Abstract
Studies have shown negative short-term effects of early weaning (EW) in finfish larvae but information on long-term effects of EW on growth and subsequent economic loss is lacking. We evaluated the short- and long-term effects of EW and late weaning (LW) on Atlantic cod. Cod larvae were fed with enriched rotifers from 2 to 35 days post-hatch (dph) and weaning carried out from 21 to 35 dph (EW) or with enriched rotifers from 2 to 29 dph followed by enriched Artemia nauplii from 25 to 56 dph and weaning carried out from 45 to 56 dph (LW). At 190 dph, 50 fish from each tank were tagged with an electronic tag and were transferred to sea cages at 10 months old. At the end of 30 months post-hatch, the weight of the fish was recorded. Our results showed a significant short-term effect of the weaning method on the growth of Atlantic cod at 65 dph, but no significant difference at 90 and 190 dph. However, fish from LW showed a significantly higher body weight compared to fish from EW at 30 months post-hatch. A cost analysis indicated substantial benefit for commercial cod farming by using LW and we recommend using LW to gain sizable financial benefit.
Key Contribution:
First study to evaluate long term effects of EW and LW in marine finfish species which showed the importance of the timing of weaning and of using Artemia in the feeding regime of the Atlantic cod larvae. Cost analysis showed that using EW; farmers tend to lose about 1.7 million USD in a sea cage farm initially stocked with 1.125 million cod juveniles.
1. Introduction
Most marine finfish larvae have a smaller mouth opening compared to salmonids and require smaller diets during start-feeding [1]. Further, they possess neither enzymes nor developed alimentary canal to digest formulated feed [2,3]. Thus, marine finfish larvae, including Atlantic cod (Gadus morhua L.), require live feed during the first feeding stage to ensure better growth and survival. While differences exist in larviculture production protocols among different marine finfish species, rotifers and Artemia are the most common cultured live feed organisms due to their smaller size and relatively easy production, especially Artemia [4]. Although easy to produce, the production of live feed is labour intensive as it requires daily production. On the other hand, the production cost of the dry diet is relatively lower than the live feed cost, and it is easy to store the dry diet in cold rooms for many months [1]. To maintain better nutritional quality, the cultured live feed must be fed with enrichment diets prior to being given to the larvae [4]. However, rotifers, and especially Artemia, catabolize these enrichment fatty acids, which presents challenges in maintaining consistent levels and proportion of the HUFA’s (DHA, EPA, ARA) in live feed [5]. In addition, differences in body composition between different batches of these live feed make it difficult to administer standard enrichment regimes and procedures [4]. This variability in live feed nutritional quality often results in unpredictable growth, survival, and quality of the juveniles. Due to these practical and economic disadvantages of live feed compared to dry diet, fish farmers tend to wean the larvae into dry feed at the earliest possible age [1]. Given the advantages of using dry diet, many studies were undertaken in recent years to wean different marine finfish larvae earlier than the usual times used in existing protocols, aiming to reduce the dependency on live feed [6,7,8,9].
Atlantic cod has been an important commercial groundfish species for centuries in North Atlantic countries such as Norway, Scotland, Iceland, Canada, Faroe Islands, and USA [10]. With declining wild Atlantic cod stocks in the 1980s and late 1990s in Norway and North America, efforts to commercially culture this species were undertaken at those times and cod was considered as the next “new species” for aquaculture in these countries. During this time, basic Atlantic cod larviculture protocols were developed and this resulted in improved performance of the larvae [10]. However, several biological problems still existed, such as poor-quality juveniles, variable growth and survival during larval and early juvenile stages, early maturation, and poor understanding of nutritional requirements of all life stages [11]. Around 2010, an increase in wild cod stock quota and economic turmoil in European countries made market prices of cod plummet, thus making the commercial cod farming unprofitable [12].
While commercial production of cod juveniles slowly vanished in Norway from 2010 onwards, the National Cod Breeding Program in Norway continued to work on selective breeding of cod and produced the 6th generation of cod in 2022. Selection for faster growth traits and increased disease resistance has improved the growth and survival of cod through slaughter [13,14,15]. The program has also made several improvements in husbandry protocols. These husbandry protocols include improvements in live feed production methods, live feed nutrition, weaning diet formulations and nutrition, and broodstock management [16,17,18,19,20]. Interest in cod farming has been rekindled in Norway in 2018 mainly due to higher market prices and supported by the biological improvement that has been achieved through the cod breeding program [13,14,15,16,17,18,19,20]. Currently, cod farming is undertaken only in Norway and 7–8 companies are involved with each playing different roles such as production of egg and juveniles, sea cage farming, and marketing. The companies are aiming to produce more than 50,000 tonnes by 2025 [21].
While early weaning reduces the dependency on the live feed and could possibly reduce the cost of juvenile production, the long-term effects of early and late weaning have never been studied in Atlantic cod. Most of the Artemia reduction studies carried out on cod followed the larval and juvenile performance [6,8,22] but did not follow them through to harvest size. The main aim of this study was to see if we could reduce, or even eliminate, the use of Artemia nauplii from the cod larval diet. We hypothesized that use of Artemia as live feed in the diet of larval cod could be eliminated without negatively affecting the short-term and long-term growth and survival. We have also evaluated short- (1–180 days post-hatch) and long-term (at 30 months post-hatch) effects of early and late weaning of cod larvae to formulated diets from hatching through slaughtering on growth, survival, and quality.
