Temperature Effects on Recruitment and Individual Growth of Two Antagonistic Fish Species, Perch Perca fluviatilis and Roach Rutilus rutilus, from a Climate Change Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Calculations
2.4. Statistics
3. Results
3.1. Air and Water Temperature
3.2. Fish Abundance and Size Structure
3.3. Age Structure and Year-Class Strength
3.4. Growth and Temperature
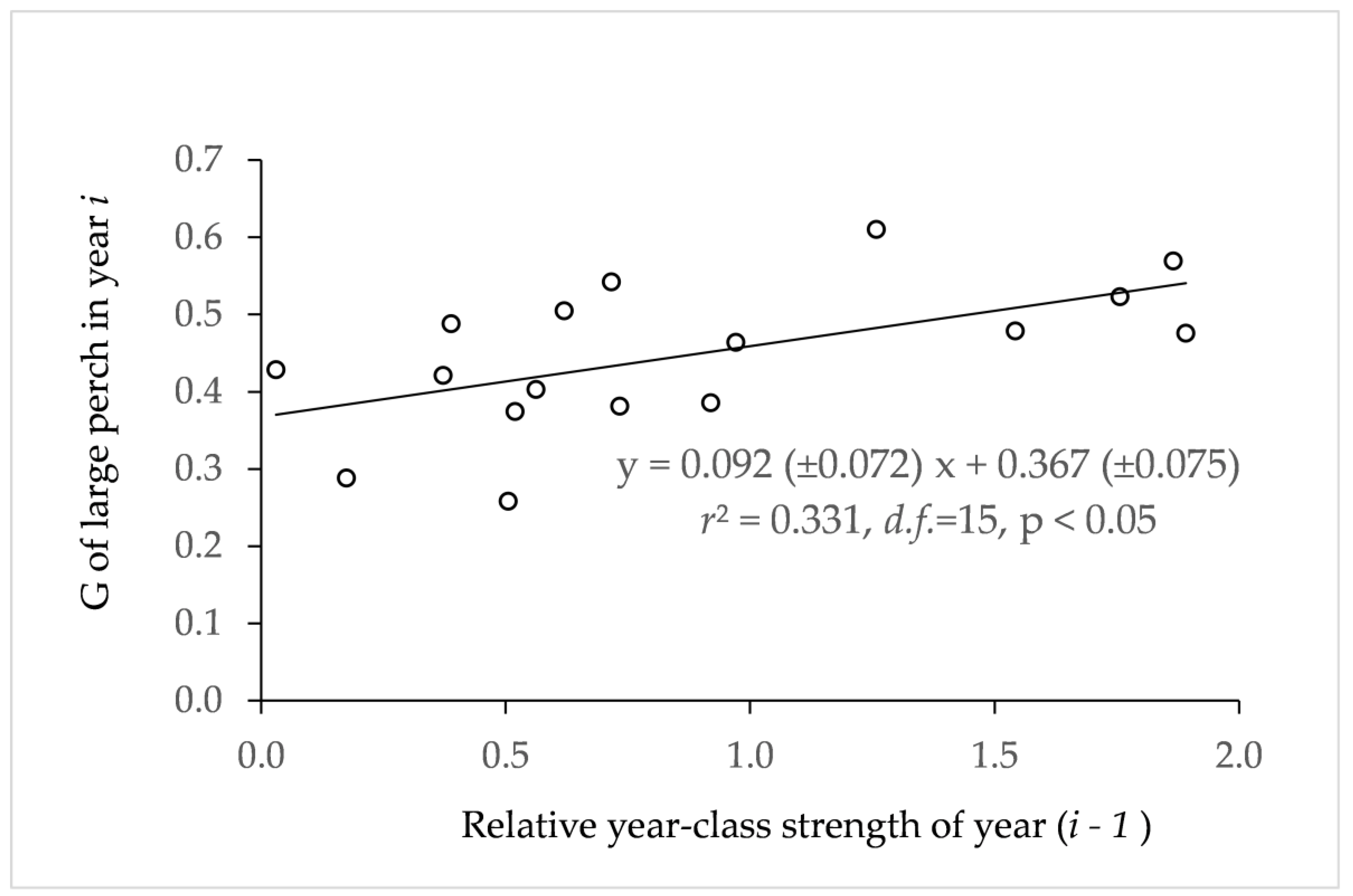
3.5. Growth Acceleration
3.6. Stomach Content
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsey, R.; Dahlman, L. Climate Change: Global Temperature. 2022. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature (accessed on 9 May 2023).
- Dore, M.H.I. Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 2005, 31, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Arunrat, N.; Sereenonchai, S.; Chaowiwat, W.; Wang, C. Climate change impact on major crop yield and water footprint under CMIP6 climate projections in repeated drought and flood areas in Thailand. Sci. Total Environ. 2022, 807, 150741. [Google Scholar] [CrossRef] [PubMed]
- EPA. U.S. Environmental Protection Agency. Climate Change Indicators: Ecosystems. 2021 02.082022. Available online: https://www.epa.gov/climate-indicators/ecosystems (accessed on 9 May 2023).
