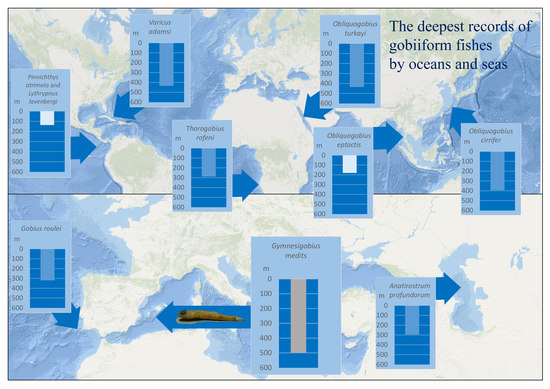
The Second Record of Gymnesigobius medits Kovačić, Ordines, Ramirez-Amaro & Schliewen, 2019, the Deepest Benthic Gobiiform Species, and the Additional Records of Gobius xoriguer Iglésias, Vukić & Šanda, 2021 (Actinopterygii: Gobiiformes: Gobiidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Morphological Identification
2.3. Genetic Analyses
3. Results
3.1. Gobius xoriguer Iglésias, Vukić & Šanda 2021
3.1.1. Studied Material
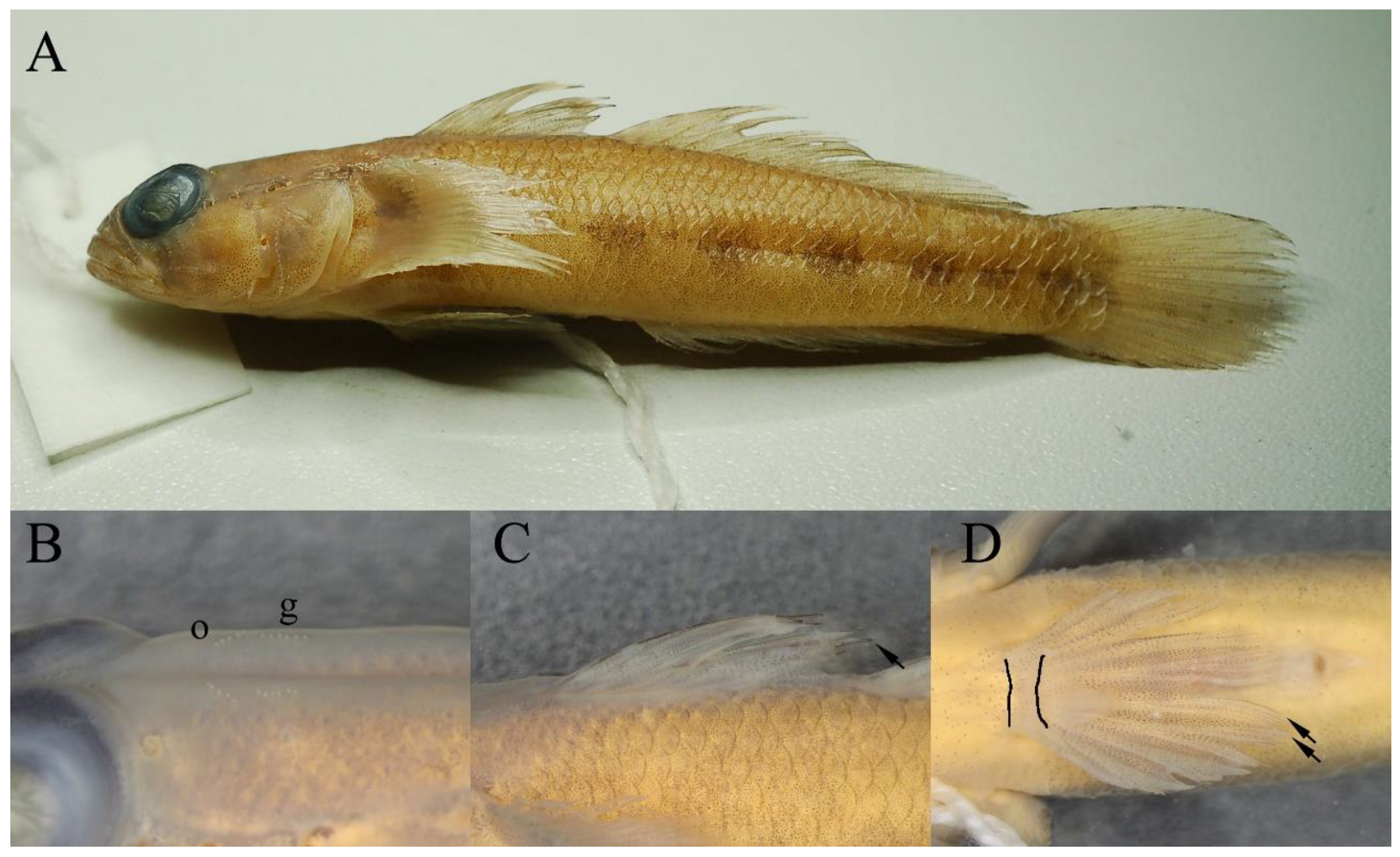
3.1.2. Identification
3.1.3. Genetics
3.1.4. Remarks
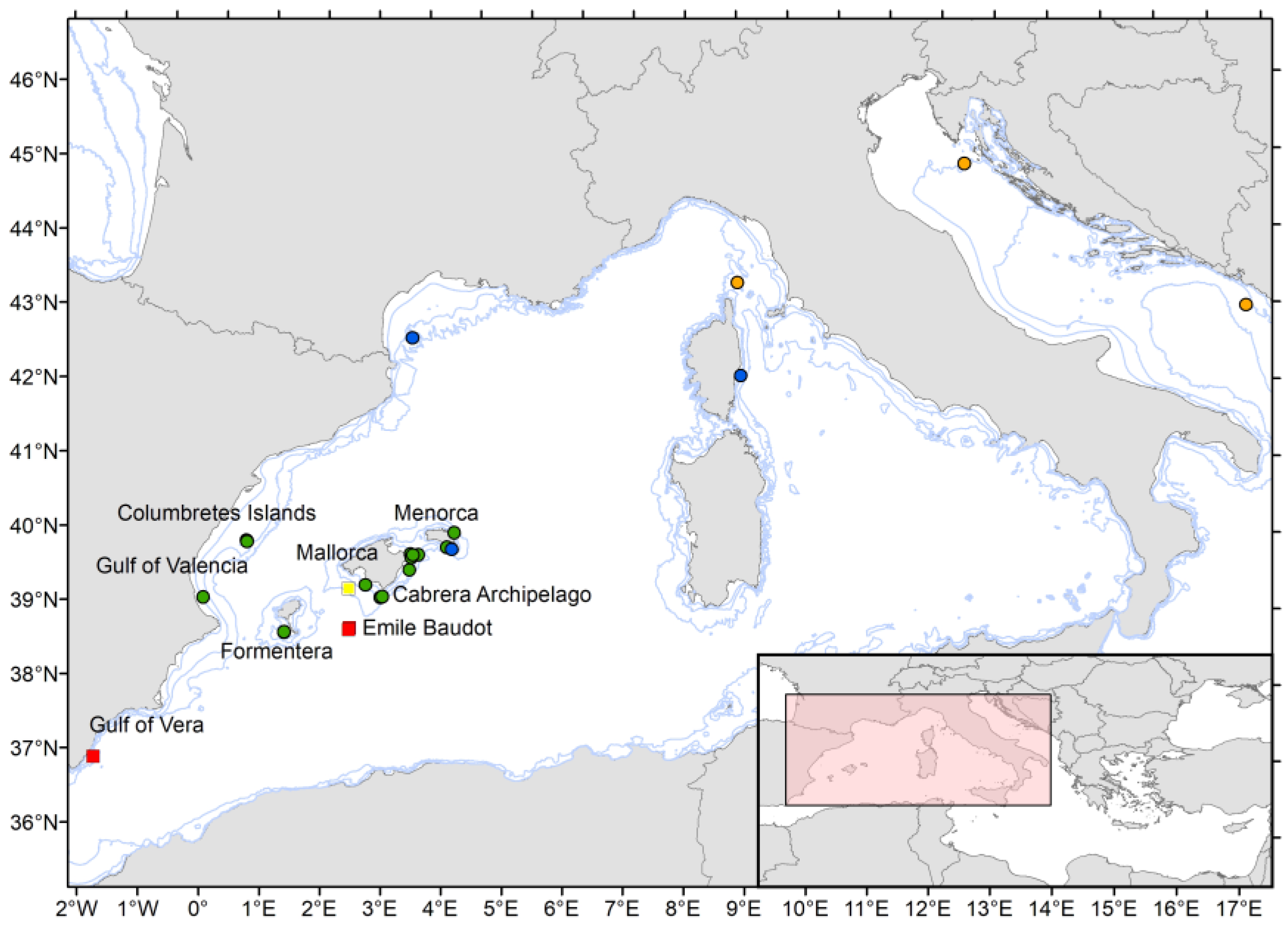
3.1.5. Ecology and Geographic Distribution
3.2. Gymnesigobius Medits Kovačić, Ordines, Ramirez-Amaro & Schliewen 2019
3.2.1. Studied Material
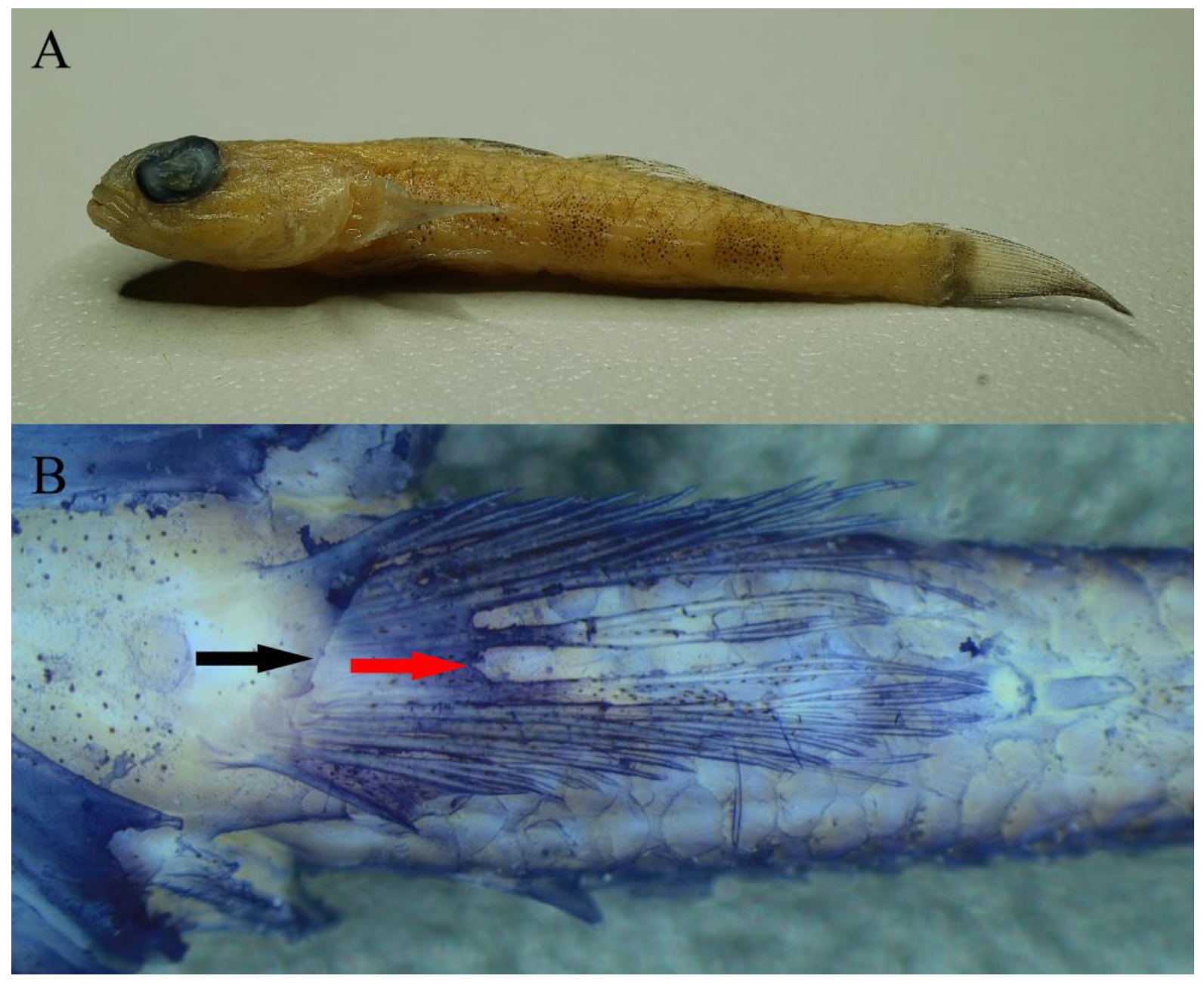
3.2.2. Identification
3.2.3. Genetics
3.2.4. Remarks
3.2.5. Ecology and Geographic Distribution
4. Discussion
| Species | Maximum Confirmed Depth | Collecting Method | Region of Collecting Site | Taxonomy & Phylogeny | Comment on Depth | Species Usual Depth Zones and Habitat | Reference(s) |
|---|---|---|---|---|---|---|---|
| European seas confirmed bathyal records and the confirmed deepest records in other oceans and seas | |||||||
| Gymnesigobius medits Kovačić, Ordines, Ramirez-Amaro & Schliewen, 2019 | 501 m (501–520 m) | Beam trawl | Mediterranean | Gobiidae, Gobiinae, Gobius-lineage | The deepest positive record of Gobiiformes, Gobiidae and Gobiinae in general, the deepest gobiid record in the Mediterranean | Restricted to bathyal mud | Present results |
| Lesueurigobius friesii (Malm, 1874) | 440 m | Trawl | Mediterranean | Gobiidae, Gobiinae, Aphia-lineage | Circalittoral and bathyal mud | [28] | |
| Lesueurigobius suerii (Risso, 1810) | 440 m | Trawl | Mediterranean | Gobiidae, Gobiinae, Aphia-lineage | Circalittoral and bathyal mud | [28] | |
