Abstract
Twenty-four adult molly fish (Poecilia sphenops, Valenciennes 1846) were collected to study the morphology and distribution of ganglia using histological, immunohistochemical, and electron microscopy and focusing on their relation to the immune cells. The ganglia were classified spatially into cranial and spinal, and functionally into sensory and autonomic. Spinal ganglia (dorsal root ganglia, DRG) contained large close ganglionic cells, enclosed by satellite cells, as well as bundles of both myelinated and non-myelinated nerve fibers. There are glial cells, immune cells and telocytes close to the ganglion. In addition, oligodendrocytes were closely related to myelinated axons. Glial fibrillary acidic protein (GFAP) expression was confined to the glia cells and the nerve fibers in the cervical ganglia next to the gills, and surprisingly, in the large ganglionic cells of the DRG. The vestibular ganglia were large, connected to the hind brain, and contained numerous neurons packed in columns. The cervical ganglia were large and observed around the pseudobranch, head kidney, and thymus. Their neurons are randomly distributed, and nerve fibers are peripherally situated. CD3-positive T-lymphocytes, dendritic cells, and CD68-positive macrophages were in close contact with the ganglia. Furthermore, the ganglia around the head kidney showed positive Iba1-expressing cells. Most ganglion cells and nerve fibers in the DRG, autonomic, and vestibular ganglia showed moderate to strong S-100 immunoreactivity. The enteric glia, CD68-expressing macrophages, and acetylcholine (Ach)-expressing neurons were observed along the muscular layer of the intestinal wall. In conclusion, different ganglia of molly fish displayed direct communication with immune cells which support and maintain healthy ganglionic cells.
Key Contribution:
The ganglia of molly fish were classified spatially into cranial and spinal, and functionally into sensory and autonomic. These ganglia displayed direct communication with immune cells which support healthy ganglionic cells.
1. Introduction
A ganglion is a cluster or group of nerve cells found in the peripheral nervous system (PNS) [1]. One characteristic that distinguishes vertebrates is the presence of neural crest cells. These multipotent neural crest cells can differentiate into a wide range of tissues and their derivatives, including pigment cells, sensory organs, peripheral ganglia, cartilage, and bone. The sensory and sympathetic ganglia, the adrenal medulla, and melanocytes all develop from the trunk neural crest [2]. The peripheral nervous system (PNS) is made up of the dorsal root ganglia, autonomic ganglia (parasympathetic, sympathetic ganglia), and enteric ganglia [3]. PNS is composed of clusters of neurons and nerves that penetrate every part of the body and transmit sensory impulses to the brain and spinal cord via afferent pathways, where they are then processed into efferent impulses that affect tegumental, glandular, or muscular structures [4].
Involuntary physiological activities including heart rate, breathing, digestion, and sexual arousal are controlled by the autonomic nervous system, a part of the peripheral nervous system. It has three anatomically and functionally distinct divisions: sympathetic, parasympathetic, and enteric. The sympathetic and the parasympathetic nervous system (PNS) contain both afferent and efferent fibers that provide sensory input and motor output, respectively, to the central nervous system (CNS) [5]. The sympathetic division often operates in situations where quick reactions are necessary. Actions that do not demand an immediate reaction are carried out by the parasympathetic division. The parasympathetic system is frequently thought of as the “rest and digest” system, whereas the sympathetic system is commonly regarded as the “fight or flight” system [6]. The sympathetic ganglia are peculiar to teleosts among vertebrates since they are situated in the cranium, and the postganglionic sympathetic fibers connect with the trigeminal, facial, glossopharyngeal, and vagus nerves to innervate numerous effector cells in the branchial tissues, heart, and stomach. The cranial sympathetic ganglia receive the preganglionic inputs from the spinal nerves. Generally, PNS includes neurons, Schwann cells, and satellite glial cells (SGCs). Each of these cells is recognized by specific protein markers that can also define their function [7]. Comparable to other vertebrates, fish also rely substantially on the activation of intrinsic neuronal circuitries that make up the enteric nervous system (ENS) to regulate the main digestive functions. The ENS is organized into two major plexuses, the submucosal plexus and myenteric plexus [8]. The ENS is frequently referred to as “the second brain” or “the intestinal brain” due to its sophisticated organization, large number of neural structures, and high degree of independence from the central nervous system [9]. Together, the immunological and nervous systems constitute an integrated physiological system. Gut neuropeptides, including gut antimicrobial neuropeptides, form a complex network between the nervous and immune systems, where they play a critical modulatory [10].
The molly fish (Poecilia sphenops) is a freshwater teleost. It lives naturally in Mexico and Colombia’s freshwater rivers. It is widely known that this fish is an herbivore that can grow swiftly and mostly consumes algae and other plant materials. In recent years, mollies have been frequently used in experimental studies [3].
As a result, the current study used histological, immunohistochemical, and electron microscopy studies to investigate the distribution and morphology of the ganglia throughout the body of the molly fish as well as the potential neuro-immune interaction.
2. Materials and Methods
The current work was performed in accordance with Egyptian laws and university guidelines for animal care. The National Ethical Committee of the Faculty of Veterinary Medicine, Assiut University, Egypt, has approved all the procedures in this study. The Ethical No. is aun/vet/4/0015.
2.1. Sample Collection
The materials employed in this study consisted of randomly obtained 24 adult molly fish (Poecilia sphenops, Valenciennes 1846). The fish were purchased from an ornamental fish shop. The specimens were 4.40 ± 4.0 cm in standard length and 10.20 ± 1.50 gm in body weight.
2.2. Histological and Histochemical Analysis
Tissues were dissected from fish immediately after death at 1 × 1 × 0.5 cm and fixed in Bouin’s fluid for 22 h. The fixed tissues were dehydrated with ethanol and cleared by methyl benzoate, then embedded in paraffin wax. Serial sagittal and transverse (5 μm thick) paraffin sections were taken and stained with Harris hematoxylin and Eosin, Grimilus silver stain, and Cresyl violet [11].
