LC-MS Based Metabolomic Profiling of Largehead Hairtail (Trichiurus japonicus) Ovary Reveals Metabolic Signatures of Ovarian Developmental Process (II–IV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. LC-MS Analysis and Data Processing
2.3. Statistics
3. Results
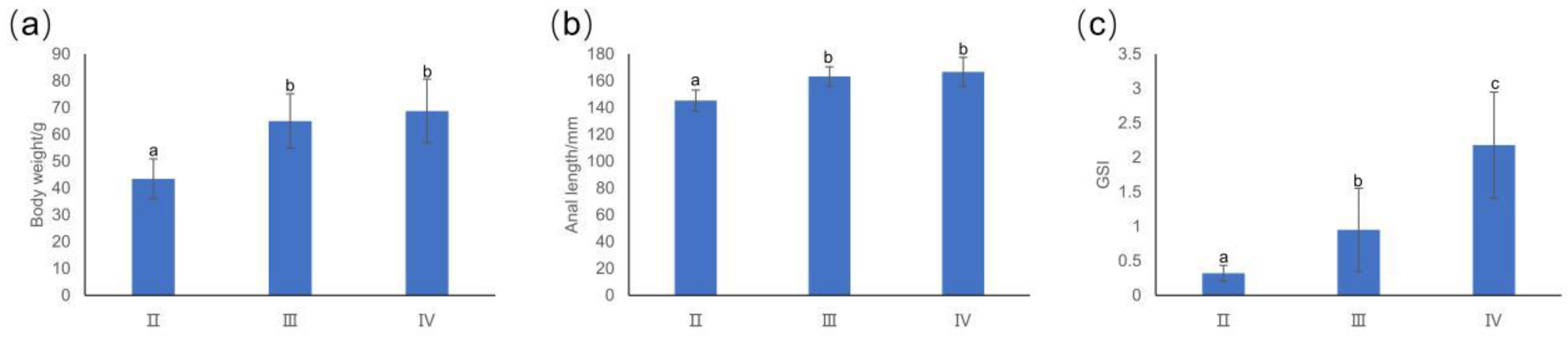
3.1. Basic Information about T. japonicus
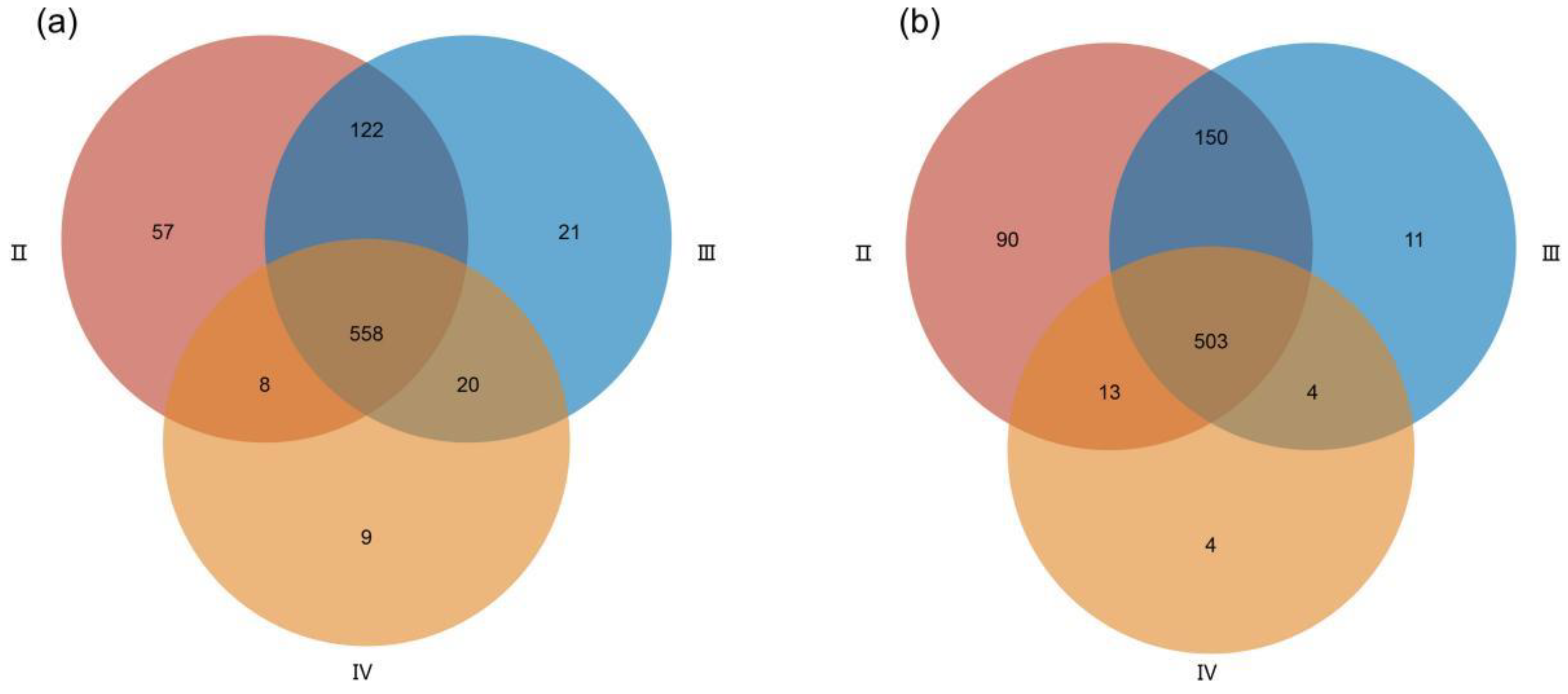
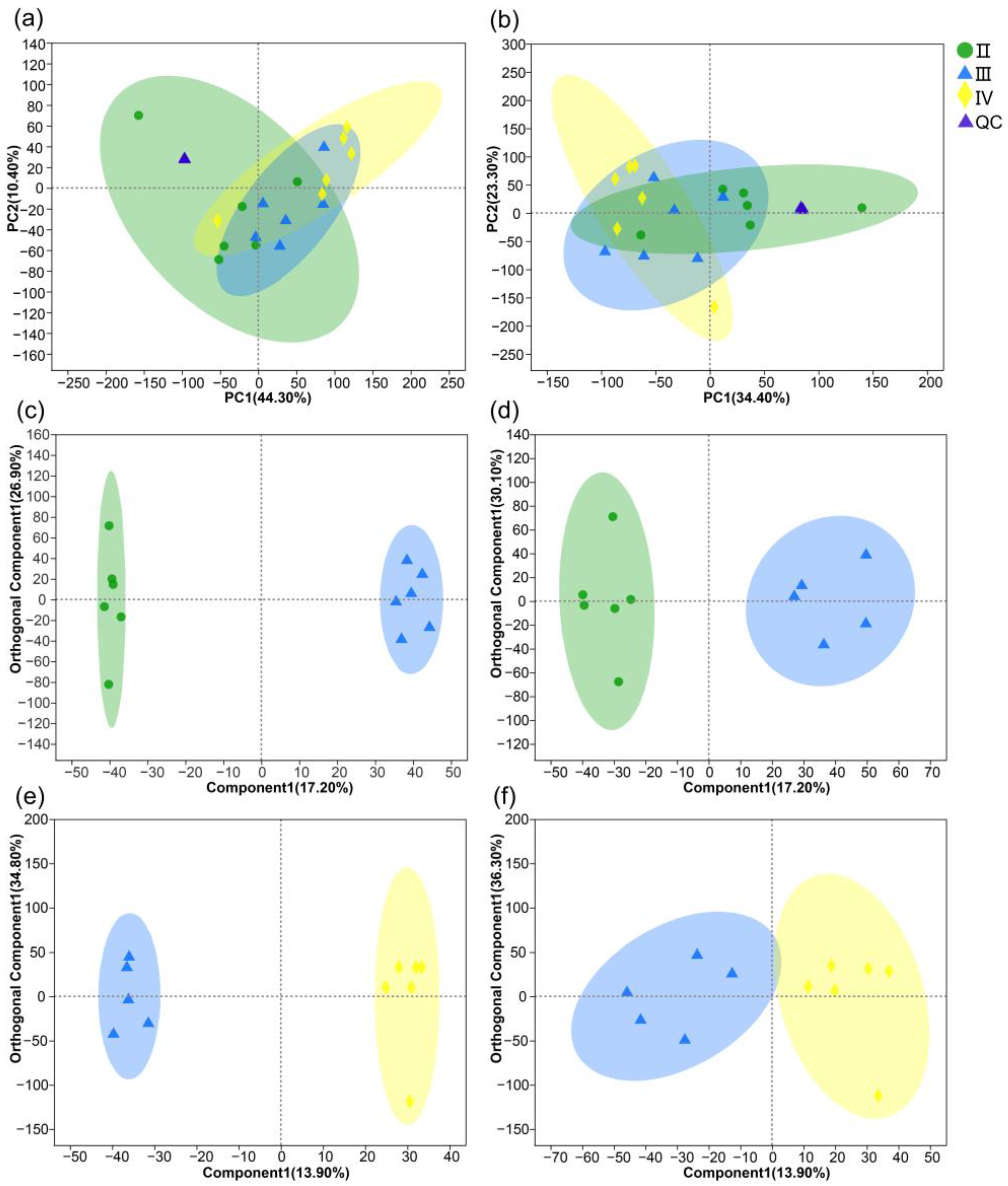
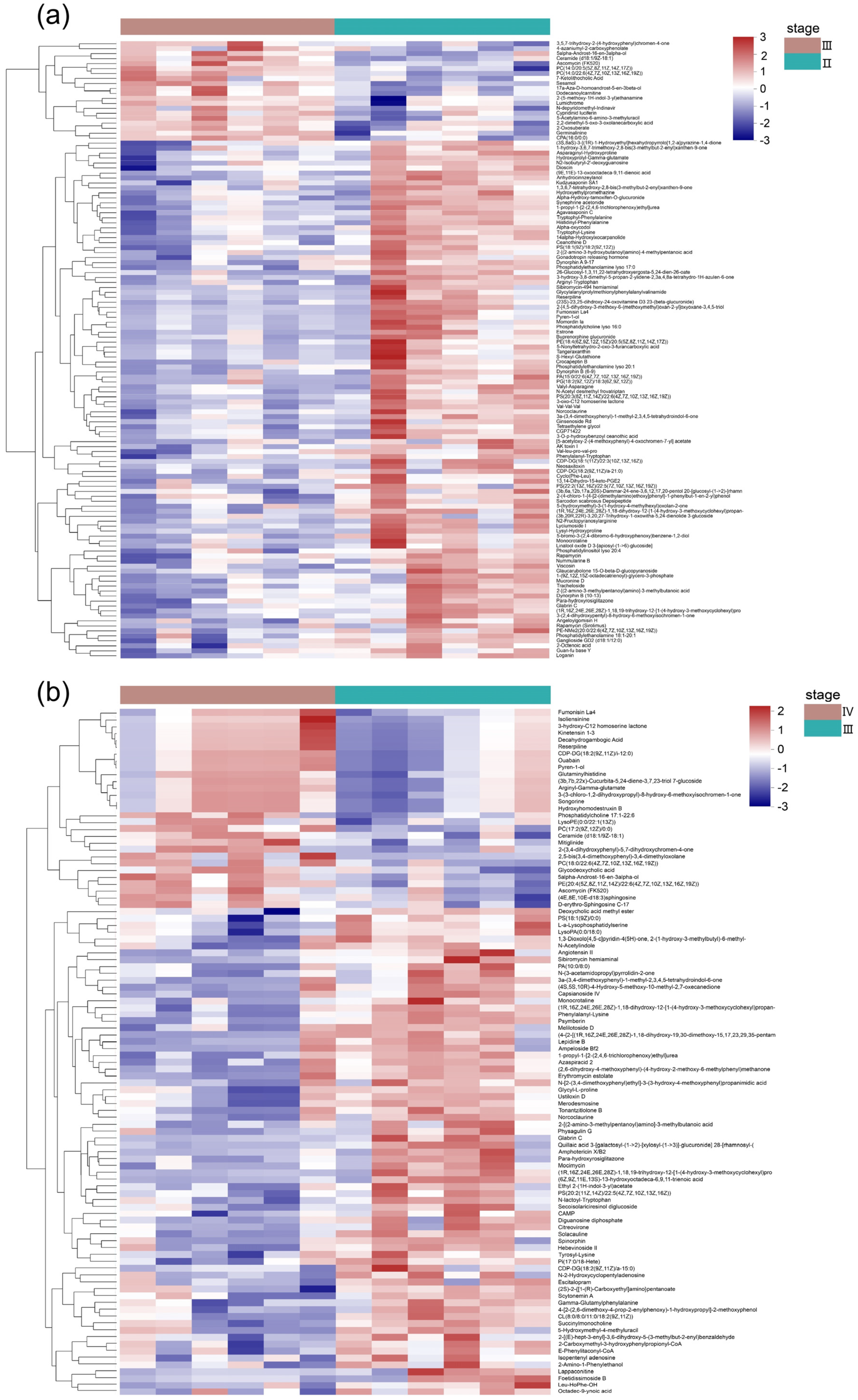
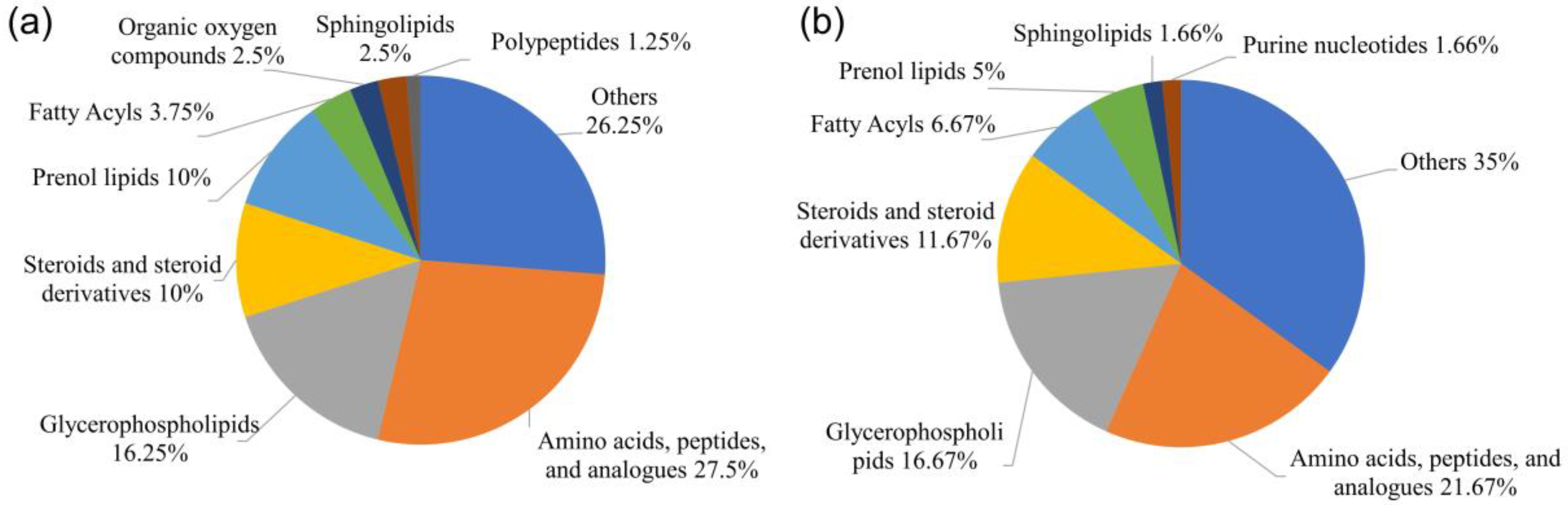
3.2. Metabolite Profiles of the Ovaries at Different Developmental Stages
3.3. Metabolic Pathways Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, China Society of Fisherie. China Fishery Statistical Yearbook; Agriculture Press: Beijing, China, 2000–2020. [Google Scholar]
- He, X.B. Spatial Distribution, Population Structure and Trophic Ecology of Common Trichiuridae Species in the Coastal Waters of China. Ph.D. Thesis, JiMei University, Xiamen, China, 2019. [Google Scholar]
- Li, C. Annual ovarian changes of Trichiurus haumela in the East China Sea. Oceanol. Limnol. Sin. 1982, 13, 461–472. [Google Scholar]
- Kwok, K.; Ni, I.-H. Reproduction of cutlassfishes Trichiurus spp. from the South China Sea. Mar. Ecol. Prog. Ser. 1999, 176, 39–47. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Q.; Fu, C.; Zhu, W.; Li, J.; Zhang, C.; Yu, H.; Sun, R.; Xu, Y.; Tian, Y. Daily growth of young-of-the-year largehead hairtail (Trichiurus japonicus) in relation to environmental variables in the East China Sea. J. Mar. Syst. 2020, 201, 103243. [Google Scholar] [CrossRef]
- Shin, S.R.; Kim, H.J.; Kim, J.W.; Kwon, D.-H.; Choi, J.; Park, J.J.; Lee, J.S. Sex Ratio, Spawning Period, and Sexual Group Maturity of the Largehead Hairtail Trichiurus japonicus (Teleostei: Trichiuridae) in Korean Waters. Fishes 2023, 8, 194. [Google Scholar] [CrossRef]
- Xu, H.X.; Liu, Z.F.; Zhou, Y.D. Variation of Trichiurus haumela productivity and recruitment in the East China Sea. J. Fish. China 2003, 27, 322–327. [Google Scholar]
- Chen, Y.L.; Shan, X.J.; Dai, F.Q.; Jin, X.S. Relative stock density and distribution of hairtail Trichiurus lepturus and its spawning stock structure in coastal waters of the East China Sea. Prog. Fish. Sci. 2013, 34, 8–15. [Google Scholar]
