Abstract
The reef manta ray, Mobula alfredi (Krefft, 1868), is a highly mobile and plankton-feeding species, classified vulnerable to extinction on the IUCN Red List for Threatened Species. Knowledge on their spatial ecology and the extent of their dispersal remain incomplete, especially within island-fragmented habitats as found in New Caledonia. Satellite telemetry was used to investigate the horizontal movement ecology of reef manta rays in New Caledonia. A total of 21 reef manta rays were tagged with pop-up satellite archival transmitting tags (21 Fastloc and 2 MiniPAT) that remained deployed for a duration ranging from 3 to 180 days (mean ± SE = 76.7 ± 50.3). Rays presented a strong site fidelity and an important affinity for coastal waters. Long-distance migrations (>300 km) were also observed, mainly through coastal and shallow water paths. Horizontal movements were compared to a home range area and classified into four distinct patterns: Fidelity, Excursion, Fidelity + Relocation and Relocation. The most dominant pattern was Fidelity, where manta rays remained within their home range for the whole duration of the tag deployment. Our findings may assist in the design of more appropriate management strategies for the species in New Caledonia and other regions worldwide.
Key Contribution:
This paper presents unique information on the horizontal movement ecology of reef manta rays (Mobula alfredi) in a context of an archipelago that combines continuous coastlines and islands separated by deep waters. The main results show a consistent use of shallow coastal waters for dispersal and that deep water might be a restraining factor but not a complete barrier to connectivity.
1. Introduction
Understanding the processes that influence a species distribution and its dynamics is particularly relevant for conservation []. This information may assist in mapping biodiversity and ecosystem services, identifying effects and potential threats of environmental changes and anthropogenic activities, enabling effective prioritization of areas for biodiversity conservation. It also provides crucial knowledge to develop tools and models used in conservation [,]. To obtain a comprehensive view of these processes, it is mandatory to understand the life history traits and the biology of the species as well as the environmental factors to which the species may respond. Marine species that are capable of large-scale movements have a high dispersal potential, especially in continuous environments such as the open ocean where physical barriers are not obvious [,]. Yet, several studies showed high residency and site fidelity patterns in marine species where movements were influenced by environmental barriers and biological factors such as mating success, access to breeding grounds or access to consistent food resources. For instance, reef fish show limited connectivity between reefs separated by large sand channels [,], killer whales display intrinsic isolation of communities due to different food resources [], bottlenose dolphins demonstrate restricted home range linked with productive habitats [] and oceanic whitetip sharks record high site fidelity driven by the consistent availability of prey []. Food resource is also a major factor that influences the movement of large-bodied filter-feeding species [,,]. Yet, this resource depends on environmental factors, resulting in different distribution patterns between regions of the world. Consequently, spatial distribution and dynamics of these highly mobile filter-feeding species may be different from one region to the next.
Reef manta rays (Mobula alfredi, (Krefft, 1868)) are filter-feeders found in tropical waters around the word. Populations are observed near coastal reefs and their movements have been documented to be driven by the availability of food resources. For instance, in the Maldives, seasonal peaks in productivity gather hundreds of individuals each year []. In Indonesia, reef manta rays perform long-distance migration triggered by monsoon shifts and the associated reduction in productivity []. In Australia, highly productive eddy events are likely to trigger the offshore movements of individuals [], and seasonal variations in temperature appear to initiate latitudinal migration over hundreds of kilometres along the east coast of Australia [,]. In contrast, when the resource is consistent throughout the year, reef manta rays seem to demonstrate strong residency patterns with only few connections between geographically close populations. In Hawaii, no connection was found over 10 years of photo-identification monitoring between two aggregation sites located only 150 km apart. Similarly, in Indonesia, acoustic telemetry suggested spatial segregation between populations that are only 150 km apart []. The discussed evidence suggests that the spatial ecology of reef manta rays can be difficult to predict, and that localised investigation might be necessary to obtain a comprehensive understanding of their movements. This is a crucial task as the species is heavily exploited in many regions of the world for their gill plates that are used in Asian medicinal trades []. In addition to this fishing pressure, reef manta rays exhibit several conservative life history traits that exacerbate this vulnerability, including small population size, low fecundity, and fragmented distribution. Their strong affinity for coastal shallow waters increases their exposition to human activities, such as bycatch fisheries [], uncontrolled mass tourism [,], habitat degradation [,,,], boat strikes [], and fish net entanglement []. Consequently, reef manta rays are classified as being vulnerable to extinction on the IUCN Red List of Threatened Species []. Providing robust information on the movement patterns and habitat use of reef manta rays would contribute to the design of effective management and conservation measures that are essential to protect the species.
Among methods that enable spatial analysis, satellite telemetry is now a common practice with an increasing number of studies deploying tags to track the movements of a wide range of species including terrestrial species, marine birds, sea turtles, marine mammals, and elasmobranchs [,,,,,,,,]. This tool allows for researchers to investigate broad-scale and fine-scale movements, diving behaviour as well as preferred depth and temperature, and sometimes revealing misconceptions or unexpected findings about the ecology of a species [,,]. Satellite tracking devices are effective tools to detect unsuspected large migrations in elasmobranchs such as the basking shark [], the six gills shark [] and the white shark [], for example.
In New Caledonia, reef manta rays have not been studied until recently [,] and basic information on the population of this emblematic and threatened species are needed. Previous findings using four years of photo-identification monitoring revealed that the population of reef manta rays was distributed in all parts of the archipelago and exhibited high long-term fidelity rates at these sites. Connectivity was also limited but possible between all sites, showing high dispersal potential []. In addition, genomic analyses found genetic differentiation between aggregation sites, which confirms the importance of site fidelity, and exacerbated the lack of connectivity between geographically close habitats [].
On this basis, we used satellite tracking to investigate the spatial ecology of M. alfredi in the fragmented environment of the New Caledonia archipelago. Our objectives were to assess the fine-scale habitat use and potential broad-scale migration patterns by addressing the following points: quantify the use of offshore versus coastal waters, show the extent and limits of the movements from aggregation sites with high fidelity rates, define the frequency and magnitude of the connectivity between studied sites, and detect potential broad-scale movements and the existence of unknown key habitats.
2. Methods
2.1. Study Sites
The archipelago of New Caledonia consists of a Main Island surrounded by a barrier reef of 1660 km that encloses shallow waters of a 16,874 km2 lagoon []. This barrier reef marks the limits of the continental shelf where the bathymetry drops to depths greater than 2000 m. Smaller islands with a relatively narrow continental shelf, named the Loyalty Islands, are located off the east coast of the Main Island separated by a 2000 m-deep channel (Figure 1).
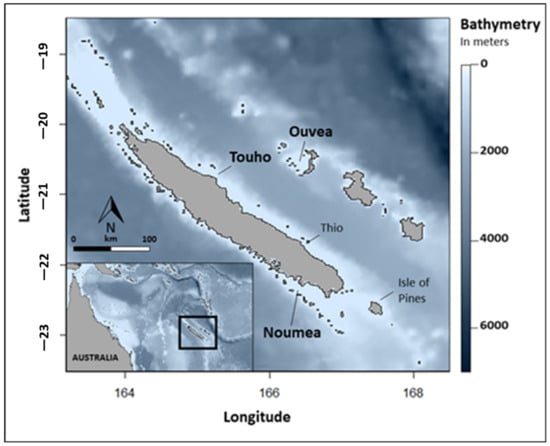
Figure 1.