2.3. Semithin Sections and TEM
Small specimens of the ganglia were fixed in a solution of 2.5% paraformaldehyde- glutaraldehyde and left overnight for fixation. Then, they were washed in 0.1 Mol/L phosphate buffer and osmicated with 1% osmium tetroxide in 0.1 Mol/L sodium-cacodylate buffer at pH 7.3. After that, the specimens were dehydrated by ethanol followed by propylene oxide and embedded in Araldite. One micrometer-thick semithin sections were stained with toluidine blue and examined under a light microscope. Ultrathin sections (70 nm) were obtained using Ultrotom-VRV (LKB Bromma, Stegen, Germany) and were stained with lead citrate and uranyl acetate. TEM images were captured with JEOL-100CX II electron microscope.
2.4. Immunohistochemical Analysis
Sections of fish were prepared for immunohistochemical analysis using an UltraTek HRP Anti-Polyvalent (DAB) Staining System (ScyTek Laboratories, West Logan, UT, USA, AMF080). The sections were deparaffinized with xylene, rehydrated in graded ethanol, and washed with distilled water. The sections were heated for 10 min in a sodium citrate buffer (0.01 M, pH 6.0) to increase epitope exposure. The sections were cooled at room temperature for 30 min and washed with PBS. The endogenous peroxidase activity was quenched with 3% H2O2 in distilled water for 15 min at RT followed by washing with PBS (2 × 5 min). The sections were blocked with the blocking solution of the kit for 5 min at RT. The sections were incubated overnight at 4 °C with the diluted (1:100) primary antibodies against S100 protein (Z0311, Dako, Glostrup, Denmark), rabbit polyclonal anti-CD3 (1:200; Abcam, Cambridge, UK, ab828), mouse monoclonal anti-CD68 (1:100; Santa Cruz, sc-17832), mouse monoclonal anti-Olig2 (1:100; Santa Cruz, Dallas, TX, USA, sc-515947(, rabbit polyclonal Nicotinic Acetylcholine R alpha 7 NACHRA7 (1:100; ABclonal, Wuhan, China, A7844), and the polyclonal glial fibrillary acidic protein (GFAP) (PA5-16291, Thermo Fisher Scientific, Waltham, MA, USA). In parallel, tissue specimens, in which S100 protein primary antibody was omitted and replaced with buffer, served as negative controls (Figures S1 and S2). Sections were rinsed (three times and 5 min each) with PBS and were incubated for 15 min with the secondary Ultra Tek HRP Anti-polyvalent antibody (goat anti-mouse, rat, rabbit and guinea pig IgG) purchased from Scy Tek, (TX, USA). Following that, the slides were washed three times for 3 min each with a wash buffer, and the tissues were incubated with the HRP for 15 min and then washed three times for 3 min each with a wash buffer. The visualization of the reaction was carried out with Diaminobenzodiazibin DAB chromogen diluted with DAB substrate (provided within the same Scy Tek Detection kit) according to the manufacturer protocol for 10–15 min until the desired staining was achieved and counterstained with Harris hematoxylin and mounted with mounting media, DPX.
3. Results
The results are summarized in Table 1. The ganglia were distributed all over the body of molly fish that could be classified according to location into spinal ganglia, cranial ganglia, cervical, and enteric ganglia. Spinal ganglia appear as swellings in the dorsal roots of the spinal nerves, close to the spinal cord (Figure 1A,B). Microscopically, the cell bodies of spinal ganglia neurons are characterized by large sizes, close together and enclosed by small nuclei of the flattened supporting satellite cells (Figure 1C). The neurons showed a positive reaction for cresyl violet (Figure 1D). The semi-thin section stained with toluidine blue showed that the ganglion is ensheathed by a capsule of dense connective tissue that branches internally into trabeculae to give a framework for the neurons. It was possible to see immune cells and telocytes close to the ganglion (Figure 1E). The cell bodies of this neuron were round to oval in shape with round vesicular nuclei. The cytoplasm of these neurons contained large amounts of Nissl’s granules. The cell body of neurons was enveloped with satellite glial cells (SGC) (Figure 1E).

Table 1.
Summary of the results of cellular elements of different ganglia in molly fish.
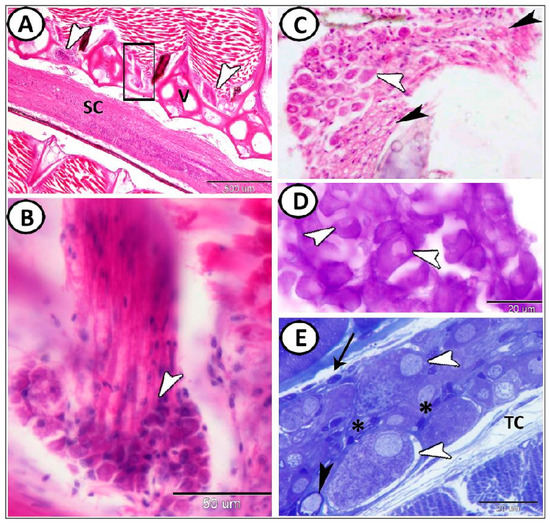
Figure 1.
Spinal ganglia in molly fish. (A,B): Spinal ganglia (arrowheads) between the vertebrae (V), and over the spinal cord (SC). Note that image (B) is a higher magnification of the boxed area in (A). (Hematoxylin and Eosin (HE)). (C) Cell bodies of the neurons (white arrowhead) are close together and surrounded by nerve fibers (black arrowheads) (HE). (D) The neurons (white arrowheads) are positive for cresyl violet. (E) Semithin section stained with toluidine blue showed neurons contained Nissl’ granules (white arrowheads) surrounded by small nuclei (asterisks) of the flattened satellite cells. Some myelinated nerve fibers were observed (black arrowhead). Note the presence of telocytes (TC) and some immune cells (arrow).
Bundles of both myelinated and non-myelinated nerve fibers could be demonstrated predominantly in the center of the ganglion (Figure 2A,B). Moreover, large ganglionic cells were demonstrated in the DRG which were arranged in rows (Figure 2A,B). Schwann cells ensheathed myelinated nerve fibers were distinct (Figure 2B). SGCs could be also observed around the neurons (Figure 2C). By electron microscopy, the ganglion cells showed large round cell bodies with large nuclei and distinct nucleolus. The cytoplasm of ganglion cells contained large amounts of rER, ribosomes, and mitochondria (Figure 2C). We observed that the satellite glia cells were completely enveloping the ganglion cell body. It has a heterochromatic nucleus and it sends cell processes that contained mitochondria (Figure 2C).