- Chao, M.; Quan, W.M.; Li, C.H.; Cheng, Y.H. Changes in trophic level of marine catches in the East China Sea region. Mar. Sci. 2005, 29, 54. [Google Scholar]
- Hutchings, J.A.; Baum, J.K. Measuring marine fish biodiversity: Temporal changes in abundance, life history and demography. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 315–338. [Google Scholar] [CrossRef]
- Mu, Y.L.; Liu, Y.; Wang, J.; Yuan, C.Y.; Dong, J. Effects of water temperature and photoperiod on maturation and spawn of flounder (Paralichthys olivaceus). J. Dalian Fish. Univ. 2016, 14, 62–65. [Google Scholar]
- Jin, Y.; Liu, Z.L.; Yan, L.P.; Yuan, X.W.; Cheng, J.H. Maturity of Hairtail Varies with Latitude and Environment in the East China Sea. Mar. Coast. Fish. 2020, 12, 395–403. [Google Scholar] [CrossRef]
- Targońska, K.; Żarski, D.; Kupren, K.; Palińska-żarska, K.; Mamcarz, A.; Kujawa, R.; Skrzypczak, A.; Furgała-Selezniow, G.; Czarkowski, T.K.; Hakuć-Błażowska, A.; et al. Influence of temperature during four following spawning seasons on the spawning effectiveness of common bream, Abramis brama (L.) under natural and controlled conditions. J. Therm. Biol. 2014, 39, 17–23. [Google Scholar] [CrossRef]
- You, H.Z.; Zheng, Y.K.; You, G.C. Research progress on the effects of different salinity on fish farming biology. Hebei Fish. 2013, 6, 47–52. [Google Scholar]
- Guo, J.J.; Ch, G.P.; Huang, Z.Y.; Lu, W.Q. Studies on growth and gonadal development of Pseudosciaena crocea in low salinity in door circulating culture system. J. Shanghai Ocean Univ. 2016, 25, 847–852. [Google Scholar] [CrossRef]
- Bapary, M.A.J.; Amin, M.N.; Takeuchi, Y.; Takemura, A. The stimulatory effects of long wavelengths of light on the ovarian development in the tropical damselfish, Chrysiptera cyanea. Aquaculture 2011, 314, 188–192. [Google Scholar] [CrossRef]
- Leclercq, E.; Taylor, J.; Sprague, M.; Migaud, H. The potential of alternative lighting-systems to suppress pre-harvest sexual maturation of 1+ Atlantic salmon (Salmo salar) post-smolts reared in commercial sea-cages. Aquac. Eng. 2011, 44, 35–47. [Google Scholar] [CrossRef]
- Xiao, D.Y. Effects of different levels of dietary vitamin A, C, E on reproductive eperformance and offspringquality of tongue sole (Cynoglossus semilaevis). Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2014. [Google Scholar]
