Abstract
Many desert fishes, which evolved in isolated aquatic “islands” with limited predation pressure, have been severely impacted by non-native predators. These impacts have been attributed to the evolutionary loss of antipredator competence, known as the predator naiveté hypothesis. Recent work provided support for this hypothesis for one desert fish species. We sought to examine the generality of the predator naiveté hypothesis by evaluating antipredator competence in five populations of Red River pupfish (Cyprinodon rubrofluviatilis), a species that occupies habitats that vary in the degree of isolation and levels of fish species richness. Fish were exposed to a conspecific chemical alarm cue released from damaged epidermal tissue as a general assay of antipredator response. We found that pupfish from all five populations exhibited antipredator behavior in response to the alarm cue, regardless of the exposure to predation risk. These data provide evidence that antipredator responses to alarm cues are conserved in Red River pupfish, even in populations isolated from piscivorous species.
Keywords:
antipredator behavior; desert fishes; multi-species refuges; non-native species; protected species Key Contribution:
Contrary to predictions of the predator naiveté hypothesis, simple ecological communities can experience sufficient levels of predation to maintain a full repertoire of antipredator responses.
1. Introduction
The predator naiveté hypothesis predicts a loss of antipredator traits for fishes that evolved in isolated habitats with reduced predation pressure [1]. This hypothesis was proposed to explain why insular fish populations have been impacted by the introduction of non-native predators [2,3]. For example, the extinction of the Ash Meadows poolfish (Empetrichthys merriami) was attributed to the introduction of red swamp crayfish (Procambarus clarkii) and American bullfrogs (Rana (Lithobates) catesbeianus) [4]. Stockwell et al. [5] recently reported a case study of predator naiveté for the closely-related Pahrump poolfish (E. latos). This species, which has been severely impacted by non-native predators [6,7,8,9], did not respond to chemical alarm cues [5]. These findings were surprising because numerous laboratory and field studies have demonstrated that fish respond behaviorally to conspecific and heterospecific alarm cues [10].
These observations led us to ask whether or not the loss/retention of antipredator behaviors is associated with the degree of isolation and community complexity. We focused on Red River pupfish (Cyprinodon rubrofluviatilis), because they are found in habitats along gradients of both spatial isolation and community complexity [11,12]. Both of these factors may contribute to the loss of antipredator traits [13,14]. Here, we tested Red River pupfish that were sourced from five populations with varying community compositions to examine the effects of predation history on antipredator behavioral responses to a conspecific alarm cue. Based on the predator naiveté hypothesis, we predicted that the strength of antipredator responses would correlate with predation risk (community complexity).
2. Materials and Methods
2.1. Population Selection and Fish Collection
Female Red River pupfish were collected from five different locations of the Red River (Figure 1; Table 1). Populations were selected based on the fish community composition outlined in Ruppel [12] to test the effects of community complexity on antipredator response intensity [15]. Red River pupfish in populations 1 and 2 co-occurred with only one species, the similarly sized small-bodied plains killifish, and these populations were ranked as having a low predation risk (Table 1). Red River pupfish in populations 3 and 4 co-occurred with two to three other species but no large predators and were ranked as having a moderate predation risk (Table 1). Red River pupfish population 5 was classified as having a high predation risk due to a complex community structure including native piscivorous fishes such as the largemouth bass (Micropterus salmoides), orange spotted sunfish (Lepomis humilis), western mosquitofish, and red shiner (Cyprinella lutrensis) (Table 1). Distances between adjacent populations ranged from 65.3 to 149.7 river km (Figure 1).
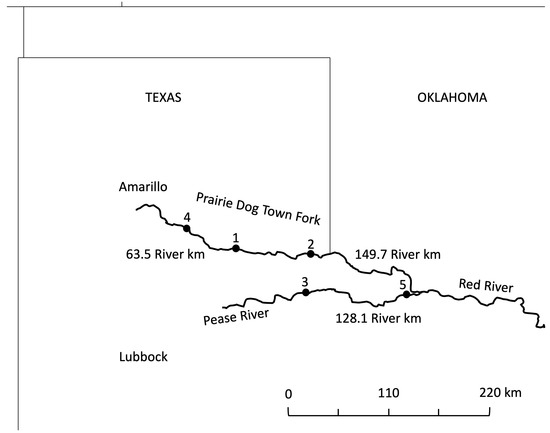
Figure 1.