Locations of the deployment of pop-up satellite tags (SPLASH10 and MiniPAT) on reef manta rays (Mobula alfredi) in New Caledonia, South Pacific. Study sites (in bold): Noumea (N = 6), Ouvea (N = 4) and Touho (N = 6).
Reef manta rays were tagged at two locations off the Main Island: Noumea in the southern part of the west coast and Touho in the northern part of the east coast. The other tagging site was off the northern island of the Loyalty Islands, Ouvea. In Noumea, tagging operations took place at two aggregation sites (24 km apart) on the barrier reef: the Boulari channel (22°29′ S, 166°26′ E) and the Dumbea channel (22°21′ S, 166°15′ E). The Boulari channel is a cleaning station on a 15 m-deep reef flat on the north tip of the channel, and the Dumbea channel is a feeding site within 30 m of the water column facing the reef crest of the south tip of the channel. In Touho (location undisclosed), the aggregation site is a cleaning station located at the north tip of the Great Channel of Touho on a 15-to-20 m reef shelf less than 5 km off the coast. In Ouvea, the deployment of the tags occurred at two aggregation sites: the Northern Pleiades (20°45′ S, 166°44′ E) and the Southern Pleiades (20°43′ S, 166°23′ E). Both sites were cleaning stations on the reef slope at a depth of 10 to 15 m on the continental shelf off the northern tip and southern tip of the island, respectively (Figure 1).
2.2. Tagging
We deployed 21 SPLASH10-F-321A PSAT tags and two MiniPAT tags (Wildlife Computers Inc.; Redmond, Washington, DC, USA) coated with Propspeed™ silicone coating to prevent fouling during the deployment period. Tag deployments were timed to coincide with sighting peaks for all sites between December and February over 4 years, from 2015 to 2020. Two additional tags were deployed opportunistically in Touho in November 2018. In Noumea and Touho, tags were deployed while scuba diving; in Ouvea, tagging was performed while free diving on free-swimming manta rays. The tag was tethered by a 30 cm stainless steel cable to a titanium dart-tip. A modified pole spear was used to apply darts into the dorsal musculature of the manta ray. All tags were programmed to remain attached for a maximum period of 180 days. Alternatively, detachment was programmed in case of the tag being recorded at a constant depth for more than 24 h (in case of mortality). SPLASH10 tags collected external temperature, light level, and pressure (depth) data every 10 s. Data were transmitted via the ARGOS satellite system (www.argos-system.org accessed on 23 June 2021) into 12 h periods. Prior to tagging, manta rays were identified using photo identification; the sex and maturity was also determined, and the size (disc width) was visually estimated to the nearest 10 cm.
2.3. Data Analysis
Locations were retrieved using the Wildlife Computers location processing systems (Fastloc GPS and GPE3). Location records were assigned with a quality rank based on the number of uplinks received per satellite pass for Argos data and on the number of connected satellites for Fastloc data. Argos data quality numbers rank from 3 to 0 and then A, B and Z, with 3 being the most accurate and Z being an invalid location. Fastloc data accuracy followed a gradient indicated by quality classes corresponding to the number of satellites, starting at 4 to 7 []. All location data were filtered according to the following steps. First, we manually removed duplicates and on-land locations, examined duplicates, and removed the less accurate ones. Second, Argos-derived locations with an accuracy class inferior to A (no accuracy estimation) and Fastloc-derived locations inferior to 5 satellites were removed. Finally, we used the sdafilter function from the R package argosfilter [] to exclude improbable locations based on speed and distance where trips exceeding a given speed (2 m·s−1) over a given distance (5000 m) were considered improbable.
Filtered datasets were analysed using R version 3.6.3 []. Maps and bathymetry data were extracted from the marmap [] package in R.
2.4. Movement Patterns Analysis
Movement patterns were defined using a threshold distance from the site of origin to determinate a home range area. This value corresponded to the distance beyond which the distribution of record frequencies shows a significant drop. Four different movement patterns were identified. First, fidelity—when manta rays remained within the home range area. Second, excursion—when manta rays extended their movement beyond the home range at least once but consistently returned to this area. Third, the relocation pattern—when manta rays moved beyond the home range area and remained outside this area more than half of the time until release of the tag. Finally, the fourth pattern, fidelity + relocation, represents manta rays that had more than half of their recorded location within the home range before moving outside of this area when the tag ceased recording or detached from the animal.
2.5. Statistical Analysis
Comparison of means tests between grouping factors were carried out using an analysis of variance (ANOVA) and Tukey’s post hoc test. Welch’s F tests were used when variances were unequal. Pairwise comparisons of means were tested with Student t-tests. The assumption of homogeneity was tested using Levene’s tests. Chi-squared (χ2) goodness-of-fit tests were used to examine the number of individuals between and among movement patterns. Z-tests of proportion were used to compare proportions. Finally, Kolmogorov–Smirnov tests were used to compare distributions and the Pearson correlation coefficient was used to test for linear relationships between variables. In this paper, mean values were followed by the standard deviation in the format: (MEAN ± SD).
2.6. Ethic Statement
Tagging was conducted with authorization from the Southern Province (permit no: 34584) and the Northern Province (permit no: 609011–33) of New Caledonia. In the Loyalty Islands Province, no permit was required by the competent authorities, though permission of the local customary representatives was granted.
3. Results
3.1. Tagging
A total of 23 tags were deployed; a total of 19 successfully transmitted data and 4 failed for unknown reasons (Table S1). Data from 16 tags were retrieved through the ARGOS server with a mean decoding rate of 83.9 ± 13.9%, and 3 tags were physically recovered, allowing for 100% of the data to be decoded. After the data filtering process, two tags were excluded from the analysis of horizontal movements due to inaccurate data. The mean retention period is 76.7 ± 50.3 days (range from 3 to 180 days). Only the MiniPAT tag detached at the term of the programmed period (180 days); all other tags popped up prematurely for unknown reasons. The majority of tagged individuals were females (57.9%), including an individual who was pregnant, and two were juveniles (one of each sex). Finally, all tagged individuals recorded only a few locations per day of deployment with an average of one record every 1.5 days. This number varied between sites from one record every 5.5 days and 0.9 days in Ouvea and Noumea, respectively. In Touho, a location every 2.7 days of deployment was recorded.
3.2. Horizontal Movements
Horizontal movements varied greatly in length and direction between individuals. Manta rays travelled an average of 4.6 ± 3.1 km d−1 (ranging from 0.2 to 12.3 km d−1) with total track lengths varying from 23 to 688 km in 100 and 53 days, respectively. The latitudinal range extended from 7.1 to 224 km with an average of 76.7 ± 74.1 km. These movements remained mostly within a relative proximity of the site of deployment, with 63.5% of the recorded locations observed within 30 km from the deployment areas. The average distance from the tagging site was 41 ± 66 km with a maximum at 311 km. Manta rays remained close to shallow waters, on average at a distance of 3.5 ± 4.4 km from depths under 10 m. They displayed limited offshore movements. Only one individual was located offshore up to 105 km from the nearest reef. During these movements, manta rays occurred at locations with highly varying bathymetry, averaging 325 ± 411 m below the surface, and a maximum was recorded at 2720 m deep.
When combining all location data from all sites, the distribution of distance from the tagging site reveals a significant drop beyond the 50 km range (Figure 2). This 50 km threshold from the site of origin will be used to identify movement patterns in and out of this range.