| Varicus adamsi Gilmore, Van Tassell & Tornabene, 2016 | 435 m | Manned submersile | Western Atlantic | Gobiidae, Gobiinae, Gobiosomatini-lineage | The deepest positive gobiid record in the Western Atlantic | Bathyal sand and rubbles | [30] |
| Obliquogobius turkayi Goren, 1992 | 434 m (434–496 m) | Trawl | Red Sea | Gobiidae, Gobiinae, lineage unknown | The deepest positive gobiid record in the Red Sea | Bathyal soft sediment | [32] |
| Obliquogobius cirrifer Shibukawa & Aonuma, 2007 | 394 m (394–404 m) | Beam trawl | Western Pacific | Gobiidae, Gobiinae, lineage unknown | The deepest positive gobiid record in the Western Pacific | Bathyal fine sand | [33] |
| Buenia lombartei Kovačić, Ordines & Schliewen, 2018 | 375 m | Trawl and beam trawl | Mediterranean | Gobiidae, Gobionellinae, Pomatoschistus- lineage | The deepest positive record of Gobionellinae in general | Restricted to bathyal mud | [7] |
| Gobius xoriguer Iglésias, Vukić & Šanda, 2021 | 374 m | Grab | Mediterranean | Gobiidae, Gobiinae, Gobius-lineage | Eurybathic, from coralline algae sea bed to bathyal | [14] | |
| Gobius roulei De Buen, 1928 | 320 m (320 –385 m) | Trawl | North Eastern Atlantic | Gobiidae, Gobiinae, Gobius-lineage | The deepest positive gobiid record in the North-Eastern Atlantic | Eurybathic, from infralittoral sands to bathyal | [34] |
| Anatirostrum profundorum (Berg, 1927) | 294 m | Trawl | Caspian Sea | Gobiidae, Gobiinae, Gobius-lineage | The deepest positive gobiid record in the Caspian Sea | Circalittoral and bathyal mud | [35] |
| Thorogobius rofeni Miller, 1988 | 288 m (288–294 m) | Trawl | South Eastern Atlantic | Gobiidae, Gobiinae, Gobius-lineage | The deepest positive gobiid record in the South-Eastern Atlantic | Bathyal, no data on the bottom composition | [36] |