Locations of focal populations along with river kilometer distances between adjacent populations. Predation risk was categorized as Low Predation (populations 1 and 2); Moderate Predation (populations 3 and 4) and High Predation (population 5). There are no barriers among sites. See Table 1 for additional details.
Female Red River pupfish were collected and shipped to NDSU overnight on the day of capture. Upon arrival, fish were transported to a field site on the North Dakota Agricultural Experiment Station where fish were acclimated to 1135 L population-specific holding tanks that had been set up two weeks prior with the salinity set at 10 ppt. Salinity was set to 10 ppt to reflect a moderate salinity level relative to the salinities measured at the sampling sites (Table 1) and to standardize salinity across all trials.

Table 1.
Red River pupfish populations, sampling locations, relative predation risk, specific conductivity, and community compositions.
Table 1.
Red River pupfish populations, sampling locations, relative predation risk, specific conductivity, and community compositions.
| Site/ Sample Size | Site Description | Location (Lat/Long) | Relative Predation Risk | Conductivity (µS/cm) | Other Fish Species Present |
|---|---|---|---|---|---|
| Site 1 n = 33 | Prairie Dog Town Fork of Red River at Hwy 256/70 | 34.628348/ −100.942 | Low | 18,159 | Plains killifish (Fundulus zebrinus) |
| Site 2 n = 22 | Prairie Dog Town Fork of Red River at Hwy 62/83 | 34.566653/ −100.196 | Low | 82,466 | Plains killifish |
| Site 3 n = 38 | Pease River 5.6 km below Hwy 62/68 | 34.194236/ −100.251 | Moderate | 24,151 | Plains killifish Red River shiner (Notropis bairdi) Plains minnow (Hypognathus placitus) |
| Site 4 n = 38 | Prairie Dog Town Fork of Red River at Hwy 207 | 34.837054/ −101.416 | Moderate | 25,403 | Plains killifish Red River shiner |
| Site 5 n = 29 | Pease River at Hwy 283 | 34.179296/ −99.2784 | High | 13,847 | Plains killifish Red River shiner Red shiner (Cyprinella lutrensis) Largemouth bass (Micropterus salmoides) Orange-spotted sunfish (Lepomis humilis) Western mosquitofish (Gambusia affinis) Bullhead minnow (Pimephales vigilax) |
2.2. Preparation of Alarm Cues and Evaluation of Fish Behavior
Chemical alarm cues were prepared following the protocol described by Wisenden [16]. Donor fish were euthanized with MS-222 (tricaine mesthanesulfonate, 500 mg/L) and via cervical dislocation before the epidermis of each fish was removed. Skin fillets from each side of the fish were laid on a flat surface and measured for total skin area, then placed in a beaker of deionized water resting on a bed of crushed ice. Once skin fillets were removed from all donor fish, the skin was blended with a handheld blender for 3 min and diluted to a final concentration of 1 cm2 of skin per 10 mL concentration. A chemical alarm cue was then aliquoted in individual 10-mL doses, then stored at −20 °C until it was needed for trials.
Trials were conducted by testing single-focal fish in 37 L glass aquaria filled with dechlorinated tap water with the salinity level at 10 ppt using Instant Ocean aquarium salt (Spectrum Brand, Blacksburg, VA, USA). All trials were completed at 24 °C as experiments occurred from June to August 2020. Lighting was set to a 16 h:8 h light: dark setting in the trial room to match the lighting conditions of the outdoor holding tanks.
A 5 × 5 cm grid was drawn on the short side of each tank to aid in scoring and opaque dividers were placed between adjacent aquaria to visually isolate the focal fish. An air stone supplied aeration to the trial tanks and a separate stimulus delivery tube was secured to the air stone to deliver test stimuli to the tank (with an alarm cue or water as a control). Focal fish were acclimated for a minimum of 20 h. Each fish was fed at least 20 min before the start of the trial to reduce overall stress.
All observations were recorded using a Canon VIZIA HF R700 video camera (Canon U.S.A. Inc., Melville, NY, USA) positioned in front of the test tank. For each trial, activity was measured by counting the total number of lines crossed by the focal fish during 5 min pre- and 5 min post-stimulus observation periods [16]. Vertical position was recorded every 10 s for both pre- and post-stimulus periods by noting the horizontal row in the grid occupied by the test subject, where a score of 1 indicated the row at the tank bottom and a score of 5 indicated the surface row.