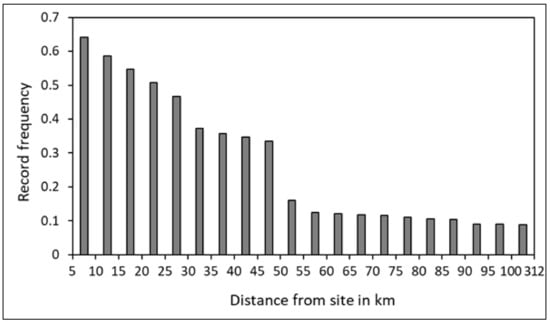
Figure 2.
Frequency distribution of the records of reef manta rays (Mobula alfredi) (N = 16) at different ranges of distance from the site of tag deployment, using satellite tags (SPLASH10), in New Caledonia.
Four different movement patterns were identified from the combined records of all sites (Figure 3 and Figure 4, Table 1). First, the fidelity pattern represents half of the individuals. Second, three individuals followed the excursion pattern. The number of excursions per deployment varied from 1 to 16 for a maximum duration ranging from 1 to 9 days (Figure S1). Third, only the movements of two individuals (12.5%) fell under the relocation pattern. Relocation movements were recorded at distances ranging from 71 to 248 km for a maximum duration of 48 and 40 days before release of the tag, respectively. Finally, the fidelity + relocation pattern describes 18.7% of individuals. The counts of individuals were not significantly different between movement pattern categories (χ2(3,16) = 4.0, p > 0.05).
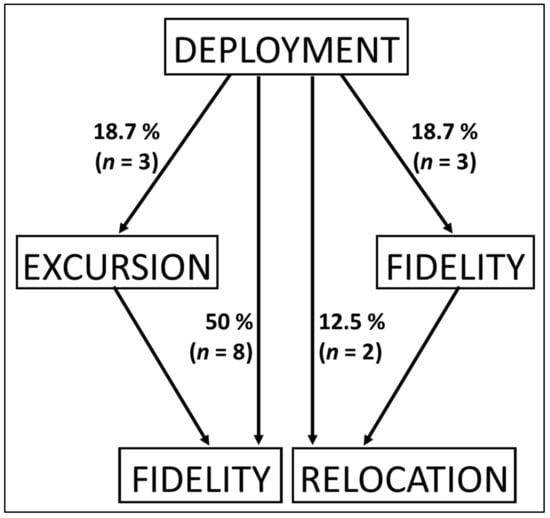
Figure 3.
Movement patterns recorded for reef manta rays (Mobula alfredi) after deployment of the tag, using satellite tags (SPLASH10), in New Caledonia. Percentage based on the total number of individuals (N = 16).
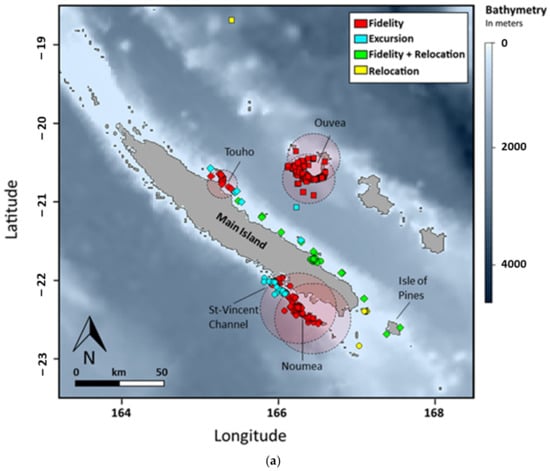
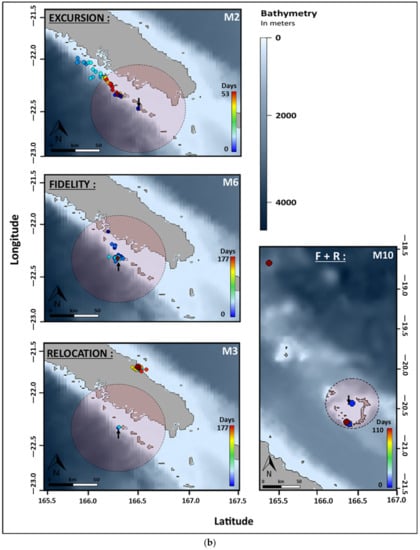
Figure 4.
(a) Movement patterns of reef manta rays (Mobula alfredi) in New Caledonia using satellite telemetry (SPLASH10). Symbol shapes indicate site of origin: circle = Noumea (N = 6), square = Ouvea (N = 4), and diamond = Touho (N = 6). (b) Examples of movement patterns of reef manta rays (Mobula alfredi) in New Caledonia using satellite telemetry (SPLASH10). Circles indicate respective home range and arrows indicate deployment location. F + R = Fidelity + Relocation and Mx indicate individual identifications.

Table 1.
Movement pattern characteristics and distribution between the sites and sex of reef manta rays (Mobula alfredi) (N = 17) in New Caledonia using satellite telemetry (SPLASH10). HR: home range.
Comparisons between sites reveal significantly different distributions of distance frequencies (Figure S2, Table S2). Home ranges were smaller in Touho (N = 19 records) and Ouvea (N = 105) with a limit at 20 and 35 km from the tagging site, respectively. In Noumea (N = 626), the home range was bigger with a limit at 50 km (Figure 4, Table 1). At this site, one third of the tagged manta rays remained within the home range perimeter. Another third recorded excursions along the barrier reef up to 86.1 km north of the deployment location. The last third relocated at 96 (M4) and 250 km (M3) at the southern tip of the lagoon of the Main Island and on the east coast of the Main Island, respectively. In Ouvea, two individuals remained within the designated home range of 35 km from the site, while another recorded an excursion halfway between the island of Ouvea and the Main Island over the 2000 m-deep channel (Figure 4, Table 1). The last manta ray relocated up to 225 km north toward the isolated reefs of Petrie and spent a maximum of 107 days outside its initial home range. In Touho, two manta rays remained within the 20 km home range (Figure 4, Table 1). Two individuals recorded excursions, including one that moved 131 km down the coast for a maximum of 82 days outside its initial home range. Finally, the two other manta rays from Touho relocated. One travelled down to the Isle of Pines (311 km) to return later toward the tagging site where the tag detached 70 km south. The other relocated at the Isle of Pines. Differences in the number of individuals per movement patterns between site were not significant (Table S3).
Overlap in space occupation between individuals tagged at different sites was observed between manta rays from Touho and Noumea in the southern tip and the southern part of the east coast of the Main Island (Figure 4). Among individuals tagged at the same site, overlap of movements outside the tagging sites only occurs off Noumea within an area located 50 km north of the Dumbea channel near the St-Vincent channel.
3.3. Sex
Females were recorded at a significantly farther distance from the site of tagging than males (t(781) = 5.9, p < 0.001). However, no gender was more mobile than the other, with no significant difference in distances per day (t(14) = 0.46, p > 0.05), total track lengths (t(14) = 0.14, p > 0.05), or in the extent of the latitudinal range (t(14) = 1.01, p > 0.05). The comparison of movement patterns among sex showed a significant difference in the distribution of frequencies of recorded distance from sites (K-S: D(21) = 0.67, p < 0.001). Females (N = 9 individuals) recorded a broader home range than males (N = 7) with a perimeter of 50 km against 35 km, respectively. Differences in the number of individuals per movement patterns between sex were not significant (p-values > 0.05 by Fisher’s exact test).