| Lebetus scorpioides (Collett, 1874) | 242 m | Trawl | North Eastern Atlantic | Gobiidae, Gobionellinae, Pomatoschistus- lineage | From infralittoral to bathyal at various bottoms | [3] | |
| Trypauchen vagina (Bloch & Schneider, 1801) | 200 m | Trawl | Mediterranean | Gobiidae, Amblyopinae, Periophthalmus-lineage | The deepest positive record of Amblyopinae in general, the deepest alien gobiid record in the Mediterranean | Continental shelf (circalittoral) to bathyal mud | [28] |
| Obliquogobius eptactis Fujiwara, Psomadakis, Swe & Motomura, 2021 | 181 m (181–184 m) | Trawl | Indian Ocean | Gobiidae, Gobiinae, lineage unknown | The deepest positive gobiid record in the Indian Ocean, continental shelf | Deep shelf, no data on the bottom composition | [37] |
| Pinnichthys atrimela (Bussing, 1997) | 137 m (137–146 m) | Otter trawl | Eastern Pacific | Gobiidae, Gobiinae, Gobiosomatini-lineage | The deepest positive gobiid record in the Eastern Pacific, continental shelf | Deep shelf, no data on the bottom composition | [38] |
| Lythrypnus lavenbergi Bussing, 1997 | 137 m (137–146 m) | Otter trawl | Eastern Pacific | Gobiidae, Gobiinae, Gobiosomatini-lineage | The deepest positive gobiid record in the Eastern Pacific, continental shelf | Deep shelf, no data on the bottom composition | [38] |
| Worldwide deepest doubtful records | |||||||
| Karsten totoyensis (Garman, 1903) | 1122 m | No data | Gulf of Boni, Sulawesi, East Indian Arcipelago (Indonesia), Western Pacific | Gobiidae, Amblyopinae, Periophthalmus- lineage | The deepest record of Gobiiformes, Gobiidae and Amblyopinae in general, the recorded depth is doubtful since it is so different from conspecific records from continental shelf at depths 30–55 m | Circalittoral sand mud | [26] |