A randomized block design with 24 blocks was used to standardize the evaluation of all five populations throughout time for the duration of the experiment, with each block composed of 10 aquaria. Within each block, two females from each of the five populations were randomly assigned to two treatments. A randomized block design also allowed each block of populations to be tested within a single day, which controlled for any effect of time spent in captivity.
2.3. Data Analysis
Fish that did not exhibit normal behavior during the pre-stimulus observation period were excluded from analyses because abnormal behavior would not provide a valid assessment of response to the test stimuli. We excluded one hyperactive fish that crossed more than 1300 lines during the pre-stimulus period (>4 SD from the mean). We also excluded eight trials where fish did not move within at least one of the five 1 min intervals during the pre-stimulus observation period. Fish that were inactive during the pre-observation period may have been in ill-heath or not well-acclimated to the test arena, either of which made the test subjects unsuitable for measuring behavioral responses to the perception of risk [16]. We performed data analyses with both the full and reduced data sets. There were no differences in the outcomes from the two sets of analyses, and thus we report the analyses based on the reduced data set (full data set analyses are available from the authors upon request). Sample sizes for each population are provided in Table 1. Data were analyzed using analysis of covariance (ANCOVA; JMP Pro version 17.0 software, JMP Statistical Discovery LLC, Cary, NC, USA), with Cue (alarm cue or water) and Population (1, 2, 3, 4, or 5) as categorical predictors and Pre-stimulus behavior (activity or vertical position) as a covariate. Block effects were included in the initial models but were not significant [15] and are not included in the results presented here. Significant effects of alarm cue were revealed either by a significant treatment effect of the cue or by a significant interaction of Cue × Pre-Stimulus behavior.
3. Results
The conspecific alarm cue caused a significant reduction in Red River pupfish post-stimulus activity relative to the water control treatment as reflected through a significant Cue × Pre-stimulus interaction for activity (Cue × Pre-stimulus activity F1,140 = 9.90; p = 0.002), but the direct and interactive effects of Population were not significant (Table 2A, Figure 2A). Analysis of all populations combined revealed that the conspecific alarm cue caused a significant reduction in post-stimulus activity as reflected through a significant Cue× Pre-stimulus interaction for activity (Cue× Pre-stimulus activity F1,156 = 16.735, p < 0.001; Figure 3A).

Table 2.
ANCOVA tables for Red River pupfish changes in behavior in response to conspecific alarm cue versus water (control) across five populations; (A) post-stimulus activity and (B) post-stimulus water column position.
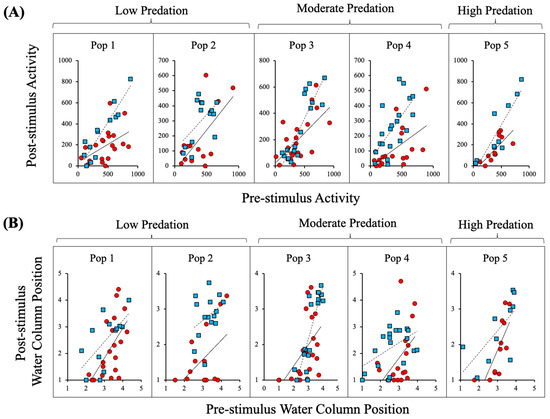
Figure 2.
Red River pupfish activity (A) and water column position (B) after introduction of alarm cue (red circles, solid lines) or water (blue squares, dashed lines) relative to pre-stimulus behavior for each of the five populations are shown. Water column values of 1 and 5, reflect the bottom and top of the water column, respectively. Populations 1 and 2 were collected from habitats with relatively low predation pressure, populations 3 and 4 were collected from habitats with relatively moderate levels of predation, and population 5 was collected from a population with relatively high predation pressure (Table 1).
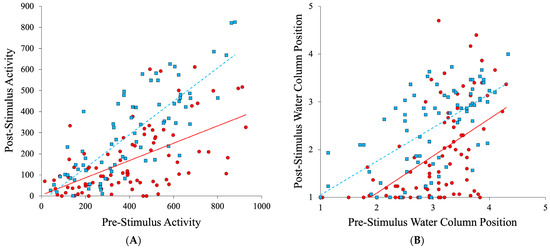
Figure 3.