- Linløkken, A.; Bergman, E.; Greenberg, L.; Seeland, P.A.H. Environmental correlates of population variables of perch (Perca fluviatilis) in boreal lakes. Environ. Biol. Fishes 2008, 82, 401–408. [Google Scholar] [CrossRef]
- Linløkken, A.N.; Bergman, E.; Greenberg, L. Effect of temperature and roach Rutilus rutilus group size on swimming speed and prey capture rate of perch Perca fluviatilis and R-rutilus. J. Fish Biol. 2010, 76, 900–912. [Google Scholar] [CrossRef]
- Neuman, E.; Thoresson, G.; Sandstrom, O. Swimming activity of perch, Perca fluviatilis, in relation to temperature, day-length and consumption. Ann. Zool. Fenn. 1996, 33, 669–678. [Google Scholar]
- Linløkken, A.; Haugen, T.O. Density and temperature dependence of gill net catch per unit effort for perch, Perca fluviatilis, and roach, Rutilus rutilus. Fish. Manag. Ecol. 2006, 13, 261–269. [Google Scholar] [CrossRef]
- Fiogbé, E.D.; Kestemont, P. Optimum daily ration for Eurasian perch Perca fluviatilis L. reared at its optimum growing temperature. Aquaculture 2003, 216, 243–252. [Google Scholar] [CrossRef]
- Hardewig, I.; Van Dijk, P.L.M. Is digestive capacity limiting growth at low temperatures in roach? J. Fish Biol. 2003, 62, 358–374. [Google Scholar] [CrossRef]
- Van Dijk, P.L.M.; Staaks, G.; Hardewig, I. The effect of fasting and refeeding on temperature preference, activity and growth of roach, Rutilus rutilus. Oecologia 2002, 130, 496–504. [Google Scholar] [CrossRef]
- Persson, L. Competition-induced switch in young of the year perch, Perca fluviatilis—An experimental test of resource limitation. Environ. Biol. Fishes 1987, 19, 235–239. [Google Scholar] [CrossRef]
- Persson, L. Asymmetries in competitive and predatory interactions in fish populations. In Size-Structured Populations: Ecology and Evolution; Ebenman, B., Persson, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- Persson, L.; Bystrom, P.; Wahlstrom, E.; Andersson, J.; Hjelm, J. Interactions among size-structured populations in a whole-lake experiment: Size- and scale-dependent processes. Oikos 1999, 87, 139–156. [Google Scholar] [CrossRef]
- Lessmark, O. Competition between Perch (Perca fluviatilis) and Roach (Rutilus rutilus) in South Swedish Lakes; Lund University: Lund, Sweden, 1983. [Google Scholar]
- Persson, L. Effects of intraspecific and interspecific competition on dynamics and size structure of a perch Perca fluviatilis and a roach Rutilus rutilus population. Oikos 1983, 41, 126–132. [Google Scholar] [CrossRef]
- Persson, L.; Greenberg, L.A. Interspecific and intraspecific size class competition affecting resource use and growth of perch, Perca fluviatilis. Oikos 1990, 59, 97–106. [Google Scholar] [CrossRef]
- Jacobsen, L.; Berg, S.; Broberg, M.; Jepsen, N.; Skov, C. Activity and food choice of piscivorous perch (Perca fluviatilis) in a eutrophic shallow lake: A radio-telemetry study. Freshw. Biol. 2002, 47, 2370–2379. [Google Scholar] [CrossRef]
- Pirhonen, J.; Muuri, L.; Kalliokoski, S.M.; Puranen, M.M.; Marjomäki, T.J. Seasonal and ontogenetic variability in stomach size of Eurasian perch (Perca fluviatilis L.). Aquac. Int. 2019, 27, 1125–1135. [Google Scholar] [CrossRef]
- Persson, L.; Byström, P.; Wahlström, E. Cannibalism and competition in Eurasian perch: Population dynamics of an ontogenetic omnivore. Ecology 2000, 81, 1058–1071. [Google Scholar] [CrossRef]
- Holmgren, K.; Appelberg, M. Effects of environmental factors on size-related growth efficiency of perch, Perca fluviatilis. Ecol. Freshw. Fish 2001, 10, 247–256. [Google Scholar] [CrossRef]
- Persson, L.; Diehl, S.; Johansson, L.; Andersson, G.; Hamrin, S.F. Trophic Interactions In Temperate Lake Ecosystems—A Test of Food-Chain Theory. Am. Nat. 1992, 140, 59–84. [Google Scholar] [CrossRef]
- Olin, M.; Vinni, M.; Lehtonen, H.; Rask, M.; Ruuhijärvi, J.; Saulamo, K.; Ala-Opas, P. Environmental factors regulate the effects of roach Rutilus rutilus and pike Esox lucius on perch Perca fluviatilis populations in small boreal forest lakes. J. Fish Biol. 2010, 76, 1277–1293. [Google Scholar] [CrossRef]
- Linløkken, A.N.; Hesthagen, T. The interactions of abiotic and biotic factors influencing perch Perca fluviatilis and roach Rutilus rutilus populations in small acidified boreal lakes. J. Fish Biol. 2011, 79, 431–448. [Google Scholar] [CrossRef]
- Le Cren, E.D. Exceptionally big individual perch (Perca fluviatilis L.) and their growth. J. Fish Biol. 1992, 40, 599–625. [Google Scholar] [CrossRef]
- Stenson, J.A.E. Predator-prey relations between fish and invertebrate prey in some forest lakes. Rep. Inst. Freshw. Res. Drottningholm 1979, 58, 166–183. [Google Scholar]
- Linløkken, A.N. Effects of Lake Productivity on Density and Size Structure of Pelagic Fish Estimated by Means of Echosounding in 17 Lakes in Southeast Norway. Sensors 2021, 21, 3391. [Google Scholar] [CrossRef] [PubMed]