4. Discussion
In New Caledonia, reef manta rays tend to remain close to coasts and reefs. Except for one male individual, all records were within 35 km off the closest reef. Several studies that documented movements of reef manta rays reported consistent use of coastal and reef areas in Australia [,], in the Red Sea [,], in the Seychelles [], and in the British Indian Ocean Territory MPA []. The use of coastal habitat by reef manta rays and other planktivorous elasmobranchs has been associated with food availability [,,,,,,]. Coastal areas of islands surrounded by deep waters are prone to assembling the conditions to generate primary productivity hotspots combining upwellings and land inputs and generating reliable food resources which might result in strong residency patterns in reef manta rays’ behaviour [,]. In New Caledonia, such conditions exist with little annual variations [,]. Limited offshore movements are likely due to the foraging ground being coastal and the probable lower productivity of the adjacent oceanic waters []. Other than food resources, coastal areas and shallow waters of the lagoon offer more protection from predators such as large sharks, and hence, represent a suitable environment for potential nurseries. In New Caledonia, the detection of juvenile individuals remains sporadic, and neonates are even scarcer, although lagoons are places of nursery grounds for reef manta rays in Raja Ampat, Indonesia [] and for other elasmobranchs [,,]. Further investigations in suspected adequate nursery grounds using aerial surveys and accurate measurement methods to quantify maturation stages might lead to the discovery of nurseries in New Caledonia. In addition, coral reefs are also home to numerous cleaning stations that are essential to manta rays’ well-being and health [], as well as mating and socialization [,]. Sheltered waters also act as a thermal refuge where enclosed shallow waters have a significantly higher temperature than oceanic waters. In this study, manta rays were observed to favour relatively high temperatures (27 to 29 °C), but were also able to face much colder water, for instance, when deep diving (7.6 °C). Basking in warm shallow waters after a deep dive would allow for the regulation of body temperature. Thermoregulation is an important part of the behaviour of mobulids [,] and other elasmobranchs [,], and the presence of warm-sheltered water in proximity of deep feeding grounds might be essential.
The use of offshore waters by one individual recorded in this study could be interpreted as a transiting trip toward potential alternative foraging grounds near isolated reefs. The presence of reef manta rays at isolated reefs have already been recorded during aerial surveys []. Further investigation in these areas might reveal new aggregations. Offshore movements by reef manta rays were also documented using satellite telemetry and were associated with foraging opportunities. In the east coast of Australia, [] recorded most of the tagged rays in the offshore Capricorn Eddy Region and attributed this behaviour to food-related issues and the high productivity generated by the eddy. In the Red Sea, [] observed offshore excursions to deeper water at night where manta rays performed deep dives supposedly to exploit pelagic planktonic resources.
Despite their demonstrated coastal affinity, reef manta rays have been observed over waters up to 2720 m deep. The archipelago of New Caledonia has a relatively narrow continental shelf, especially on the east coast of the Main Island and around the Loyalty Islands, beyond which the water depth drops rapidly. Even though manta rays spent most of their time within the first 50 m, our results suggest that this species is able to use deeper water to transit between areas (e.g., to connect with an isolated reef) or to access to demersal food resources []. This supports previous findings that recorded reef manta rays commuting between islands chain-separated by deep water in Indonesia [,] or transiting by deep water to access food resources in east Australia [] and the Maldives []. However, even if there is evidence that deep water is not a strict barrier to their movements, large expanses of deep water might still be a restricting factor to connectivity. For instance, in this study, spatial occupancy overlapped only for individuals originating from sites sharing the same coastline and not for individuals separated by a 2000 m-deep channel. Other evidence through different spatial and temporal scales using photo identification [] and genomics [] showed that these connections occur but were limited, highlighting the lack of connectivity over this deep-water channel. Other studies taking place in atolls were consistent with these observations, with populations recording no or only few connections between islands separated by deep waters in Hawaii [,], in the Maldives [], in French Polynesia [], or in the Seychelles []. On the contrary, reef manta rays showed long-distance movements along continuous coastlines, up to 1150 km in east Australia [], but also along the west coast of Australia (up to 700 km, []) or the coast of southern Mozambique (up to 350 km, []).
Horizontal movements were classified into four distinct patterns. The most dominant pattern was Fidelity, where manta rays remained within their home range for the whole duration of the tag deployment. Manta rays also displayed excursions, occasionally travelling relatively long distances, as far as 131 km away from the site of origin. Moreover, total track lengths were relatively small (maximum of 688 km) in comparison to previous findings, averaging 839 km in western Australia [], 1169 km in east Australia [], and 1074 km in the Seychelles []. Our results describe a behaviour that corresponds to site fidelity, as defined by Chapman et al. (2015): the return of an individual to a location where it previously resided after an absence as long as or longer than the residency period []. Additional evidence of such behaviour was found in New Caledonia using long-term photo-identification data to find high re-sighting rates for each site [] and genomics analysis to detect genetic structure between sites []. Site fidelity is often motivated by consistent foraging opportunities over time within an area []. This behaviour has been largely documented for reef manta rays in Hawaii [], Mozambique [], east Australia [], Indonesia [,], or French Polynesia []. Although most of the tagged individuals displayed constrained movements near the aggregation site of origin, manta rays of New Caledonia demonstrate the ability to potentially connect with all parts of the archipelago, at least parts that are linked by coastlines or shallow waters. In particular, almost a third of the individuals showed Relocation and Fidelity + Relocation patterns, connecting with sites up to 311 km from the site of origin. Coupled with return trips over 200 km away from the deployment location, these observations suggest that manta rays connect to intermediate sites. For instance, while no connection was observed between studied sites during this study, overlap occurred for individuals from Touho and Noumea at in-between sites along the east coast and at the southern tip of the Main Island. Sightings of manta rays using photo identification over five years revealed different aggregation sites throughout the archipelago, with multiple re-sightings from individuals originating from different sites []. The present results suggest the potential existence of additional aggregation sites where manta rays from different sites may encounter each other, although no field operation could be undertaken to confirm it.
The present results present a partial short-term overview of the spatial ecology of the reef manta rays of New Caledonia. The average deployment duration in this study was short (approx. 77 days) compared to what was achieved in other satellite telemetry studies on reef manta rays (e.g., 92 days, []; 147 days, []; 116 days, []). This might also explain the relatively smaller track lengths recorded in this study than at other locations in the world. To complement this finding, long-term monitoring of the movements and geographically extended sampling effort might help deciding whether these observations are sporadic, frequent, seasonal, or to what extent long-term site fidelity is supported. They may as well reveal new connections and new potential aggregation sites. To this regard, photo identification offers such perspectives, and acoustic telemetry may narrow the gap between photo identification and satellite telemetry by generating spatially and temporally intermediate data. In addition, genomics would provide a greater picture of the evolutionary processes that shape the population.