- Afzal Khan, M.; Jafri, A.; Chadha, N. Effects of varying dietary protein levels on growth, reproductive performance, body and egg composition of rohu, Labeo rohita (Hamilton). Aquac. Nutr. 2005, 11, 11–17. [Google Scholar] [CrossRef]
- Shan, X.; Hu, Z.; Shao, C. Progress in the study of fishing-induced evolution of fish biological characteristics. Prog. Fish. Sci. 2020, 41, 165–175. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.-A.; Dufour, S.; Karlsen, Ø.; Norberg, B. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef]
- Terasawa, E.; Fernandez, D.L. Neurobiological mechanisms of the onset of puberty in primates. Endocr. Rev. 2001, 22, 111–151. [Google Scholar] [CrossRef]
- Sisk, C.L.; Foster, D.L. The neural basis of puberty and adolescence. Nat. Neurosci. 2004, 7, 1040–1047. [Google Scholar] [CrossRef]
- Yaron, Z.; Gur, G.; Melamed, P.; Rosenfeld, H.; Elizur, A.; Levavi-Sivan, B. Regulation of fish gonadotropins. Int. Rev. Cytol. 2003, 225, 131–185. [Google Scholar] [PubMed]
- Zhou, L.-F.; Zhao, B.-W.; Guan, N.-N.; Wang, W.-M.; Gao, Z.-X. Plasma metabolomics profiling for fish maturation in blunt snout bream. Metabolomics 2017, 13, 40. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Leng, X.; Du, H.; Wu, J.; He, S.; Luo, J.; Liang, X.; Liu, H.; Wei, Q. Metabolomics and gene expressions revealed the metabolic changes of lipid and amino acids and the related energetic mechanism in response to ovary development of Chinese sturgeon (Acipenser sinensis). PLoS ONE 2020, 15, e0235043. [Google Scholar] [CrossRef] [PubMed]
- Tamrakar, S.; Huerta, B.; Chung-Davidson, Y.-W.; Li, W. Plasma metabolomic profiles reveal sex-and maturation-dependent metabolic strategies in sea lamprey (Petromyzon marinus). Metabolomics 2022, 18, 90. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic analysis in food science: A review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Zhang, Q.H. Fishery resources and their sustainable use in the East China Sea; Fudan University Press: Shanghai, China, 2007. [Google Scholar]
- Yan, L.P.; Hu, F.; Li, J.S.; Liu, Y.; Chen, J.H. Age and growth of Trichiurus haumela in the East China Sea. Mar. Fish. 2005, 27, 139–142. [Google Scholar]
- Htun-Han, M. The reproductive biology of the dab Limanda limanda (L.) in the North Sea: Gonosomatic index, hepatosomatic index and condition factor. J. Fish Biol. 1978, 13, 369–378. [Google Scholar] [CrossRef]
