenate were assayed colorimetrically, in triplicate, according to the Sigma-Aldrich company’s protocol (Burlington, NJ, USA), at 405 nm for amylase, where 1 unit is the amount of amylase that cleaves ethylidene-pNP-G7 to generate 1.0 µmole of p-nitrophenol per minute at 25 °C. Lipase was assayed at 570 nm, where 1 unit of lipase is the amount of enzyme that will generate 1.0 μmole of glycerol from triglycerides per minute at 37 °C. Protease was measured at 525 nm according to the manufacturer’s protocol.
2.5. Immune Parameters
Immunoglobulin M (IgM) mg/dl was assayed in 50 μL plasma/sample at 450 nm within 10 min using a colorimetric CUSABIO kit (Wuhan, China) and the manufacturer’s protocol. Plasma nitrous oxide and lysozyme levels were colorimetrically measured at a wavelength of 570 and 450 nm using Sigma-Aldrich kit protocols (Burlington, NJ, USA) following our previous studies [29]. Nitrous oxide was determined using oxidized nitrite to NO amount with 84 µL Griess reagent. Lysozyme activity was measured using a suspension of Micrococcus lysodeikticus as substrate, in a 2.6 mL reaction mixture.
2.6. Biochemical Assays
Plasma proteins were assayed in triplicate using the company’s protocol (Spinreact, Girona, Spain) through a chemistry analyzer RA-50, Bayer (Budapest, Hungary). Liver enzymes (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)), glucose, and cortisol levels were assayed spectrophotometrically in plasma using BioMed Diagnostic commercial kits (Cairo, Egypt) at 340 nm, according to the process outlined by Liu et al. [30] and Huang et al. [31].
2.7. Antioxidants
In total, 9 liver samples were obtained from each group (3 samples/replicate) and placed in phosphate-buffered saline of pH 7.4 for assessing glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde MDA levels according to the process described by Aebi [32]. GPx was evaluated at 340 nm by estimating the enzyme amount that oxidized 1.0 nmol of NADPH/min. at 26 °C. SOD was measured at 340 nm using Biodiagnostic Co. commercial kits (Cairo, Egypt) by estimating the enzyme activity that converted the methosulphate using the indicator tetrazolium blue dye. MDA was quantified colorimetrically at 532 nm using Abcam, MA 02453 commercial kits (Eugene, OR, USA), depending on the reaction of the sample with thiobarbituric acid (TBA) to produce an MDA-TBA adduct.
2.8. Intestinal Morphometric Analysis
Intestinal samples were collected from different groups and then fixed in 10% neutral buffered formalin. After dehydration and clearance, the tissues were embedded in paraffin, 5 µm sectioned, and stained with Hematoxylin and Eosin [33]. Histomorphometric analysis was performed using National Institutes of Health ImageJ analysis software (Bethesda, MD, USA). The intestinal villi length, width, and inter-villi space were measured by ImageJ analysis software v1.31 and expressed as µm.
2.9. Real-Time PCR Analysis of igf-1 and cc5
Liver samples (9 fish/group) were collected in Ambion RNAlater (Waltham, MA, USA) and subjected to Trizol total RNA isolation using a method (1 mL Trizol/50 mg sample) following the company working protocol (Invitrogen, Carlsbad, CA, USA). A real-time PCR study was performed following our previous studies [34]. RNA samples were quantified using NanoDrop spectrophotometry; the 260/280 nm ratio was assessed with purity confirmation at 1.80:2.00. cDNA reverse transcription (Invitrogen, Carlsbad, CA, USA) was then performed, and expression of genes’ primers (Metabion, Planegg, Germany) (Table 2) was performed using Stratagene MX3005P. Reaction conditions included 1 cycle (94 °C for 15 min), 40 cycles (94 °C for 5 min), 62 °C for 30 s, and 72 °C for 30 s. The relative expression was estimated via the “2−ΔΔCt” method [35].

Table 2.
Primer sequences of targeted genes in O. niloticus for the gene expression study.
2.10. Statistical Analysis
The result was statistically analyzed using one-way analysis of variance (ANOVA) by the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 22.0, released 2013, IBM Corp., Armonk, NY, USA). Duncan’s multiple range test was used to identify the significant differences among means at a probability level of p < 0.05. Data are expressed as means ± standard error.