5. Conclusions
This work used satellite telemetry and confirmed that reef manta rays are resident to coastal water and show strong site fidelity in New Caledonia. Yet, this species is capable of relatively long-distance migrations seemingly favouring, but not limiting them to, coastal and shallow water paths. Deep water might be a restraining factor but not a complete barrier to connectivity. These results raise concerns regarding threats associated with habitat degradation, human exploitation, and disturbance. The home range of this species may also extend over greater distances than previously thought, especially in habitat fragmented by deep waters, which raises concerns regarding potential movements outside areas under protective jurisdictions. In New Caledonia, concerns regarding the species conservation are limited since reef manta rays are not targeted by fisheries and human activity remains relatively low. Such favourable context is scarce, which makes the population of reef manta rays in New Caledonia a reference to be preserved 19]. Therefore, preventive precautions should be taken at a local level where coastal development is rapidly expanding and might threaten critical habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8060328/s1, Figure S1: Horizontal movements of reef manta rays (Mobula alfredi) recorded using pop-up satellite tags (SPLASH10) in New Caledonia.; Table S1: Summary of satellite tag deployments on reef manta rays (Mobula alfredi) in New Caledonia.; Figure S2: Movement patterns recorded for reef manta rays (Mobula alfredi) after deployment of the tag, using satellite tag (SPLASH10), in Noumea, New Caledonia.; Table S2: Kolmogorov-Smirnov (K-S) test statistics of differences in distributions of frequencies of distance from sites records of reef manta rays (Mobula alfredi) (N = 16) in New Caledonia.; Table S3: Chi-Square test statistics of differences number of individuals per movement patterns of reef manta rays (Mobula alfredi) (N = 16) in New Caledonia.; Table S4: Horizontal and vertical movement metrics for reef manta rays (Mobula alfredi) tracked using satellite tags in New Caledonia.; Table S5: Z-test statistics of differences in proportion of dive below 300 m between movement patterns recorded for reef manta rays (Mobula alfredi) (N = 16) in New Caledonia.
Author Contributions
Conceptualization, H.L., L.W. and O.C.; methodology, H.L. and L.W.; validation, H.L., L.W. and O.C.; formal analysis, H.L. and L.W.; investigation, H.L.; resources, H.L., L.W. and O.C.; data curation, H.L.; writing—original draft preparation, H.L.; writing—review and editing, H.L., L.W. and O.C.; visualization, H.L., L.W. and O.C.; supervision, L.W. and O.C.; project administration, H.L. and L.W.; funding acquisition, H.L., L.W. and O.C. All authors have read and agreed to the published version of the manuscript.
Funding
We express particular thanks to the Keidanren Nature Conservation Fund (KNCF) who funded the study through the SATO YAMA UMI Project and Conservation International. We thank the Southern Province of New Caledonia for their financial support in the form of a scholarship to H.L. We also gratefully acknowledge the financial support of MAC3 Impact Philanthropies, William Brooks, Pam Rorke Levy, Audrey and Shannon Wong, Daniel Roozen and Kris Norvig in sponsoring satellite tags, and we thank OceanMax for supplying the Propspeed antifouling coating for our tags. Conservation International played a role in the study design and data collection, but had no role in the analysis, decision to publish, or preparation of the manuscript. The remaining funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
This study was conducted with authorizations from the Southern Province (permit no: 34584 approved on November 2017) and the Northern Province (permit no: 609011-33 approved on October 2018) of New Caledonia. In the Loyalty Islands Province, no permit was required by the local authorities, though permission of the local customary representatives was granted.
Data Availability Statement
We have presented extensive data generated by this study in the tables and Supplementary Materials. Contact the corresponding author for further requests.
Acknowledgments
We thank Conservation International, the Aquarium des Lagons, MAC3 Impact Philanthropies and The Manta Trust for their overall support for the study. We especially thank Mark Erdmann for unvaluable guidance and help on the field and continuing overall support. Special thanks to Franck Bouilleret, Mael Imirizaldu, and Thomas Auger for their priceless assistance in field operations, as well as Jean-Christophe Lefeuvre and François Tron for their support. We also thank the Hô-üt Association and its members as well as all those involved in the field operations for their valuable help (Pierre Kaouma, Marino Tiaou, Jean-Baptiste Badiou, Ludovic Mazens, Abyss Plongée, Sarah Lewis, Schannel van Dijken, Minori Matsuoka). We finally gratefully acknowledge the customary representatives of Ouvéa and Touho, and the three Provinces for allowing such operations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fletcher, R.; Fortin, M. Spatial Ecology and Conservation Modeling; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-030-01988-4. [Google Scholar]
- Graham, R.T.; Witt, M.J.; Castellanos, D.W.; Remolina, F.; Maxwell, S.; Godley, B.J.; Hawkes, L.A. Satellite tracking of manta rays highlights challenges to their conservation. PLoS ONE 2012, 7, e36834. [Google Scholar] [CrossRef] [PubMed]
- Palumbi, S.R. Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Evol. Syst. 2014, 25, 547–572. [Google Scholar] [CrossRef]
- Palumbi, S.R. Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl. 2003, 13 (Suppl. S1), 146–158. [Google Scholar] [CrossRef]
- Chateau, O.; Wantiez, L. Movement patterns of four coral reef fish species in a fragmented habitat in New Caledonia: Implications for the design of marine protected area networks. ICES Mar. Sci. Symp. 2009, 66, 50–55. [Google Scholar] [CrossRef]
- Meyer, C.G.; Papastamatiou, Y.P.; Clark, T.B. Differential movement patterns and site fidelity among trophic groups of reef fishes in a Hawaiian marine protected area. Mar. Biol. 2010, 157, 1499–1511. [Google Scholar] [CrossRef]
- Foote, A.D.; Similä, T.; Víkingsson, G.A.; Stevick, P.T. Movement, site fidelity and connectivity in a top marine predator, the killer whale. Evol. Ecol. 2010, 24, 803–814. [Google Scholar] [CrossRef]
- Passadore, C.; Möller, L.; Diaz-Aguirre, F.; Parra, G.J. High site fidelity and restricted ranging patterns in southern Australian bottlenose dolphins. Ecol. Evol. 2018, 8, 242–256. [Google Scholar] [CrossRef]
