Abstract
High stocking density is used in aquaculture to optimize farming. However, such strategies can stress territorial fish by increasing aggression, thus harming the fish welfare and productive performance. Here, we tested the effect of long-term tactile body stimulation (TS) on alleviating the impact of high stocking density in Nile tilapia (Oreochromis niloticus). Ten males were grouped for 21 days into four treatments (10 replicates each one) combining TS with high (1.2 kg·m³) or low (0.6 kg·m³) densities: (1) without TS and high density; (2) without TS and low density; (3) with TS and high density; and (4) with TS and low density. A rectangular PVC frame fitted with vertical plastic sticks sided with silicone bristles was placed in the center of aquarium. The fish receive TS when passing through the bristles. An apparatus without bristles was used as control. We found higher cortisol levels under TS conditions. All parameters of productive performance (growth, food efficiency, and condition factor) were higher in the treatment with TS and low density, lower in the treatment without TS and high density, and similar between treatments high density with TS and low density without TS. The results suggest that TS improves productive performance of Nile tilapia, counteracting the negative effects of high stocking density.
Key Contribution:
Long-term TS counteracts the impacts of high stocking density and, when combined with low stocking density, it has better performance. Therefore, TS is a tool that can improve fish productivity and welfare.
1. Introduction
Global fish production has steadily increased over the last few years, with Nile tilapia (Oreochromis niloticus) ranking third among fish species in the world in terms of production quantity [1]. This increase in production is followed by the necessity for larger spaces in fish farms to raise the fish adequately. However, instead of increasing space, high stocking density is usually carried out by farmers. This condition affects food competition and consumption, growth, and can generate non-adaptive stress, therefore affecting the fish’s quality of life and welfare [2]. Consequently, concerns about the impact of increased production on the species have also grown [3,4].
Stocking density is represented as the biomass or number of individuals for the volume of the tank at any time during rearing [5]. Both high as well as low densities can impair animal welfare based on the natural behavior of the species [6]. For example, for species that naturally live in large shoals, low densities can be detrimental, whereas, for territorial species (such as Nile tilapia and salmonids), high densities can have negative effects as it increases aggressive interaction [3]. High densities can affect fish welfare, especially when associated with limited spaces used to increase productivity by area [3,7].
High stocking density is a very common stressor in the production of Nile tilapia [8], associated with several effects, such as: decreased growth and feed efficiency [9,10,11,12,13,14]; decreased condition factor (K) [14]; increased cortisol levels [10,15]; decreased immune capacity [16]; increased mortality [12,13,14,17]; and worse economic performance in biofloc [17] and cages culture systems [13]. Nile tilapia is a social fish that establishes social rank and whose dominant male defends a reproductive territory by fights [8]. Thus, the number of individuals in a group is associated with the probability of encounters. As a result, we would expect that the higher the stocking density, the higher the probability of fights. Hence, studies combining the effect of stocking density and social aggressive interactions should be conducted to allow us to better understand its impact on Nile tilapia’s welfare [8]. At the same time, finding alternatives to minimize the negative effects of rearing on fish welfare are urgent.
A strategy that has been increasingly considered to improve fish quality of life in rearing systems is environmental enrichment. It is a way to increase environmental complexity by adding physical structures and providing several types of motor or sensorial stimuli [18,19]. The main goal of environmental enrichment is providing elements to reduce maladaptive traits [18], trigger fish’s nature-based behavior [20], as well as meet their physiological, behavioral and psychological needs [19], which can improve animal welfare.
In this context, tactile stimulation (TS) emerges as a sensorial enrichment to improve animal welfare. Several studies on mammals have demonstrated that some kind of touch, caress, or “massage” promotes positive effects in animals. For example, Field et al. [21] demonstrated that TS decreases stress and reduces anxiety in humans, decreases heart rate in piglets [22], increases weight gain and decreases aggression in isolated rodents [23]. In fish, short-term TS (10 days or less) has been reported to decrease stress in reef fish (Ctenochaetus striatus) [24] and reduce aggression in previously isolated male Nile tilapia [25]. In the long term (21 days), TS decreases aggressiveness and increase productive performance in social groups of male Nile tilapia [26]. As high stocking density is associated with aggressive interactions and social stress in territorial fish, we aimed to investigate whether long-term TS alleviates the negative effects of high stocking density on the welfare of Nile tilapia.
There is no universal definition of animal welfare; however, according to Huntingford et al. [27], there are several indicators of welfare in fish (and other animals) depending on the approach. An animal is considered as being in a good welfare state if it is healthy and shows adequate feeding, growth, and reproduction (function-based approach). The same if animal shows absence of fear, pain, and suffering (feeling-based approach), as well as can express its natural behavior repertoire (nature-based approach). In animal production science and aquaculture, the function-based approach is more used, but all the different approaches are not exclusive. While working with one approach, other approaches can also be proposed, particularly by offering conditions that bring positive valence to the individuals [28]. Here, we hypothesized that TS counteract the negative effects of high density. We predicted that individuals in contact with TS would exhibit reduced stress and increased productive performance parameters, such as growth rate, feed efficiency, and condition factor, thus considering the welfare function-based approach.