3. Results
3.1. Characterization of Curcumin Nanoparticles
Scheme 1A illustrates the fingerprint XRD pattern of Cur without any peaks of other materials or shifts of Cur peaks. The SEM and TEM images, as illustrated in Scheme 1B,C, show the spherical to subspherical shape of Cur nanoparticles with no shape or size control; this may have occurred due to the oil coating. The size of Cur nanoparticles was about 35 nm without any agglomeration of particles. Size determination using DLS (Scheme 1D,E) recorded a 59 nm size with a −12 mV zeta potential.

Scheme 1.
XRD pattern (A), TEM (B), SEM (C), DLS size (D), and zeta potential (E) of Cur nanoparticles.
3.2. Growth Performance
Growth performance was significantly influenced by NCur and NCur/Ch supplementation. Growth parameters, including final weight and length, body mass gain (BMG), specific growth (SGR) and length gain (LGR) rates, and feed conversion ratio (FCR), were improved in the supplemented groups compared with the control. Ncur at a level of 0.0125 and NCur/Ch at a level of 0.00625 + 0.5 g/kg diet induced the most significant (p < 0.05) improvement in growth performance (Table 3).

Table 3.
Growth parameters of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed.
3.3. Digestive Enzymes
A diet of 0.00625 g NCur/kg showed the highest significant amylase enzyme activity values. However, protease activity did not improve in the supplemented groups over the control, and lipase activity did not reveal significant (p < 0.05) differences (Figure 1).
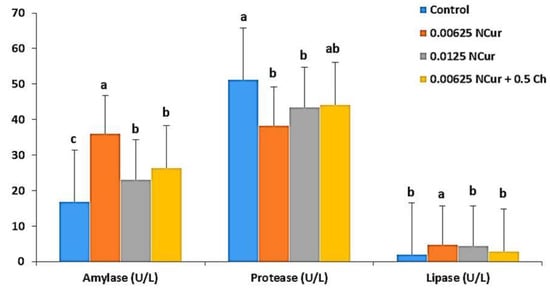
Figure 1.
Digestive enzymes (amylase, lipase, and protease) activities in O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed. Data are presented as mean (n = 9) ± SE. Values marked with superscript letters are significantly different (p < 0.05).
3.4. Immune Response
NCur incorporation at a level of 0.00625 g produced the most significant (p < 0.05) enhanced IgM, nitrous oxide (NO), and lysozyme activity, while the other 2 dosages (NCur 0.0125 and 0.00625 + 0.5 NCur/Ch g/kg diet) did not produce significant differences in immune parameters compared to the control group, except for NO activity, where the 2 dosages produced lower activities than the control (Figure 2).

Figure 2.
IgM, nitrous oxide, and lysozyme activities of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed. Data are presented as mean (n = 9) ± SE. Values marked with different superscript letters are significantly different (p < 0.05).
3.5. Biochemical Profile and Plasma Proteins
The levels of ALT and AST significantly (p < 0.05) decreased in the NCur- and NCur/Ch-supplemented groups compared with the control. The lowest (p < 0.05) levels of ALT and AST in plasma samples were detected for the group receiving 0.00625 + 0.5 NCur/Ch g/kg diet (10.6 ± 0.002 and 22 ± 0.001). The lowest (p < 0.05) levels of ALT and AST in liver samples were detected for both NCur 0.0125 (22.4 ± 0.002 and 33.9 ± 0.005) and NCur/Ch 0.00625 + 0.5 groups (28.3 ± 0.002 and 25.3 ± 0.002) (Figure 3).
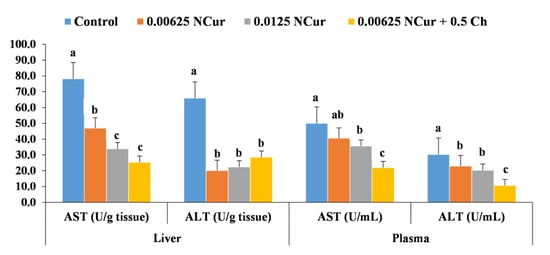
Figure 3.
Kidney function enzymes of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed. Data are presented as mean (n = 9) ± SE. Values showing different superscript letters are significantly different at (p < 0.05).
In addition, glucose and cortisol levels were significantly (p < 0.05) decreased in the supplemented groups over the control, with the lowest (p < 0.05) values for NCur 0.0125 and NCur/Ch 0.00625 + 0.5 groups (Table 4). Plasma albumin and total protein were significantly (p < 0.05) increased, mainly in the 0.00625 g NCur/kg diet, while the supplemented groups did not show a significant difference in globulin level compared to the control (Table 4).