2. Materials and Methods
2.1. Gamete Collection and Egg Incubation
Cultured adult Atlantic cod originated from the 2010 year class (2nd generation) were transferred from the sea cages of the Centre for Marine Aquaculture (CMA) at Røsnes (69°48′0′′ N 19°16′0′′ E) to the land facility of CMA in Kraknes, Tromsø, Norway (69°45′21.9′′ N 19°01′33.7′′ E), in January 2013. They were kept in 25 m3 circular tanks with ambient water supply at 250 L min−1 and a temperature of 3.5 ± 0.5 °C. In late March, gametes were collected by hand stripping and were fertilized using the standard dry fertilization method [23]. Briefly, after stripping, eggs and sperm were mixed in a beaker at a ratio of 5:1, respectively. Then, 500 mL of sea water was added to the gamete mixture and was gently mixed and allowed 5 min for fertilization. After 5 min, the fertilized eggs were washed with sea water to remove excess sperm. The fertilized eggs were incubated in 25 L upwelling incubators at 4 °C with gentle aeration in a flow-through water system.
2.2. Larval Rearing
Larvae hatched in 21–22 days (≈90° days). At 2 days post-hatch (dph), newly hatched larvae (15,000) were transferred to six 190 L circular fiberglass tanks. The water temperature was kept at 4 °C from 2 to 5 dph and gradually increased to 10 °C from 5 to 10 dph. Two weaning regimes were used with three replicates (Figure 1). In the early weaning (EW) regime, larvae were fed with enriched rotifers from 2 to 35 dph and weaning onto dry feed started at 21 dph and completed at 35 dph (Table S1). For the late weaning (LW) regime, larvae were fed with enriched rotifers from 2 to 29 dph, followed by enriched Artemia from 25 to 56 dph and weaning onto dry feed started from 45 dph and completed at 56 dph (Table S2). Rotifers were enriched with a mixture of enrichment (0.25 g per million rotifer) products: Multigain (Biomar AS, Myre, Norway), PhosphoNorse (Trofi, Tromsø, Norway), Pavlova (Microalgae AS, Vigra, Norway), Chlorella (Pacific Trading Aquaculture Ltd., Dublin, Ireland) for one hour prior to feeding. Artemia nauplii were enriched with a mixture of Multigain, MicroNorse (Trofi, Tromsø, Norway), and PhosphoNorse at 36 h post-hatch of the nauplii (see [20] for fatty acid profiles of live feed). Both enriched rotifers and Artemia were stored at 4 °C and the feeding of live and dry feed was carried out by an automated robot feeder (Storvik Aqua AS, Sunndalsøra, Norway). Prior to the commencement of weaning, a co-feeding strategy was also employed where dry feed was introduced to the larvae just before the rotifer or Artemia feeding from 15 to 20 dph (EW) or 38–45 dph (LW), respectively. In both regimes, during weaning, one live feed per day was withdrawn every 2/3 days and replaced by formulated dry diet. During weaning and after the completion of weaning, both groups were fed with the same dry diet. Different sizes of AlgoNorse extra (Trofi, Tromsø, Norway) were used as weaning and post-weaning diet and the nutrient content of the dry feed is given in Table 1. At 90 dph, the larval survival was measured by counting the individuals from each tank. At, 90 dph, the number of fish kept in each tank was standardized to 300 to minimize density related effects on growth [24]. At 190 dph, 50 fish from each tank were tagged intraperitoneally using Passive Integrated Transponders (PIT, Sokymat SA, Valais, Switzerland) tags and reared in the same tank until transferred to two sea cages (7.5 × 7.5 × 10 m) in February 2014. From the weaning experiment, 150 of the fish were stocked in each of these two sea cages, along with 5000 fish from the Atlantic cod breeding program, and 350 fish from another two experiments. All the fish were individually PIT tagged, so that fish from different experiments can be identified using a PIT tag reader. At the end of 30 months post-hatch (October 2015), all the fish from the weaning experiment were killed and weight and deformities were recorded.
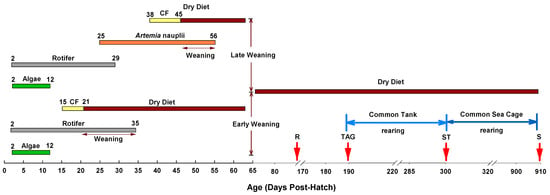
Figure 1.
Schematic diagram showing the experimental set-up. Numbers in the diagram represent the age of the larvae. CF—co-feeding of live feed and dry diet. R—reduction in fish numbers to 300 in all tanks at 90 dph. TAG—inserting individual electronic tags into the abdominal cavity of each juvenile at 190 dph. ST—sea cage transfer at 300 dph. S—slaughter at 910 dph.

Table 1.
Nutritional content and HUFA (highly unsaturated fatty acid) lipid classes of the dry feed, Algonorse extra. DHA—Docosahexaenoic acid; EPA—Eicosapentaenoic acid; ARA—Arachidonic acid; PL—Polar lipid; PC—Phosphatidylcholine; 2-LPC—Lysophosphatidylcholine, PI—Phosphatidylinositol; PE—Phosphatidylethanolamine; PA—Phosphatidic acid.
2.3. Data Collection and Analysis
Live feed samples were collected and kept frozen at −25 °C until further analysis for free amino acids (FAA). FAA levels in live feed were analysed by high-pressure liquid chromatography (HPLC) in a Pico-Tag Amino Acid Analysis System (Waters Alliance 2695 separation module, Milford, CT, USA). Samples were homogenized with internal standard solution (norleucine) containing 0.05 M HCl [25]. The homogenized sample was filtered and phenylisothiocyanate (PITC) was used for precolumn derivatization of FAAs according to the procedures described by Cohen, Meys, Tarvin [26]. Reverse phase HPLC equipped with a 30 cm Pico-Tag column, 2487 UV/Vis detector, and Empower software was used for separation and detection of the resulting peaks (Waters, Milford, CT, USA).
At 2, 12, 22, 43 and 65 dph, 10 larvae from each tank (30 per treatment) were sampled and anaesthetized with MS 222. The standard length of each larva was measured using a stereo microscope (LeicaMZ125 stereoscope, Oslo, Norway) equipped with a micrometre in the eye piece. The number of juveniles was counted in each tank at 90 dph. The total weight of all surviving fish in each tank was also recorded and the average weight of a juvenile was calculated. At 190 dph (during tagging), the weight of 50 fish (150 for treatment) from each tank was recorded. During this time, the occurrence of skeletal deformities (externally visible—lordosis, scoliosis, kyphosis, jaw deformity, head deformity, fused vertebrae) were also recorded. After tagging, juveniles from all tanks were kept in a common tank until transferred to two sea cages at 300 dph. At the end of the experiment (30 months post-hatch), the rounded whole weight of individual fish and survival were recorded.
