Abstract
The effect of different main dietary compositions on growth, anticipatory digestive enzyme activities, and oxidative status was studied in the proximal intestine of juvenile European sea bass. A control diet (C, 44% protein, 17.6% lipid, and 20% starch), three diets with increasing starch levels to test protein sparing (P36S36, P40S29, and P43S24), and two diets with high lipid content (L20S13 and L22S7) were tested. After 20 weeks, growth, digestive enzyme activities, lipid peroxidation, antioxidant enzyme activities, and G6PDH activity were measured after a 24-h fast. Sea bass fed P43S24 and L20S13 maintained an oxidative status like C fish, up-regulated CAT activity, and adjusted anticipatory protease activity. Instead, the lipid peroxidation increased in the L22S7 group, although CAT activity increased, whereas anticipatory total protease activity was downregulated. P40S29 also triggered LPO and CAT activity, but G6PDH levels diminished significantly. Moreover, an up-regulation in digestive enzyme activities was found. Finally, P36S36 fish showed less antioxidant enzyme activity and G6PDH, although their LPO tended to increase and their lipase and α-amylase activities were upregulated. In conclusion, the inclusion of carbohydrates up to 24% or lipids up to 20% is possible for this species if protein requirements are met without negative effects on growth.
Key Contribution:
Moderate changes in macronutrient composition affect anticipatory digestive enzyme activities and LPO in the intestine. Up to 24% carbohydrates or 20% lipid inclusion in diets for sea bass is possible if protein requirements are met.
1. Introduction
One of the most significant production costs in aquaculture is feed. This is even more critical in carnivorous species because of their high protein requirements, considering it is the most expensive macronutrient [1,2]. Both fish meal (FM) and fish oil (FO) are the most balanced sources of nutrients for fish because of their amino acid and fatty acid profiles, but overexploitation of these sources and the steady rise of aquaculture have caused their prices to increase [1].
Alternative sources like vegetable ingredients, insects, and algae are considered viable candidates, and important advances have already been made [3,4,5,6,7,8]. It has been estimated that around 80–95% of FM and FO could be replaced by plant sources without considerably affecting performance if essential amino acid and fatty acid requirements are met [9]. Moreover, alternative sources come with their own set of problems, such as high cellulose and chitin contents, anti-nutritional factors, and deficient amino and fatty acid profiles [5,9,10,11]. Furthermore, several environmental and biotic factors and their interactions determine whether the organism uses nutrients efficiently [12,13,14]. The efficiency with which a feed is used is highly dependent on the digestive enzyme activities and its nutrient absorption capacity, which are closely related to intestinal integrity. Moreover, the production of reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, and hydroxyl radicals, as a byproduct of normal oxygen metabolism [15,16], has the potential to cause cell damage affecting intestinal integrity. This ROS production can be natural or promoted by external factors such as stressful situations and even nutritional imbalances [15,17]. Thus, organisms have evolved defensive mechanisms to prevent cellular damage, such as low molecular weight molecules such as vitamins and enzymatic antioxidants [17].
The digestive process is a combination of complex processes, from the physical trituration of feed to the absorption of nutrients by the brush border membrane of the intestinal tract. It starts in the stomach, where pepsin initiates the enzymatic hydrolysis of the protein. As the chyme reaches the pyloric caeca, cholecystokinin stimulates both the gall bladder to secrete bile and the pancreas to secrete bicarbonate to neutralize the acid from the stomach and digestive enzymes such as proteases (e.g., trypsin and chymotrypsin), carbohydrases (e.g., α-amylase), and lipases (lipase and colipase) [18]. Moreover, the activity of these enzymes is affected by a multitude of factors, such as age, physiological state, temperature, pH, seasonality, and even the composition of the feed itself [12,14,19,20]. Further, not all fish species have the same digestive enzyme profile [21,22,23], suggesting that enzymatic digestive capacities are species-specific.
Diet replacement by plant feedstuffs impacts the digestive and absorptive processes of other commercially relevant marine species, such as gilthead sea bream (Sparus aurata), as has been widely studied [12,20,21,24,25,26,27,28]. Nonetheless, despite being one of the most important farmed fish species in the Mediterranean and the fourth in Europe, little is known about the effects on digestive processes in European sea bass. As a marine carnivorous species, protein requirements for optimum growth in sea bass are high when compared to other fish [29,30,31]. Regarding lipid requirements, the data is not very clear, but it appears that an inclusion level between 12 and 18% delivers the best growth performance [13,19]. Regarding carbohydrate utilization, Enes et al. [32] concluded that starch digestibility in sea bass juveniles is high and can be further improved by processing. They also found that an inclusion level of around 20% digestible carbohydrates ensures high growth and feed utilization.
One of the most serious impacts of ROS is lipid peroxidation (LPO). Polyunsaturated fatty acids, especially phospholipids from membranes [33], start a chain reaction in which serious cell damage occurs. The action mechanisms of the main antioxidants are well known [15,17,34]. Briefly, superoxide dismutase (SOD) accelerates the dismutation of the superoxide radical into hydrogen peroxide and oxygen. Catalase (CAT) and glutathione peroxidase (GPx) both act on H2O2, but through different pathways. CAT catalyzes H2O2 to water and oxygen or by causing the oxidation of other reduced compounds with H2O2. GPx catalyzes the oxidation of reduced glutathione (GSH). Glucose-6-phosphate dehydrogenase (G6PDH) was also related to antioxidant defense modulation as NADPH generated by this enzyme in the pentose phosphate pathway is essential for the reduction of oxidized glutathione (GSSG) [35]. It is known that dietary composition can affect peroxidation levels. Thus, the lipid content of the diet and its level of unsaturation increase LPO [36]. Moreover, a 20% dietary carbohydrate inclusion did not promote significant changes in LPO at the intestinal level in sea bass and sea bream; however, in the liver, it had a protective effect [17,37].