Table 4.
Plasma biochemical parameters of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed.
3.6. Antioxidants
The group receiving NCur/Ch 0.00625 + 0.5 was the only group to reveal a significantly (p < 0.05) increased GPx level. For SOD level, only the NCur 0.0125 group showed a marked increase over the control group. While all the incorporated groups showed a significant (p < 0.05) decrease in MDA level, the most pronounced value was in the NCur/Ch 0.00625 + 0.5 group (Figure 4).

Figure 4.
Antioxidant enzyme activities of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed. Data are presented as mean ± SE (n = 9). Values showing different superscripts are significantly different (p < 0.05).
3.7. Intestinal Morphometric Analysis
The effect of the incorporation of NCur and NCur/Ch on the intestinal morphometric parameters of O. niloticus was also analyzed, including the villus length, width, inter-space, and goblet cell abundance (Table 5 and Scheme 2). Supplemented groups exhibited significantly (p < 0.05) increased villus length, width, and goblet cell count in the anterior part of the intestine, with the most pronounced increase (p < 0.05), compared with the control group, observed in the group fed the diet containing NCur 0.0125. The inter-villus spaces of the anterior part of the intestine were significantly (p < 0.05) smaller in the supplemented groups in comparison with the control. Similarly, the villus length of the middle part of the intestine and goblet cell number were significantly enhanced (p < 0.05) in the supplemented groups, with the highest (p < 0.05) increase in villus length and the highest (p < 0.05) decrease in goblet cells number also observed for the NCur 0.0125 group. Meanwhile, villus width did not show significant (p < 0.05) differences among supplemented groups. For the posterior part of the intestine, results were similar to those of the middle part, except for the villus width, which showed the highest (p < 0.05) increase in NCur 0.00625 groups.

Table 5.
Intestinal morphometry of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed.

Scheme 2.
Photomicrograph of anterior, middle, and posterior parts of the intestine of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed.
3.8. Gene Expression of igf-1 and cc5
The groups that received NCur and NCur/Ch supplemented diets displayed (p < 0.05) noticeable upregulation in the expression pattern of both igf-1 and cc5 genes, with the most marked increase, compared to the control, in the NCur/Ch 0.00625 + 0.5 group (Figure 5).
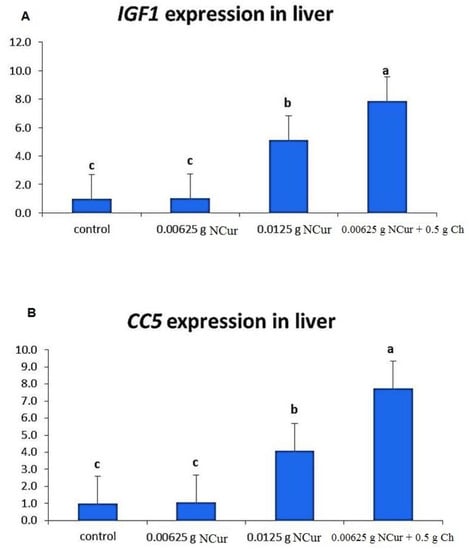
Figure 5.
igf-1 (A) and cc5 (B) gene expression of O. niloticus fed diets supplemented with nano-curcumin (NCur) and nano-curcumin/chitosan blend (NCur/Ch) feed. Data are presented as mean (n = 9) ±SE. Values with different letter superscripts are significantly different (p < 0.05).
4. Discussion
The current study indicated that NCur and an Ncur/Ch blend could be used as a dietary supplement to promote growth, digestion, innate immunity, and related gene expression in Nile tilapia. A diet of Ncur 0.0125 and Ncur/Ch 0.00625 + 0.5 g/kg produced the best growth performance. These findings were similar to prior studies reporting positive effects of Cur in C. carpio [7,38], O. niloticus [16,39], and O. mossambicus [40]. These activities are mostly attributed to the attractive flavor of Cur, which possibly improves feed palatability and, consequently, feed intake and utilization, leading to improved feed efficiency [41]. The incorporation of NCur and NCur/Ch also enhanced the digestion process, as represented by increased activity of amylase, with the highest significant activity (p < 0.05) in the group supplemented with 0.00625 g NCur. Furthermore, NCur and NCur/Ch improved intestinal morphometric parameters, including increased villus width and length, which, in turn, increased absorption area and improved nutrient utilization. In the same vein, the dietary Cur activity improved the digestion process through improved trypsin, lipase, and amylase activities; thus, it may improve the growth performance of crucian carp, Carassius auratus [42]. Regarding Ch dietary supplementation, Nile tilapia, gibel carp (C. auratus gibelio), and copia (Rachycentron canadum) fed Ch- or Ch nanoparticle-supplemented diets had preferred growth performance, feed utilization, intestinal morphology, intestine microbiota, and disease resistance compared to the control group [26,43,44,45].