| Platygobiopsis tansei Okiyama, 2008 | 960 m (960–970 m) | No data | Southern Japan, Western Pacific | Gobiidae, Gobiinae, lineage unknown | The deepest record of Gobiinae. The recorded depth has already been questioned by Okiyama (2008) and is doubtful since it is so different from conspecific records from the continental shelf at depths 65–128 m | Circalittoral muddy sand bottoms | [27] |
| European seas unconfirmed bathyal records | |||||||
| Crystallogobius linearis (Düben, 1845) | 400 m | No data | North Eastern Atlantic | Gobiidae, Gobionellinae, Pomatoschistus- lineage | The deepest gobiid record in Eastern Atlantic, the depth not positively confirmed, mentioned for the species by Miller (1986) without any exact data, details or cited reference | From infralittoral to bathyal, at various bottoms, from coarse sand to mud | [18] |
| Lebetus scorpioides (Collett 1874) | 375 m | No data | North Eastern Atlantic | Gobiidae, Gobionellinae, Pomatoschistus- lineage | The depth not positively confirmed, mentioned for the species by Miller (1986) without any exact data, details or cited reference | From infralittoral to bathyal at various bottoms | [18] |
| Buenia jeffreysii (Günther, 1867) | 330 | No data | North Eastern Atlantic | Gobiidae, Gobionellinae, Pomatoschistus- lineage | The depth not positively confirmed, mentioned for the species by Miller (1986) without any exact data, details or cited reference | From infralittoral to bathyal at various bottoms | [18] |
| Pomatoschistus norvegicus (Collett, 1902) | 325 m | No data | North Eastern Atlantic | Gobiidae, Gobionellinae, Pomatoschistus- lineage | The depth not positively confirmed, mentioned for the species by Miller (1986) without any exact data, details or cited reference | From infralittoral to bathyal, at various bottoms, from coarse sand to mud | [18] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera/Species by Family/Subfamily. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp (accessed on 25 April 2023).