2. Methods
2.1. Fish Housing
Males of the genetically improved farmed tilapia (GIFT) strain, Oreochromis niloticus (L.), from a commercial supplier (Projeto Peixe fish farming, Sales Oliveira, Brazil) were acclimated for 20 days in polypropylene tanks (ca. 500 L, 1 fish/10 L) with water at 27 °C and 12L:12D light regime (7:00 a.m. to 7:00 p.m.). The fish were fed twice a day (9:00 a.m. and 3:00 p.m.) with commercial fish food (AGROMIX®, Jaboticabal, Brazil—32% crude protein, to apparent satiety). Water quality was maintained using biological filters (400 L/h) and constant aeration. The tanks were siphoned weekly to remove leftover food and feces. However, it was ensured that no more than 25% of the water was replaced so that there was minimal harm to the animals, e.g., [29,30].
2.2. Tactile Stimulation
TS was provided by an apparatus previously tested in our lab [25,31]. It was composed of vertical plastic sticks filled with silicone bristles on each side, fixed in a rectangular polyvinyl chloride (PVC) structure (Figure 1A). A similar apparatus without silicone bristles was used as control, as it did not provide TS (Figure 1B). Both apparatuses, each in their respective treatments, were introduced in the middle of the glass tank (Figure 1C); thus, when passing through the center of the glass tank, the fish in the TS treatment had contact with the silicone bristles (Figure 1D). The TS apparatus does not remove the mucus lining the bodies of animals, nor does it cause injury [25,26,31]. Moreover, Nile tilapia individuals spontaneously cross through this apparatus and overcome an aversive stimulus to access it [31], meaning it is not aversive to the fish.
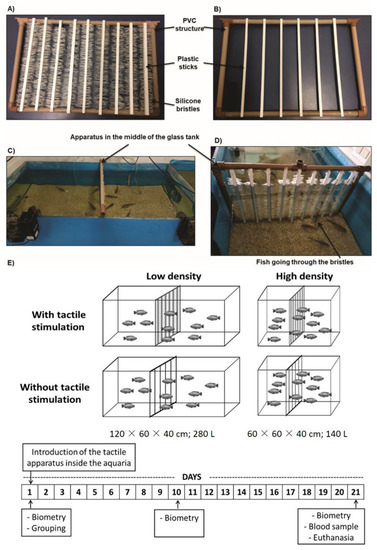
Figure 1.
Tactile stimulation device and experimental design. The apparatus for tactile stimulation (TS) made of a rectangular PVC structure with silicone bristles attached to plastic sticks (A), and a device without silicone bristles for control (B). An apparatus positioned in the middle of the glass tank (C) and fish receiving body TS (D). The experimental design and experimental schedule are shown in (E). We combined low (10 fish/280 L—0.6 kg·m³) or high (10 fish/140 L—1.2 kg·m³) stocking density with presence or absence of TS for 21 days, therefore setting 4 independent treatments (N = 10 replicates each one). Photos: A.C.S. Gauy.
2.3. Experimental Design
We tested the effect of tactile stimulation on fish’s productive performance under high (1.2 kg·m³) and low (0.6 kg·m³) stocking densities. We used groups of 10 males tested in four treatments: (1) Without TS and high density; (2) Without TS and low density; (3) With TS and high density; (4) With TS and low density. There were 10 replicates (glass tanks with groups of 10 animals) per treatment; therefore, 400 animals were used. A summary of the experimental protocol is shown in Figure 1E.
To obtain different stocking densities, we reduced the available space rather than increasing the number of animals in the same space (Figure 1E). Thus, we standardized the number of animals in all treatments, as different numbers of individuals in a group can interfere with aggressive interactions between them [32]. Thus, the animals were grouped into tanks of different lengths and similar height: 60 × 60 × 40 cm; ca. 140 L for the high-density treatments and 120 × 60 × 40 cm; ca. 280 L for the low-density treatments. All sides of the tanks were covered with opaque blue plastic to avoid visual contact with animals from the neighboring tanks. Blue was used because it prevents stress in Nile tilapia [33]. Small gravel substrate was added to the glass tanks because Nile tilapia prefer this condition than an environment without substrate [34].
All the fish were anesthetized with benzocaine (Ethyl 4-aminobenzoate, Sigma Aldrich®, São Paulo, Brazil) at 0.03 g/L, measured, weighed, grouped, and assigned to one of the treatments for 21 days. The 21-day period was chosen as the long-term period, as it corresponds to three times the time used to provide TS to Nile tilapia [25]. Furthermore, it has already been shown by Gauy et al. [26] that 21 days is sufficient to observe a cumulative effect of TS on reducing fights in this species.
2.4. Feeding Procedures
The animals were fed at the same time and with the same feed provided during the acclimatization period, but with 4% of the group biomass. The amount of feed provided throughout the experiment was not statistically different between treatments (mean ± standard error of feed provided (g)—treatment without TS and high density: 131.15 ± 16.95; treatment without TS and low density: 127.48 ± 11.87; treatment with TS and high density: 131.42 ± 15.06; treatment with TS and low density: 154.72 ± 18.14; two-way ANOVA, between treatments: F(1,36) = 0.77, p = 0.39; between densities: F(1,36) = 0.39, p = 0.53; no interaction between treatment and density: F(1,36) = 0.74, p = 0.40).