Differences between the treatments in FAA of live feed, growth, survival, and deformity at different life stages of cod were analysed using one-way ANOVA, and the strength of one-way ANOVA was tested with an alpha test. Residuals were examined for the assumption of independence, homogeneity, and normality by plotting a frequency distribution of the data and visually compared to a normal distribution and used Levene’s test of equality of error variance. The difference in growth (SL) between 2 and 65 dph was analysed using t-test. The standard-length data of the larvae was rank transformed [27], and Bonferroni/Tukey post hoc pairwise multiple comparisons test was carried out. Significant difference was set at 0.05.
3. Results
The FAA contents in enriched rotifers and Artemia are shown in Table 2. Among all the FAA detected in the analysis, enriched Artemia had higher, or equal amount of FAA compared to the rotifers except for citrulline. Artemia had significantly higher (p < 0.001) taurine levels compared to rotifers (Table 2).

Table 2.
Free fatty acid (FAA) content of enriched rotifers and Artemia. ND—Not detected. * denotes significant difference at 0.05.
Overall, there was no significant effect (p < 0.268) of the treatment on standard length of the larvae (Figure 2). However, post hoc comparison showed that larvae from EW treatment were significantly larger at 12 and 22 dph than the LW group (p < 0.0001 for both ages). The significant effect of treatment between the two groups of larvae disappeared at 43 dph (p < 0.316), and by 65 dph larvae from LW treatment became significantly larger than the EW larvae (p < 0.0001). The growth difference in SL between 2 and 65 dph was significantly higher for LW larvae than larvae from EW (p < 0.015).
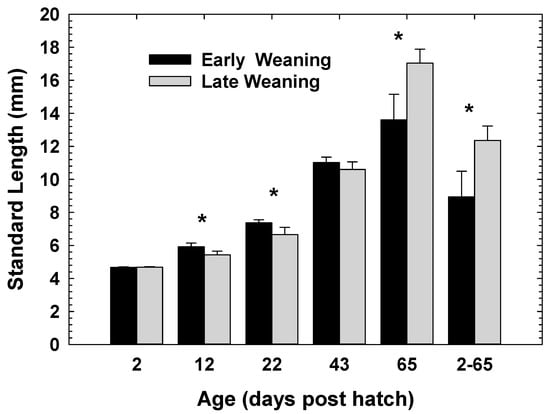
Figure 2.
Standard length of EW and LW Atlantic cod larvae. * represents significant differences at p < 0.05. Values are mean ± SD.
At 90 dph, body weight was not significantly different between the treatment groups (p < 0.466). Similarly, survival was also not significantly different (p < 0.524; LW—0.71 ± 0.15 g; EW—0.62 ± 0.07 g; Figure 3A) between the two treatments and the average survival was 10.1 ± 1.6% and 9.2 ± 1.2% for LW and EW, respectively (Figure 3B).
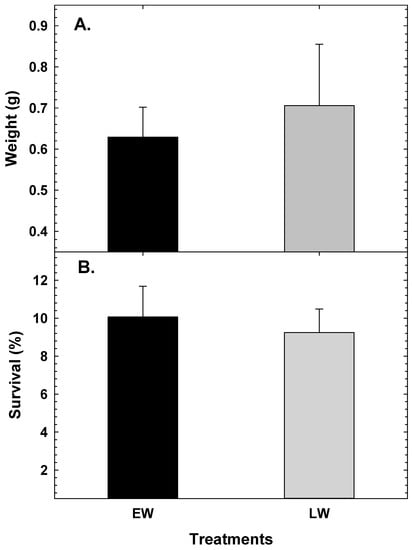
Figure 3.
Average weight (A) and survival (B) of early juveniles of Atlantic cod larvae raised with EW and LW at 90 dph. Values are mean ± SD.
There were no significant differences (p < 0.185) in bodyweight during tagging at 190 dph between the two treatments with juveniles from EW averaging 23.9 g (SD = 0.68) and LW averaging 25.1 g (SD = 3.2) (Figure 4A). No significant differences (p < 0.71) were found in occurrence of skeletal deformities of fish from EW (10%) and LW (8.6%) at 190 dph (Figure 4B).
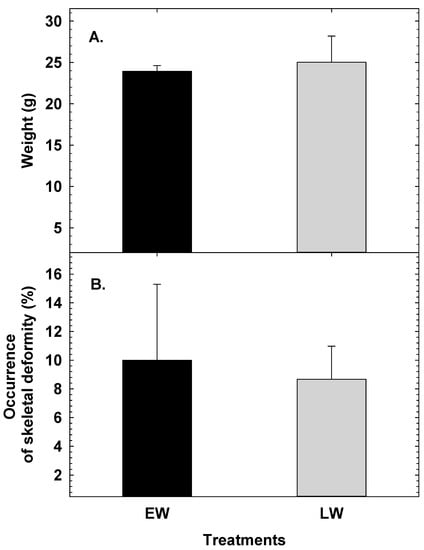
Figure 4.
Average weight (A) and occurrence of skeletal deformities (B) of Atlantic cod juveniles from EW and LW at 190 dph. Values are mean ± SD.
At 30 months, adult cod from LW were significantly heavier than the adult cod from EW (p < 0.012). During this time cod from LW were 2.48 ± 0.17 kg, while fish from EW were 2.01 ± 0.07 kg (Figure 5A). However, no significant differences were found in the survival of cod during the sea cage phase (p < 0.13) with EW cod showing 74.67 ± 8.3% and LW cod showing 85.3 ± 5.0% (Figure 5B).