- Sanni, S.; Wærvågen, S.B. Oligotrophication as a result of planktivorous fish removal with rotenone in the small, eutrophic lake Mosvatn, Norway. Hydrobiologia 1990, 200, 263–274. [Google Scholar] [CrossRef]
- Olin, M.; Rask, M.; Ruuhijärvi, J.; Keskitalo, J.; Horppila, J.; Tallberg, P. Effects of biomanipulation on fish and plankton communities in ten eutrophic lakes of southern Finland. Hydrobiologia 2006, 553, 67–88. [Google Scholar] [CrossRef]
- Kagami, M.; Yoshida, T.; Gurung, T.; Urabe, J. Direct and indirect effects of zooplankton on algal composition in situ grazing experiments. Oecologia 2002, 133, 356–363. [Google Scholar] [CrossRef]
- Ginter, K.; Blank, K.; Haberman, J.; Kangur, A.; Kangur, K. Fish predation pressure on zooplankton in a large northern temperate lake: Impact of adult predators versus juvenile predators. Proc. Est. Acad. Sci. 2018, 67, 356–367. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Ekvall, M.K.; Hansson, L.-A. Controlling Harmful Cyanobacteria: Taxa-Specific Responses of Cyanobacteria to Grazing by Large-Bodied Daphnia in a Biomanipulation Scenario. PLoS ONE 2016, 11, e0153032. [Google Scholar] [CrossRef]
- Persson, L. Predator-Mediated Competition In Prey Refuges—The Importance of Habitat Dependent Prey Resources. Oikos 1993, 68, 12–22. [Google Scholar] [CrossRef]
- Claessen, D.; de Roos, A.M.; Persson, L. Dwarfs and Giants: Cannibalism and Competition in Siz… Structured Populations. Am. Nat. 2000, 155, 219–237. [Google Scholar] [CrossRef]
- Persson, L.; Diehl, S.; Johansson, L.; Andersson, G.; Hamrin, S.F. Shifts in fish communities along the productivity gradient of temperate lakes—Patterns and the importance of size-structured interactions. J. Fish Biol. 1991, 38, 281–293. [Google Scholar] [CrossRef]
- Svärdson, G. Interspecific population dominance in fish communities of Scandinavian lakes. Inst. Freshw. Res. Drottningholm Rep. 1977, 55, 144–172. [Google Scholar]
- Neuman, E. The growth and year-class strength of perch in some Baltic archipelagos, with special reference to temperature. Rep. Inst. Freshw. Res. Drottningholm 1976, 55, 51–70. [Google Scholar]
- Neuman, E. Activity of perch, Perca fluviatilis L. and roach, Rutilus rutilus (L.), in a Baltic Bay, with special reference to temperature. Inst. Freshw. Res. Drottningholm 1979, 58, 107–125. [Google Scholar]
- Le Cren, E.D. Observations on the growth of perch (Perca fluviatilis) over twenty-two years with special reference to effects of temperature and changes in population density. J. Anim. Ecol. 1958, 27, 287–334. [Google Scholar] [CrossRef]
- Le Cren, E.D.; Kipling, C.; McCormack, J.C. A Study of the Numbers, Biomass and Year-Class Strengths of Perch (Perca fluviatilis L.) in Windermere from 1941 to 1966. J. Anim. Ecol. 1977, 46, 281–307. [Google Scholar] [CrossRef]
- Linløkken, A. Temperature dependence of Eurasian perch (Perca fluviatilis) recruitment. In Percis III: The Third International Percid Fish Symposium; University of Wisconsin Sea Grant Institute: Madison, WI, USA, 2003; pp. 75–76. [Google Scholar]
- Tolonen, A.; Lappalainen, J.; Pulliainen, E. Seasonal growth and year class strength variations of perch near the northern limits of its distribution range. J. Fish Biol. 2003, 63, 176–186. [Google Scholar] [CrossRef]
- Böhling, P.; Hudd, R.; Lehtonen, H.; Karås, P.; Neuman, E.; Thoresson, G. Variations in year-class strength of different perch (Perca fluviatilis) populations in the Baltic Sea with special reference to temperature and pollution. Can. J. Fish. Aquat. Sci. 1991, 48, 1181–1187. [Google Scholar] [CrossRef]
- Karås, P. Recruitment of perch (Perca fluviatilis L) from Baltic coastal waters. Arch. Für Hydrobiol. 1996, 138, 99–121. [Google Scholar] [CrossRef]
- Britton, R.; Cowx, I.; Axford, S.; Frear, P. An overview of recruitment patterns of roach Rutilus rutilus (L.) between 1969 and 2001 in the rivers of England and their influence on population abundance. Ecohydrol. Hydrobiol. 2004, 4, 91–102. [Google Scholar]
- Grenouillet, G.; Hugueny, B.; Carrel, G.; Olivier, J.M.; Pont, D. Large-scale synchrony and inter-annual variability in roach recruitment in the Rhone River: The relative role of climatic factors and density-dependent processes. Freshw. Biol. 2001, 46, 11–26. [Google Scholar] [CrossRef]
- Barnsley, H. Factors Affecting the Growth and Recruitment of Cyprinid Populations of the River Wensum, Eastern England, with Special Reference to Roach Rutilus rutilus (L.). Master’s Thesis, Bournemouth University, Poole, UK, 2012; 17p. [Google Scholar]
- Beardsley, H.; Britton, J.R. Recruitment success in a roach Rutilus rutilus population of a hydrologically stable chalk river: Relative influences of temperature and flow. Ecol. Freshw. Fish 2012, 21, 168–171. [Google Scholar] [CrossRef]
- Beardsley, H.; Britton, J.R. Contribution of temperature and nutrient loading to growth rate variation of three cyprinid fishes in a lowland river. Aquat. Ecol. 2012, 46, 143–152. [Google Scholar] [CrossRef]