2.5. Productive Performance Parameters
All animals (n = 10 groups each treatment, total 400 animals) underwent biometry on the 1st, 10th, and 21st day of the experiments. Biometry on day 10 was used to adjust the proportion of feed offered to the animals (4% of biomass/day, divided into two meals per day, 9:00 a.m. and 3:00 p.m.) (Figure 1E). Six parameters of productive performance were evaluated for each group:
- -
- Length gain = final standard length − initial standard length;
- -
- Weight gain = final weight − initial weight;
- -
- Coefficient of size variation in the group = (standard deviation/mean) × 100;
- -
- Specific growth rate (SGR) = (ln final weight − ln initial weight/time) × 100;
- -
- Apparent feed efficiency ratio (FER) = weight gain/feed intake, which reflects the association between food consumed and weight gain [35]. As we could not evaluate individual food consumption, we estimated the FER as a proportion of weight gain and food received each day. We assumed that feed efficiency improves with increase in apparent FER [35];
- -
- Condition factor (Fulton’s K) = (weight/length3) × 100, which reflects the physiological state of the fish in relation to its welfare [36], and was calculated according to [37,38]. K shows if fish grow with a balanced association between length and weight and can be used as welfare indicator [39].
2.6. Blood Sampling and Cortisol Assay
Blood samples were collected from all animals (n = 10 groups each treatment, total 400 animals) on the 21st day of the experiment (Figure 1E) for further analysis of plasma cortisol levels, as cortisol is a physiological indicator of stress in fish [2,40,41]. The animals were anesthetized (benzocaine, 0.09 g/L) and blood samples were collected from the caudal vein using hypodermic needles and heparinized syringes. Handling was performed for less than 2 min to avoid interference with hormone levels [42]. Blood was centrifuged at 3000 rpm for 10 min, and the plasma was frozen at −20 °C for later hormone assays. Cortisol assays were performed by enzyme-linked immunosorbent assay (ELISA), using commercial kits (IBL—Immuno Biological Laboratories, Hamburg, Germany), already validated for Nile tilapia [26,43]. The analysis was performed on all fish in the experiment in duplicate and without dilution of the samples. The intra- and inter-assay coefficients of variation were 5.7% and 11.5%, respectively.
2.7. Control of Abiotic Variables
The photoperiod was 12L:12D (7:00 a.m. to 7:00 p.m.). External charcoal filters were used to aerate and maintain water quality. Water parameters were monitored on days 1, 10, and 21, and the mean ± standard error of the days in each treatment is shown in Table 1.

Table 1.
Mean ± S. E. of water parameters on the three measurement days in each treatment (n = 10 each treatment).
2.8. Statistical Analysis
Data were tested for normality and homoscedasticity using Kolmogorov–Smirnov and Fmax tests, respectively [44]. The analyzes were carried out with the mean of the 10 fishes per replicate, resulting in 10 data points per treatment. Two-way ANOVA was used to compare initial and final standard lengths and weights, group coefficients of variation, length gain, weight gain, SGR, FER, and group cortisol level (dependent variables) between TS and density (categorical variables). Repeated measures MANOVA was used to compare the initial and final K (dependent variables) between TS and density (categorical variables). Tukey HSD test was used as a post hoc test. Statistical significance was set at p ≤ 0.05.
2.9. Ethical Note
This study followed the Ethical Principles adopted by the National Council for the Control of Animal Experimentation (CONCEA/Brazil) and was approved by the Committee on Ethics in Animal Use of the Instituto de Biociências, Letras e Ciências Exatas, UNESP, São José do Rio Preto (CEUA-IBILCE/UNESP), permit #213/2019. procedures also followed the ARRIVE’s guidelines, such as animal allocation in adequate aquaria, containing small gravel substrate, external filters and monitoring of pH, ammonium, and nitrite to maintain water quality. Temperature was also controlled according to the species, and specific food for cichlids was provided. Handling for biometry and blood collection was always gentle and preceded by anesthesia in adequate concentration. At the end of the experiment, they were euthanized by deepening anesthesia, according to the CONCEA guidelines and ARRIVE’s recommendations. The number of replicates was adequate to answer our questions. Altogether, we avoided suffering and pain while valuing the life of fish, as well as the quality of our study.
3. Results
There was no difference in the initial standard length and weight between treatments, or in the respective group coefficients of variation. However, there was a difference in these parameters at the end of the period between the treatments, except for the group coefficients of variation (Table 2). There were also differences in length gain, weight gain, SGR, FER, and cortisol levels among treatments (two-way ANOVA; Table 3; Figure 2A–E). Taking these variables into consideration, the animals had lower performance in the treatment without TS and high density (p < 0.0003), and higher performance in the treatment with TS and low density than in the others (p < 0.0001). In addition, animals in the treatments without TS and low density, and with TS and high density, performed similarly (p = 0.96). The cortisol level was lower in the treatment without TS and high density (p < 0.03) and similar in the other treatments (p = 0.22). No mortality was observed in any of the treatments.

Table 2.
Standard length (SL) and weight (W) of the animals at the beginning and end of the 21 days. The coefficient of variation (CV) for SL and W in each treatment is also shown. Data are mean ± S. E. (n = 10 groups each treatment, total 400 animals).

Table 3.
Two-way ANOVA comparison of length gain, weight gain, Specific Growth Rate (SGR), Apparent Feed Efficiency Ratio (FER), and cortisol level between treatments (n = 10 groups each treatment, total 400 animals).
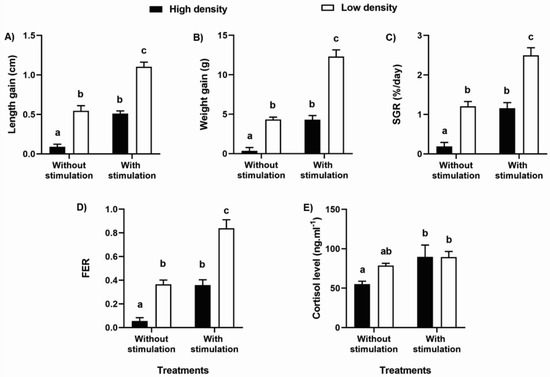
Figure 2.