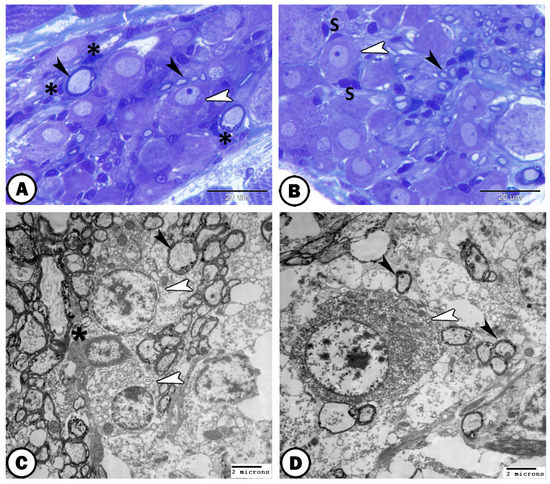
Figure 2.
Semithin section and ultrastructure of ganglia. (A,B) Semithin section stained with toluidine blue showing the structure of the spinal ganglia that contained large neurons (white arrowheads) and nerve fibers (black arrowheads). Note the presence of Schwann cells (asterisks) and satellite glia cells (S). (C) TEM images show oligodendrocytes (white arrowheads) that possess a large round euchromatic nucleus tending to be eccentrically located in the cytoplasm, and myelinated nerve fibers (black arrowhead). Note the surrounding satellite cells (asterisk). (D) TEM images showing neurons (white arrowhead) filled with Nissl’s granules. Note the surrounding myelinated nerve fibers (black arrowhead).
Myelinated axons in both longitudinal and cross sections can be demonstrated within the ganglion (Figure 2C,D). It was possible to see that oligodendrocytes were closely related to myelinated axons. They were characterized by their large vesicular nucleus and sparse cytoplasm, which included ribosomes and mitochondria (Figure 2C).
GFAP (glial fibrillary acidic protein) expression could be observed in both satellite glia cells and nerve fibers (Figure 3A,B). Surprisingly, the expression of GFAP could be detected in the large ganglion cells of the DRG (Figure 3C,D).
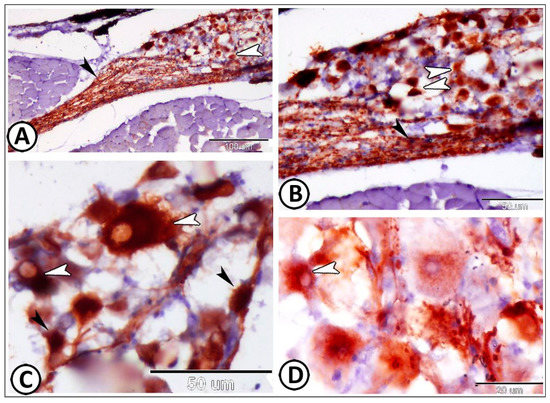
Figure 3.
GFAP expression in spinal ganglia. (A,B) GFAP expression could be observed in both satellite glia cells (white arrowheads) and nerve fibers (black arrowheads). (C,D) GFAP could be detected in the large ganglion cells of the DRG (white arrowheads). Note that satellite glia cells expressed GFAP (black arrowheads).
However, the expression of GFAP was confined to the glia cells (Figure 4A–C) and the longitudinally oriented nerve fibers in the cervical ganglia that were observed near the gills (Figure 4D,E). The enteric glia could be observed along the muscular layer of the intestinal wall. The nerve fibers and these glia cells showed strong immunoreactivity for GFAP (Figure 5A–D).
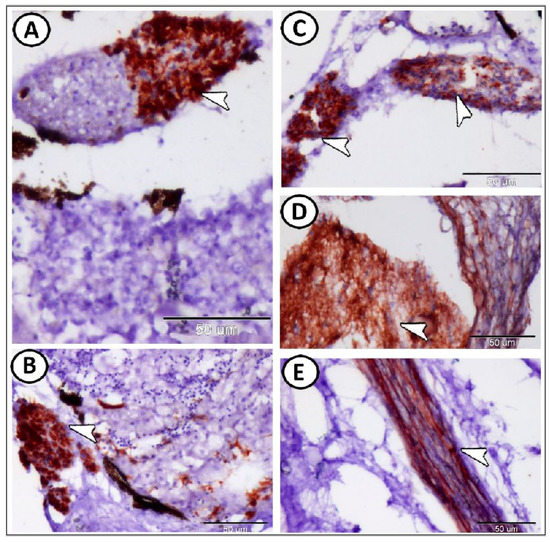
Figure 4.
GFAP expression in cervical ganglia. (A–C) The expression of GFAP was confined to the glia cells (arrowheads). (D,E) GFAP was expressed in the longitudinally oriented nerve fibers in the cervical ganglia that were observed near the gills (arrowheads).
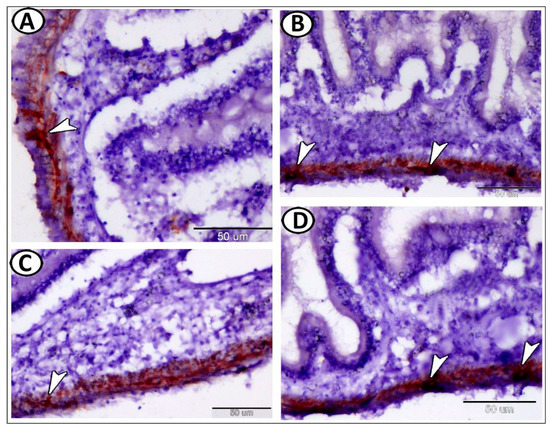
Figure 5.
GFAP expression in the enteric glia of the intestine. (A–D) The nerve fibers and ganglia cells showed strong immunoreactivity for GFAP (arrowheads).
Macrophages expressed CD68 around the glia cells in the tunica muscularis of the intestine (Figure 6A,B). In addition, Ach was expressed in the neurons in the intestinal muscularis layer (Figure 6C,D).