- Lehtonen, H. Potential effects of global warming on northern European freshwater fish and fisheries. Fish. Manag. Ecol. 1996, 3, 59–71. [Google Scholar] [CrossRef]
- Cowx, I.G.; Frear, P.A. Assessment of year class strength in freshwater recreational fish populations. Fish. Manag. Ecol. 2004, 11, 117–123. [Google Scholar] [CrossRef]
- Linløkken, A.N.; Sandlund, O.T. Recruitment of sympatric vendace (Coregonus albula) and whitefish (C. lavaretus) is affected by different environmental factors. Ecol. Freshw. Fish 2015, 25, 652–663. [Google Scholar] [CrossRef]
- Karås, P. Basic abiotic conditions for production of perch (Perca fluviatilis L) young-of-the-year in the Gulf of Bothnia. Ann. Zool. Fenn. 1996, 33, 371–381. [Google Scholar]
- Karås, P.; Thoresson, G. An application of a bioenergetics model to Eurasian perch (Perca fluviatilis L). J. Fish Biol. 1992, 41, 217–230. [Google Scholar] [CrossRef]
- Linløkken, A.; Kleiven, E.; Matzow, D. Population-Structure, Growth and Fecundity of Perch (Perca-fluviatilis L) in an Acidified River System in Southern Norway. Hydrobiologia 1991, 220, 179–188. [Google Scholar] [CrossRef]
- Allen, K.R. The food and migration of perch (Perca fluviatilis) in Windermere. J. Anim. Ecol. 1935, 4, 264–273. [Google Scholar] [CrossRef]
- Persson, L.; Claessen, D.; De Roos, A.M.; Bystrom, P.; Sjogren, S.; Svanback, R.; Wahlstrom, E.; Westman, E. Cannibalism in a size-structured population: Energy extraction and control. Ecol. Monogr. 2004, 74, 135–157. [Google Scholar] [CrossRef]
- Yazıcıoğlu, O.; Yılmaz, S.; Yazıcı, R.; Erbaşaran, M.; Polat, N. Feeding ecology and prey selection of European perch, Perca fluviatilis inhabiting a eutrophic lake in northern Turkey. J. Freshw. Ecol. 2016, 31, 641–651. [Google Scholar] [CrossRef]
- Appelberg, M.; Berger, H.M.; Hesthagen, T.; Kleiven, E.; Kurkilahti, M.; Raitaniemi, J.; Rask, M. Development and intercalibration of methods in Nordic freshwater fish monitoring. Water Air Soil Pollut. 1995, 85, 401–406. [Google Scholar] [CrossRef]
- Linløkken, A. Vertical distribution of brown trout (Salmo trutta) and perch (Perca fluviatilis) in an acidified lake. Water Air Soil Pollut. 1988, 40, 203–213. [Google Scholar] [CrossRef]
- Le Cren, E.D. The determination of the age and growth of perch (Perca fluviatilis) from opercular bone. J. Anim. Ecol. 1947, 16, 188–204. [Google Scholar] [CrossRef]
- Horppila, J.; Nyberg, K. The validity of different methods in the backcalculation of the lengths of roach—A comparison between scales and cleithra. J. Fish Biol. 1999, 54, 489–498. [Google Scholar]
- Coporation, O.C. Onset Data Loggers. 2021. Available online: https://www.onsetcomp.com/products/data-loggers?gclid=EAIaIQobChMIzumHieS88wIVAeuyCh2dUweBEAAYASAAEgK3ZvD_BwE (accessed on 9 May 2023).
- Nunn, A.D.; Frear, P.A.; Lee, M.; Cowx, I.G. Is there evidence for a shift in fish growth and recruitment success linked to climate change? J. Fish Biol. 2010, 77, 1780–1792. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. Bull. 1975, 191, 382. [Google Scholar]
- Robson, D.S.; Chapman, D.G. Catch Curves and Mortality Rates. Trans. Am. Fish. Soc. 1961, 90, 181–189. [Google Scholar] [CrossRef]
- Bagenal, T.B. Methods for Assessment of Fish Production in Fresh Waters; IBP Handbook; Blackwell: London, UK, 1978; Volume 3, 365p. [Google Scholar]
- Linløkken, A.; Seeland, P.A.H. Growth and production of perch (Perca fluviatilis L) responding to biomass removal. Ann. Zool. Fenn. 1996, 33, 427–435. [Google Scholar]
- Microsoft Excel 365. 2023. Available online: https://www.microsoft.com/en-us/microsoft-365/excel (accessed on 9 May 2023).
- R-Core-Team. R—A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 9 May 2023).
- Crawley, M.J. The R Book; Wiley: Chichester, UK, 2007. [Google Scholar]
- Mehner, T.; Schultz, H.; Bauer, D.; Herbst, R.; Voigt, H.; Benndorf, J. Intraguild predation and cannibalism in age-0 perch (Perca fluviatilis) and age-0 zander (Stizostedion lucioperca): Interactions with zooplankton succession, prey fish availability and temperature. Ann. Zool. Fenn. 1996, 33, 353–361. [Google Scholar]
- Willemsen, J. Population Dynamics of Percids in Lake IJssel and some Smaller Lakes in The Netherlands. J. Fish. Res. Board Can. 1977, 34, 1710–1719. [Google Scholar] [CrossRef]
- Buijse, A.D. Dynamics and Exploitation of an Unstable Percid Population. Ph.D. Thesis, University of Wageningen, Wageningen, The Netherlands, 1992; 168p. [Google Scholar]
- Dörner, H.; Berg, S.; Jacobsen, L.; Hülsmann, S.; Brojerg, M.; Wagner, A. The feeding behaviour of large perch Perca fluviatilis (L.) in relation to food availability: A comparative study. Hydrobiologia 2003, 506, 427–434. [Google Scholar] [CrossRef]
- NOAA. Monthly Global Climate Report for Annual 2020. State of the Climate 2022. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202013 (accessed on 14 June 2022).