Productive performance and stress indicators. (A) length gain, (B) weight gain, (C) specific growth rate—SGR, (D) apparent feed efficiency rate—FER and (E) cortisol level of the group in each treatment. Comparison between treatments by Two-way ANOVA, followed by Tukey HSD. Different letters indicate statistical differences between treatments. Bars with at least one same letter are statistically similar. Data are mean ± S.E.
There was also a difference in K between treatments at the beginning and end of the period (MANOVA for repeated measures, with interaction between treatment and density: F(1,36) = 4.40, p = 0.04; interaction between K and density: F(1,36) = 15.39, p = 0.0004; interaction between K and treatment: F(1,36) = 17.52, p = 0.0002; no interaction between K, density, and treatment: F(1,36) = 1.84, p = 0.18; Figure 3). There was no difference in the initial K values between treatments (p = 0.77). The final K was not statistically different among treatments (p = 0.37), except for the treatment with TS and low density, which was higher than that of the others (p < 0.002). Furthermore, there was an increase in K at the beginning and end of the period in the treatment with TS and low density (p = 0.0001), which did not occur in the other treatments, where K remained the same (p > 0.09).
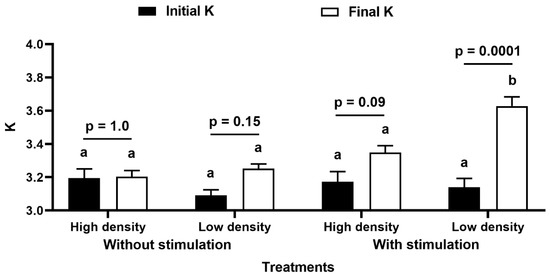
Figure 3.
Condition factor. Condition factor (K) at the beginning and the end of the 21 days of experimental period compared by MANOVA for repeated measures followed by Tukey HSD. p values indicate significant differences within treatment while letters compare between treatments. Different letters indicate statistical difference. Data are mean ± S.E.
4. Discussion
In this study, we observed that long-term TS improved the productive performance of Nile tilapia by increasing their growth, feed efficiency, and K, thereby counteracting the negative effects of high stocking density. Furthermore, long-term TS combined with low stocking density showed the best performance among all treatments, even when cortisol levels were higher compared to treatment without TS and high density. Therefore, TS can be a tool used in fish farming to increase productivity by improving fish welfare.
At the start of all treatment, animals had a similar standard length and weight conditions. Growth was observed in all of them. However, in the presence of TS, this growth was greater. Furthermore, all treatments had good quality of water, which could have likely influenced the performance of animals at high densities [5,45]. Therefore, we suggest that all parameters analyzed here had a clear influence on TS at the two stocking densities as all abiotic variables were adequately controlled.
There are several studies showing that structurally enriched tanks improve productive performance and welfare in fishes [19]. For example, suspended conduit used as enrichment improved weight gain and feed conversion ratio in rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta) [46]. Arechavala-Lopez et al. [47] demonstrated that ropes suspended in the tank can reduce aggressiveness in juvenile seabream (Sparus aurata), although no effects were found on fish condition, growth and mortality. In our study, we used a structure that allowed fish to receive TS when passing through the bristles, therefore characterizing not only a structural, but also a sensorial enrichment. It is probable that this enrichment combination intensifies the results. However, the structure was necessary to provide TS for fish, and the structure itself was used as a control. Therefore, the following discussion will focus on TS.
In this study, we did not evaluate aggressiveness in each treatment, as the number of fish per group hindered this analysis. Nevertheless, one possible mechanism that explains the effect of TS on productive parameters is the reduction in aggressive interaction. In previous studies with Nile tilapia, we showed that TS decreases aggression in both isolated [25] and grouped animals [26]. Aggressive interactions, in turn, demand high energy expenditure for fish [48], which can interfere with growth in Nile tilapia [49]. This can likely be attributed to the increase in serotonin levels by TS, as reported in humans [21]. The manipulation of the serotoninergic system has been shown to decrease aggression in rainbow trout [50] and in males Nile tilapia fed a diet enriched with tryptophan (a serotonin precursor) [51]. The effect of TS on serotonin pathways, however, has not yet been studied in fish.
Fish under TS treatment may also have presented increased growth by lowering the activity of the following pathways: (1) In fight or flight situations, the sympathetic nervous system is activated, increasing the secretion of adrenaline by chromaffin cells [52]. In turn, adrenaline increases the breakdown of energy substrate stores, such as liver glycogen and fat [52]. (2) In conditions of social stress, the hypothalamic-pituitary-interrenal (HPI) axis is activated in fish, increasing cortisol secretion [52,53]. Cortisol is a catabolic hormone that facilitates lipolysis and, under chronic conditions, promotes protein breakdown [52]. These two mechanisms act directly on animal growth as weight and length gain are anabolic events and are favored by less activation of the sympathetic nervous system and HPI axis. Furthermore, cortisol can interfere with the action of growth hormone (GH), either directly by influencing GH production [54], or indirectly by inhibiting its IGF-1 receptors in the liver, with a consequent reduction in muscle growth [55,56]. In addition, cortisol can inhibit appetite and interfere with nutrient absorption in fish [56]. Thus, both growth and feed efficiency may be associated with improved food intake and assimilation if TS reduces social stress.