- Chen, X.J. Fishery Resources and Fishery Oceanography; China Ocean Press: Beijing, China, 2004. [Google Scholar]
- Yang, P.; Yao, W.; Wang, Y.; Li, M.; Li, X.; Leng, X. Dietary effects of fish meal substitution with Clostridium autoethanogenum on flesh quality and metabolomics of largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 23, 101012. [Google Scholar] [CrossRef]
- Chen, X.; Hou, X.; Yang, M.; Wang, J.; Wang, C. Differential metabolomic profiles of two closely related Leuciscinae fish, the species Ctenopharyngodon idellus and Leuciscus idus. Aquac. Rep. 2021, 20, 100650. [Google Scholar] [CrossRef]
- Zhou, H.; Leng, X.Q.; Tan, Q.S.; Du, H.; Wu, J.P.; Liang, X.F.; Wei, Q.W. Identification of key nutrients for gonadal development by comparative analysis of proximate composition and fatty/amino acid profile in tissues and eggs of Chinese sturgeon (Acipenser sinensis Gray, 1835). J. Appl. Ichthyol. 2017, 33, 885–891. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Gong, B.; Bao, F.; Wang, Y.; Liu, H.; Xiao, M.; He, J. Metabonomics study on the effect of traditional Chinese medicines feed addition on growth performance and serum metabolic profile of juvenile Chinese softshell turtle (Pelodiscus sinensis Wiegmann). Aquac. Rep. 2021, 20, 100632. [Google Scholar] [CrossRef]
- Xu, G.; Du, F.; Li, Y.; Nie, Z.; Xu, P. Integrated application of transcriptomics and metabolomics yields insights into population-asynchronous ovary development in Coilia nasus. Sci. Rep. 2016, 6, 31835. [Google Scholar] [CrossRef] [PubMed]
- Mommsen, T.P.; Walsh, P.J. 5 Vitellogenesis and oocyte assembly. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 11, pp. 347–406. [Google Scholar] [CrossRef]
- Nowosad, J.; Kucharczyk, D.; Łuczyńska, J.; Targońska, K.; Czarkowski, T.K.; Biłas, M.; Krejszeff, S.; Horváth, L.; Müller, T. Changes in European eel ovary development and body and ovary chemistry during stimulated maturation under controlled conditions: Preliminary data. Aquac. Int. 2015, 23, 13–27. [Google Scholar] [CrossRef]
- Luo, L.; Ai, L.; Li, T.; Xue, M.; Wang, J.; Li, W.; Liang, X. The impact of dietary DHA/EPA ratio on spawning performance, egg and offspring quality in Siberian sturgeon (Acipenser baeri). Aquaculture 2015, 437, 140–145. [Google Scholar] [CrossRef]