Immunoglobulin M (IgM), NO, and lysozyme levels are principal indicators that reflect a fish’s immune system [46]. In the present study, a diet of NCur at a level of 0.00625 g/kg produced the most enhanced IgM, nitrous oxide (NO), and lysozyme activities. The obtained data is in accordance with the findings of Ashry et al. [41] and Yonar et al. [47], who recorded significantly improved immune parameters, including phagocytic activity, in gilthead seabream (Sparus aurata) and rainbow trout (O. mykiss) supplemented with Cur-enriched diets. Furthermore, in Nile tilapia, the Cur immune-stimulatory effect might occur due to the stabilizing ability of Cur in lysozyme production [48,49]. Furthermore, it was found that Cur incorporation into the diet at a rate of 50 and 100 mg Cu kg−1 notably enhanced plasma lysozyme activity and increased immunoglobin concentrations [39]. Thus, it enhanced both the innate and humoral immune responses in Nile tilapia. Likewise, Gheytasi et al. [50] reported an elevation of lysozyme activity and complement C3 level upon feeding lemon essential oil loaded with Ch nanoparticles to rainbow trout.
Curcumin is characterized by a hepatoprotective effect, indicated by a sharp decrease in ALT and AST activities. Further, curcumin is characterized by the glucose and cortisol levels in supplemented groups, with the lowest levels for the NCur 0.0125 and NCur/Ch 0.00625 + 0.5 groups. This decrease might be due to the ability of Cur to stabilize the membrane of hepatocytes, which stops intracellular enzyme leakage, and its hepatoprotective role [51]. These findings are in agreement with Gheytasi et al. [50], who reported a decrease in serum ALT activity in 1% lemon-loaded Ch nanoparticles of rainbow trout. Further, serum ALT activity decreased in Nile tilapia fed a Cur-supplemented diet [48].
Plasma proteins might be affected by dietary supplementation in finfish [52,53]. The current study showed that albumin and total protein were augmented mainly for the 0.00625 g NCur /kg diet. The reduced lipoprotein levels could be due to the inhibition of cholesterol production, resulting in a reduction of intracellular liver sterols [54].
Antioxidant activity is a key indicator of the overall status of a fish [39]. In the present study, dietary inclusion with NCur/Ch 0.00625 + 0.5 and NCur 0.0125 improved GPx and SOD levels. All the incorporated groups shared this improvement and demonstrated a significant decrease in MDA level. The improved antioxidant response of previous groups might be attributed to the hepatoprotective effects of Cur [51] and Ch [55]. Similarly, a Cur-supplemented diet, at a dose of 1%, significantly increased SOD and CAT activities in Nile tilapia [16]. The same finding was reported by Ji et al. [56] in yellow croaker, and by Yonar et al. [47] in rainbow trout. The improved results could be because of the hepatoprotective properties of Cur and its ability to protect the hepatocytes [51].
The present study demonstrated that the group receiving the diet supplemented with NCur/Ch revealed significantly increased villi length, width, and goblet cell count in the intestine. The improved intestinal morphometric characteristics may explain the improved growth performance of Nile tilapia receiving the supplemented diets, since both parameters are correlated [57]. In the same instance, feeding Nile tilapia with dietary Cur nanoparticle diets resulted in a higher villi length/width and absorption area [17]. This enhancement may be attributed to the administration of phenolic compounds, such as Cur, which could decrease intestinal inflammation, causing improvements in nutrient digestibility and absorption [58].
On the gene expression level, igf-I expression levels reflect growth patterns in animals [59]. Additionally, cc5 is an immune response-modulating cytokine that could be affected by Cur supplementation [16]. Current data shows that supplemented diets display noticeable upregulation in the expression of igf-1 and cc5 genes, with the most marked increase in the NCur/Ch 0.00625 + 0.5 group. Moreover, immune-related genes, including IL-1β and cc5, were enhanced by Cur incorporation in Nile tilapia [7]. This can be attributed to the hepatoprotective, immunostimulating, and antioxidative activities of Cur in fish [17].