Although previous studies have shown that TS reduces cortisol levels in mammals [21,57,58] and reef fish [24], such reduction was not observed in the present study. Gauy et al. [26] also did not observe a reduction in cortisol levels in groups of Nile tilapia in contact with TS. Contrary to our prediction, we observed higher cortisol levels in the treatments with higher animal growth, which contradicts the cortisol-influenced growth mechanisms cited above. However, the cortisol level observed in the present study may not necessarily suggest that the animals were stressed as cortisol is necessary for the maintenance of all organisms [59]. Here, we used the GIFT strain, which has been selected for increased growth performance [60,61]. It is possible that individuals more reactive to stress may have been selected, and these selected individuals may have higher cortisol levels compared to the common Nile tilapia, e.g., [62] with rainbow trout.
Some studies have reported basal levels of 90–110 ng/mL−1 for isolated GIFT Nile tilapia [25] and 70–90 ng/mL−1 for grouped animals [15,16,26]. Thus, the cortisol levels in the TS treatments in this study were within the basal levels for this strain. Moreover, such levels were similar to those found by Gauy et al. [26] in which the animals in contact with the long-term TS showed less aggressiveness and better productive performance than the control ones. Furthermore, the lower cortisol level observed in the treatment without TS and high density can be explained by the fact that chronic elevation of cortisol level is regulated by negative feedback from the HPI axis [51], reflecting a lower cortisol peak in this situation, e.g., [63,64]. Thus, the lower cortisol levels found in the treatment without TS and high density does not necessarily imply that animals were less stressed in these conditions than in the treatment with TS and low density. Although cortisol level is a standard stress indicator in fish, complementary indicators of metabolic activity (such as blood glucose and liver glycogen) could be used to better address stress levels [65]. On the other hand, reducing growth results from reserve substrate breakdown and protein degradation when an individual reaches the distress phase [59]. Here, it is possible that none of the fish entered the distress phase, as there was no negative effect on the SGR.
In addition to growth, feed efficiency was also improved in the presence of TS at both densities. This parameter indicates the conversion of feed to weight, that is, the better the feed efficiency, the less feed was consumed per gram of fish produced [35]. Comparing the results of the treatments, we can suggest that a similar amount of feed can be converted into three times more weight when animals are in contact with TS. Furthermore, improving the growth rate and feed efficiency of animals can have a positive impact on fish farming, as these characteristics cannot only reduce the environmental impacts of this practice but also reduce the total production costs [35,66,67].
The association of TS and low density had the best performance also in K values. K can be considered a welfare indicator in Nile tilapia [39] and is related to several animal performance characteristics, such as growth and nutritional quality [37]. In this species, Wu et al. [14] observed higher K at low stocking densities (K = 3.37) and Gauy et al. [26] reported higher K in animals in contact with long-term TS than the control ones (K = 3.4). Herein, we found a very high K, which suggested the fish had eaten more and gained more weight than length. However, the length gain in low density conditions and with TS was also higher than in the other treatments. Thus, we suggest that the combination of TS and low stocking density has better performance in terms of body condition.
The results of the present study showed that TS had a positive effect on the high stocking density treatment, increasing productive parameters to levels similar to those found in the treatment without TS and low density. Low-density treatments, both with and without TS, showed better performance in productive parameters than high-density treatments. Some studies on Nile tilapia have demonstrated that high densities have a negative impact on the production of this species as they are associated with lower growth and feed efficiency [9,11,12,13] as well as lower K, meat quality [14], and survival [12,13,14,17]. Barcellos et al. [10] and Jun et al. [15] reported elevated cortisol levels at higher densities. These negative effects may occur because high densities can influence social interactions and suppress animal growth [3,5]. In fact, all the above-mentioned studies that reported the impacts of high density on Nile tilapia discussed the influence of social interactions, such as aggressive interactions, on the results. Thus, we suggest that long-term TS reduces the negative impacts of high stocking density on Nile tilapia, likely by reducing aggressiveness in the group, e.g., [26].
5. Conclusions
The negative impacts of high stocking density depend on the species as well as the way fish are reared and managed [7]. Thus, environmental enrichment has been suggested as a potential strategy to improve the welfare of captive fish [19]. In this scenario, we conclude that the sensorial enrichment provided by long-term TS can be a promising tool to increase productivity in fish farming, particularly when associated with low densities, and to counteract the negative effects of high stocking densities. However, further studies are required to confirm its efficiency in tanks with larger numbers of animals. This tool can improve production in the future, as to meet the global demand for human feeding, fish farming will need to produce larger quantities using less food, water, and space [45,68].
Author Contributions
Conceptualization, A.C.d.S.G. and E.G.-d.-F.; Formal analysis, A.C.d.S.G. and E.G.-d.-F.; Investigation, A.C.d.S.G. and M.C.B.; Methodology, A.C.d.S.G. and E.G.-d.-F.; Supervision, E.G.-d.-F.; Validation, A.C.d.S.G.; Writing—original draft, A.C.d.S.G. and E.G.-d.-F.; Writing—review and editing, A.C.d.S.G., M.C.B. and E.G.-d.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “National Council of Technological and Scientific Development”—CNPq [grant numbers: A.C.d.S. Gauy Ph.D. scholarship—#154975/2016-8; E. Gonçalves-de-Freitas research—#428296/2016-5 and #312410/2019-0].