- Madigan, D.J.; Brooks, E.J.; Bond, M.E.; Gelsleichter, J.; Howey, L.A.; Abercrombie, D.L.; Brooks, A.; Chapman, D.D. Diet shift and site-fidelity of oceanic whitetip sharks Carcharhinus longimanus along the Great Bahama Bank. Mar. Ecol. Prog. 2015, 529, 185–197. [Google Scholar] [CrossRef]
- Sims, D.W.; Quayle, V.A. Selective foraging behaviour of basking sharks on zooplankton in a small-scale front. Nature 1998, 393, 460–464. [Google Scholar] [CrossRef]
- Heyman, W.D.; Graham, R.T.; Kjerfve, B.; Johannes, R.E. Whale sharks Rhincodon typus aggregate to feed on fish spawn in Belize. Mar. Ecol. Prog. 2001, 215, 275–282. [Google Scholar] [CrossRef]
- Graham, R.T.; Roberts, C.M.; Smart, J.C. Diving behaviour of whale sharks in relation to a predictable food pulse. J. R. Soc. Interface 2006, 3, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Adam, M.S.; Goes, J.I. From monsoons to mantas: Seasonal distribution of Manta alfredi in the Maldives. Fish. Oceanogr. 2011, 20, 104–113. [Google Scholar] [CrossRef]
- Germanov, E.S.; Marshall, A.D. Running the gauntlet: Regional movement patterns of Manta alfredi through a complex of parks and fisheries. PLoS ONE 2014, 9, e110071. [Google Scholar] [CrossRef] [PubMed]
- Jaine, F.R.A.; Rohner, C.A.; Weeks, S.J.; Couturier, L.I.E.; Bennett, M.B.; Townsend, K.A.; Richardson, A.J. Movements and habitat use of reef manta rays off eastern Australia: Offshore excursions, deep diving and eddy affinity revealed by satellite telemetry. Mar. Ecol. Prog. 2014, 510, 73–86. [Google Scholar] [CrossRef]
- Jaine, F.R.; Couturier, L.I.; Weeks, S.J.; Townsend, K.A.; Bennett, M.B.; Fiora, K.; Richardson, A.J. When giants turn up: Sighting trends, environmental influences and habitat use of the manta ray Manta alfredi at a coral reef. PLoS ONE 2012, 7, e46170. [Google Scholar] [CrossRef]
- Couturier, L.I.; Dudgeon, C.L.; Pollock, K.H.; Jaine, F.R.A.; Bennett, M.B.; Townsend, K.A.; Weeks, S.J.; Richardson, A.J. Population dynamics of the reef manta ray Manta alfredi in eastern Australia. Coral Reefs 2014, 33, 329–342. [Google Scholar] [CrossRef]
- Setyawan, E.; Sianipar, A.B.; Erdmann, M.; Fischer, A.M.; Haddy, J.A.; Beale, C.S.; Lewis, S.A.; Mambrasar, R. Site fidelity and movement patterns of reef manta rays (Mobula alfredi: Mobulidae) using passive acoustic telemetry in northern Raja Ampat, Indonesia. Nat. Conserv. Res. 2018, 3, 17–31. [Google Scholar] [CrossRef]
- O’Malley, M.P.; Townsend, K.A.; Hilton, P.; Heinrichs, S.; Stewart, J.D. Characterization of the trade in manta and devil ray gill plates in China and South-east Asia through trader surveys. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 394–413. [Google Scholar] [CrossRef]
- White, W.T.; Giles, J.; Potter, I.C. Data on the bycatch fishery and reproductive biology of mobulid rays (Myliobatiformes) in Indonesia. Fish. Res. 2006, 82, 65–73. [Google Scholar] [CrossRef]
- O’Malley, M.P.; Lee-Brooks, K.; Medd, H.B. The global economic impact of manta ray watching tourism. PLoS ONE 2013, 8, e65051. [Google Scholar] [CrossRef]
- Venables, S.; McGregor, F.; Brain, L.; van Keulen, M. Manta ray tourism management, precautionary strategies for a growing industry: A case study from the Ningaloo Marine Park, Western Australia. Pac. Conserv. Biol. 2016, 22, 295–300. [Google Scholar] [CrossRef]
- Ward-Paige, C.A.; Davis, B.; Worm, B. Global population trends and human use patterns of Manta and Mobula rays. PLoS ONE 2013, 8, e74835. [Google Scholar] [CrossRef] [PubMed]
- Rohner, C.A.; Pierce, S.J.; Marshall, A.D.; Weeks, S.J.; Bennett, M.B.; Richardson, A.J. Trends in sightings and environmental influences on a coastal aggregation of manta rays and whale sharks. Mar. Ecol. Prog. 2013, 482, 153–168. [Google Scholar] [CrossRef]
- Croll, D.A.; Dewar, H.; Dulvy, N.K.; Fernando, D.; Francis, M.P.; Galván-Magaña, F.; Hall, M.; Heinrichs, S.; Marshall, A.; White, W.T. Vulnerabilities and fisheries impacts: The uncertain future of manta and devil rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 562–575. [Google Scholar] [CrossRef]
- Lawson, J.M.; Fordham, S.V.; O’Malley, M.P.; Davidson, L.N.; Walls, R.H.; Heupel, M.R.; Stevens, G.; Fernando, D.; Budziak, A.; Simpfendorfer, C.A.; et al. Sympathy for the devil: A conservation strategy for devil and manta rays. PeerJ 2017, 5, e3027. [Google Scholar] [CrossRef]
- McGregor, F.; Richardson, A.J.; Armstrong, A.J.; Armstrong, A.O.; Dudgeon, C.L. Rapid wound healing in a reef manta ray masks the extent of vessel strike. PLoS ONE 2019, 14, e0225681. [Google Scholar] [CrossRef]
- Stewart, J.D.; Jaine, F.R.A.; Armstrong, A.J.; Armstrong, A.O.; Bennett, M.B.; Burgess, K.B.; Couturier, L.I.E.; Croll, D.A.; Cronin, M.R.; Deakos, M.H.; et al. Research priorities to support effective manta and devil ray conservation. Front. Mar. Sci. 2018, 5, 314. [Google Scholar] [CrossRef]
- Marshall, A.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.; Jabado, R.W.; Liu, K.M.; Pacoureau, N.; et al. Mobula alfredi (amended version of 2019 assessment). IUCN Red List. Threat. Species 2022, e.T195459A214395983. IUCN Red List. Threat. Species 2022, e.T195459A214395983. [Google Scholar] [CrossRef]
- Baird, R.W.; Schorr, G.S.; Webster, D.L.; Mahaffy, S.D.; McSweeney, D.J.; Hanson, M.B.; Andrews, R.D. Open-ocean movements of a satellite-tagged Blainvi’le’s beaked whale (Mesoplodon densirostris): Evidence for an offshore population in Hawaii? Aquat. Mamm. 2011, 37, 506–511. [Google Scholar] [CrossRef]
- Block, B.A.; Costa, D.P.; Boehlert, G.W.; Kochevar, R.E. Revealing pelagic habitat use: The tagging of Pacific pelagics program. Oceanol. Acta 2002, 25, 255–266. [Google Scholar] [CrossRef]
- Hart, K.M.; Hyrenbach, K.D. Satellite telemetry of marine megavertebrates: The coming of age of an experimental science. Endanger. Species Res. 2009, 10, 9–20. [Google Scholar] [CrossRef]
- Mate, B.R.; Best, P.B.; Lagerquist, B.A.; Winsor, M.H. Coastal, offshore, and migratory movements of South African right whales revealed by satellite telemetry. Mar. Mamm. Sci. 2011, 27, 455–476. [Google Scholar] [CrossRef]
- Phillips, E.M.; Horne, J.K.; Adams, J.; Zamon, J.E. Selective occupancy of a persistent yet variable coastal river plume by two seabird species. Mar. Ecol. Prog. Ser. 2018, 594, 245–261. [Google Scholar] [CrossRef]
- Hofman, M.P.G.; Hayward, M.W.; Heim, M.; Marchand, P.; Rolandsen, C.M.; Mattisson, J.; Urbano, F.; Heurich, M.; Mysterud, A.; Melzheimer, J.; et al. Right on track? Performance of satellite telemetry in terrestrial wildlife research. PLoS ONE 2019, 14, e0216223. [Google Scholar] [CrossRef] [PubMed]