- O’Reilly, C.M.; Sharma, S.; Gray, D.K.; Hampton, S.E.; Read, J.S.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; McIntyre, P.B.; Kraemer, B.M.; et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 10773–10781. [Google Scholar] [CrossRef]
- Adámek, Z.; Mikl, L.; Šlapanský, L.; Jurajda, P.; Halačka, K. The diet of predatory fish in drinking water reservoirs—How can they contribute to biomanipulation efforts? Folia Zool. 2019, 68, 215–224, 210. [Google Scholar] [CrossRef]
- Persson, L.; Eklöv, P. Prey Refuges Affecting Interactions between Piscivorous Perch and Juvenile Perch and Roach. Ecology 1995, 76, 70–81. [Google Scholar] [CrossRef]
- Fjeld, E.; Rognerud, S. Mercury in Freshwater Fish from the South Norway in 1998–2002, Levels and Temporal Development; Norwegian Institute of Water Research: Oslo, Norway, 2004; p. 57. [Google Scholar]
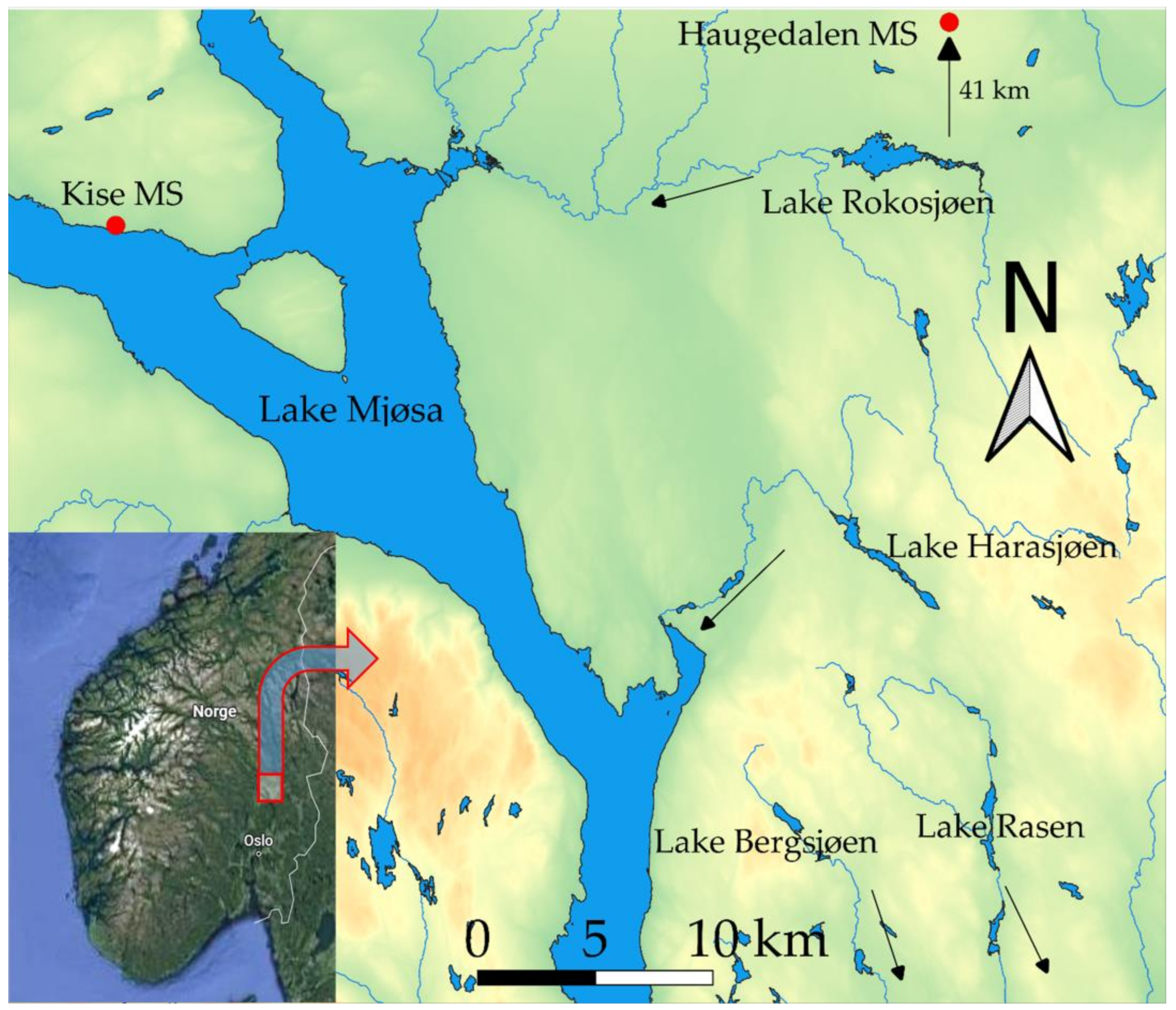

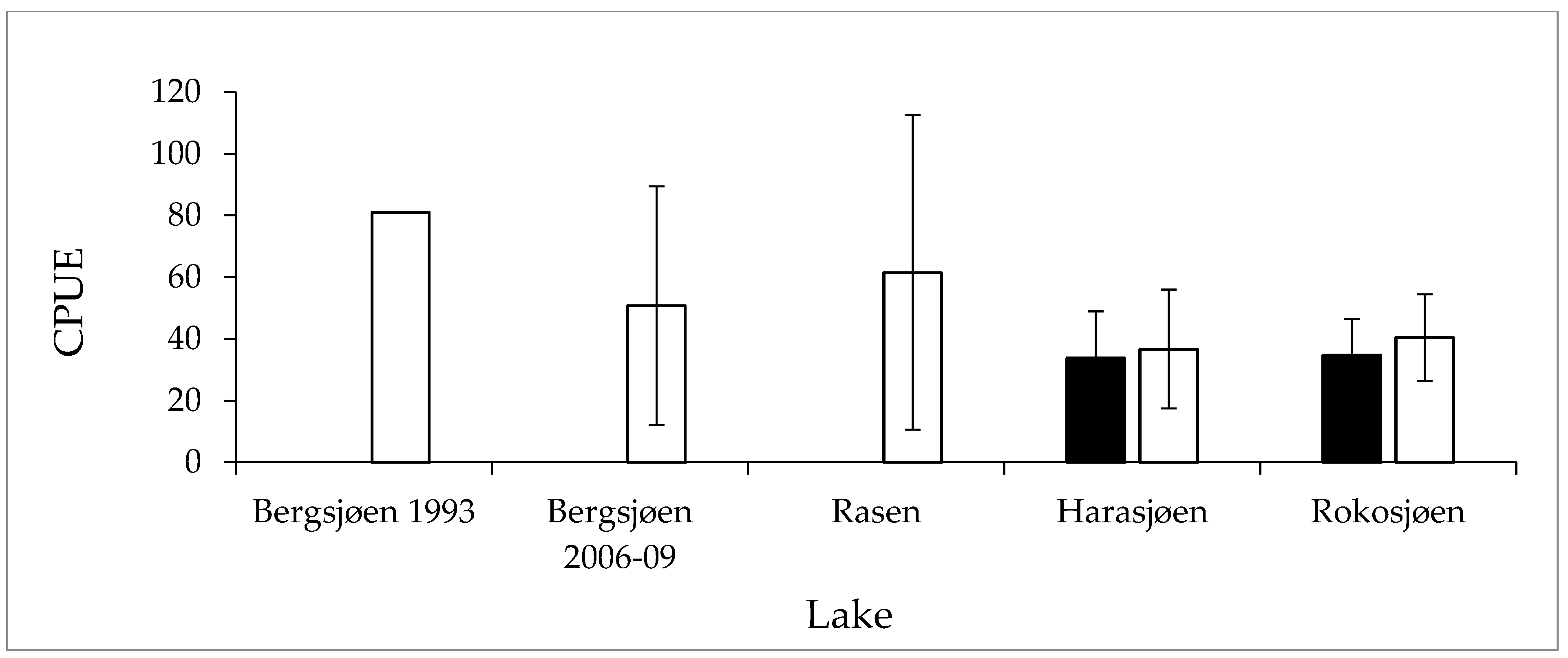
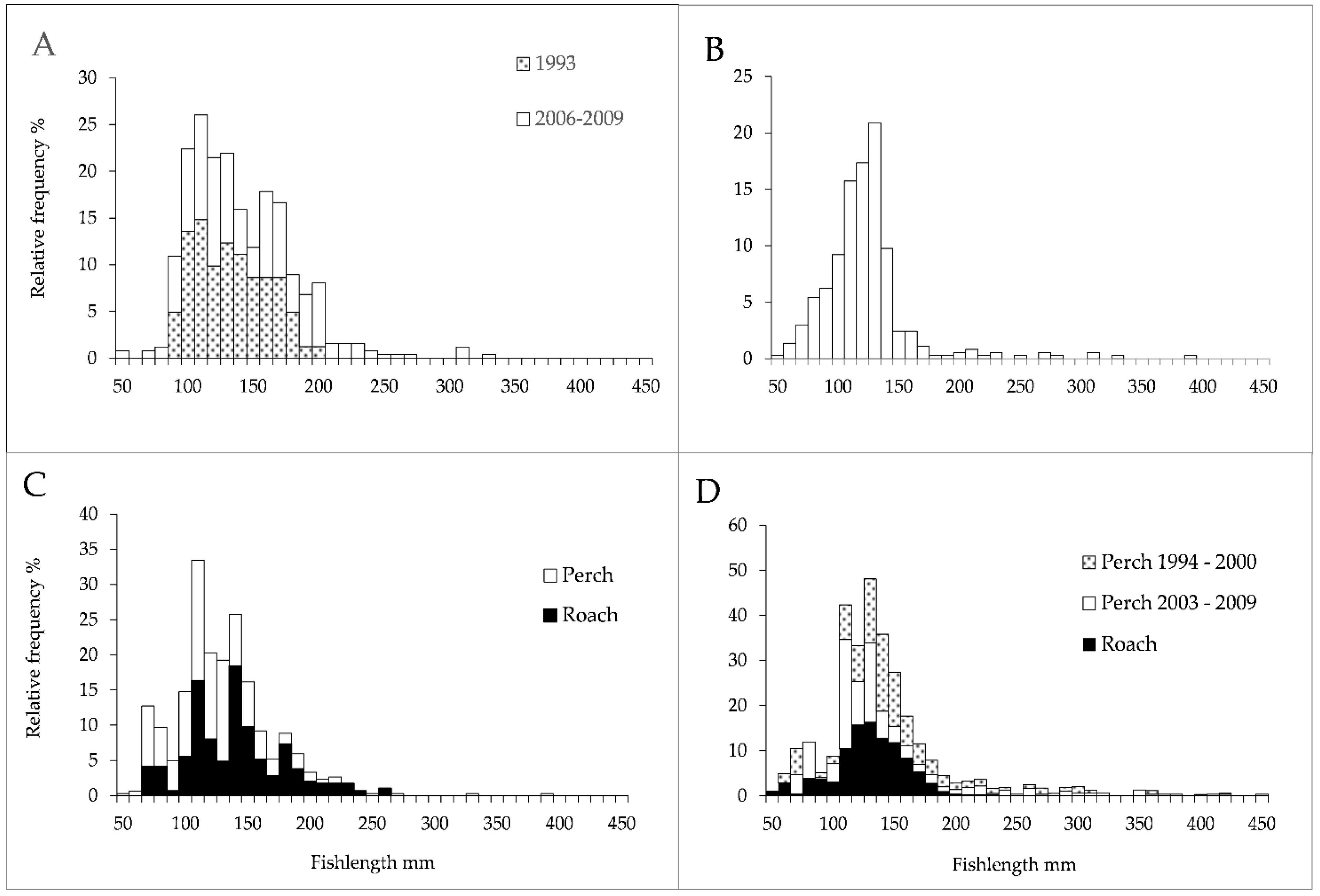
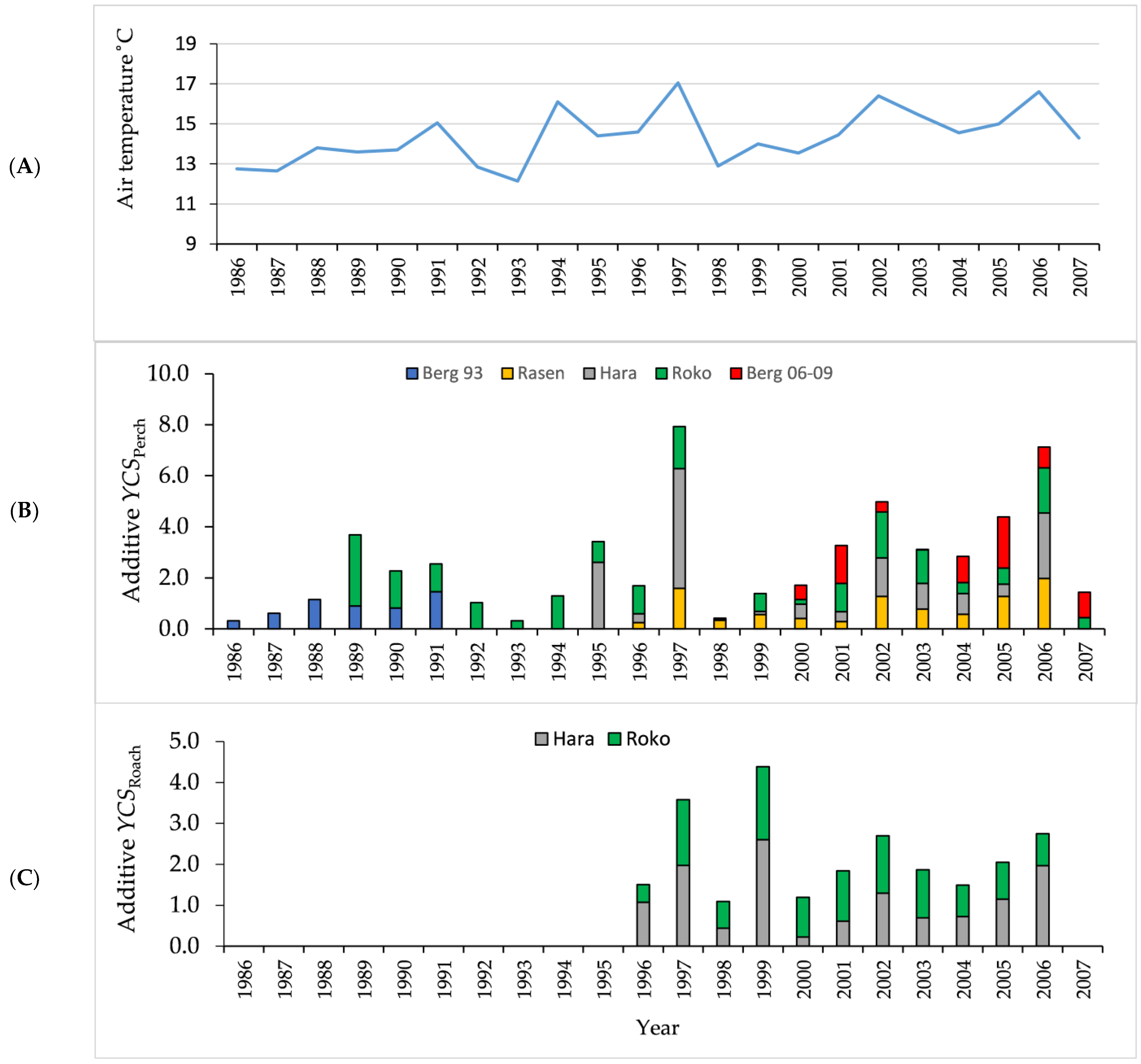


| Lake | Area | Altitude | Depthmax | pH | Conductivity | Color | Tot P |
|---|---|---|---|---|---|---|---|
| ha | m o.s.l. | m | mS m−1 | mg Pt/L | µg L−1 | ||
| Bergsjøen before liming | 57 | 366 | 34 | 5.17 | 2.1 | 45 | - |
| Bergsjøen after liming | 6.54 | 3.0 | 56 | 8 | |||
| Rasen | 93 | 246 | 20 | 5.98 | 3.2 | 110 | 8 |
| Harasjøen | 182 | 280 | 30 | 6.32 | 3.3 | 70 | 10 |
| Rokosjøen | 377 | 209 | 30 | 6.32 | 4.1 | 75 | 11 |
| Year | Lake Bergsjøen | Lake Rasen | Lake Harasjøen | Lake Rokosjøen | ||
|---|---|---|---|---|---|---|
| Perch | Perch | Perch | Roach | Perch | Roach | |