Red River pupfish activity (A) and water column position (B) after introduction of alarm cue (red circles, solid line) or water (blue squares dashed line) relative to pre-stimulus behavior for all five populations combined. Water column values of 1 and 5 reflect the bottom and top of the water column, respectively.
The Cue × Pre-stimulus behavior interaction was not significant for water column position (Cue× Pre-stimulus activity F1,140 = 0.39, p = 0.533). Alarm cue caused a significant reduction in the post-stimulus water column position (Cue F1,140 = 21.170, p < 0.001; Table 2B), but the direct and interactive effects of Population were not significant (Table 2B, Figure 2B). Analysis of all populations combined revealed that alarm cue caused a significant reduction in the post-stimulus water column position (Cue F1,156 = 23.138, p < 0.001; Figure 3B).
4. Discussion
Red River pupfish populations were selected to encompass a range of community complexity and predation pressure to test predictions of the evolutionary predator naiveté hypothesis. Red River pupfish occur in communities ranging from simple to complex ones throughout the Red River watershed. The evolutionary naiveté hypothesis predicts that populations evolving in the absence of predators or with limited predation pressure may behave naïvely towards introduced predators [17]. Thus, this hypothesis predicted that Red River pupfish responses should positively correlate with the level of predation risk with which they live. Our data are not consistent with the predictions of the predator naiveté hypothesis for inter-population comparisons across a range of ecological conditions for Red River pupfish. Instead, our findings are consistent with general antipredator behavioral responses to conspecific alarm cues observed in many other fishes [10].
Predator naiveté can result from multiple ecological and evolutionary sequences of sympatry and allopatry between prey and predator populations [18]. Fish in freshwater systems are particularly likely to find themselves isolated from predators [1,17]. However, reduced predation risk may not result in naiveté if the mechanism for detecting risk and acquiring predator recognition through associative learning is maintained. Fish use chemical alarm cues for detecting the presence of an actively foraging predator. Fish can also detect alarm cues and/or alarm cue metabolites in the feces of the predator [19,20,21]. In both cases, chemical alarm cues can be used by fish to facilitate releaser-induced recognition learning [22]. When novel stimuli such as a predator’s odor or appearance are paired with chemical alarm cues, prey learn to associate risk with the novel stimulus. A single pairing of novel stimuli and alarm cues is sufficient to allow long-term recognition of correlates of predation with risk [10]. Thus, fish possess a flexible mechanism to accommodate the spatial and temporary heterogeneity of predator identity in freshwater systems. The data presented here suggest that Red River pupfish would be able to use injury-released alarm cues to reduce predation risk from an actively foraging predator and presumably would also be able to use alarm cues to acquire recognition of introduced predators.
Our findings are consistent with the observed alarm cue responses recently reported for two other pupfish species that evolved in simple communities [15,23,24]. Both the Shoshone pupfish (C. nevadensis shoshone) and the Amargosa pupfish (C. n. amargosae) have been presumably isolated since the end of the Pleistocene [25]. The Shoshone pupfish is the only fish species within its biological community, while the Amargosa pupfish co-occurs with two small-bodied fish, the native Amargosa Canyon speckled dace (Rhinichthyes osculus nevadensis) and the non-native western mosquitofish. Both of these pupfish species displayed antipredator behaviors in response to conspecific alarm cues [15,24]. Further, when conspecific alarm cues were paired with the odor of largemouth bass Micropterus salmoides, both pupfishes acquired recognition of bass odor as an indicator of danger [24]. However, they did not respond to alarm cues in feces of a bass that had preyed on pupfish which suggested partial naiveté [24]. Future work will be needed to determine if the inability to detect and learn from dietary alarm cues is restricted to Shoshone and Amargosa pupfish or is more widespread among Cyprinodon and other insular fishes.