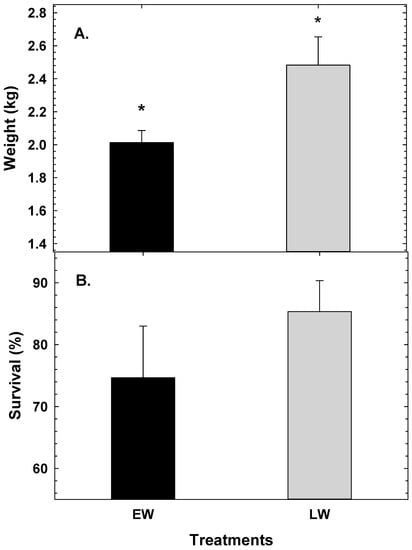
Figure 5.
Average weight (A) and survival (B) of early juveniles of Atlantic cod larvae raised with EW and LW at 30 months post-hatch. Values are mean ± SD. * represents significant differences at p < 0.05.
The cost analysis for Artemia production is shown in Table 3. Our calculations indicate that the production cost of Artemia nauplii to produce 1.125 million 25 g cod juveniles will be USD 26,240, including labour, capital, and energy cost. Capital and running cost for rotifer is not included because both weaning methods require rotifer, thus the cost will be similar. The number of rotifers used in early and late weaning were 206 and 186 million, respectively and this cost difference was negligible and was not included in the calculations. Similarly, the weaning diet cost was also not included because both LW and EW protocols used the same diet and similar amount. The profit analysis of stocking sea cages with LW juvenile cod in comparison to stocking EW juveniles is shown in Table 4. With an average of 0.47 kg difference between adult cod from EW and LW at 30 months post-hatch and with 80% survival in the sea cages (our data) and a gate price of USD 4 per kg round weight, the difference in profit margin for using juveniles from LW for cage farming would be USD 1.665 million.

Table 3.
Production cost of Artemia nauplii to produce 1.125 million cod juveniles with an assumption of 15% survival of cod larvae from hatching to 25 g juveniles.

Table 4.
Estimated profit of using LW protocol in comparison to EW protocol. It is assumed that the survival of the cod juveniles to adult will be 80% in sea cages as shown in our results.
4. Discussion
Larvae raised using the LW protocol using Artemia nauplii were significantly heavier at 65 dph and had a greater harvest weight compared to the larvae raised using EW protocol. The major goal of weaning early is to reduce the dependency on live feed for marine finfish larviculture (in Atlantic cod [6,8,22]; in Halibut [7]). Most of these above-mentioned studies were mainly aiming to remove the Artemia nauplii from the diet of the larvae. While all these studies were successful in reducing or eliminating the Artemia nauplii from the larval diet, there were consequences of the reduction/elimination in Artemia nauplii with the larvae showing suppressed growth and/or survival [6,7,8,22,28,29].
Bonaldo, Parma, Badiani, Serratore, Gatta [9] reported comparable survival and proper metamorphosis but lower growth in common sole (Solea solea L.) when sole larvae were weaned early at 13 dph. Early weaning, however, gave better tank hygiene in their study. Mata-Sotres, Lazo, Baron-Sevilla [30] reported better growth but lower survival in totoaba (Totoaba macdonaldi) larvae in an early weaning regime compared to late weaning. However, the authors indicated that higher cannibalism in the early weaning tanks was responsible for the low survival. Similarly, Nhu, Dierckens, Nguyen, Hoang, Le, Tran, Nys, Sorgeloos [31] reported better growth, but not survival, of cobia (Rachycentron canadum) larvae in early weaning compared to late weaning larvae while Nguyen, Reinertsen, Wold, Tran, Kjørsvik [32] reported reduced growth, survival rate, and gut maturation index in early weaned cobia larvae. Nhu, Dierckens, Nguyen, Hoang, Le, Tran, Nys, Sorgeloos [31] did not mention any cannibalism but cobia is a fast-growing cannibalistic species. Cannibalistic fish are, in general, larger, and selectively prey on smaller fish. Further, cannibalistic fish grow larger as they consume the smaller tank mates [33,34]. Thus, size selective mortality could be the reason for the better growth in early weaning groups. So, in Mata-Sotres, Lazo, Baron-Sevilla [30] study, the higher growth rate could have been caused by a combination of selective mortality of smaller fish and the better nutritious food that larger fish received through eating smaller fish.
In our study, we employed a co-feeding strategy in both EW and LW treatments, where the dry feed was introduced in small amounts to the larvae just before each live feed feeding, so that they would become familiar with dry feed during the weaning process. Further, this would also slowly induce gut and digestive enzyme development [35]. We did not evaluate the success of the co-feeding in the current study; however, co-feeding strategy, in general, provided better growth and survival in many finfish larvae (in sole Solea senegalensis [35]; in Atlantic cod [20,22]). Pousão-Ferreira, Santos, Carvalho, Morais, Narciso [36] showed that early weaning of gilthead seabream (Sparus aurata L.) larvae resulted in lower growth and survival and concluded that complete replacement of live feed is not possible with currently available microdiets. As in our study, they have also suggested using combinations of microdiet and live prey and employing late weaning.