| 1993 | 81 | |||||
| 1994 | 119 | 35 | ||||
| 1995 | 23 | 23 | ||||
| 1996 | 46 | 59 | ||||
| 1997 | 33 | 24 | ||||
| 1998 | ||||||
| 1999 | ||||||
| 2000 | 56 | 16 | ||||
| 2001 | ||||||
| 2002 | ||||||
| 2003 | 95 | 42 | 55 | 50 | ||
| 2004 | 28 | 18 | 31 | 26 | ||
| 2005 | 47 | 31 | 33 | |||
| 2006 | 34 | 42 | 56 | 20 | 47 | |
| 2007 | 69 | 50 | 37 | 49 | ||
| 2008 | 31 | 138 | 60 | 75 | 41 | 99 |
| 2009 | 65 | 61 | 35 | 34 | 94 | 83 |
| Total | 280 | 296 | 297 | 274 | 549 | 495 |
| Lake Harasjøen | Coefficient | S.E. | t | r2 | p |
|---|---|---|---|---|---|
| Total model: Ln(GRoach) = + b1 × factor(Age) + b2 × T | AAIC = 23.86, F2,8 = 4.11 | 0.507 | 0.06 | ||
| Intercept | −3.308 | 2.121 | −1.560 | >0.05 | |
| Ln(ln(1 + YCS1+perch)) perch | −0.3581 | 0.178 | −2.017 | 0.251 | 0.08 |
| TJulAug | 0.258 | 0.141 | 1.970 | 0.256 | 0.08 |
| Lake Rokosjøen | Coefficient | S.E. | t | r2 | p |
| Total model: AIC = 11.70, F2,8 = 3.65 | 0.477 | 0.07 | |||
| Intercept | −1.385 | 1.278 | −1.083 | >0.05 | |
| Ln(ln(1 + YCS1+perch)) perch | −0.268 | 0.112 | −2.391 | 0.374 | <0.05 |
| TJulAug | 0.149 | 0.084 | 1.787 | 0.104 | >0.05 |
| (A) | |||||
|---|---|---|---|---|---|
| Coefficients | Estimate | S.E. | t-Value | R2/r2 | p |
| Model: Ln(GPerch) = +b1 × Sex + b2 × factor(Age) + b3 × T | |||||
| Lake Bergsjøen | AIC = 15.01 F5,18 = 32.64 | F5,18 = 32.64 | 0.901 | <0.001 | |
| Intercept | −2.476 | 1.094 | −2.264 | <0.05 | |
| Sex (female) | 0.245 | 0.119 | 2.052 | 0.021 | 0.07 |
| Factor (Age) | 0.839 | ||||
| Age 3+ | −0.823 | 0.156 | −5.274 | <0.0001 | |
| Age 4+ | −1.439 | 0.164 | −8.752 | <0.0001 | |