The findings from the experimental work with Red River pupfish could have resulted from several non-mutually exclusive factors. First, it is possible that our classification of predation pressure at each site did not reflect the actual predation pressure. Communities defined as having relatively low predation pressure co-occurred with another small-bodied fish, the plains killifish. Plains killifish may directly compete with Red River pupfish and may prey on pupfish eggs and/or larvae [11]. Second, because fish communities may change during high-flow events [26], community structure and associated risk may not be static. Third, high-flow regimes would facilitate gene flow among populations, which could limit divergence among populations [27,28,29]. For instance, Storfer and Sih [27] reported evidence of outbreeding suppression reflected by the fact that isolated populations that co-occurred with fish predators gave a stronger response to fish predators than did larvae from populations that co-occurred with fish predators but were not genetically isolated from fishless populations. Fourth, other sources of predation, from invertebrate predators such as odonate nymphs, hemipterans and coleopterans, and birds, which we did not quantify, could influence the maintenance of, and variation in, antipredator responses. Any or all of these factors may be sufficient to maintain behavioral responses to conspecific alarm cues.
Our findings are not consistent with the well-studied population-level effects of antipredator behavior found in guppies Poecilia reticulata as a function of evolutionary history with predators [30]. These differences persist even after several generations under lab conditions, implying genetically driven behavioral differences [31]. Our findings also contrast recent work on the endangered Pahrump poolfish, which did not respond to a conspecific alarm cue [5]. However, Pahrump poolfish have evolved with limited piscivorous predation pressure since the end of the Pleistocene, while the temporal scales of isolation for various Red River pupfish populations are not known. Thus, work on additional pupfish species/populations that evolved over long periods of isolation is warranted. We have initiated work to evaluate the alarm cue responses of the White Sands pupfish (C. tularosa) because this species has been isolated from other pupfish species for approximately 2.5 million years [32], which is similar to the conditions associated with predator naiveté in Pahrump poolfish [5]. In fact, Rogowski and Stockwell [33] reported that western mosquitofish virtually eliminated larval production for experimental populations of White Sands pupfish, begging the question of whether or not White Sands pupfish have lost antipredator traits.
We recognize that ecological factors such as salinity may indirectly confound the effect of predation pressure because piscivorous fishes are generally intolerant to salinity. Craig [34] summarized 15 independent studies for fish communities in the Red River system and reported that piscivores were limited to salinity levels below 15,000 μs/cm. However, salinity is unlikely to directly affect the efficacy of alarm cues because alarm reactions occur in both freshwater and marine systems [10,35]. Further, there was not a straightforward link between community complexity and specific conductivity (Table 1), from which salinity levels are inferred. For example, while one of the low-predation habitats had high specific conductivity (~82,000 µS/cm); conductivities for the other low-predation site and the high-predation site were relatively similar (18,000 µS/cm and 13,000 µS/cm, respectively; Table 1). Finally, despite the inter-habitat variation in salinity, antipredator behavioral responses did not vary among the associated populations.
This work opens the door to additional behavioral questions about the antipredator competence of pupfish. The absence of piscivorous fish species does not by itself equate to the absence of predation risk. Aquatic insects and avian predators may be sufficient to maintain antipredator responses in fishes in these isolated populations. Although other insular fish may be evolutionarily naïve, behavioral responses to alarm cues appear to be conserved in Red River pupfish even for populations from simple communities with relaxed selection from fish predators.
5. Conclusions
Because they occur across a gradient of habitats that vary in community complexity and associated predation risk, Red River pupfish provided an ideal opportunity to test the predator naiveté hypothesis. Contrary to our predictions, pupfish antipredator responses did not differ among the five tested populations. In fact, pupfish from all five test populations responded with antipredator behaviors to chemical alarm cues typically observed in small-bodied fishes [10]. These responses included a reduced water column position and reduced activity. These data provide evidence that antipredator responses to alarm cues are conserved in Red River pupfish despite the high inter-habitat variation in ecological factors such as salinity and community complexity, including the presence/absence of piscivorous predators.
Author Contributions
Conceptualization, C.M.A., B.D.W., C.A.C. and C.A.S.; methodology, C.M.A., B.D.W. and C.A.S.; validation, C.M.A.; formal analysis, C.M.A., B.D.W. and C.A.S.; investigation C.M.A. and C.A.C.; resources; C.A.S.; data curation C.A.S.; writing–original draft preparation, C.M.A.; writing—review and editing, C.M.A., B.D.W., C.A.S. and C.A.C.; visualization, C.M.A.; supervision, C.A.S. and B.D.W.; project administration, C.A.S.; funding acquisition, C.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding but C.M.A. was supported by a research assistantship funded by North Dakota State University’s Environmental & Conservation Sciences Graduate Program.