- Liang, M.Q.; Lu, Q.K.; Qian, C.; Zheng, K.K.; Wang, X.X. Effects of dietary n-3 to n-6 fatty acid ratios on spawning performance and larval quality in tongue sole Cynoglossus semilaevis. Aquac. Nutr. 2014, 20, 79–89. [Google Scholar] [CrossRef]
- Mazorra, C.; Bruce, M.; Bell, J.; Davie, A.; Alorend, E.; Jordan, N.; Rees, J.; Papanikos, N.; Porter, M.; Bromage, N. Dietary lipid enhancement of broodstock reproductive performance and egg and larval quality in Atlantic halibut (Hippoglossus hippoglossus). Aquaculture 2003, 227, 21–33. [Google Scholar] [CrossRef]
- Wiegand, M.D. Composition, accumulation and utilization of yolk lipids in teleost fish. Rev. Fish Biol. Fish. 1996, 6, 259–286. [Google Scholar] [CrossRef]
- Shi, L.D.; Ren, T.J.; Han, Y.Z. Research progress of nutrients regulating reproductive performance of aquaculture animals: A review. J. Dalian Ocean Univ. 2020, 35, 620–630. [Google Scholar] [CrossRef]
- Rønnestad, I.; Thorsen, A.; Finn, R.N. Fish larval nutrition: A review of recent advances in the roles of amino acids. Aquaculture 1999, 177, 201–216. [Google Scholar] [CrossRef]
- Chong, A.S.; Hashim, R.; Ali, A.; Hara, K. Amino acid profile of various body tissues and eggs of discus fish, Symphysodon aequifasciata. J. Appl. Aquac. 2004, 16, 157–168. [Google Scholar] [CrossRef]
- Lanes, C.F.C.; Bizuayehu, T.T.; Bolla, S.; Martins, C.; de Oliveira Fernandes, J.M.; Bianchini, A.; Kiron, V.; Babiak, I. Biochemical composition and performance of Atlantic cod (Gadus morhua L.) eggs and larvae obtained from farmed and wild broodstocks. Aquaculture 2012, 324, 267–275. [Google Scholar] [CrossRef]
- Li, W.; Wei, Q.; Shen, L. Biochemical comparison between eggs from female Chinese sturgeon (Acipenser sinensis Gray, 1835) reconditioned in freshwater and eggs from wild females: Evaluation of female reconditioning as a conservation culture technique. J. Appl. Ichthyol. 2014, 30, 1237–1242. [Google Scholar] [CrossRef]
- Ősz, K.; Várnagy, K.; Süli-Vargha, H.; Csámpay, A.; Sanna, D.; Micera, G.; Sóvágó, I. Acid–base properties and copper (II) complexes of dipeptides containing histidine and additional chelating bis (imidazol-2-yl) residues. J. Inorg. Biochem. 2004, 98, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shen, S.T. The effect of L-arginine on animal reproductive function. Feed Livest. 2013, 10, 5–6. [Google Scholar]