| Age 5+ | −1.717 | 0.166 | −10.337 | <0.0001 | |
| TJulAug | 0.189 | 0.070 | 2.713 | 0.041 | <0.05 |
| Lake Rasen | AIC = −16.22, F5,14 = 99.37, F5,14 = 99.37 | 0.973 | <0.00001 | ||
| Intercept | −1.441 | 0.587 | −2.456 | <0.05 | |
| Sex | 0.024 | 0.062 | 0.387 | 0.00 | >0.05 |
| Factor (Age) | 0.956 | ||||
| Age 3+ | −0.761 | 0.091 | −8.386 | <0.0001 | |
| Age 4+ | −−1.612 | 0.096 | −16.712 | <0.0001 | |
| Age 5+ | −1.879 | 0.096 | −19.475 | <0.0001 | |
| TJul | 0.116 | 0.040 | 2.887 | 0.016 | <0.05 |
| Lake Harasjøen | AIC = 35.48, F5,30 = 16.71 | 0.736 | <0.0001 | ||
| Intercept | −2.895 | 1.513 | −1.913 | >0.05 | |
| Sex | −0.136 | 0.123 | −1.101 | 0.018 | >0.05 |
| Factor (Age) | 0.670 | ||||
| Age 3+ | −08902 | 0.153 | −5.818 | <0.0001 | |
| Age 4+ | −1.024 | 0.164 | −6.231 | <0.0001 | |
| Age 5+ | −1.575 | 0.202 | −7.789 | <0.0001 | |
| TJulAug | 0.221 | 0.095 | 2.325 | 0.048 | <0.05 |
| Lake Rokosjøen | AIC = 14.93, F5,35 = 62.2 | 0.899 | <0.0001 | ||
| Intercept | −1.920 | 0.746 | −2.572 | <0.05 | |
| Sex | −0.0512 | 0.083 | −0.616 | 0.0006 | >0.05 |
| Factor (Age) | 0.8694 | ||||
| Age 3+ | −0.810 | 0.110 | −7.354 | <0.0001 | |
| Age 4+ | −1.545 | 0.108 | −14.279 | <0.0001 | |
| Age 5+ | −1.924 | 0.145 | −13.267 | <0.0001 | |
| TJulAug | 0.154 | 0.049 | 3.165 | 0.029 | <0.01 |
| (B) | |||||
| Coefficients | Estimate | S.E. | t-Value | R2/r2 | p |
| Lake Harasjøen | |||||
| Total model: AIC = −1.681, F4,14 = 28.04 | 0.889 | >0.05 | |||
| Intercept | 0.039 | 0.472 | 0.083 | ||
| Factor (Age) | 0.878 | ||||
| Age 3+ | −0.955 | 0.119 | −7.996 | <0.00001 | |