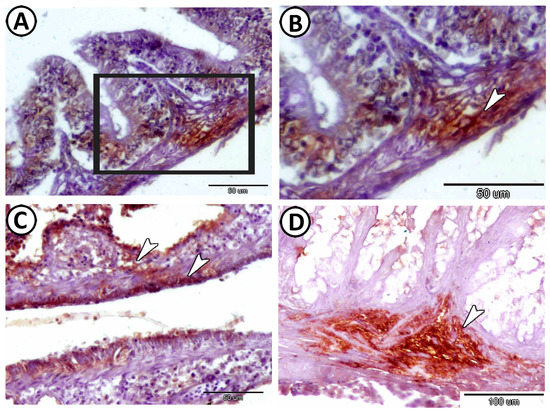
Figure 6.
Expression of CD68 and acetylcholine (Ach) in the intestine. (A,B) Macrophages expressed CD68 around the glia cells in the tunica muscularis of the intestine (arrowheads). Note that image (B) is a higher magnification of the boxed area in (A). (C,D) Ach was expressed in the neurons in the intestinal muscularis layer (arrowheads).
The vestibular ganglia were observed close to the hind brain (Figure 7A). They are the largest ganglia with the vestibular ganglion cells arranged in numerous compact columns (Figure 7B). The vestibular ganglion cells are either ovoid or round and have central or eccentric nuclei. These cells vary considerably in size and a few glial cells were distributed between them. The nerve fibers are scanty within the vestibular ganglion (Figure 7C,D).
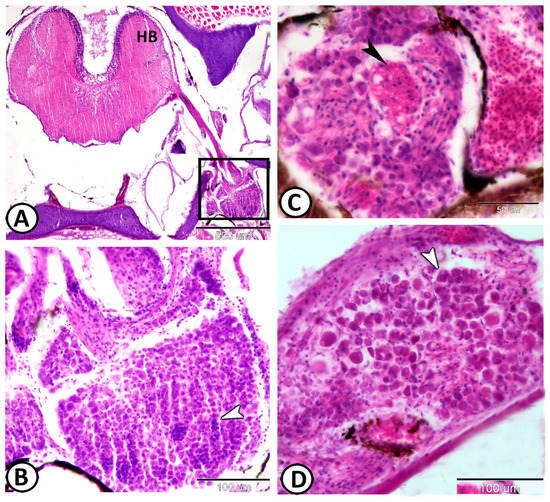
Figure 7.
Vestibular ganglia stained with HE. (A) Ganglia (boxed area) in the hindbrain (HB). (B) A higher magnification of the boxed area in (A) shows a large cluster of neuronal cell bodies in the head of molly (arrowhead). (C,D) The nerve fibers (black arrowhead) are arranged in between the neurons (white arrowhead).
The cervical ganglia were large ganglia observed around the pseudobranch (Figure 8), head kidney, and thymus (Figure 9). These are characterized by randomly distributed neurons and peripherally situated nerve fibers (Figure 8A–E). The ganglia located beside the immune organs (thymus and head kidney) show abundant neurons (Figure 9A–C).
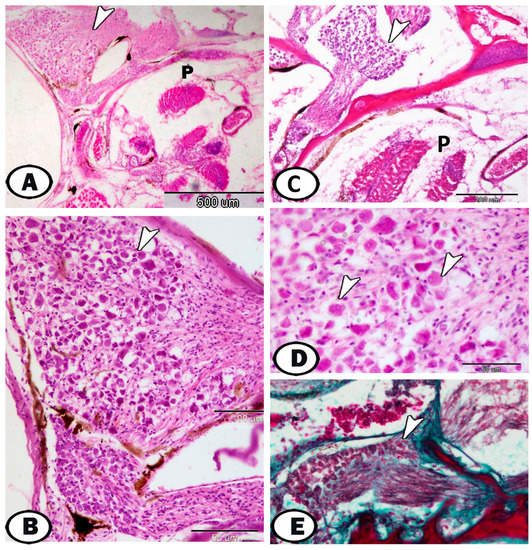
Figure 8.
Cervical ganglia around the pseudobranch. (A–C) The cervical ganglia (arrowheads) are observed around the pseudobranch (P) (HE). (D) Higher magnification showed the random distribution of neurons (arrowheads) (HE). (E) Ganglia stained with Crossmon’s trichrome showed neurons and peripherally situated nerve fibers (arrowhead).
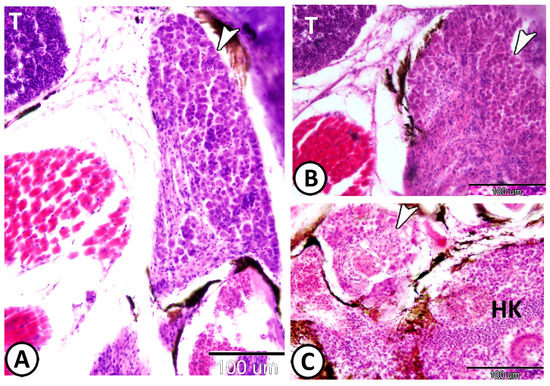
Figure 9.
Cervical ganglia around thymus and head kidney stained with HE. (A,B) The cervical ganglia (arrowhead) are observed around the thymus (T). (C) The ganglia (arrowhead) beside the head kidney (HK).
The ganglia along the trunk kidney exhibit cell bodies more widely spaced than in spinal and vestibular ganglia because of the presence of numerous neurites (axons and dendrites) in between (Figure 10A–D) that most of them showed positive reaction for silver stain (Figure 10E). The semi-thin sections to these ganglia revealed the presence of many types of immune cells around the ganglia. Numerous lymphocytes, dendritic cells, and macrophages could be identified in close contact with the ganglia (Figure 11A–C). Moreover, telocytes with distinct telopodes were oriented around the ganglia (Figure 11D). These distributed T-lymphocytes around the ganglia expressed CD3 (Figure 12A,B).