The positive findings of the NCur/Ch blend in the present study could reveal a synergistic effect of NCur and Ch in improving fish response. This could reflect the role of Ch in the efficient delivery of NCur to target sites in the animal body. Cur in bulk or nano-form has metabolic limitations such as poor absorption, low availability, fast metabolism, and excretion [4,14,18]. Meanwhile, NCur blended with Ch, in the present study, may have improved absorption, biodegradability, and biocompatibility and increased Cur systemic levels [28].
In addition, the dietary incorporation of NCur is more profitable and economic than the traditional forms, as it produces better actions with lesser amounts (0.0125 g nano-form/kg diet compared with 1–2 g traditional form/kg diet) of Cur. In the current study, around 2 g was required to achieve the required dose of NCur throughout the experiment.
5. Conclusions
Based on the study outcomes, we recommend the use of nano-curcumin NCur and a nano-curcumin/chitosan blend as dietary supplements for promising growth, feed utilization, innate immunity, hepatic functions, and associated gene expression in Nile tilapia. Moreover, these supplements can be used to improve digestion by enhancing intestinal morphometric biomarkers, including increased villi width/length, which reflect improved nutrient absorption and reduce the required amounts of traditional forms of the same supplements. Future studies are required to elucidate the mode of action of nano-curcumin (NCur) and a nano-curcumin/chitosan blend, as well as to test its efficacy on other fish species and at higher doses.
Author Contributions
Conceptualization, H.E., H.H.M., S.M.R.S., A.M.A. (Abdelwahab M. Abdelwahab), M.S., S.H.I., A.M.A. (Abdelfattah M. Abdelfattah), A.K., A.T.M., H.S.H. and H.Y.; methodology, H.E., H.Y., H.H.M., S.M.R.S., M.S., S.H.I., A.M.A. (Abdelwahab M. Abdelwahab), A.K., H.S.H. and K.M.A.; investigation, H.E., H.Y., H.H.M., S.M.R.S., M.S., S.H.I., A.M.A. (Abdelfattah M. Abdelfattah), A.K. and H.S.H.; funding acquisition, K.M.A.; resources, H.E., H.Y., H.H.M., S.M.R.S., M.S., S.H.I., A.M.A. (Abdelfattah M. Abdelfattah), A.K., H.S.H., A.T.M. and K.M.A.; validation, H.E., H.H.M., H.S.H. and A.T.M.; software, K.M.A., A.T.M., A.M.A. (Abdelfattah M. Abdelfattah), A.K. and H.S.H.; writing—original draft preparation, H.E.; writing—review and editing, H.E., H.H.M. and A.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R402), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
All experimental procedures with live fish were approved by the animal welfare and ethical review committee of Faculty of Veterinary Medicine, Benha University, Egypt BUFVTM 07-10-22.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon request.
Acknowledgments
The authors gratefully acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R402), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sallam, A.E.; Mansour, A.T.; Alsaqufi, A.S.; Salem, M.E.-S.; El-Feky, M.M. Growth performance, antioxidative status, innate immunity, and ammonia stress resistance of Siganus rivulatus fed diet supplemented with zinc and zinc nanoparticles. Aquac. Rep. 2020, 18, 100410. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Ashour, M.; Labena, A.; Alsaqufi, A.S.; Mansour, A.T.; Abbas, E.M. Effects of dietary Arthrospira platensis nanoparticles on growth performance, feed utilization, and growth-related gene expression of Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2022, 551, 737905. [Google Scholar] [CrossRef]
- Elabd, H.; Youssuf, H.; Mahboub, H.H.; Salem, S.M.; Husseiny, W.A.; Khalid, A.; El-Desouky, H.S.; Faggio, C. Growth, hemato-biochemical, immune-antioxidant response, and gene expression in Nile tilapia (Oreochromis niloticus) received nano iron oxide-incorporated diets. Fish Shellfish. Immunol. 2022, 128, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, H.; Sourinejad, I.; Johari, S.A. Growth performance, haemato-immunological responses and antioxidant status of Pacific white shrimp Penaeus vannamei fed with turmeric powder, curcumin and curcumin nanomicelles. Aquac. Nutr. 2021, 27, 2294–2306. [Google Scholar] [CrossRef]