Institutional Review Board Statement
This study is in accordance with the Ethical Principles of the National Council for the Control of Animal Experimentation (CONCEA/Brazil) and was approved by the Committee on Ethics in Animal Use, IBILCE, UNESP, São José do Rio Preto, permit #213/2019.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Felipe Dorigão Guimarães and Manuela Lombardi Brandão for technical assistance during data collection, and Carlos Eduardo de Sousa and Roselene Costa Ferreira for technical support in the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; p. 236. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I.M. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Arechavala-Lopez, P. Welfare of fish-no longer the elephant in the room. Fishes 2019, 4, 39. [Google Scholar] [CrossRef]
- Ellis, T.; North, B.; Scott, A.P.; Bromage, N.R.; Porter, M.; Gadd, D. The relationships between stocking density and welfare in farmed rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Kadri, S. Defining, assessing and promoting the welfare of farmed fish. Rev. Off. Int. Epizoot. 2014, 33, 233–244. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Rachinas-Lopes, P.; Arechavala-Lopez, P. Finding the “golden stocking density”: A balance between fish welfare and farmers’ perspectives. Front. Vet. Sci. 2022, 9, 930221. [Google Scholar] [CrossRef]
- Gonçalves-de-Freitas, E.; Bolognesi, M.C.; Gauy, A.C.S.; Brandão, M.L.; Giaquinto, P.C.; Fernandes-Castilho, M. Social behavior and welfare in Nile tilapia. Fishes 2019, 4, 23. [Google Scholar] [CrossRef]
- Azaza, M.S.; Assad, A.; Maghrbi, W.; El-Cafsi, M. The effects of rearing density on growth, size heterogeneity and inter-individual variation of feed intake in monosex male Nile tilapia Oreochromis niloticus L. Animal 2013, 7, 1865–1874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barcellos, L.J.G.; Nicolaiewsky, S.; De Souza, S.M.G.; Lulhier, F. The effects of stocking density and social interaction on acute stress response in Nile tilapia Oreochromis niloticus (L.) fingerlings. Aquac. Res. 1999, 30, 887–892. [Google Scholar] [CrossRef]
- Garcia, F.; Romera, D.M.; Gozi, K.S.; Onaka, E.M.; Fonseca, F.S.; Schalch, S.H.C.; Candeira, P.G.; Guerra, L.O.M.; Carmo, F.J.; Carneiro, D.J.; et al. Stocking density of Nile tilapia in cages placed in a hydroelectric reservoir. Aquaculture 2013, 410–411, 51–56. [Google Scholar] [CrossRef]
- Manduca, L.G.; Silva, M.A.; Alvarenga, E.R.; Alves, G.F.O.; Fernandes, A.F.A.; Assumpção, A.F.; Cardoso, C.C.; Sales, S.C.M.; Teixeira, E.A.; Silva, M.A.; et al. Effects of a zero exchange biofloc system on the growth performance and health of Nile tilapia at different stocking densities. Aquaculture 2020, 521, 735064. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Uddin, K.B.; Basak, S.; Mahmud, Y.; Zaher, M.; Bai, S.C. Effects of stocking density on growth, body composition, yield and economic returns of Monosex Tilapia (Oreochromis niloticus L.) under cage culture system in Kaptai Lake of Bangladesh. J. Aquac. Res. Dev. 2015, 6, 357–363. [Google Scholar] [CrossRef]
- Wu, F.; Wen, H.; Tian, J.; Jiang, M.; Liu, W.; Yang, C.; Yu, L.; Lu, X. Effect of stocking density on growth performance, serum biochemical parameters, and muscle texture properties of genetically improved farm tilapia, Oreochromis niloticus. Aquac. Int. 2018, 26, 1247–1259. [Google Scholar] [CrossRef]
- Jun, Q.; Hong, Y.; Hui, W.; Didlyn, K.M.; Jie, H.; Pao, X. Physiological responses and HSP70 mRNA expression in GIFT tilapia juveniles, Oreochromis niloticus under short-term crowding. Aquac. Res. 2013, 46, 335–345. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Xu, P.; Habte-Tsion, H.M.; Ma, X.Y.; Zhu, Z.X. The changes in cortisol and expression of immune genes of GIFT tilapia Oreochromis niloticus (L.) at different rearing densities under Streptococcus iniae infection. Aquac. Int. 2016, 24, 1365–1378. [Google Scholar] [CrossRef]
- Manduca, L.G.; Silva, M.A.; Alvarenga, E.R.; Alves, G.F.O.; Ferreira, N.H.; Teixeira, E.A.; Fernandes, A.F.A.; Silva, M.A.; Turra, E.M. Effects of different stocking densities on Nile tilapia performance and profitability of a biofloc system with a minimum water exchange. Aquaculture 2021, 530, 735814. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabreba-Álvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Rev. Aquac. 2021, 14, 704–728. [Google Scholar] [CrossRef]
- Brandão, M.L.; Dorigão-Guimarães, F.; Bolognesi, M.C.; Gauy, A.C.S.; Pereira, A.V.S.; Vian, L.; Carvalho, T.B.; Gonçalves-de-Freitas, E. Understanding behaviour to improve the welfare of an ornamental fish. J. Fish Biol. 2021, 99, 726–739. [Google Scholar] [CrossRef]
- Field, T.; Hernandez-Reif, M.; Diego, M.; Schanberg, S.; Kuhn, C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef]