Studies have shown that early weaning impairs the development of internal gut structures (brush border cells, microvilli, etc.) and digestive enzymes which subsequently affects the growth, survival, and quality of the juveniles (in seabass [37]; in southern flounder Paralichthys lethostigma [28]; in common sole [29]; in yellowtail kingfish Seriola lalandi [38]). Ma, Qin, Hutchinson, Chen, Song [38] suggested that timing of weaning is important for proper development of digestive enzymes and early weaning would affect growth and survival in yellowtail kingfish. Parma, Bonaldo, Massi, Yúfera, Martínez-Rodríguez, Gatta [29] showed that early weaning of common sole larvae would significantly affect the ontogeny of enzyme development and associated gene expression profiles, which subsequently reduced the growth and survival. Faulk, Holt [28] reported decreased growth and higher incidence of deformities in southern flounder along with reduced digestive enzyme activities and absence of gastric digestion. We did not see any significant differences in skeletal deformities of juveniles from EW and LW treatments. Guerreiro, de Vareilles, Pousão-Ferreira, Rodrigues, Dinis, Ribeiro [39] in white seabream (Diplodus sargus) larvae, reported lower growth and initial low enzyme activity but early maturation of brush border cells. They also reported that the enzyme activities restored at later larval stages [39], but long-term effects of these delays and lost growth opportunities are not known. In our study, we did not investigate the enzyme activities. However, most of the published data on early weaning indicates reduced enzyme activities in larval fish and subsequent reduction on growth, survival, and quality.
Studies have suggested that including Artemia nauplii in the feed improved growth and survival through increase the production of bombesin in gilthead seabream, S. aurata, larvae [40,41,42] and in seabass, Dicentrarchus labrax, larvae [2]. Bombesin is a pituitary neuropeptide hormone similar to gastrin releasing peptide which is produced in the digestive system [43]. Thomdyke, Holmgren [44] reported the presence of bombesin in the adult stages of Atlantic cod and rainbow trout, Oncohynchus mykiss. In mammals, bombesin influences digestion by activating the peristaltic movement of the gut and the release of HCl as well as increasing blood circulation to the gut wall [45]. Kolkovski, Koven, Tandler [40] showed that production of bombesin was increased threefold in gilthead seabream larvae that were fed exclusively with Artemia nauplii compared to the larvae fed only microdiet. Hansen, Puvanendran, Jøstensen, Falk-Petersen [20] showed that Atlantic cod larvae co-fed with Artemia nauplii and a microdiet had better growth and increased gut length and foregut villus circumference compared to larvae fed with rotifers and microdiet. Similarly, Curnow, King, Partridge, Kolkovski [46] showed that use of Artemia nauplii with microdiet improved the growth and survival of barramundi (Lates calcarifer Bloch) larvae. In our study, cod larvae in the LW treatment were fed with Artemia nauplii while larvae in the EW were not. Although an increased presence of bombesin in larvae fed with Artemia nauplii and in Atlantic cod adults has been shown by studies [40,44], and their role in digestion is explained [43,44], the direct involvement of bombesin in growth and/or long-term effects of Artemia (bombesin) nauplii on growth has not been established.
Two studies involving Atlantic cod larvae that were fed with copepods and rotifers showed the importance of optimal live feed during the early larval stage which affected the performance of the late juvenile [47] and adult [48] growth performance. Koedijk, Folkvord, Foss, Pittman, Stefansson, Handeland, Imsland [47] showed that there is a critical period at 22 dph in cod larval development and larvae require more nutritious feed than rotifers beyond this stage. They suggested starting weaning beyond 22 dph (similar to EW in our study) to satisfy the nutritional requirement of cod larvae. In our study, EW resulted in no major differences in growth in cod juveniles compared to LW juveniles at 190 dph; however, a major difference in growth was evident between adult cod from EW and LW at 30 months post-hatch. As in our study, both studies did not examine the effects of different live feed on the digestive enzymes and peptide hormone. However, both studies support our finding that optimal live feed during the early larval stages could have a long-term effect on the growth of Atlantic cod.
In our study, the FAA in enriched rotifers was significantly lower compared to enriched Artemia nauplii (Table 2). However, studies have shown that starving or unenriched Artemia have lower levels of FAA compared to rotifers while Artemia enriched with algae has higher FAA [49]. Artemia catabolizes the FAA and fatty acids at much faster rates than rotifers [50]. In our study, the Artemia and rotifers were kept at 4 °C until they were fed to the cod larvae and this lower storage temperature minimizes the catabolism of the nutrients [4]. Studies have shown that different FAAs promote the growth of the fish larvae and juveniles through digestive enzyme modulation, osmotic regulation, and the maintenance of acid-base balance ([51] and references therein). Thus, the significantly higher FAA levels in enriched Artemia in our study could have been one of the reasons for the enhanced the performance of the larvae in LW compared to the larvae in EW.
Taurine is a simple free amino acid (β-AA) and important for cysteinesulfinate decarboxylase (CSD) and cysteine dioxygenase (CDO) activity in fish [52]. These enzymes promote growth in several teleosts [52], such as Japanese flounder [53], red sea bream [54], and yellowtail [55]. Although most marine fish species have high levels of taurine, they cannot naturally synthesize it [53]. Thus, it needs to be present or incorporated into the feed of the marine finfish. Our results showed that enriched Artemia nauplii had three times more taurine compared to enriched rotifers and similar results were reported by Aragão, Conceição, Dinis, Fyhn [49]. Other studies showed copepods have more taurine compared to Artemia and rotifers [56]. In our study, larvae from EW treatments received only rotifers as live feed, while larvae from LW treatments received both rotifers and Artemia. Thus, larvae from LW received higher amounts of taurine during the critical period (22 dph and beyond, [47]), which would have provided the boost for an improved growth.
Considerable progress in the development of formulated larval feeds and feeding protocols has been made over the past decades, with the goal to reduce the dependency on live feed for marine finfish larviculture. However, most marine finfish still require live feed for better quality, growth, and survival. In our study, our initial focus was to determine if we could eliminate the Artemia nauplii from the feeding protocol of Atlantic cod larvae without affecting the growth. Although it seems to be possible at juvenile stage (no significant difference in growth at this stage), growth in sea cages showed a significant difference in weight between the adults from the EW and LW treatments. A crude analysis of the cost effectiveness of both methods revealed that cod farmers may lose a substantial income (USD 1.665 million per million slaughtered cod) if they use EW juveniles instead of LW juveniles. A similar cost estimation for Artemia production cost was also presented by Sutherland [57] in his proposal for economic potential for production of Atlantic cod.