- Rather, M.; Sharma, R.; Aklakur, M.; Ahmad, S.; Kumar, N.; Khan, M.; Ramya, V. Nanotechnology: A novel tool for aquaculture and fisheries development. A prospective mini-review. Fish. Aquac. J. 2011, 16, 3. [Google Scholar]
- Rashidi, L. Different nano-delivery systems for delivery of nutraceuticals. Food Biosci. 2021, 43, 101258. [Google Scholar] [CrossRef]
- Pirani, F.; Moradi, S.; Ashouri, S.; Johari, S.A.; Ghaderi, E.; Kim, H.P.; Yu, I.J. Dietary supplementation with curcumin nanomicelles, curcumin, and turmeric affects growth performance and silver nanoparticle toxicity in Cyprinus carpio. Environ. Sci. Pollut. Res. 2021, 28, 64706–64718. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Anter, R.G.; Arisha, A.H.; Mansour, A.T.; Safhi, F.A.; Alwutayd, K.M.; Elshopakey, G.E.; Abd El-Hakim, Y.M.; Mohamed, E.M. Growth Retardation, Oxidative Stress, Immunosuppression, and Inflammatory Disturbances Induced by Herbicide Exposure of Catfish, Clarias gariepinus, and the Alleviation Effect of Dietary Wormwood, Artemisia cina. Fishes 2023, 8, 297. [Google Scholar] [CrossRef]
- Shahin, S.A.; Mansour, A.T.; Abdel-Rahim, M.M.; El-Dahhar, A.A.; El Basuini, M.F.; Elhetawy, A.I. Improving survival, growth, feed utilization, antioxidant status, and fatty acids profile of European seabass, larvae fed silymarin, supplemented weaning diet. Ann. Anim. Sci. 2022, 23, 253–264. [Google Scholar] [CrossRef]
- Elumalai, P.; Kurian, A.; Lakshmi, S.; Faggio, C.; Esteban, M.A.; Ringø, E. Herbal immunomodulators in aquaculture. Rev. Fish. Sci. Aquac. 2020, 29, 33–57. [Google Scholar] [CrossRef]
- Mansour, A.T.; Hamed, H.S.; El-Beltagi, H.S.; Mohamed, W.F. Modulatory Effect of Papaya Extract against Chlorpyrifos-Induced Oxidative Stress, Immune Suppression, Endocrine Disruption, and DNA Damage in Female Clarias gariepinus. Int. J. Environ. Res. Public Health 2022, 19, 4640. [Google Scholar] [CrossRef]
- Mansour, A.T.; Mahboub, H.H.; Amen, R.M.; El-Beltagy, M.A.; Ramah, A.; Abdelfattah, A.M.; El-Beltagi, H.S.; Shalaby, T.A.; Ghazzawy, H.S.; Ramadan, K.M.; et al. Ameliorative Effect of Quercetin against Abamectin-Induced Hemato-Biochemical Alterations and Hepatorenal Oxidative Damage in Nile Tilapia, Oreochromis niloticus. Animals 2022, 12, 3429. [Google Scholar] [CrossRef] [PubMed]
- Almarri, S.H.; Khalil, A.A.; Mansour, A.T.; El-Houseiny, W. Antioxidant, Immunostimulant, and Growth-Promoting Effects of Dietary Annona squamosa Leaf Extract on Nile Tilapia, Oreochromis niloticus, and Its Tolerance to Thermal Stress and Aeromonas sobria Infection. Animals 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- El-abd, H.; El-latif, A.; Shaheen, A. Effect of curcumin on growth performance and antioxidant stress status of Nile tilapia (Oreochromis niloticus). Iran. J. Fish. Sci. 2021, 20, 1234–1246. [Google Scholar]
- Abdel-Tawwab, M.; Eissa, E.-S.H.; Tawfik, W.A.; Abd Elnabi, H.E.; Saadony, S.; Bazina, W.K.; Ahmed, R.A. Dietary curcumin nanoparticles promoted the performance, antioxidant activity, and humoral immunity, and modulated the hepatic and intestinal histology of Nile tilapia fingerlings. Fish Physiol. Biochem. 2022, 48, 585–601. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.-I. Recent advances in nanomicelles delivery systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; Salem, M.E.S. Effects of dietary chitosan supplementation on farmed fish: A review. Rev. Aquac. 2020, 12, 438–452. [Google Scholar] [CrossRef]
- El-Naggar, M.; Medhat, F.; Taha, A. Applications of chitosan and chitosan nanoparticles in fish aquaculture. Egypt. J. Aquat. Biol. Fish. 2022, 26, 23–43. [Google Scholar] [CrossRef]