- Haywood, J.C.; Fuller, W.J.; Godley, B.J.; Margaritoulis, D.; Shutler, J.D.; Snape, R.T.; Widdicombe, S.; Zbinden, J.A.; Broderick, A.C. Spatial ecology of loggerhead turtles: Insights from stable isotope markers and satellite telemetry. Divers. Distrib. 2020, 26, 368–381. [Google Scholar] [CrossRef]
- Meyers, M.M.; Francis, M.P.; Erdmann, M.; Constantine, R.; Sianipar, A. Movement patterns of whale sharks in Cenderawasih Bay, Indonesia, revealed through long-term satellite tagging. Pac. Conserv. Biol. 2020, 26, 353–364. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A.; Collins, A.B.; Tyminski, J.P. Residency and movement patterns of bonnethead sharks, Sphyrna tiburo, in a large Florida estuary. Environ. Biol. Fishes 2006, 76, 47–67. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Gallagher, A.J.; Lazarre, D.M. A review of shark satellite tagging studies. J. Exp. Mar. Biol. Ecol. 2011, 398, 1–8. [Google Scholar] [CrossRef]
- Crossin, G.T.; Heupel, M.R.; Holbrook, C.M.; Hussey, N.E.; Lowerre-Barbieri, S.K.; Nguyen, V.M.; Raby, G.D.; Cooke, S.J. Acoustic telemetry and fisheries management. Ecol Appl. 2017, 27, 1031–1049. [Google Scholar] [CrossRef]
- Sims, D.W.; Southall, E.J.; Tarling, G.A.; Metcalfe, J.D. Habitat-specific normal and reverse diel vertical migration in the plankton-feeding basking shark. J. Anim. Ecol. 2005, 74, 755–761. [Google Scholar] [CrossRef]
- Andrews, K.S.; Williams, G.D.; Levin, P.S. Seasonal and ontogenetic changes in movement patterns of sixgill sharks. PLoS ONE 2010, 5, e12549. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, R.; Meÿer, M.; Scholl, M.C.; Johnson, R.; O’Brien, S.; Oosthuizen, H.; Swanson, S.; Kotze, D.; Paterson, M. Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science 2005, 310, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Lassauce, H.; Chateau, O.; Erdmann, M.V.; Wantiez, L. Diving behavior of the reef manta ray (Mobula alfredi) in New Caledonia: More frequent and deeper night-time diving to 672 meters. PLoS ONE 2020, 15, e0228815. [Google Scholar] [CrossRef] [PubMed]
- Lassauce, H.; Dudgeon, C.L.; Armstrong, A.J.; Wantiez, L.; Carroll, E.L. Evidence of fine-scale genetic structure for reef manta rays Mobula alfredi in New Caledonia. Endanger. Species Res. 2022, 47, 249–264. [Google Scholar] [CrossRef]
- Lassauce, H.; Chateau, O.; Erdmann, M.V.; Wantiez, L. Citizen Science to Infer Characteristics and Habitat Use of the Population of Reef Manta Rays (Mobula alfredi) in New Caledonia; Manuscript in Preparation; University of New Caledonia: Noumea, New Caledonia, 2023. [Google Scholar]
- Andréfouët, S.; Cabioch, G.; Flamand, B.; Pelletier, B. A reappraisal of the diversity of geomorphological and genetic processes of New Caledonian coral reefs: A synthesis from optical remote sensing, coring and acoustic multibeam observations. Coral Reefs 2009, 28, 691–707. [Google Scholar] [CrossRef]
- Van Canneyt, O.; Dorémus, G.; Laran, S.; Ridoux, V.; Watremez, P. Distribution et Abondance de la Mégafaune Marine Dans le sud-ouest du Pacifique. 2015 (Scientific Campaign Report). Available online: https://www.observatoire-pelagis.cnrs.fr/wp-content/uploads/2021/05/9-RAPPORT_REMMOA_SOP_INTER_2015.pdf (accessed on 22 May 2020).
- Freitas, C.; Lydersen, C.; Fedak, M.A.; Kovacs, K.M. A simple new algorithm to filter marine mammal Argos locations. Mar. Mamm. Sci. 2008, 24, 315–325. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://www.R-project.org/ (accessed on 28 January 2021).
- Pante, E.; Simon-Bouhet, B. marmap: A package for importing, plotting and analyzing bathymetric and topographic data in R. PLoS ONE 2013, 8, e73051. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Armstrong, A.O.; McGregor, F.; Richardson, A.J.; Bennett, M.B.; Townsend, K.A.; Hays, G.C.; van Keulen, M.; Smith, J.; Dudgeon, C.L. Satellite tagging and photographic identification reveal connectivity between two UNESCO World Heritage Areas for reef manta rays. Front. Mar. Sci. 2020, 7, 725. [Google Scholar] [CrossRef]
- Braun, C.D.; Skomal, G.B.; Thorrold, S.R.; Berumen, M.L. Movements of the reef manta ray (Manta alfredi) in the Red Sea using satellite and acoustic telemetry. Mar. Biol. 2015, 162, 2351–2362. [Google Scholar] [CrossRef]
- Kessel, S.T.; Elamin, N.A.; Yurkowski, D.J.; Chekchak, T.; Walter, R.P.; Klaus, R.; Hill, G.; Hussey, N.E.; Hussey, N.E. Conservation of reef manta rays (Manta alfredi) in a UNESCO World Heritage Site: Large-scale island development or sustainable tourism? PLoS ONE 2017, 12, e0185419. [Google Scholar] [CrossRef]
- Peel, L.R.; Stevens, G.M.; Daly, R.; Keating Daly, C.A.; Collin, S.P.; Nogués, J.; Meekan, M.G. Regional movements of reef manta rays (Mobula alfredi) in Seychelles waters. Front. Mar. Sci. 2020, 7, 558. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Chapple, T.K.; Curnick, D.J.; Carlisle, A.B.; Castleton, M.; Jacoby, D.M.; Peel, L.R.; Schallert, R.J.; Tickler, D.M.; Block, B.A. Individual variation in residency and regional movements of reef manta rays Mobula alfredi in a large marine protected area. Mar. Ecol. Prog. Ser. 2020, 639, 137–153. [Google Scholar] [CrossRef]
- Dewar, H.; Mous, P.; Domeier, M.; Muljadi, A.; Pet, J.; Whitty, J. Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar. Biol. 2008, 155, 121–133. [Google Scholar] [CrossRef]
- Couturier, L.I.; Jaine, F.R.; Townsend, K.A.; Weeks, S.J.; Richardson, A.J.; Bennett, M.B. Distribution, site affinity and regional movements of the manta ray, Manta alfredi (Krefft, 1868), along the east coast of Australia. Mar. Freshw. Res. 2008, 62, 628–637. [Google Scholar] [CrossRef]
- Couturier, L.I.E.; Newman, P.; Jaine, F.R.A.; Bennett, M.B.; Venables, W.N.; Cagua, E.F.; Townsend, K.A.; Weeks, S.J.; Richardson, A.J. Variation in occupancy and habitat use of Mobula alfredi at a major aggregation site. Mar. Ecol. Prog. Ser. 2018, 599, 125–145. [Google Scholar] [CrossRef]
- Armstrong, A.O.; Armstrong, A.J.; Jaine, F.R.; Couturier, L.I.; Fiora, K.; Uribe-Palomino, J.; Weeks, S.J.; Townsend, K.A.; Bennett, M.B.; Richardson, A.J. Prey density threshold and tidal influence on reef manta ray foraging at an aggregation site on the Great Barrier Reef. PLoS ONE 2016, 11, e0153393. [Google Scholar] [CrossRef]
- McCoy, E.; Burce, R.; David, D.; Aca, E.Q.; Hardy, J.; Labaja, J.; Snow, S.J.; Ponzo, A.; Araujo, G. Long-term photo-identification reveals the population dynamics and strong site fidelity of adult whale sharks to the coastal waters of Donsol, Philippines. Front. Mar. Sci. 2018, 5, 271. [Google Scholar] [CrossRef]