- Kovačić, M. Checklist of gobies (Teleostei: Gobiidae) of the Mediterranean Sea and a key for species identification. Zootaxa 2020, 4877, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Byrkjedal, I.; Wienerroither, R.; Jensen, G. Lebetus scorpioides and Buenia jeffreysii (Teleostei: Gobiidae) found north of the Arctic Circle. Fauna Nor. 2016, 36, 47–50. [Google Scholar] [CrossRef]
- Kovačić, M.; Svensen, R. Northern extension of Lesueurigobius friesii (Malm, 1874) (Pisces: Gobiidae) distribution and the gobiid diversity decline along the Norwegian coast. Acta Adriat. 2019, 60, 147–156. [Google Scholar] [CrossRef]
- Kovačić, M.; Ordines, F.; Schliewen, U.K. A new species of Speleogobius (Teleostei: Gobiidae) from the Red Sea. Zootaxa 2016, 4066, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, M.; Ordines, F.; Schliewen, U.K. A new species of Buenia (Teleostei: Gobiidae) from the western Mediterranean Sea, with the description of this genus. Zootaxa 2017, 4250, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, M.; Ordines, F.; Schliewen, U.K. A new species of Buenia (Perciformes: Gobiidae) from the western Mediterranean slope bottoms, the redescription of Buenia jeffreysi and the first Balearic record of Buenia affinis. Zootaxa 2018, 4392, 267–288. [Google Scholar] [CrossRef]
- Kovačić, M.; Ordines, F.; Ramirez-Amaro, S.; Schliewen, U.K. Gymnesigobius medits (Teleostei: Gobiidae), a new gobiid genus and species from the western Mediterranean slope bottoms. Zootaxa 2019, 4651, 513–530. [Google Scholar] [CrossRef]
- Schliewen, U.K.; Kovačić, M.; Gerwenka, A.F.; Svensen, R.; Ordines, F. Lebetus patzneri (Teleostei: Gobiidae), a new goby species from the Balearic Islands, western Mediterranean, with first records of Lebetus guilleti (Le Danois, 1913) from this area and Norway, and with notes on its biology. Zootaxa 2019, 4706, 231–254. [Google Scholar] [CrossRef]
- Iglésias, S.P.; Vukić, J.; Sellos, D.Y.; Soukupová, T.; Šanda, R. Gobius xoriguer, a new offshore Mediterranean goby (Gobiidae), and phylogenetic relationships within the genus Gobius. Ichthyol. Res. 2021, 68, 445–449. [Google Scholar] [CrossRef]
- Kovačić, M.; Glavičić, I. The first Adriatic finding of Speleogobius llorisi (Actinopterygii: Gobiiformes: Gobiidae). Acta Ichthyol. Piscat. 2019, 49, 181–184. [Google Scholar] [CrossRef]
- Dulčić, J.; Lepen Pleić, I.; Zorica, B.; Bušelić, I.; Šestanović, M.; Kovačić, M. Fish larvae DNA barcoding indicated the potential appearance of rare species: Buenia massutii Kovačić, Ordines, and Schliewen, 2017 in the Adriatic Sea. Acta Adriat. 2022, 63, 45–52. [Google Scholar] [CrossRef]
- Seyhan Öztürk, D.; Oruç, A.Ç. The new distribution records of recently described gobies; Aegean Sea records for Pomatoschistus nanus Engin & Seyhan Öztürk, 2017 and easternmost records of Buenia massutii Kovačić, Ordines & Schliewen, 2017. J. Mar. Biol. Assoc. UK 2023, 103, e40. [Google Scholar]
- Kovačić, M.; Froglia, C. The Adriatic record of Gobius xoriguer Iglésias, Vukić & Šanda, 2021 and additional records of Vanneaugobius dollfusi Brownell, 1978 (Actinopterygii: Gobiiformes: Gobiidae). Cybium, 2023; (early view). [Google Scholar] [CrossRef]