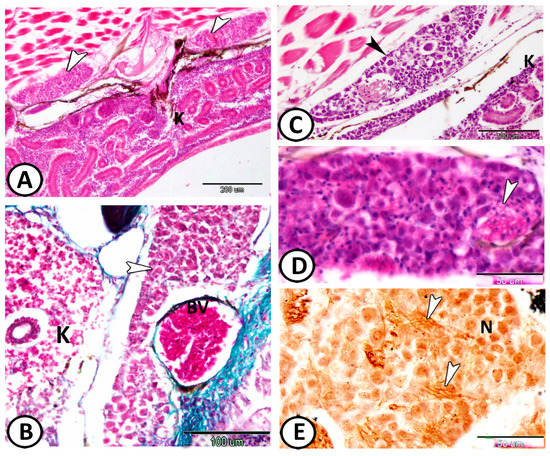
Figure 10.
The ganglia along the trunk kidney. (A,B) The ganglia (arrowheads) are along the trunk kidney (K). Note the presence of a large blood vessel (BV) (HE, Crosmmon’s trichrome, respectively). (C,D) These ganglia exhibit cell bodies (black arrowhead) more widely spaced than in spinal and cranial ganglia because of the presence of numerous neurites (white arrowhead) in between. (E) The neurons (N) and nerve fibers (arrowheads) showed a positive reaction to the silver stain.
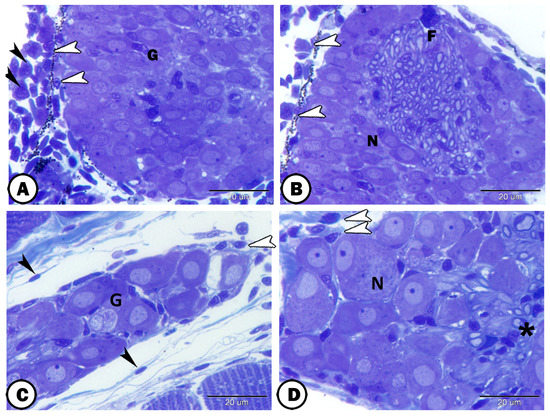
Figure 11.
Semithin section of the ganglia in association with immune cells. (A,B) Ganglia (G) were observed in neighboring to lymphocytes (white arrowheads) and macrophages (black arrowheads). These ganglia consisted of neurons (N) and nerve fibers (F). (C) Telocytes (black arrowheads) and dendritic cells (white arrowhead) are observed around the ganglia (G). (D) Dendritic cells (arrowheads) are seen in close contact with the neurons (N). Note the satellite glia cells (asterisk).
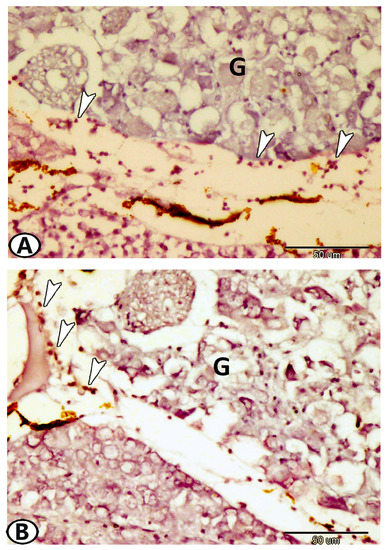
Figure 12.
Expression of CD3. (A,B) T-lymphocytes (arrowheads) expressed CD3 around the ganglia (G).
Additionally, the macrophages expressed CD68 that were distributed around the ganglia of the kidney (Figure 13A) and ovary (Figure 13B). On the other hand, the neurons and nerve fibers in the ganglia around the trunk kidney expressed Ach (Figure 13C,D). Furthermore, the ganglia around the head kidney showed positive expression of Iba1 in microglia and macrophages around the ganglia (Figure 14D). Most ganglion cells and nerve fibers in the DRG, autonomic, and vestibular ganglia showed moderate to strong immunoreactivity for S-100 protein (Figure 15).
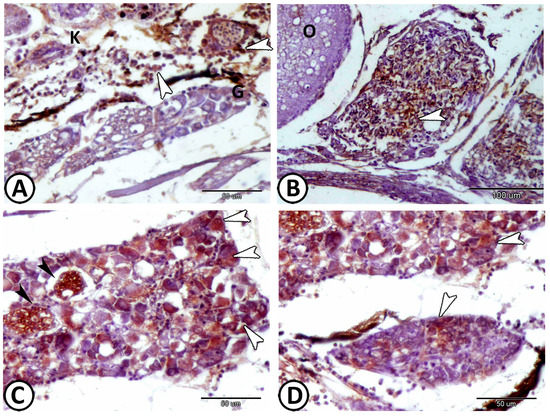
Figure 13.
Expression of CD68 and Ach. (A,B) The macrophages (arrowheads) expressed CD68 that are distributed around the ganglia (G) of the kidney (K) and ovary (O). (C,D) The neurons (white arrowheads) and nerve fibers (black arrowheads) in the ganglia around the trunk kidney expressed Ach.
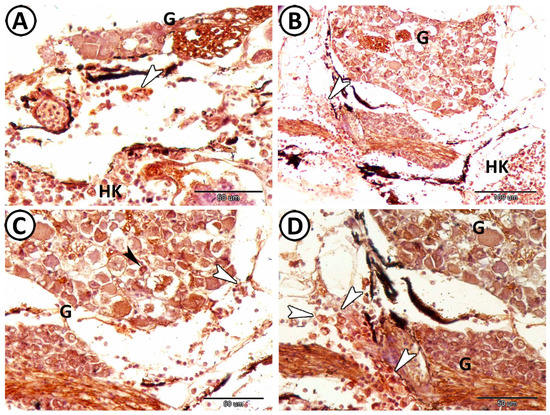
Figure 14.
Expression of Iba1 in microglia of the ganglia and macrophages. (A,B) The ganglia (G) around the head kidney (HK) showed macrophages (arrowheads) that expressed Iba1, which were distributed around the neurons in the ganglia. (C,D) Higher magnifications showed positive expression of Iba1 in microglia (black arrowhead) and macrophages (white arrowheads) around the ganglia (G).