| Age 4+ | −0.984 | 0.124 | −7.913 | <0.00001 | |
| Age 5+ | −1.325 | 0.145 | −9.160 | <0.00001 | |
| TAug | 0.038 | 0.031 | 1.616 | 0.0193 | >0.05 |
| Lake Rokosjøen | |||||
| Total model: AIC = 10.19, F4,24 = 35.77 | 0.856 | <0.00001 | |||
| Intercept | −0.559 | 0.369 | −1.412 | >0.05 | |
| Factor (Age) | 0.786 | ||||
| Age 3+ | −0.610 | 0.136 | −4.474 | <0.001 | |
| Age 4+ | −1.016 | 0.140 | −7.251 | <0.00001 | |
| Age 5+ | −1.406 | 0.132 | −10.684 | <0.00001 | |
| TAug | 0.096 | 0.028 | 3.436 | 0.071 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linløkken, A.N. Temperature Effects on Recruitment and Individual Growth of Two Antagonistic Fish Species, Perch Perca fluviatilis and Roach Rutilus rutilus, from a Climate Change Perspective. Fishes 2023, 8, 295. https://doi.org/10.3390/fishes8060295
Linløkken AN. Temperature Effects on Recruitment and Individual Growth of Two Antagonistic Fish Species, Perch Perca fluviatilis and Roach Rutilus rutilus, from a Climate Change Perspective. Fishes. 2023; 8(6):295. https://doi.org/10.3390/fishes8060295
Chicago/Turabian StyleLinløkken, Arne N. 2023. "Temperature Effects on Recruitment and Individual Growth of Two Antagonistic Fish Species, Perch Perca fluviatilis and Roach Rutilus rutilus, from a Climate Change Perspective" Fishes 8, no. 6: 295. https://doi.org/10.3390/fishes8060295
APA StyleLinløkken, A. N. (2023). Temperature Effects on Recruitment and Individual Growth of Two Antagonistic Fish Species, Perch Perca fluviatilis and Roach Rutilus rutilus, from a Climate Change Perspective. Fishes, 8(6), 295. https://doi.org/10.3390/fishes8060295