- Qi, C.L. The nutritional and physiological functions of argininein Chinese mitten crab Eriocheir sinensis at juvenile and gonad maturation stage. Ph.D. Thesis, East China Normal University, Shanghai, China, 2020. [Google Scholar]
- Jessus, C.; Munro, C.; Houliston, E. Managing the oocyte meiotic arrest—Lessons from frogs and jellyfish. Cells 2020, 9, 1150. [Google Scholar] [CrossRef]
- Senthilkumaran, B.; Yoshikuni, M.; Nagahama, Y. A shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol. Cell. Endocrinol. 2004, 215, 11–18. [Google Scholar] [CrossRef]
- Van Bohemen, C.G.; Lambert, J.; Van Oordt, P. Vitellogenin induction by estradiol in estrone-primed rainbow trout, Salmo gairdneri. Gen. Comp. Endocrinol. 1982, 46, 136–139. [Google Scholar] [CrossRef]
- Sampath Kumar, R.; Ijiri, S.; Trant, J.M. Changes in the expression of genes encoding steroidogenic enzymes in the channel catfish (Ictalurus punctatus) ovary throughout a reproductive cycle. Biol. Reprod. 2000, 63, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C. Aromatase—A brief overview. Annu. Rev. Physiol. 2002, 64, 93. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, G.; Senthilkumaran, B. A role for cytochrome P450 17α-hydroxylase/c17-20 lyase during shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. J. Steroid Biochem. Mol. Biol. 2009, 115, 77–85. [Google Scholar] [CrossRef]
- Wang, A.M. Electron microscopic observation on the yolk formation of Oreochomis mossambicus. Acta Hydrobiol. Sinica. 1994, 18, 26–31. [Google Scholar]
- Nagahama, Y. Endocrine regulation of gametogenesis in fish. Int. J. Dev. Biol. 2002, 38, 217–229. [Google Scholar] [CrossRef]
- Downs, S.M. Regulation of the G2/M transition in rodent oocytes. Mol. Reprod. Dev. 2010, 77, 566–585. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, M. Transduction mechanisms for gonadotrophin-induced oocyte maturation in mammals. Zygote 1994, 2, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Richani, D.; Lavea, C.F.; Kanakkaparambil, R.; Riepsamen, A.H.; Bertoldo, M.J.; Bustamante, S.; Gilchrist, R.B. Participation of the adenosine salvage pathway and cyclic AMP modulation in oocyte energy metabolism. Sci. Rep. 2019, 9, 18395. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef]
- Alhajeri, M.M.; Alkhanjari, R.R.; Hodeify, R.; Khraibi, A.; Hamdan, H. Neurotransmitters, neuropeptides and calcium in oocyte maturation and early development. Front. Cell Dev. Biol. 2022, 10, 980219. [Google Scholar] [CrossRef]