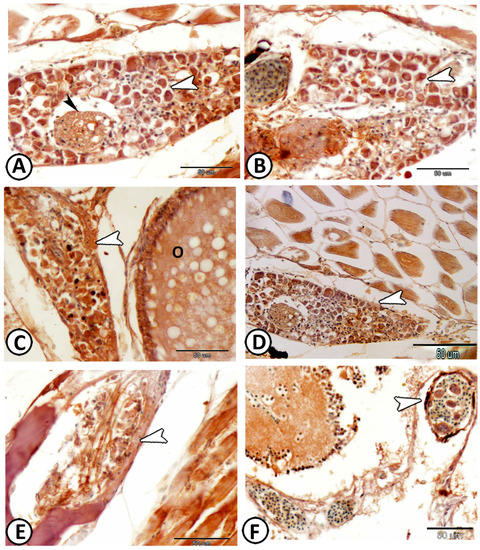
Figure 15.
Expression of s100 protein in the ganglia. (A,B) The ganglion cells (white arrowheads) and nerve fibers (black arrowhead) expressed s100 protein in the ganglia around the kidney. (C) The ganglia (arrowhead) expressed s100 protein around the ovary (O). (D,E) DRG ganglia showed moderate to strong immunoreactivity for S-100 protein (arrowheads). (F) Cranial ganglia (arrowhead) expressed s100 protein.
4. Discussion
In the current work, we used histomorphological, immunohistochemical, and ultra-structural approaches to characterize the ganglion throughout the body of the molly fish. We were able to identify a variety of ganglion types observed in molly fish, such as the DRG, enteric ganglia, and autonomic ganglia. The precise coordination of the various cellular components that make up the gut wall requires the enteric nervous system (ENS), which provides local control of the gastrointestinal tract. In most fish species ENS is capable of carrying out a number of CNS-independent tasks, including gut peristalsis, homeostasis, hormone secretion from the stomach, and osmoregulation [12,13]. In mammals, the enteric ganglia are made up of two major layers: an outer myenteric plexus and an inner submucosal plexus [14]. However, Zebrafish and the currently investigated molly fish only have one myenteric plexus [15]. ENS ganglia exist as a collection of neurons and glia that are arranged in a series of plexuses throughout the gastrointestinal tract. The main enteric nerve cell types, sensory, inter, and motorneurons, are located in the myenteric plexus [16]. Enteric glia, astrocyte-like cells that surround enteric neurons, regulate enteric neurons’ output. Indeed, there are molecular similarities between astrocytes and enteric glia as they both express similar proteins, such as the intermediate filament glial fibrillary acidic protein (GFAP), and have similar electrophysiological properties [17]. GFAP is a major constituent of glial intermediary filaments that are expressed in astrocytes of the central nervous system and in the peripheral nervous system, and enteric glial cells [18,19]. In mammals and birds, glial cells outnumber neurons by an average of up to four.
Glial cells have a variety of functions in the mammalian gut; including protecting and supporting the neurons and they can also participate in neurotransmission [20]. Recent investigations have shown putative glial cells which are immunoreactive to GFAP in the gut of various teleost species [21]. In fish, the ENS ganglia contain neurons that are sparsely distributed along the surface of the gut and these neurons do not cluster to form ganglia as mammals [16]. The same observation has been reported in zebrafish [22] and the currently investigated study. We investigated that these neurons express the neurotransmitter choline acetyltransferase (ChAT). Nonetheless, in zebrafish, the enteric neurons express several neurochemical and neurotransmitters markers; including serotonin (5HT), tyrosine hydroxylase (TH), vasoactive intestinal peptide (VIP), calbindin (CB), calretinin (CR), choline acetyltransferase (ChAT) and nitric oxide (NO) [23,24,25,26]. Both sympathetic and sensory ganglia contain satellite glia and Schwann cells, two prominent glial cell types that are closely related to their neuronal neighbors and significantly affect a wide range of neuronal processes [27]. Schwann cells are connected to peripheral axons and play well-known functions in myelination, axon regeneration, the trophic and metabolic support of neurons [28,29].
The sensory ganglia found in the dorsal roots of the spinal cord are made up of afferent neurons, and satellite glial cells (SGCs) that encase them [30]. SGCs and DRG neurons are intimately associated, forming a unique structural unit that supports strong bidirectional communication. SGCs control the ionic and neurotransmitter concentrations, stimulate neuronal morphogenesis, control synaptic transmission, and serve as a functional substitute for the deficient blood-brain barrier, just as astrocytes do in the CNS [30,31,32,33]. Additionally, they are capable of forming perikaryal myelin sheaths and even participating in phagocytic activity, which is characteristic of oligodendrocytes and microglia, respectively [34,35]. In the neurons of the DRG and sympathetic ganglia of molly fish, we demonstrated the expression of S-100 protein, which constitutes the largest subgroup of calcium-binding proteins. A similar observation has been shown in a subpopulation of sensory and sympathetic neurons, and it was not observed in the enteric nervous system of adult zebrafish [36]. Since S-100 protein has primarily been found in the satellite glial cells of both sympathetic and sensory ganglia in higher vertebrates, especially mammals, the expression of S-100 protein in molly fish and zebrafish clearly differs from that in these species [37].
It is suggested that the immune system of zebrafish is very close to mammals and has innate and adaptive immune cells such as B cells, T cells, macrophages, and neutrophils. In addition to immune mediators like cytokines and complement proteins [38]. Moreover, the sensory ganglia, including the DRG, host several types of immune cells allowing for local neuroimmune communication which is important for neuropathic pain [39]. It is well-known that in most vertebrates, the immune system contributes to the normal nervous system development from cellular to behavioral levels. At the cellular level, neuronal apoptosis is a critical mechanism promoting normal neural connectivity in the development of the nervous system [40].
A study investigating human ganglia found a population of local macrophages around 5–20% of the total cells present. In addition to low numbers of CD3 and CD8 lymphocytes were detectable in sensory and autonomic ganglia [41]. It was observed that human trigeminal ganglia-resident SGC (TGSGC) equally expressed the common leukocyte marker CD45 and the macrophage markers CD14, CD68, and CD11b, as well as dendritic cell (DC) marker CD11c, the T-cell costimulatory molecules CD40, CD54, CD80, and CD86 and MHC class II [34].