5. Conclusions
While the development of weaning microdiets needs to be continued until it meets the nutritional and physical property requirements of the developing larvae, our results showed that removing Artemia from the diet of Atlantic cod larvae and producing cod juveniles with an early weaning regime will affect the long-term growth and significantly reduce the slaughter weight. From a scientific point of view, the mechanisms behind these growth differences should be understood and more focus should be given to understand the role of bombesin and taurine. Based on our results, we recommend using a late weaning method for cod larvae until better enrichment products and protocols for rotifers and better weaning diet for larvae are developed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8060312/s1, Table S1: Detailed feeding table of Atlantic cod larvae from start feeding to end of weaning using late weaning strategy. Rotifer numbers are in million and dry feed amount is in grams. Table S2: Detailed feeding table of Atlantic cod larvae from start feeding to end of weaning using early weaning strategy. Rotifer numbers are in million and dry feed amount is in grams.
Author Contributions
Conceptualization, V.P. and Ø.J.H.; Methodology, V.P. and Ø.J.H.; Investigation, V.P.; Data collection and analysis, V.P.; Writing original draft, V.P.; Writing, reviewing, and editing, V.P. and Ø.J.H.; Project administration, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
It was supported through an internal funding from the National Cod Breeding Program.
Institutional Review Board Statement
The experiment was carried out in accordance with the national rules and regulations at the site of the experiments and all efforts were undertaken to minimize stress and suffering of the animals. The experimental protocol was approved by the facility manager of Centre for Marine Aquaculture (CMA) in accordance with the Norwegian Animal Research Authority (Forsøksdyrutvalget) to carry out the experiment (FOTS ID 14130 and 14124 and internal ID H17/17 and H17/16, respectively).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material. A detailed data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank the staff at the Centre for Marine Aquaculture’s land-based facility at Kraknes and the sea cage at Røsnes, Norway for their help in taking care of the fish during this experiment. We also thank Nivetha Puvanendran for the grammatical editing of this manuscript. This work was supported by the National Cod Breeding Program which is funded by the Norwegian Ministry of Trade, Industry and Fisheries (Norwegian: Nærings-og fiskeridepartementet).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cahu, C.; Infante, J.Z. Substitution of live food by formulated diets in marine fish larvae. Aquaculture 2001, 200, 161–180. [Google Scholar] [CrossRef]
- Kolkovski, S.; Tandler, A.; Izquierdo, M.S. The effects of live food and dietary digestive enzymes on the efficiency of microdiets for seabass Dicentrarchus labrax. larvae. Aquaculture 1997, 148, 313–322. [Google Scholar] [CrossRef]
- Moguel-Hernández, I.; Peña, R.; Andree, K.B.; Tovar-Ramirez, D.; Bonacic, K.; Dumas, S.; Gisbert, E. Ontogeny changes and weaning effects in gene expression patterns of digestive enzymes and regulatory digestive factors in spotted rose snapper (Lutjanus guttatus) larvae. Fish Physiol. Biochem. 2016, 42, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Sorgeloos, P.; Dhert, P.; Candreva, P. Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture 2001, 200, 147–159. [Google Scholar]
- Navarro, J.C.; Henderson, R.J.; McEvoy, L.A.; Bell, M.V.; Amat, F. Lipid conversions during enrichment of Artemia. Aquaculture 1999, 174, 155–166. [Google Scholar] [CrossRef]
- Baskerville-Bridges, B.; Kling, L.J. Early weaning of Atlantic cod (Gadus morhua) larvae onto a microparticulate diet. Aquaculture 2000, 189, 109–117. [Google Scholar]
- Næss, T.; Hamre, K.; Holm, J.C. Successful early weaning of Atlantic halibut (Hippoglossus hippoglossus L.) in small shallow raceway systems. Aquacult. Res. 2001, 32, 163–168. [Google Scholar]
- Fletcher Jr, R.C.; Roy, W.; Davie, A.; Taylor, J.; Robertson, D.; Migaud, H. Evaluation of new microparticulate diets for early weaning of Atlantic cod (Gadus morhua): Implications on larval performances and tank hygiene. Aquaculture 2007, 263, 35–51. [Google Scholar]
- Bonaldo, A.; Parma, L.; Badiani, A.; Serratore, P.; Gatta, P.P. Very early weaning of common sole (Solea solea L.) larvae by means of different feeding regimes and three commercial microdiets: Influence on performances, metamorphosis development and tank hygiene. Aquaculture 2011, 321, 237–244. [Google Scholar] [CrossRef]
- Brown, J.A.; Minkoff, G.; Puvanendran, V. Larviculture of Atlantic cod (Gadus morhua)—Progress, protocols and problems. Aquaculture 2003, 227, 357–372. [Google Scholar] [CrossRef]
- Rosenlund, G.; Halldórsson, Ó. Cod juvenile production: Research and commercial developments. Aquaculture 2007, 268, 188–194. [Google Scholar] [CrossRef]
- Polanco, J.F.; Bjørndal, T. Aquaculture diversification in Europe: The Kingdom of Spain and the Kingdom of Norway. In Planning for Aquaculture Diversification: The Importance of Climate Change and Other Driver; Harvey, B., Soto, D., Carolsfeld, J., Beveridge, M., Bartley, D.M., Eds.; FAO Fisheries and Aquaculture Proceedings No. 47; FAO: Rome, Italy, 2017; pp. 37–45. [Google Scholar]