- Tallet, C.; Sy, K.; Prunier, A.; Nowak, R.; Boissy, A.; Boivin, X. Behavioural and physiological reactions of piglets to gentle tactile interactions vary according to their previous experience with humans. Livest. Sci. 2014, 167, 331–341. [Google Scholar] [CrossRef]
- Wei, B.; Tai, F.; Liu, X.; Ma, L.; Yang, X.; Jia, R.; Zhang, X. Neonatal tactile stimulation alleviates the negative effects of neonatal isolation on novel object recognition, sociability and neuroendocrine levels in male adult mandarin voles (Microtus mandarinus). Physiol. Behav. 2013, 112–113, 14–22. [Google Scholar] [CrossRef]
- Soares, M.C.; Oliveira, R.F.; Ros, A.F.H.; Grutter, A.S.; Bshary, R. Tactile stimulation lowers stress in fish. Nat. Commun. 2011, 2, 534. [Google Scholar] [CrossRef]
- Bolognesi, M.C.; Gauy, A.C.S.; Gonçalves-de-Freitas, E. Tactile stimulation reduces aggressiveness but does not lower stress in a territorial fish. Sci. Rep. 2019, 9, 40. [Google Scholar] [CrossRef]
- Gauy, A.C.S.; Bolognesi, M.C.; Gonçalves-de-Freitas, E. Long-term body tactile stimulation reduces aggression and improves productive performance in Nile tilapia groups. Sci. Rep. 2022, 12, 20239. [Google Scholar] [CrossRef]
- Huntingford, F.; Kadri, S.; Jobling, M. Introduction: Aquaculture and behavior. In Aquaculture and Behavior; Huntingford, F., Jobling, M., Kadri, S., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 1–35. [Google Scholar]
- Fife-Cook, I.; Franks, B. Positive welfare for fishes: Rationale and areas for future study. Fishes 2019, 4, 31. [Google Scholar] [CrossRef]
- Gonçalves-de-Freitas, E.; Teresa, F.B.; Gomes, F.S.; Giaquinto, P.C. Effect of water renewal on dominance hierarchy of the Nile tilapia. Appl. Anim. Behav. Sci. 2008, 112, 187–195. [Google Scholar] [CrossRef]
- Gauy, A.C.S.; Boscolo, C.N.P.; Gonçalves-de-Freitas, E. Less water renewal reduces effects on social aggression of the cichlid Pterophyllum scalare. Appl. Anim. Behav. Sci. 2018, 198, 121–126. [Google Scholar] [CrossRef]
- Gauy, A.C.S.; Bolognesi, M.C.; Martins, G.D.; Gonçalves-de-Freitas, E. Preference and motivation tests for body tactile stimulation in fish. Animals 2021, 11, 2042. [Google Scholar] [CrossRef] [PubMed]
- Falsarella, L.N.; Brandão, M.L.; Gonçalves-de-Freitas, E. Fish adjust aggressive behavior to audience size with limited information on bystanders’ fighting ability. Behav. Process. 2017, 142, 116–118. [Google Scholar] [CrossRef]
- Maia, C.M.; Volpato, G.L. Environmental light color affects the stress response of Nile tilapia. Zoology 2013, 116, 64–66. [Google Scholar] [CrossRef]
- Mendonça, F.Z.; Volpato, G.L.; Costa-Ferreira, R.S.; Gonçalves-de-Freitas, E. Substratum choice for nesting in male Nile tilapia Oreochromis niloticus. J. Fish Biol. 2010, 77, 1439–1445. [Google Scholar] [CrossRef]
- De Verdal, H.; Komen, H.; Quillet, E.; Chatain, B.; Allal, F.; Benzie, J.A.H.; Vandeputte, M. Improving feed efficiency in fish using selective breeding: A review. Rev. Aquac. 2017, 10, 833–851. [Google Scholar] [CrossRef]
- Ighwela, K.A.; Ahmed, A.B.; Abol-Munafi, A.B. Condition factor as an indicator of growth and feeding intensity of Nile tilapia fingerlings (Oreochromis niloticus) feed on different levels of maltose. Am. Euras. J. Agric. Environ. Sci. 2011, 11, 559–563. [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Fulton, T.W. The rate of growth of fishes. In Twenty-Second Annual Report; Fisheries Board of Scotland: Edinburgh, UK, 1904; Volume 3, pp. 141–241. [Google Scholar]
- Flores-García, L.; Camargo-Castellanos, J.C.; Pascual-Jímenez, C.; Almazán-Rueda, P.; Monroy-López, J.F.; Albertos-Alpuche, P.J.; Martínez-Yáñez, R. Welfare Indicators in Tilapia: An Epidemiological Approach. Front. Vet. Sci. 2022, 9, 882567. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandøe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish Biol. 2006, 68, 332–372. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Galhardo, L.; Noble, C.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Pottinger, T.G. The stress response in fish-mechanisms, effects and measurement. In Fish Welfare; Branson, E.J., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 32–48. [Google Scholar] [CrossRef]
- Boscolo, C.N.P.; Morais, R.N.; Gonçalves-de-Freitas, E. Same-sized fish groups increase aggressive interaction of sex-reversed males Nile tilapia GIFT strain. Appl. Anim. Behav. Sci. 2011, 135, 154–159. [Google Scholar] [CrossRef]
- Ha, R.R.; Ha, J.C. Integrative Statistics for the Social and Behavioral Sciences, 1st ed.; SAGE Publications Inc.: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Huntingford, F.A.; Turnbull, J.F.; Kadri, S. Methods to increase fish production: Welfare and sustainability implications. In Are We Pushing Animals to Their Biological Limits? Welfare and Ethical Implications; Grandin, T., Whiting, M., Eds.; CAB International: Wallingford, UK, 2018; pp. 89–121. [Google Scholar] [CrossRef]