A study using zebrafish embryos as a model host for Mycobacterium lepra (M. leprae) infection detected bacterial, glial, neural and immune cell interactions during M. leprae infection. M. leprae were able to alter the myelin structure of glial cells surrounding axons within the spinal cord by stimulating macrophages production of reactive nitrogen species causing mitochondrial and axonal damage in both myelinated and non-myelinated axons [42].
Dendritic cells are part of innate immune cells that play a key role in linking innate and adaptive immunity. They recognize and respond to pathogen-associated and danger-associated signals, and shape the acute inflammatory response [43]. It was found that following peripheral nerve injury, there is an accumulation of dendritic cells (DCs) within the dorsal root leptomeninges (DRLs).
A study in mice infected with Herpes simplex virus type 1 (HSV-1), which is a type of infection, is controlled mainly by the immune response within the trigeminal ganglia (TG). This study results that dendritic cells (DCs) and macrophages were the key sources of Interleukin 1 beta (IL-1β) and Inducible nitric oxide synthase (iNOS), respectively which both are essential for the immune response against HSV-1 [44].
Several studies indicated several functions of T-cells in fish, such as activating macrophages to initiate their microbicidal activity and B-cells to produce antibodies, in addition to enhancing cell-mediated immunity [45]. A study characterized the immune response in ganglia after infection of primary simian varicella virus (SVV) in African green monkeys (AGMs) found that local reduction in viral load within ganglia was correlated with increased infiltrating of T cells, suggesting intragSupplanglionic immunity involves controlling SVV proliferation [46].
We noted that the vestibular ganglion in molly fish is large and the vestibular ganglion cells arranged in a very compact, linearly arranged mass of cells similar to that described in cats [47]. The large size of the ganglion and the high density of its cells is related to a greater number of hair cells in the inner ear, a larger nerve and a greater size of the primary central area [48]. It has been reported from a pioneer study on cats, guinea pigs and squirrel monkeys that each cell size receives fibers from specific parts of the vestibular sensory regions [49]. Upon injury, monocyte/macrophage /microglia are recruited to the ganglia. Both M1 and M2 macrophages, which secrete IL-1β and Arginase 1, respectively, were reported [50].
5. Conclusions
Different ganglia of molly fish displayed direct communication with immune cells which support and maintain healthy ganglionic cells. This study enhances our understanding of the possible cells underlying the normal support and the possible peripheral neuropathy events following injury or infection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8060289/s1, Figures S1 and S2: Negative controls.
Author Contributions
Conceptualization, methodology, formal analysis, data curation, D.M.M., A.A. (Abdelraheim Attaai) and M.T.H.; resources, M.T.H.; writing—original draft preparation, D.M.M., A.A. (Abdelraheim Attaai) and M.T.H.; writing—review and editing, G.Z., A.A. (Alessio Alesci), and R.A.; validation, M.T.H.; investigation—supervision, D.M.M. and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Researcher Supporting Project, Number (RSPD2023R591), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The animal study protocol was approved by the National Ethics Committee of the Faculty of Veterinary Medicine, Assiut University, Egypt, has approved all the procedures in this study. Approval code: aun/vet/4/0015. Approval date: 6 July 2022.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors thank the technical support provided from the department of cell and tissues, Faculty of Veterinary medicine, and Electron Microscopy unit in Assiut University, Egypt.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Crane, J.F.; Trainor, P.A. Neural crest stem and progenitor cells. Annu. Rev. Cell Dev. Biol. 2006, 22, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Bronner, M.E. Formation and migration of neural crest cells in the vertebrate embryo. Histochem. Cell Biol. 2012, 138, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Osório, J.; Rétaux, S. The lamprey in evolutionary studies. Dev. Genes Evol. 2008, 218, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Jänig, W. Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Jänig, W.; Häbler, H.-J. Chapter 25—Specificity in the organization of the autonomic nervous system: A basis for precise neural regulation of homeostatic and protective body functions. In Progress in Brain Research; Mayer, E.A., Saper, C.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 122, pp. 351–367. [Google Scholar]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Microb. Endocrinol. Microbiota-Gut-Brain Axis Health Dis. 2014, 817, 39–71. [Google Scholar]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected roles for the second brain: Enteric nervous system as master regulator of bowel function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef]
- Aresti Sanz, J.; El Aidy, S. Microbiota and gut neuropeptides: A dual action of antimicrobial activity and neuroimmune response. Psychopharmacology 2019, 236, 1597–1609. [Google Scholar] [CrossRef]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed; Churchill Livingstone Pub: Edinburgh, Scotland, 2002; Volume 172, pp. 593–620. [Google Scholar]
- Furness, J.B. The enteric nervous system: Normal functions and enteric neuropathies. Neurogastroenterol. Motil. 2008, 20 (Suppl. S1), 32–38. [Google Scholar] [CrossRef]
- Burns, A.J.; Pachnis, V. Development of the enteric nervous system: Bringing together cells, signals and genes. Neurogastroenterol. Motil. 2009, 21, 100–102. [Google Scholar] [CrossRef]
- Wallace, A.S.; Burns, A.J. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005, 319, 367–382. [Google Scholar] [CrossRef]
- Wallace, K.N.; Akhter, S.; Smith, E.M.; Lorent, K.; Pack, M. Intestinal growth and differentiation in zebrafish. Mech. Dev. 2005, 122, 157–173. [Google Scholar] [CrossRef]