- Yan, J.; Guo, C.; Dawood, M.; Gao, J. Effects of dietary chitosan on growth, lipid metabolism, immune response and antioxidant-related gene expression in Misgurnus anguillicaudatus. Benef. Microbes 2017, 8, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elala, N.M.; Mohamed, S.H.; Zaki, M.M.; Eissa, A.E. Assessment of the immune-modulatory and antimicrobial effects of dietary chitosan on Nile tilapia (Oreochrmis niloticus) with special emphasis to its bio-remediating impacts. Fish Shellfish. Immunol. 2015, 46, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Singh, P.; Samoon, M.H.; Balange, A.K. Effect of dietary chitosan on non-specific immune response and growth of Cyprinus carpio challenged with Aeromonas hydrophila. Int. Aquat. Res. 2010, 2, 77. [Google Scholar]
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish. Immunol. 2019, 88, 254–258. [Google Scholar] [CrossRef]
- Saleh, M.; Essawy, E.; Shaalan, M.; Osman, S.; Ahmed, F.; El-Matbouli, M. Therapeutic Intervention with Dietary Chitosan Nanoparticles Alleviates Fish Pathological and Molecular Systemic Inflammatory Responses against Infections. Mar. Drugs 2022, 20, 425. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-based drug delivery system: Applications in fish biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.-P.; Shaheen, A.; Yao, H.; Abbass, A. Anti-oxidative effects of some dietary supplements on Yellow perch (Perca flavescens) exposed to different physical stressors. Aquac. Rep. 2017, 8, 21–30. [Google Scholar] [CrossRef]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase-old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K.; Im, H.-S.; Yarimaga, O.; Yoon, E.; Kim, H.-S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Aebi, H. Methods in Enzymology Oxygen Radical in Biological System; Academic Press, Inc.: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Theory and Practice of Histological Techniqueseighth; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Elabd, H.; Wang, H.-P.; Shaheen, A.; Yao, H.; Abbass, A. Feeding Glycyrrhiza glabra (liquorice) and Astragalus membranaceus (AM) alters innate immune and physiological responses in yellow perch (Perca flavescens). Fish Shellfish. Immunol. 2016, 54, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- Gröner, F.; Ziková, A.; Kloas, W. Effects of the pharmaceuticals diclofenac and metoprolol on gene expression levels of enzymes of biotransformation, excretion pathways and estrogenicity in primary hepatocytes of Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. 2015, 167, 51–57. [Google Scholar] [CrossRef]
- Cruz, E.V.; Brown, C. Influence of the photoperiod on growth rate and insulin-like growth factor-I gene expression in Nile tilapia Oreochromis niloticus. J. Fish Biol. 2009, 75, 130–141. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abbass, F.E. Turmeric powder, Curcuma longa L., in common carp, Cyprinus carpio L., diets: Growth performance, innate immunity, and challenge against pathogenic Aeromonas hydrophila infection. J. World Aquac. Soc. 2017, 48, 303–312. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Sruthi, M.; Nair, A.B.; Arun, D.; Thushara, V.; Sheeja, C.; Vijayasree, A.S.; Oommen, O.V.; Divya, L. Dietary curcumin influences leptin, growth hormone and hepatic growth factors in Tilapia (Oreochromis mossambicus). Aquaculture 2018, 496, 105–111. [Google Scholar] [CrossRef]
- Ashry, A.M.; Hassan, A.M.; Habiba, M.M.; El-Zayat, A.; El-Sharnouby, M.E.; Sewilam, H.; Dawood, M.A. The impact of dietary curcumin on the growth performance, intestinal antibacterial capacity, and haemato-biochemical parameters of gilthead seabream (Sparus aurata). Animals 2021, 11, 1779. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, X.-Y.; Zhou, X.-Q.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, Y. Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture 2016, 463, 174–180. [Google Scholar] [CrossRef]