- Yamada, J.; Sugimoto, Y.; Yoshikawa, T.; Horisaka, K. Hyperglycemia induced by the 5-HT receptor agonist, 5-methoxytryptamine, in rats: Involvement of the peripheral 5-HT2A receptor. Eur. J. Pharmacol. 1997, 323, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.; Eglen, R.; Walsh, L.; Perkins, L.; Whiting, R.; Clarke, D. 5-Methoxytryptamine and 2-methyl-5-hydroxytryptamine-induced desensitization as a discriminative tool for the 5-HT3 and putative 5-HT4 receptors in guinea pig ileum. Naunyn-Schmiedebergs Arch. Pharmacol. 1990, 342, 9–16. [Google Scholar] [CrossRef]
- Wada, K.; Hu, L.; Mores, N.; Navarro, C.E.; Fuda, H.; Krsmanovic, L.Z.; Catt, K.J. Serotonin (5-HT) receptor subtypes mediate specific modes of 5-HT-induced signaling and regulation of neurosecretion in gonadotropin-releasing hormone neurons. Mol. Endocrinol. 2006, 20, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, E.M.; Franci, C.R. Involvement of serotonin 5HT1 and 5HT2 receptors and nitric oxide synthase in the medial preoptic area on gonadotropin secretion. Brain Res. Bull. 2004, 63, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Vega, J.A.; Ricci, A.; Collier, W.L. Localization of 5-hydroxytryptamine-like immunoreactive cells and nerve fibers in the rat female reproductive system. Anat. Rec. -Adv. Integr. Anat. Evol. Biol. 2010, 233, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Kishimoto, T.; Kadam, A.L.; Kanatani, H.; Koide, S.S. Induction of spawning and oocyte maturation by 5-hydroxytryptamine in the surf clam. J. Exp. Zool. 1988, 245, 318–321. [Google Scholar] [CrossRef]
- Tanabe, T.; Osada, M.; Kyozuka, K.; Inaba, K.; Kijima, A. A novel oocyte maturation arresting factor in the central nervous system of scallops inhibits serotonin-induced oocyte maturation and spawning of bivalve mollusks. Gen. Comp. Endocrinol. 2006, 147, 352–361. [Google Scholar] [CrossRef]
- Romero-Reyes, J.; Cárdenas, M.; Damián-Matsumura, P.; Domínguez, R.; Ayala, M.E. Inhibition of serotonin reuptake in the prepubertal rat ovary by fluoxetine and effects on ovarian functions. Reprod. Toxicol. 2016, 59, 80–88. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides–lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef]