- Jennings, S.; Lancaster, J.; Woolmer, A.; Cotter, J. Distribution, diversity and abundance of epibenthic fauna in the North Sea. J. Mar. Biol. Assoc. UK 1999, 79, 385–399. [Google Scholar] [CrossRef]
- Bertrand, J.; De Sola, L.; Papaconstantinou, C.; Relini, G.; Souplet, A. The general specifications of the MEDITS surveys. Sci. Mar. 2002, 66, 9–17. [Google Scholar] [CrossRef]
- Sanzo, L. Distribuzione delle papille cutanee (organi ciatiforme) e suo valore sistematico nei Gobi. Mitt. Zool. Stn. Neapel 1911, 20, 249–328. [Google Scholar]
- Miller, P.J. Gobiidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; Unesco: Paris, France, 1986; Volume 3, pp. 1019–1085. [Google Scholar]
- Saruwatari, T.; López, J.A.; Pietsch, T.W. Cyanine blue: A versatile and harmless stain for specimen observation. Copeia 1997, 1997, 840–841. [Google Scholar] [CrossRef]
- Agorreta, A.; San Mauro, D.; Schliewen, U.; Van Tassell, J.L.; Kovačić, M.; Zardoya, R.; Ruber, L. Molecular phylogenetics of Gobioidei and phylogenetic placement of European gobies. Mol. Phylogenetics Evol. 2013, 69, 619–633. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Hall, T. ABioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Miller, P.J. A new species of Gobius (Teleostei: Gobiidae) from the western English Channel, with a key to related species in the British and Irish fauna. J. Zool. 1974, 174, 467–480. [Google Scholar] [CrossRef]
- Murdy, E.O. Karsten: A new genus of eel goby (Gobiidae: Amblyopinae) with a key to “Trypauchen” group genera. Copeia 2002, 2002, 787–791. [Google Scholar] [CrossRef]
- Okiyama, M. Platygobiopsis tansei, a new species of dorso-ventrally flattened gobiid fish from Southern Japan. Bull. Natl. Mus. Nat. Sci. Ser. A 2008, Series A, 85–96. [Google Scholar]
- Goren, M.; Danovaro, R.; Rothman, S.B.S.; Mienis, H.K.; Galil, B.S. Snapshot of the upper slope macro- and megafauna of the Southeastern Mediterranean Sea: Ecological diversity and protection. Vie Milieu 2019, 69, 233–248. [Google Scholar]
- Ahnelt, H.; Sauberer, M. Deep-water, offshore, and new records of Schindler’s fishes, Schindleria (Teleostei, Gobiidae), from the Indo-west Pacific collected during the Dana-Expedition, 1928–1930. Zootaxa 2020, 4731, 451–470. [Google Scholar] [CrossRef]
- Tornabene, L.; Van Tassell, J.L.; Gilmore, R.G.; Robertson, D.R.; Young, F.; Baldwin, C.C. Molecular phylogeny, analysis of character evolution, and submersible collections enable a new classification for a diverse group of gobies (Teleostei: Gobiidae: Nes subgroup), including nine new species and four new genera. Zool. J. Linn. Soc. 2016, 177, 764–812. [Google Scholar] [CrossRef]
- Tornabene, L.; Manning, R.; Robertson, D.R.; Van Tassell, J.L.; Baldwin, C.C. A new lineage of deep-reef gobies from the Caribbean, including two new species and one new genus (Teleostei: Gobiidae: Gobiosomatini). Zool. J. Linn. Soc. 2022, 197, 322–343. [Google Scholar] [CrossRef]
- Goren, M. Obliquogobius turkayi, a new species of gobiid fish from the deep water of the central Red Sea. Senckenberg. Marit. 1992, 22, 265–270. [Google Scholar]