Institutional Review Board Statement
Experimental protocols for this study were reviewed and approved by the North Dakota State University Animal Care and Use Committee with the approval code: #A18054 and an approval date of 23 April 2018. Red River pupfish were collected under Texas Parks and Wildlife permit # SPR-0520-069.
Data Availability Statement
The data file is archived in DYRAD: https://doi.org/10.5061/dryad.ngf1vhhxz (Published 20 April 2023).
Acknowledgments
We thank B. Gillis, A. Bauer, K. Spartz, and B. Scraper for assisting with fish care and the experiments. In addition, T. Bonner, C. Maldonado, and G. Linam assisted with the permit and fish collection in Texas. Research space to maintain our holding tanks on the North Dakota Agricultural Experiment Stations was graciously provided by G. Lardy and D. Ritchison. We thank M. Orr and J. Waraniak for the consultations on the statistical analyses. We thank P. Branco, E. Gillam, K. Hu, M. Johnson, Sekhar MA, M. Orr, B. Scraper, J. Waraniak and three anonymous reviewers for their insightful comments on earlier versions of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anton, A.; Geraldi, N.R.; Ricciardi, A.; Dick, J.T.A. Global determinants of prey naiveté to exotic predators. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192978. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R. Man and the changing fish fauna of the southwestern United States. Mich. Acad. Sci. Arts. Lett. 1961, 46, 365–404. [Google Scholar]
- Minckley, W.L.; Deacon, J.E. Southwestern fishes and the enigma of “endangered species”. Science 1968, 159, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R.; Williams, J.D.; Williams, J.E. Extinctions of North American fishes during the past century. Fisheries 1989, 14, 22–38. [Google Scholar] [CrossRef]
- Stockwell, C.A.; Schmelzer, M.; Gillis, B.; Anderson, C.; Wisenden, B.D. Ignorance is not bliss: Evolutionary naiveté in an endangered desert fish and implications for conservation. Proc. R. Soc. B 2022, 289, 20220752. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.; Goodchild, S.C.; Stockwell, C.A.; Lema, S.C. Characterization and phylogenetic analysis of complete mitochondrial genomes for two desert cyprinodontoid fishes, Empetrichthys latos and Crenichthys baileyi. Gene 2017, 626, 163–172. [Google Scholar] [CrossRef]
- Guadalupe, K.; (Nevada Department of Wildlife, Las Vegas, NV, USA). Personal Communication, 2018.
- Goodchild, S.C.; Stockwell, C.A. An experimental test of novel ecological communities of imperiled and invasive species. Trans. Am. Fish. Soc. 2016, 145, 264–268. [Google Scholar] [CrossRef]
- Paulson, B. Ex Situ Analysis of Non-Native Species Impacts on Imperiled Desert Fishes. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2019. [Google Scholar]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator-prey interactions in aquatic ecosystems: A review and prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Echelle, A.A.; Echelle, A.F.; Hill, L.G. Interspecific interactions and limiting factors of abundance and distribution in the Red River Pupfish, Cyprinodon rubrofluviatilis. Am. Midl. Nat. 1972, 88, 109–130. [Google Scholar] [CrossRef]
- Ruppel, D.S. Factors Influencing Community Structure of Riverine Organisms: Implications for Imperiled Species Management. Ph.D. Dissertation, Texas State University, San Marcos, TX, USA, 2019. [Google Scholar]
- Nosil, P.; Crespi, B.J. Experimental evidence that predation promotes divergence in adaptive radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 9090–9095. [Google Scholar] [CrossRef]
- Langerhans, R.B.; Gifford, M.E.; Joseph, E.O. Ecological speciation in Gambusia fishes. Evolution 2007, 61, 2056–2074. [Google Scholar] [CrossRef]
- Anderson, C.M. Pupfishes as a System to Test the Predator Naiveté Hypothesis. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2022. [Google Scholar]