- Setyawan, E.; Erdmann, M.V.; Lewis, S.A.; Mambrasar, R.; Hasan, A.W.; Templeton, S.; Beale, C.S.; Sianipar, A.B.; Shidqi, R.; Heuschkel, H.; et al. Natural history of manta rays in the Bird’s Head Seascape, Indonesia, with an analysis of the demography and spatial ecology of Mobula alfredi (Elasmobranchii: Mobulidae). J. Ocean Sci. Found. 2020, 36, 49–83. [Google Scholar]
- Torréton, J.P.; Rochelle-Newall, E.; Pringault, O.; Jacquet, S.; Faure, V.; Briand, E. Variability of primary and bacterial production in a coral reef lagoon (New Caledonia). Mar. Pollut. Bull. 2010, 61, 335–348. [Google Scholar] [CrossRef]
- Le Borgne, R.; Douillet, P.; Fichez, R.; Torréton, J.P. Hydrography and plankton temporal variabilities at different time scales in the southwest lagoon of New Caledonia: A review. Mar. Pollut. Bull. 2010, 61, 297–308. [Google Scholar] [CrossRef]
- McCauley, D.J.; DeSalles, P.A.; Young, H.S.; Papastamatiou, Y.P.; Caselle, J.E.; Deakos, M.H.; Gardner, J.P.A.; Garton, D.W.; Collen, J.D.; Micheli, F. Reliance of mobile species on sensitive habitats: A case study of manta rays (Manta alfredi) and lagoons. Mar. Biol. 2014, 161, 1987–1998. [Google Scholar] [CrossRef]
- Heupel, M.R.; Carlson, J.K.; Simpfendorfer, C.A. Shark nursery areas: Concepts, definition, characterization, and assumptions. Mar. Ecol. Prog. Ser. 2007, 337, 287–297. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Lowe, C.G.; Caselle, J.E.; Friedlander, A.M. Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 2009, 90, 996–1008. [Google Scholar] [CrossRef]
- Dale, J.I.; Wallsgrove, N.J.; Popp, B.N.; Holland, K.N. Nursery habitat use and foraging ecology of the brown stingray Dasyatis lata determined from stomach contents, bulk and amino acid stable isotopes. Mar. Ecol. Prog. Ser. 2011, 433, 221–236. [Google Scholar] [CrossRef]
- Barr, Y.; Abelson, A. Feeding–Cleaning Trade-Off: Manta Ray “Decision-Making” as a Conservation Tool. Front. Mar. Sci. 2019, 6, 88. [Google Scholar] [CrossRef]
- Stevens, G.M.W. Conservation and Population Ecology of Manta Rays in the Maldives. Doctoral Dissertation, University of York, York, UK, 2016; pp. 67–88. [Google Scholar]
- Perryman, R.J.; Venables, S.K.; Tapilatu, R.F.; Marshall, A.D.; Brown, C.; Franks, D.W. Social preferences and network structure in a population of reef manta rays. Behav. Ecol. Sociobiol. 2019, 73, 114. [Google Scholar] [CrossRef]
- Thorrold, S.R.; Afonso, P.; Fontes, J.; Braun, C.D.; Santos, R.S.; Skomal, G.B.; Berumen, M.L. Extreme diving behaviour in devil rays links surface waters and the deep ocean. Nat. Commun. 2014, 5, 4274. [Google Scholar] [CrossRef]
- Stewart, J.D.; Hoyos-Padilla, E.M.; Kumli, K.R.; Rubin, R.D. Deep-water feeding and behavioral plasticity in Manta birostris revealed by archival tags and submersible observations. Zoology 2016, 119, 406–413. [Google Scholar] [CrossRef]
- Thums, M.; Meekan, M.; Stevens, J.; Wilson, S.; Polovina, J. Evidence for behavioural thermoregulation by the world’s largest fish. J. R. Soc. Interface 2013, 10, 20120477. [Google Scholar] [CrossRef]
- Speed, C.W.; Meekan, M.G.; Field, I.C.; McMahon, C.R.; Harcourt, R.G.; Stevens, J.D.; Babcock, R.C.; Pillans, R.D.; Bradshaw, C.J.A. Reef shark movements relative to a coastal marine protected area. Reg. Stud. Mar. Sci. 2016, 3, 58–66. [Google Scholar] [CrossRef]
- Laran, S.; Doremus, G.; Mannocci, L.; Van Canneyt, O.; Watremez, P.; Cadinouche, A.; Dulau-Drouot, V.; Mayer, F.M.; Monthy, D.; Andrianarivelo, N.; et al. Progress of the REMMOA Aerial Surveys Conducted in the French EEZ and Adjacent Waters: Contrasted Cetacean Habitats in the Southwest Indian Ocean. Report, 2016. Available online: https://library.wcs.org/doi/ctl/view/mid/33065/pubid/PUB14164.aspx (accessed on 26 June 2020).
- Lassauce, H.; Chateau, O.; Wantiez, L. Spatial ecology of the population of reef manta rays (Mobula alfredi) in New Caledonia using satellite telemetry: 2-Vertical behaviour. Fishes 2023, in press. [Google Scholar]
- Harris, J.L.; McGregor, P.K.; Oates, Y.; Stevens, G.M. Gone with the wind: Seasonal distribution and habitat use by the reef manta ray (Mobula alfredi) in the Maldives, implications for conservation. Aquatic Conservation: Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1649–1664. [Google Scholar] [CrossRef]
- Clark, T.B. Abundance, Home Range, and Movement Patterns of Manta Rays (Manta alfredi, M. birostris) in Hawaii. Doctoral Dissertation, University of Hawaii at Manoa, Honolulu, HI, USA, December 2010. [Google Scholar]
- Deakos, M.H.; Baker, J.D.; Bejder, L. Characteristics of a manta ray Manta alfredi population off Maui, Hawaii, and implications for management. Mar. Ecol. Prog. Ser. 2011, 429, 245–260. [Google Scholar] [CrossRef]
- Kitchen-Wheeler, A.M.; Ari, C.; Edwards, A.J. Population estimates of Alfred mantas (Manta alfredi) in central Maldives atolls: North Male, Ari and Baa. Environ. Biol. Fishes 2012, 93, 557–575. [Google Scholar] [CrossRef]
- Carpentier, A.S.; Berthe, C.; Ender, I.; Jaine, F.R.; Mourier, J.; Stevens, G.; De Rosemont, M.; Clua, E. Preliminary insights into the population characteristics and distribution of reef (Mobula alfredi) and oceanic (M. birostris) manta rays in French Polynesia. Coral Reefs 2019, 38, 1197–1210. [Google Scholar] [CrossRef]
- Armstrong, A.O.; Armstrong, A.J.; Bennett, M.B.; Richardson, A.J.; Townsend, K.A.; Dudgeon, C.L. Photographic identification and citizen science combine to reveal long distance movements of individual reef manta rays Mobula alfredi along Australia’s east coast. Mar. Biodivers. Rec. 2019, 12, 14. [Google Scholar] [CrossRef]
- Venables, S.K.; van Duinkerken, D.I.; Rohner, C.A.; Marshall, A.D. Habitat use and movement patterns of reef manta rays Mobula alfredi in southern Mozambique. Mar. Ecol. Prog. Ser. 2020, 634, 99–114. [Google Scholar] [CrossRef]
- Chapman, D.D.; Feldheim, K.A.; Papastamatiou, Y.P.; Hueter, R.E. There and back again: A review of residency and return migrations in sharks, with implications for population structure and management. Annu. Rev. Mar. Sci. 2015, 7, 547–570. [Google Scholar] [CrossRef]
- Marshall, A.D.; Pierce, S.J.; Bennett, M.B. Morphological measurements of manta rays (Manta birostris) with a description of a foetus from the east coast of Southern Africa. Zootaxa 2008, 1717, 24–30. [Google Scholar] [CrossRef]
- Germanov, E.S.; Bejder, L.; Chabanne, D.B.; Dharmadi, D.; Hendrawan, I.G.; Marshall, A.D.; Pierce, S.J.; van Keulen, M.; Loneragan, N.R. Contrasting habitat use and population dynamics of reef manta rays within the Nusa Penida marine protected area, Indonesia. Front. Mar. Sci. 2019, 6, 215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).