- Bangera, R.; Ødegård, J.; Præbel, A.K.; Mortensen, A.; Nielsen, H.M. Genetic correlations between growth rate and resistance to vibriosis and viral nervous necrosis in Atlantic cod (Gadus morhua L.). Aquaculture 2011, 317, 67–73. [Google Scholar] [CrossRef]
- Bangera, R.; Ødegård, J.; Mikkelsen, H.; Nielsen, H.M.; Seppola, M.; Puvanendran, V.; Gjøen, H.M.; Hansen, Ø.J.; Mortensen, A. Genetic analysis of francisellosis field outbreak in Atlantic cod (Gadus morhua L.) using an ordinal threshold model. Aquaculture 2014, 420, S50–S56. [Google Scholar] [CrossRef]
- Bangera, R.; Drangsholt, T.M.; Nielsen, H.M.; Sae-Lim, P.; Ødegård, J.; Puvanendran, V.; Hansen, Ø.J.; Mortensen, A. Genotype by environment interaction for growth in Atlantic cod (Gadus morhua L.) in four farms of Norway. J. Mar. Sci. Eng. 2015, 3, 412–427. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V.; Jøstensen, J.P.; Ous, C. Effects of dietary levels and ratio of phosphatidylcholine and phosphatidylinositol on the growth, survival and deformity levels of Atlantic cod larvae and early juveniles. Aquacult. Res. 2011, 42, 1026–1033. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V.; Mortensen, A. Importance of broodstock holding temperature on fecundity and egg quality in three groups of photo-manipulated Atlantic cod broodstock. Aquacult. Res. 2012, 44, 140–150. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V.; Bangera, R. Do maternal age and experience contribute to better growth, survival and disease resistance of offspring in Atlantic cod (Gadus morhua)? Aquacult. Int. 2015, 23, 1157–1164. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V.; Bangera, R. Broodstock diet with water and astaxanthin improve condition and egg output of brood fish and larval survival in Atlantic cod, Gadus morhua L. Aquacult. Res. 2016, 47, 819–829. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V.; Jøstensen, J.P.; Falk-Petersen, I.B. Early introduction of an inert diet and unenriched Artemia enhances growth and quality of Atlantic cod (Gadus morhua) larvae. Aquacult. Nutr. 2018, 24, 102–111. [Google Scholar] [CrossRef]
- Puvanendran, V.; Mortensen, A.; Johansen, L.H.; Kettunen, A.; Hansen, Ø.J.; Henriksen, E.; Heide, M. Development of cod farming in Norway: Past and current biological and market status and future prospects and directions. Rev. Aquacult. 2022, 14, 308–342. [Google Scholar] [CrossRef]
- Callan, C.; Jordaan, A.; Kling, L.J. Reducing Artemia use in the culture of Atlantic cod (Gadus morhua). Aquaculture 2003, 219, 585–595. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V. Fertilization success and blastomere morphology as predictors of egg and juvenile quality for domesticated Atlantic cod, Gadus morhua, broodstock. Aquacult. Res. 2010, 41, 1791–1798. [Google Scholar] [CrossRef]
- Björnsson, B.; Ólafsdóttir, S.R. Effects of water quality and stocking density on growth performance of juvenile cod (Gadus morhua L.). ICES J. Mar. Sci. 2006, 63, 326–334. [Google Scholar] [CrossRef]
- Brekken, B. Kvantitativ Bestemmelse av Frie Aminosyrer; SSF-Rapport: Stockholm, Sweden, 1989; A-142; 13p. [Google Scholar]
- Cohen, S.A.; Meys, M.; Tarvin, T.L. The Pico Tag® Method. A Manual of Advanced Techniques for Amino Acid Analysis. In Water Division of Millipore WM02, Rev 1; Millipore Corporation: Milford, MA, USA, 1989; 123p. [Google Scholar]
- Conover, W.J.; Iman, R.L. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 1981, 35, 124–129. [Google Scholar]
- Faulk, C.K.; Holt, G.J. Early weaning of southern flounder, Paralichthys lethostigma, larvae and ontogeny of selected digestive enzymes. Aquaculture 2009, 296, 213–218. [Google Scholar] [CrossRef]
- Parma, L.; Bonaldo, A.; Massi, P.; Yúfera, M.; Martínez-Rodríguez, G.; Gatta, P.P. Different early weaning protocols in common sole (Solea solea L.) larvae: Implications on the performances and molecular ontogeny of digestive enzyme precursors. Aquaculture 2013, 414, 26–35. [Google Scholar] [CrossRef]
- Mata-Sotres, J.A.; Lazo, J.P.; Baron-Sevilla, B. Effect of age on weaning success in totoaba (Totoaba macdonaldi) larval culture. Aquaculture 2015, 437, 292–296. [Google Scholar] [CrossRef]
- Nhu, V.C.; Dierckens, K.; Nguyen, H.T.; Hoang, T.M.T.; Le, T.L.; Tran, M.T.; Nys, C.; Sorgeloos, P. Effect of early co-feeding and different weaning diets on the performance of cobia (Rachycentron canadum) larvae and juveniles. Aquaculture 2010, 305, 52–58. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Reinertsen, H.; Wold, P.A.; Tran, T.M.; Kjørsvik, E. Effects of early weaning strategies on growth, survival and digestive enzyme activities in cobia (Rachycentron canadum L.) larvae. Aquacult. Int. 2011, 19, 63–78. [Google Scholar] [CrossRef]
- Folkvord, A.; Otterå, H. Effects of initial size distribution, day length, and feeding frequency on growth, survival, and cannibalism in juvenile Atlantic cod (Gadus morhua L.). Aquaculture 1993, 114, 243–260. [Google Scholar] [CrossRef]
- Puvanendran, V.; Laurel, B.J.; Brown, J.A. Cannibalism of Atlantic cod Gadus morhua larvae and juveniles on first-week larvae. Aquat. Biol. 2008, 2, 113–118. [Google Scholar] [CrossRef]
- Canavate, J.P.; Fernández-Dıaz, C. Influence of co-feeding larvae with live and inert diets on weaning the sole Solea senegalensis onto commercial dry feeds. Aquaculture 1999, 174, 255–263. [Google Scholar] [CrossRef]