- Olsson, C. Autonomic innervation of the fish gut. Acta Histochem. 2009, 111, 185–195. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980, 286, 736–737. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; Apple Academic Press: Oakville, ON, Canada, 2021. [Google Scholar]
- Mokhtar, D.M.; Sayed, R.K.; Zaccone, G.; Albano, M.; Hussein, M.T. Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies. Cells 2022, 11, 2659. [Google Scholar] [CrossRef]
- Rühl, A.; Nasser, Y.; Sharkey, K. Enteric glia. Neurogastroenterol. Motil. 2004, 16, 44–49. [Google Scholar] [CrossRef]
- Hagström, C.; Olsson, C. Glial cells revealed by GFAP immunoreactivity in fish gut. Cell Tissue Res. 2010, 341, 73–81. [Google Scholar] [CrossRef]
- Baker, P.A.; Meyer, M.D.; Tsang, A.; Uribe, R.A. Immunohistochemical and ultrastructural analysis of the maturing larval zebrafish enteric nervous system reveals the formation of a neuropil pattern. Sci. Rep. 2019, 9, 6941. [Google Scholar] [CrossRef]
- Holmberg, A.; Olsson, C.; Holmgren, S. The effects of endogenous and exogenous nitric oxide on gut motility in zebrafish Danio rerio embryos and larvae. J. Exp. Biol. 2006, 209, 2472–2479. [Google Scholar] [CrossRef]
- Holmberg, A.; Schwerte, T.; Pelster, B.; Holmgren, S. Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J. Exp. Biol. 2004, 207, 4085–4094. [Google Scholar] [CrossRef]
- Olden, T.; Akhtar, T.; Beckman, S.A.; Wallace, K.N. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis 2008, 46, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, L.; Shepherd, I.T.; Harrisson, F.; Hubens, G.; Blust, R.; Timmermans, J.P.; Van Nassauw, L. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio). J. Comp. Neurol. 2010, 518, 4419–4438. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- Monk, K.R.; Feltri, M.L.; Taveggia, C. New insights on Schwann cell development. Glia 2015, 63, 1376–1393. [Google Scholar] [CrossRef]
- Hanani, M. Satellite glial cells in sensory ganglia: From form to function. Brain Res. Rev. 2005, 48, 457–476. [Google Scholar] [CrossRef]
- Pannese, E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. 2010, 6, 3–10. [Google Scholar] [CrossRef]
- Avraham, O.; Feng, R.; Ewan, E.E.; Rustenhoven, J.; Zhao, G.; Cavalli, V. Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair. eLife 2021, 10, e68457. [Google Scholar] [CrossRef]
- Enes, J.; Haburčák, M.; Sona, S.; Gerard, N.; Mitchell, A.C.; Fu, W.; Birren, S.J. Satellite glial cells modulate cholinergic transmission between sympathetic neurons. PLoS ONE 2020, 15, e0218643. [Google Scholar] [CrossRef]
- van Velzen, M.; Laman, J.D.; KleinJan, A.; Poot, A.; Osterhaus, A.D.; Verjans, G.M. Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J. Immunol. 2009, 183, 2456–2461. [Google Scholar] [CrossRef]
- Wu, H.-H.; Bellmunt, E.; Scheib, J.L.; Venegas, V.; Burkert, C.; Reichardt, L.F.; Zhou, Z.; Farinas, I.; Carter, B.D. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci. 2009, 12, 1534–1541. [Google Scholar] [CrossRef]
- Germanà, A.; Marino, F.; Guerrera, M.C.; Campo, S.; De Girolamo, P.; Montalbano, G.; Germanà, G.P.; Ochoa-Erena, F.J.; Ciriaco, E.; Vega, J. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 2008, 71, 248–255. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, T.; Perez-Pinera, P.; Díaz-Esnal, B.; Vega, J. S-100 proteins in the human peripheral nervous system. Microsc. Res. Tech. 2003, 60, 633–638. [Google Scholar] [CrossRef]
- Meeker, N.D.; Trede, N.S. Immunology and zebrafish: Spawning new models of human disease. Dev. Comp. Immunol. 2008, 32, 745–757. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Zhang, J.; Shanahan, H.; Tonello, R.; Lee, S.H.; Strong, J.A.; Berta, T.; Zhang, J.M. Key role of CCR2-expressing macrophages in a mouse model of low back pain and radiculopathy. Brain Behav. Immun. 2021, 91, 556–567. [Google Scholar] [CrossRef]
- Herbomel, P.; Thisse, B.; Thisse, C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev. Biol. 2001, 238, 274–288. [Google Scholar] [CrossRef]
- Esiri, M.M.; Reading, M.C. Macrophages, lymphocytes and major histocompatibility complex (HLA) class II antigens in adult human sensory and sympathetic ganglia. J. Neuroimmunol. 1989, 23, 187–193. [Google Scholar] [CrossRef]
- Madigan, C.A.; Cambier, C.; Kelly-Scumpia, K.M.; Scumpia, P.O.; Cheng, T.-Y.; Zailaa, J.; Bloom, B.R.; Moody, D.B.; Smale, S.T.; Sagasti, A. A macrophage response to Mycobacterium leprae phenolic glycolipid initiates nerve damage in leprosy. Cell 2017, 170, 973–985.e10. [Google Scholar] [CrossRef]
- Collin, M.; Ginhoux, F. Human dendritic cells. Semin. Cell Dev. Biol. 2019, 86, 1–2. [Google Scholar] [CrossRef]
- Lucinda, N.; Figueiredo, M.M.; Pessoa, N.L.; Santos, B.S.Á.d.S.; Lima, G.K.; Freitas, A.M.; Machado, A.M.V.; Kroon, E.G.; Antonelli, L.R.d.V.; Campos, M.A. Dendritic cells, macrophages, NK and CD8+ T lymphocytes play pivotal roles in controlling HSV-1 in the trigeminal ganglia by producing IL1-beta, iNOS and granzyme B. Virol. J. 2017, 14, 37. [Google Scholar] [CrossRef]
- Robertsen, B. The interferon system of teleost fish. Fish Shellfish Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Ouwendijk, W.J.D.; Getu, S.; Mahalingam, R.; Gilden, D.; Osterhaus, A.D.M.E.; Verjans, G.M.G.M. Characterization of the immune response in ganglia after primary simian varicella virus infection. J. NeuroVirol. 2016, 22, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Gacek, R.R. The course and central termination of first order neurons supplying vestibular end organs in the cat. Acta Otorhinolaryngol. Suppl. 1969, 254, 1–66. [Google Scholar]
- Weston, J.K. Observations on the comparative anatomy of the VIIIth nerve complex. Acta Oto-Laryngol. 1939, 27, 457–498. [Google Scholar] [CrossRef]
- Ballantyne, J.; Engström, H. Morphology of the vestibular ganglion cells. J. Laryngol. Otol. 1969, 83, 19–42. [Google Scholar] [CrossRef]
- Bas, E.; Goncalves, S.; Adams, M.; Dinh, C.T.; Bas, J.M.; Van De Water, T.R.; Eshraghi, A.A. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front. Cell. Neurosci. 2015, 9, 303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).