- White, S.C.; Krebs, E.; Huysman, N.; Voorhees, J.M.; Barnes, M.E. Use of suspended plastic conduit arrays during brown trout and rainbow trout rearing in circular tanks. North Am. J. Aquac. 2019, 81, 101–106. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Diaz-Gil, C.; Saraiva, J.L.; Moranta, D.; Castanheira, M.F.; Nuñez-Velázquez, S.; Ledesma-Corvi, S.; Mora-Ruiz, M.R.; Grau, A. Effects of structural environmental enrichment on welfare of juvenile seabream (Sparus aurata). Aquac. Rep. 2019, 15, 100224. [Google Scholar] [CrossRef]
- Alvarenga, C.M.D.; Volpato, G.L. Agonistic profile and metabolism in alevins of the Nile tilapia. Physiol. Behav. 1995, 57, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.O.; Volpato, G.L. Heterogeneous growth in the Nile tilapia: Social stress and carbohydrate metabolism. Physiol. Behav. 1993, 54, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Lepage, O.; Larson, E.T.; Mayer, I.; Winberg, S. Serotonin, but not melatonin, plays a role in shaping dominant-subordinate relationships and aggression in rainbow trout. Horm. Behav. 2005, 48, 233–242. [Google Scholar] [CrossRef]
- Vieira, B.R.M.; Guermandi, I.I.; Bellot, M.S.; Camargo-dos-Santos, B.; Favero-Neto, J.; Giaquinto, P.C. The effects of tryptophan supplementation on stress and aggression in Nile tilapia. J. Appl. Ichthyo. 2021, 37, 578–584. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Winberg, S.; Höglund, E.; Øverli, Ø. Variation in the neuroendocrine stress response. In Biology of Stress in Fish; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 35–74. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y.S. Modulation of fish growth hormone levels by salinity, temperature, pollutants and aquaculture related stress: A review. Rev. Fish Biol. Fish. 2009, 19, 97–120. [Google Scholar] [CrossRef]
- Kajimura, S.; Hirano, T.; Visitacion, N.; Moriyama, S.; Aida, K.; Grau, E.G. Dual mode of cortisol action on GH/IGF-I/IGF binding proteins in the tilapia, Oreochromis mossambicus. J. Endocrinol. 2003, 178, 91–99. [Google Scholar] [CrossRef]
- Sadoul, B.; Vijayan, M.M. Stress and growth. In Biology of Stress in Fish; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 167–205. [Google Scholar] [CrossRef]
- Freitas, D.; Antoniazzi, C.T.D.; Segat, H.J.; Metz, V.G.; Vey, L.T.; Barcelos, R.C.S.; Duarte, T.; Duarte, M.M.M.F.; Burger, M.E. Neonatal tactile stimulation decreases depression-like and anxiety-like behaviors and potentiates sertraline action in young rats. Int. J. Dev. Neurosci. 2015, 47, 192–197. [Google Scholar] [CrossRef]
- Rehn, T.; Handlin, L.; Uvnäs-Moberg, K.; Keeling, L.J. Dog’s endocrine and behavioural responses at reunion are affected by how the human initiates contact. Physiol. Behav. 2014, 124, 45–53. [Google Scholar] [CrossRef]
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef]
- Dey, M.M.; Eknath, A.E.; Sifa, L.; Hussain, M.G.; Thien, T.M.; Hao, N.V.; Aypa, S.; Pongthana, N. Performance and nature of genetically improved farmed tilapia: A bioeconomic analysis. Aquac. Econ. Manag. 2000, 4, 83–106. [Google Scholar] [CrossRef]
- Gupta, M.V.; Acosta, B.O. From drawing board to dining table: The success story of the GIFT project. Naga World Fish Cent. Q. 2004, 27, 4–14. Available online: https://hdl.handle.net/20.500.12348/2057 (accessed on 27 April 2023).
- Pottinger, T.G.; Moran, T.A.; Morgan, J.A.W. Primary and secondary indices of stress in the progeny of rainbow trout (Oncorhynchus mykiss) selected for high and low responsiveness to stress. J. Fish Biol. 1994, 44, 149–163. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Nicolaiewsky, S.; De Souza, S.M.G.; Lulhier, F. Plasmatic levels of cortisol in the response to acute stress in Nile tilapia, Oreochromis niloticus (L.), previously exposed to chronic stress. Aquac. Res. 1999, 30, 437–444. [Google Scholar] [CrossRef]
- Salonius, K.; Iwama, G.K. Effects of early rearing environment on stress response, immune function, and disease resistance in juvenile Coho (Oncorhynchus kisutch) and Chinook Salmon (O. tshawytscha). Can. J. Fish. Aquat. Sci. 1993, 50, 759–766. [Google Scholar] [CrossRef]
- Corrêa, S.A.; Fernandes, M.O.; Iseki, K.K.; Negrão, J.A. Effect of the establishment of dominance relationships on cortisol and other metabolic parameters in Nile tilapia (Oreochromis niloticus). Braz. J. Med. Biol. Res. 2003, 36, 1725–1731. [Google Scholar] [CrossRef]
- Besson, M.; Aubin, J.; Komen, H.; Poelman, M.; Quillet, E.; Vandeputte, M.; Van Arendonk, J.A.M.; De Boer, I.J.M. Environmental impacts of genetic improvement of growth rate and feed conversion ratio in fish farming under rearing density and nitrogen output limitations. J. Clean. Prod. 2016, 116, 100–109. [Google Scholar] [CrossRef]
- Garcia, F.; Romera, D.M.; Sousa, N.S.; Paiva-Ramos, I.; Onaka, E.M. The potential of periphyton-based cage culture of Nile tilapia in a Brazilian reservoir. Aquaculture 2016, 464, 229–235. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).