- Nikolova-Karakashian, M.N.; Rozenova, K.A. Ceramide in stress response. In Sphingolipids as Signaling and Regulatory Molecules; Springer: Berlin/Heidelberg, Germany, 2010; pp. 86–108. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, G.; Gao, X.; Wu, D.; Carrasco, P.; Casals, N.; Hegardt, F.G.; Moran, T.H.; Lopaschuk, G.D. Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc. Natl. Acad. Sci. USA 2011, 108, 9691–9696. [Google Scholar] [CrossRef]
- Ramírez, S.; Martins, L.; Jacas, J.; Carrasco, P.; Pozo, M.; Clotet, J.; Serra, D.; Hegardt, F.G.; Diéguez, C.; López, M. Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes 2013, 62, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Heras, V.; Castellano, J.M.; Fernandois, D.; Velasco, I.; Tena-Sempere, M. Central Ceramide Signaling Mediates Obesity-Induced Precocious Puberty. Cell Metab. 2020, 32, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.F.; Zhang, J.P.; Yuan, X.P.; Liu, G.Q. Metabonomics analysis of ovaries of Coilia nasus in seawater and freshwater based on liquid chromatography-mass spectrometry. South China Fish. Sci. 2022, 18, 68–75. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.-Y.; Yan, L.-P.; Li, R.-W.; Li, S.-F.; Cheng, J.-H.; Jin, Y. LC-MS Based Metabolomic Profiling of Largehead Hairtail (Trichiurus japonicus) Ovary Reveals Metabolic Signatures of Ovarian Developmental Process (II–IV). Fishes 2023, 8, 262. https://doi.org/10.3390/fishes8050262
Feng L-Y, Yan L-P, Li R-W, Li S-F, Cheng J-H, Jin Y. LC-MS Based Metabolomic Profiling of Largehead Hairtail (Trichiurus japonicus) Ovary Reveals Metabolic Signatures of Ovarian Developmental Process (II–IV). Fishes. 2023; 8(5):262. https://doi.org/10.3390/fishes8050262
Chicago/Turabian StyleFeng, Liu-Ying, Li-Ping Yan, Run-Wei Li, Sheng-Fa Li, Jia-Hua Cheng, and Yan Jin. 2023. "LC-MS Based Metabolomic Profiling of Largehead Hairtail (Trichiurus japonicus) Ovary Reveals Metabolic Signatures of Ovarian Developmental Process (II–IV)" Fishes 8, no. 5: 262. https://doi.org/10.3390/fishes8050262
APA StyleFeng, L.-Y., Yan, L.-P., Li, R.-W., Li, S.-F., Cheng, J.-H., & Jin, Y. (2023). LC-MS Based Metabolomic Profiling of Largehead Hairtail (Trichiurus japonicus) Ovary Reveals Metabolic Signatures of Ovarian Developmental Process (II–IV). Fishes, 8(5), 262. https://doi.org/10.3390/fishes8050262