- Shibukawa, K.; Aonuma, Y. Three New Species of the Deep-dwelling Goby Genus Obliquogobius (Perciformes: Gobiidae: Gobiinae) from Japan, with Comments on the Limits of the Genus. Bull. Natl. Mus. Nat. Sci. Ser. A 2007, Series A, 137–152. [Google Scholar]
- Maul, G.E. The fishes taken in bottom trawls by R.V. “Meteor” during the 1967 Seamounts Cruises in the Northeast Atlantic. Meteor Forsch. Erebnisse 1976, 22, 1–69. [Google Scholar]
- Ahnelt, H.; Abdoli, A.; Naderi, M.; Coad, B.W. Anatirostrum profundorum: A rare deep-water gobiid species from the Caspian Sea. Cybium 2000, 24, 139–159. [Google Scholar]
- Sauberer, M.; Ahnelt, H. First record of the rare deep-water gobiid Thorogobius rofeni (Gobiidae) from the Southeast Atlantic. Cybium 2008, 32, 277–278. [Google Scholar]
- Fujiwara, K.; Psomadakis, P.N.; Swe, T.Y.Y.; Motomura, H. Description of a new species of Obliquogobius (Teleostei: Gobiidae) from the Andaman Sea (northeastern Indian Ocean). Raffles Bull. Zool. 2021, 69, 541–547. [Google Scholar]
- Bussing, W.A. Chirolepis atrimelum (Gobiidae) a new species of gobiid fish from Isla del Coco, Costa Rica. Rev. Biol. Trop. 1997, 45, 1547–1552. [Google Scholar]

| N | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gobius xoriguer OQ874680 | 2 | 3 | 1 | 3 | 4 | 3 | 56 | 57 | 57 | 57 | 83 | 84 | 81 | 83 | 85 | 85 | 83 | 83 | |
| 2 | Gobius xoriguer OQ874681 | 0.33 | 1 | 1 | 1 | 2 | 1 | 54 | 55 | 55 | 55 | 83 | 84 | 81 | 83 | 85 | 85 | 83 | 83 | |
| 3 | Gobius xoriguer OQ874682 | 0.49 | 0.16 | 2 | 2 | 3 | 2 | 55 | 56 | 56 | 56 | 84 | 85 | 82 | 84 | 86 | 86 | 84 | 84 | |
| 4 | Gobius xoriguer OQ874683 | 0.16 | 0.16 | 0.33 | 2 | 3 | 2 | 55 | 56 | 56 | 56 | 82 | 83 | 80 | 82 | 86 | 86 | 84 | 84 | |
| 5 | Gobius xoriguer OQ874684 | 0.49 | 0.16 | 0.33 | 0.33 | 3 | 2 | 53 | 54 | 54 | 54 | 82 | 83 | 80 | 82 | 84 | 84 | 82 | 82 | |
| 6 | Gobius xoriguer KR914773 | 0.66 | 0.33 | 0.49 | 0.49 | 0.49 | 3 | 55 | 56 | 56 | 56 | 82 | 83 | 80 | 82 | 86 | 86 | 84 | 84 | |
| 7 | Gobius xoriguer KR914774 | 0.49 | 0.16 | 0.33 | 0.33 | 0.33 | 0.49 | 55 | 56 | 56 | 56 | 84 | 83 | 82 | 84 | 84 | 84 | 82 | 82 | |
| 8 | Gobius gasteveni KR914770 | 9.20 | 8.87 | 9.03 | 9.03 | 8.70 | 9.03 | 9.03 | 3 | 4 | 3 | 89 | 90 | 87 | 89 | 89 | 89 | 86 | 88 | |
| 9 | Gobius gasteveni MT670218 | 9.36 | 9.03 | 9.20 | 9.20 | 8.87 | 9.20 | 9.20 | 0.49 | 1 | 0 | 88 | 89 | 86 | 88 | 88 | 88 | 85 | 87 | |
| 10 | Gobius gasteveni MT670220 | 9.36 | 9.03 | 9.20 | 9.20 | 8.87 | 9.20 | 9.20 | 0.66 | 0.16 | 1 | 87 | 88 | 85 | 87 | 87 | 87 | 84 | 86 | |
| 11 | Gobius gasteveni MT670222 | 9.36 | 9.03 | 9.20 | 9.20 | 8.87 | 9.20 | 9.20 | 0.49 | 0.00 | 0.16 | 88 | 89 | 86 | 88 | 88 | 88 | 85 | 87 | |
| 12 | Gobius niger MT670242 | 13.63 | 13.63 | 13.79 | 13.46 | 13.46 | 13.46 | 13.79 | 14.61 | 14.45 | 14.29 | 14.45 | 12 | 8 | 11 | 75 | 75 | 77 | 75 | |
| 13 | Gobius niger MT670243 | 13.79 | 13.79 | 13.96 | 13.63 | 13.63 | 13.63 | 13.63 | 14.78 | 14.61 | 14.45 | 14.61 | 1.97 | 6 | 9 | 78 | 78 | 78 | 76 | |
| 14 | Gobius niger MT670244 | 13.30 | 13.30 | 13.46 | 13.14 | 13.14 | 13.14 | 13.46 | 14.29 | 14.12 | 13.96 | 14.12 | 1.31 | 0.99 | 5 | 75 | 75 | 75 | 73 | |
| 15 | Gobius niger MT670245 | 13.63 | 13.63 | 13.79 | 13.46 | 13.46 | 13.46 | 13.79 | 14.61 | 14.45 | 14.29 | 14.45 | 1.81 | 1.48 | 0.82 | 79 | 79 | 79 | 77 | |