- Pousão-Ferreira, P.; Santos, P.; Carvalho, A.P.; Morais, S.; Narciso, L. Effect of an experimental microparticulate diet on the growth, survival and fatty acid profile of gilthead seabream (Sparus aurata L.) larvae. Aquacult. Int. 2003, 11, 491–504. [Google Scholar] [CrossRef]
- Cahu, C.L.; Infante, J.Z. Early weaning of sea bass (Dicentrarchus labrax) larvae with a compound diet: Effect on digestive enzymes. Comp. Biochem. Physiol. 1994, 109, 213–222. [Google Scholar] [CrossRef]
- Ma, Z.; Qin, J.G.; Hutchinson, W.; Chen, B.N.; Song, L. Responses of digestive enzymes and body lipids to weaning times in yellowtail kingfish Seriola lalandi (Valenciennes, 1833) larvae. Aquacult. Res. 2014, 45, 973–982. [Google Scholar] [CrossRef]
- Guerreiro, I.; de Vareilles, M.; Pousão-Ferreira, P.; Rodrigues, V.; Dinis, M.T.; Ribeiro, L. Effect of age-at-weaning on digestive capacity of white seabream (Diplodus sargus). Aquaculture 2010, 300, 194–205. [Google Scholar] [CrossRef]
- Kolkovski, S.; Koven, W.; Tandler, A. The mode of action of Artemia in enhancing utilization of microdiet by gilthead seabream Sparus aurata larvae. Aquaculture 1997, 155, 193–205. [Google Scholar] [CrossRef]
- Naz, M.; Turkmen, M. Digestive enzymes and hormones in gilthead seabream larvae (Sparus aurata) fed Artemia nauplii enriched with free histidine. Isr. J. Aquac. 2008, 60, 230–236. [Google Scholar] [CrossRef]
- Naz, M.; Türkmen, M. The changes in digestive enzymes and hormones of gilthead seabream larvae (Sparus aurata, L 1758) fed on Artemia nauplii enriched with free methionine. Aquacult. Int. 2009, 17, 243–256. [Google Scholar] [CrossRef]
- Batten, T.F.C.; Camere, M.L.; Moons, L.; Vandesande, F. Comparative distribution of neuropeptide-immunoreactive systems in the brain of the greenmolly, Poecillia latipinna. J. Comp. Neurol. 1990, 302, 893–919. [Google Scholar] [CrossRef]
- Thorndyke, M.; Holmgren, S. Bombesin potentiates the effect of acetylcholine on isolated strips of fish stomach. Regul. Pept. 1990, 30, 125–135. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.J.; Jornvall, H.; Nilsson, G.; Vagne, M.; Ghatei, M.; Bloom, S.R.; Mutt, V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979, 90, 227–233. [Google Scholar] [CrossRef]
- Curnow, J.; King, J.; Partridge, G.; Kolkovski, S. Effects of two commercial microdiets on growth and survival of barramundi (Lates calcarifer Bloch) larvae within various early weaning protocols. Aquacult. Nutr. 2006, 12, 247–255. [Google Scholar] [CrossRef]
- Koedijk, R.M.; Folkvord, A.; Foss, A.; Pittman, K.; Stefansson, S.O.; Handeland, S.; Imsland, A.K. The influence of first-feeding diet on the Atlantic cod Gadus morhua phenotype: Survival, development and long-term consequences for growth. J. Fish Biol. 2010, 77, 1–19. [Google Scholar] [CrossRef]
- Imsland, A.K.; Foss, A.; Koedijk, R.; Folkvord, A.; Stefansson, S.O.; Jonassen, T.M. Short-and long-term differences in growth, feed conversion efficiency and deformities in juvenile Atlantic cod (Gadus morhua) startfed on rotifers or zooplankton. Aquacult. Res. 2006, 37, 1015–1027. [Google Scholar] [CrossRef]
- Aragão, C.; Conceição, L.E.; Dinis, M.T.; Fyhn, H.J. Amino acid pools of rotifers and Artemia under different conditions: Nutritional implications for fish larvae. Aquaculture 2004, 234, 429–445. [Google Scholar] [CrossRef]
- Naz, M. The changes in the biochemical compositions and enzymatic activities of rotifer (Brachionus plicatilis, Müller) and Artemia during the enrichment and starvation periods. Fish Physiol. Biochem. 2008, 34, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, S.; Wu, G. Nutrition and functions of amino acids in fish. In Amino Acids in Nutrition and Health, Advances in Experimental Medicine and Biology; Wu, G., Ed.; Springer: Cham, Switzerland, 2021; pp. 133–168. [Google Scholar]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Kim, S.-K.; Takeuchi, T.; Akimoto, A.; Furuita, H.; Yamamoto, T.; Yokoyama, M.; Murata, Y. Effect of taurine supplemented practical diet on growth performance and taurine contents in whole body and tissues of juvenile Japanese flounder Paralichthys olivaceus. Fish. Sci. 2005, 71, 627–632. [Google Scholar] [CrossRef]
- Matsunari, H.; Yamamoto, T.; Kim, S.-K.; Goto, T.; Takeuchi, T. Optimum dietary taurine level in casein-based diet for juvenile red sea bream Pagrus major. Fish. Sci. 2008, 74, 347–353. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, H.; Goto, T.; Endo, M.; Yamashita, H.; Ukawa, M. Taurine is an essential nutrient for yellowtail Seriola quinqueradiata fed non-fish meal diets based on soy protein concentrate. Aquaculture 2008, 280, 198–205. [Google Scholar] [CrossRef]
- Karlsen, Ø.; van der Meeren, T.; Rønnestad, I.; Mangor-Jensen, A.; Galloway, T.F.; Kjørsvik, E.; Hamre, K. Copepods enhance nutritional status, growth and development in Atlantic cod (Gadus morhua L.) larvae—Can we identify the underlying factors? PeerJ 2015, 3, e902. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R. Economics of Potential Systems for Farmed Production of Cod. 1998. 36p. Available online: https://www.seafish.org/media/Publications/EconomicsOfPotentialforFarmedProductionofCod.pdf (accessed on 25 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).