- Wisenden, B.D. Quantifying anti-predator responses to chemical alarm cues. In Zebrafish Behavioral Protocols; Kalueff, A.V., Hart, P., LaPorte, J., Eds.; Humana Press Springer Science: New York, NY, USA, 2011; pp. 49–60. [Google Scholar]
- Cox, J.G.; Lima, S.L. Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 2006, 21, 674–680. [Google Scholar] [CrossRef]
- Carthey, A.J.; Blumstein, D.T. Predicting predator recognition in a changing world. Trends Ecol. Evol. 2018, 33, 106–115. [Google Scholar] [CrossRef]
- Mathis, A.; Smith, R.J.F. Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike’s diet. Anim. Behav. 1993, 46, 645–656. [Google Scholar] [CrossRef]
- Chivers, D.P.; Mirza, R.S. Predator diet cues and the assessment of predation risk by aquatic vertebrates: A review and prospectus. Chem. Sig. Vert. 2002, 9, 277–284. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Chivers, D.P.; McCormick, M.I.; Ferrari, M.C. Learning to distinguish between predators and non-predators: Understanding the critical role of diet cues and predator odours in generalisation. Sci. Rep. 2015, 5, 13918. [Google Scholar] [CrossRef]
- Suboski, M.D. Releaser-induced recognition learning. Psychol. Rev. 1990, 97, 271. [Google Scholar] [CrossRef]
- Snider, M. Antipredator Behavior and Morphology in Isolated Cyprinodont Fishes. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2019. [Google Scholar]
- Wisenden, B.D.; Anderson, C.M.; Hanson, K.; Johnson, M.; Stockwell, C.A. A sniff and a whiff: Acquired predator recognition via epidermal alarm cues but not dietary alarm cues by isolated pupfish. Roy. Soc. Open Sci. 2023; submitted. [Google Scholar]
- Miller, R.R. The Cyprinodont Fishes of the Death Valley System of Eastern California and Southwestern Nevada; Miscellaneous Publications, University of Michigan, Museum of Zoology: Ann Arbor, MI, USA, 1948; Volume 68, pp. 1–55. [Google Scholar]
- Stoffels, R.J.; Rehwinkle, R.A.; Price, A.E.; Fagan, W.F. Dynamics of fish dispersal during river-floodplain connectivity and its implications for community assembly. Aquat. Sci. 2016, 78, 355–365. [Google Scholar] [CrossRef]
- Storfer, A.; Sih, A. Gene flow and ineffective antipredator behavior in a stream-breeding salamander. Evolution 1998, 52, 558–565. [Google Scholar] [CrossRef]
- Lenormand, T. Gene flow and the limits to natural selection. Trends Ecol. Evol. 2002, 17, 183–189. [Google Scholar] [CrossRef]
- Hendry, A.P.; Taylor, E.B.; McPhail, J.D. Adaptive divergence and the balance between selection and gene flow: Lake and stream stickleback in the Misty system. Evolution 2007, 56, 1199–1216. [Google Scholar] [CrossRef]
- Seghers, B.H. Schooling behavior in the guppy (Poecilia reticulata): An evolutionary response to predation. Evolution 1974, 28, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.L.; Magurran, A.E. Effects of relaxed predation pressure on visual predator recognition in the guppy. Behav. Ecol. Sociobiol. 2003, 54, 225–232. [Google Scholar] [CrossRef]
- Hoagstrom, C.W.; Osborne, M. Biogeography of Cyprinodon across the Great Plains-Chihuahuan Desert region and adjacent areas. In Proceedings of the Desert Fishes Council Proceedings 2021:20-76, St. George, UT, USA, 17–21 November 2021; Desert Fishes Council: Austin, TX, USA, 2021. [Google Scholar]
- Rogowski, D.L.; Stockwell, C.A. Assessment of potential impacts of exotic species on populations of a threatened species, White Sands pupfish, Cyprinodon tularosa. Biol. Invasions 2006, 8, 79–87. [Google Scholar] [CrossRef]
- Craig, C. Descriptions, Classifications, and Explanations of Processes and Patterns Structuring and Maintaining Inland Fish Communities. Ph.D. Dissertation, Texas State University-San Marcos, San Marcos, TX, USA, 2020. [Google Scholar]
- McCormick, M.I.; Larson, J.K. Field verification of the use of chemical alarm cues in a coral reef fish. Coral Reefs 2007, 26, 571–576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).