| 16 | Gobius roulei MT670259 | 13.96 | 13.96 | 14.12 | 14.12 | 13.79 | 14.12 | 13.79 | 14.61 | 14.45 | 14.29 | 14.45 | 12.32 | 12.81 | 12.32 | 12.97 | 2 | 4 | 4 | |
| 17 | Gobius roulei MT670260 | 13.96 | 13.96 | 14.12 | 14.12 | 13.79 | 14.12 | 13.79 | 14.61 | 14.45 | 14.29 | 14.45 | 12.32 | 12.81 | 12.32 | 12.97 | 0.33 | 4 | 2 | |
| 18 | Gobius roulei MT670261 | 13.63 | 13.63 | 13.79 | 13.79 | 13.46 | 13.79 | 13.46 | 14.12 | 13.96 | 13.79 | 13.96 | 12.64 | 12.81 | 12.32 | 12.97 | 0.66 | 0.66 | 2 | |
| 19 | Gobius roulei MT670262 | 13.63 | 13.63 | 13.79 | 13.79 | 13.46 | 13.79 | 13.46 | 14.45 | 14.29 | 14.12 | 14.29 | 12.32 | 12.48 | 11.99 | 12.64 | 0.66 | 0.33 | 0.33 |
| N | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gymnesigobius medits OQ874685 | 6 | 5 | 5 | 5 | 100 | 97 | 104 | 108 | |
| 2 | Gymnesigobius medits OQ874686 | 1.05 | 5 | 5 | 3 | 101 | 98 | 105 | 106 | |
| 3 | Gymnesigobius medits OQ874687 | 0.88 | 0.53 | 2 | 0 | 100 | 98 | 104 | 108 | |
| 4 | Gymnesigobius medits OQ874688 | 0.88 | 0.53 | 0.35 | 2 | 100 | 97 | 104 | 108 | |
| 5 | Gymnesigobius medits MK628514 | 0.88 | 0.53 | 0.00 | 0.35 | 98 | 96 | 102 | 106 | |
| 6 | Odondebuenia balearica MK628520 | 17.54 | 17.72 | 17.54 | 17.54 | 17.19 | 90 | 88 | 98 | |
| 7 | Vanneaugobius dollfusi MK628516 | 17.02 | 17.19 | 17.19 | 17.02 | 16.84 | 15.83 | 104 | 102 | |
| 8 | Wheelerigobius wirtzi MK628521 | 18.25 | 18.42 | 18.25 | 18.25 | 17.89 | 15.44 | 18.16 | 68 | |
| 9 | Wheelerigobius maltzani MK628522 | 18.95 | 18.60 | 18.95 | 18.95 | 18.60 | 17.19 | 17.85 | 11.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačić, M.; Ramírez-Amaro, S.; Farriols, M.T.; Ordines, F. The Second Record of Gymnesigobius medits Kovačić, Ordines, Ramirez-Amaro & Schliewen, 2019, the Deepest Benthic Gobiiform Species, and the Additional Records of Gobius xoriguer Iglésias, Vukić & Šanda, 2021 (Actinopterygii: Gobiiformes: Gobiidae). Fishes 2023, 8, 331. https://doi.org/10.3390/fishes8060331
Kovačić M, Ramírez-Amaro S, Farriols MT, Ordines F. The Second Record of Gymnesigobius medits Kovačić, Ordines, Ramirez-Amaro & Schliewen, 2019, the Deepest Benthic Gobiiform Species, and the Additional Records of Gobius xoriguer Iglésias, Vukić & Šanda, 2021 (Actinopterygii: Gobiiformes: Gobiidae). Fishes. 2023; 8(6):331. https://doi.org/10.3390/fishes8060331
Chicago/Turabian StyleKovačić, Marcelo, Sergio Ramírez-Amaro, Maria Teresa Farriols, and Francesc Ordines. 2023. "The Second Record of Gymnesigobius medits Kovačić, Ordines, Ramirez-Amaro & Schliewen, 2019, the Deepest Benthic Gobiiform Species, and the Additional Records of Gobius xoriguer Iglésias, Vukić & Šanda, 2021 (Actinopterygii: Gobiiformes: Gobiidae)" Fishes 8, no. 6: 331. https://doi.org/10.3390/fishes8060331
APA StyleKovačić, M., Ramírez-Amaro, S., Farriols, M. T., & Ordines, F. (2023). The Second Record of Gymnesigobius medits Kovačić, Ordines, Ramirez-Amaro & Schliewen, 2019, the Deepest Benthic Gobiiform Species, and the Additional Records of Gobius xoriguer Iglésias, Vukić & Šanda, 2021 (Actinopterygii: Gobiiformes: Gobiidae). Fishes, 8(6), 331. https://doi.org/10.3390/fishes8060331