- Fadl, S.E.; El-Gammal, G.A.; Abdo, W.S.; Barakat, M.; Sakr, O.A.; Nassef, E.; Gad, D.M.; El-Sheshtawy, H.S. Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia (Oreochromis niloticus) as well as the resistance to Streptococcus agalactiae infection. Aquac. Res. 2020, 51, 1120–1132. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Yang, Y.; Han, D.; Jin, J.; Xie, S. Effect of dietary chitosan on growth performance, haematology, immune response, intestine morphology, intestine microbiota and disease resistance in gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2014, 20, 532–546. [Google Scholar] [CrossRef]
- Geng, X.; Dong, X.-H.; Tan, B.-P.; Yang, Q.-H.; Chi, S.-Y.; Liu, H.-Y.; Liu, X.-Q. Effects of dietary chitosan and Bacillus subtilis on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Fish Shellfish. Immunol. 2011, 31, 400–406. [Google Scholar] [CrossRef]
- Ming, J.; Ye, J.; Zhang, Y.; Xu, Q.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish. Immunol. 2020, 97, 540–553. [Google Scholar] [CrossRef]
- Yonar, M.E.; Yonar, S.M.; İspir, Ü.; Ural, M.Ş. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish. Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Abdelkhalek, N.; El-Adl, M.; El-Ashram, A.; Othman, M.; Gadallah, H.; El-Diasty, M.; Dawood, M.A.; Almeer, R.; Abdel Daim, M. Immunological and antioxidant role of curcumin in ameliorating fipronil toxicity in Nile tilapia (Oreochromis niloticus). Aquac. Res. 2021, 52, 2791–2801. [Google Scholar] [CrossRef]
- Kohli, K.; Ali, J.; Ansari, M.; Raheman, Z. Curcumin: A natural antiinflammatory agent. Indian J. Pharmacol. 2005, 37, 141. [Google Scholar] [CrossRef]
- Gheytasi, A.; Shekarabi, S.P.H.; Islami, H.R.; Mehrgan, M.S. Feeding rainbow trout, Oncorhynchus mykiss, with lemon essential oil loaded in chitosan nanoparticles: Effect on growth performance, serum hemato-immunological parameters, and body composition. Aquac. Int. 2021, 29, 2207–2221. [Google Scholar] [CrossRef]
- Yousef, M.I.; El-Demerdash, F.M.; Radwan, F.M. Sodium arsenite induced biochemical perturbations in rats: Ameliorating effect of curcumin. Food Chem. Toxicol. 2008, 46, 3506–3511. [Google Scholar] [CrossRef] [PubMed]
- Mansour, W.A.; Abdelsalam, N.R.; Tanekhy, M.; Khaled, A.A.; Mansour, A.T. Toxicity, inflammatory and antioxidant genes expression, and physiological changes of green synthesis silver nanoparticles on Nile tilapia (Oreochromis niloticus) fingerlings. Comp. Biochem. Physiol. C Toxicol. 2021, 247, 109068. [Google Scholar] [CrossRef]
- Faheem, M.; Khaliq, S.; Abbas, R.Z.; Mansour, A.T. Moringa oleifera alleviated oxidative stress, physiological and molecular disruption induced by acute thermal stress in grass carp, Ctenopharyngodon idella. Fish Physiol. Biochem. 2022, 48, 1463–1473. [Google Scholar] [CrossRef]
- Mehta, K.; Balaraman, R.; Amin, A.; Bafna, P.; Gulati, O. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef]
- Ibrahim, D.; Neamat-Allah, A.N.; Ibrahim, S.M.; Eissa, H.M.; Fawzey, M.; Mostafa, D.I.; Abd El-Kader, S.A.; Khater, S.; Khater, S.I. Dual effect of Selenium loaded Chitosan Nanoparticles on growth, antioxidant, immune related genes expression, transcriptomics modulation of caspase 1, cytochrome P450 and heat shock protein and Aeromonas hydrophila resistance of Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 110, 91–99. [Google Scholar]
- Ji, R.; Xiang, X.; Li, X.; Mai, K.; Ai, Q. Effects of dietary curcumin on growth, antioxidant capacity, fatty acid composition and expression of lipid metabolism-related genes of large yellow croaker fed a high-fat diet. Br. J. Nutr. 2021, 126, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O.; Dawood, M.A.; Eltholth, M. Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac. Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Giannenas, I.; Pappas, I.; Mavridis, S.; Kontopidis, G.; Skoufos, J.; Kyriazakis, I. Performance and antioxidant status of broiler chickens supplemented with dried mushrooms (Agaricus bisporus) in their diet. Poult. Sci. 2010, 89, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.C.; Waldbieser, G.C.; Bilodeau, L. IGF-I and IGF-II mRNA expression in slow and fast growing families of USDA103 channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 139, 317–323. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).