The aim of this study was to evaluate growth performance, the digestive process, and intestinal oxidative status in sea bass fed diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%. Analysis was focused on the proximal intestine to study total and individual proteases, amylases, and lipases activities, a traditional marker of oxidative stress such as LPO, some markers of the lipid-soluble antioxidant system (SOD, CAT, GPx), and G6PDH enzyme activities.
2. Materials and Methods
2.1. Trial and Diets
The trial was carried out at Institut de Recerca i Tecnologia Agroalimentàries (IRTA) facilities in La Rápita (Tarragona, Spain), where 450 sea bass juveniles (Dicentrarchus labrax) with an initial weight of 95.4 g ± 0.4 g were distributed randomly in 18 tanks of 400 L with a final stocking density of 12.8 kg·m−3. The trial was carried out between February and June and spanned 142 days with a 2-week acclimatization period and a 12 L/12 D photoperiod during the whole experiment. Water quality indicators were monitored and kept at an oxygen concentration of 8 mg·mL−1, a salinity of 35‰, and a temperature of 22 °C. Skretting ARC (Norway) formulated and produced six diets; their ingredients and nutritional composition are presented in Table 1.

Table 1.
The proximate composition and ingredients of the experimental diets when protein was replaced by starch at levels above 20% or where lipid was included above 18%. Diets were formulated and produced by Skretting ARC (Norway).
The C diet was considered a control due to its high crude protein and lipid content (44% and 18%, respectively) according to Dias et al. [29] and Peres and Oliva-Teles [31]. The C diet also included the maximum starch content recommended for this species (20%) [32]. On three diets, protein and lipids were partially replaced by starch at levels above 20%. These diets contained 16% lipid and were named according to their crude protein and starch contents as P36S36, P40S29, and P43S24. Furthermore, two 44% protein diets with high lipid content were formulated, L20S13 and L22S7, whose amounts of lipids and starch gave them their names. Cellulose was used as an inert filler. All dietary treatments were run in triplicate, and fish were fed twice a day by hand until visual satiety.
2.2. Sampling and Performance Indicators
At the end of the growth trial and 24 h post-feeding, all fish per treatment were anesthetized (with phenoxyethanol at 100 ppm), measured, weighed, and sacrificed by severing their spinal cord. The proximal intestine from nine fish per treatment was removed, and the first 1.5 cm of this segment was rapidly frozen in liquid nitrogen and stored at −80 °C. All fish-handling procedures complied with the European guidelines for animal care (Directive 2010/63/EU).
The specific growth rate (SGR) was calculated as: ((lnWfin(g) − lnWini(g)) × t−1) × 100, where Wfin and Wini represent the final and initial weights, respectively, and t is the duration of the trial in days. The condition factor (K) was calculated as: (final body weight × standard length−3) × 100. Protein retention efficiency (PER) was calculated as: g weight gain × g ingested protein−1. The feed conversion ratio (FCR) was calculated as: g feed intake × g weight gain−1. Voluntary feed intake (VFI) was calculated according to Lupatsch et al. [38] as: g × BW × day−1, where BW was calculated as: . In addition, the hepatosomatic (HSI) and mesenteric fat (MFI) indices were calculated: HSI = (liver weight/FBW) × 100 and MFI = (visceral fat weight/FBW) × 100, where FBW represent final body weight.
2.3. PH of Intestinal Duct and Sample Homogenization
Proximal intestine samples were thawed on ice, and the intestinal duct pH was measured (Crison, Micro pH 2000). After that, samples were individually homogenized (Polytron 2000, Sorvall TC) in Tris-HCl, 50 mM, pH 7.5, to a final concentration of 250 mg·mL−1. Next, homogenates were centrifuged for 15 min (1100× g, 4 °C, Jouan CR411), and aliquots of the supernatant were then stored at −80 °C for future digestive enzyme activity determination and for LPO, antioxidant, and G6PDH activity analysis.
2.4. Digestive Enzyme Analysis
Total protease activity (TPA) was measured according to the end-point assay described by Santigosa et al. [21]. Briefly, samples and standards were reacted with 1% casein (w/v) in 50 mM Tris-HCl buffer at the pH of the intestinal duct. After 30 min, the reaction was stopped with trichloroacetic acid (TCA) 12%, the samples were stored at 4 °C for 60 min, and then centrifuged for 5 min at 7500× g and 4 °C. The supernatant absorbance was measured at 280 nm (Tecan Infinite 200 PRO, Tecan Austria, Grodig, Austria), with blanks established for each sample and the standard. TPA was calculated as BAEE units, and bovine trypsin (Sigma Aldrich, Madrid, Spain, T9935, 12100 BAEE U·mg protein−1) was used as the standard. Activity was reported as BAEE U per mg of protein.
To characterize individual proteases (trypsin-like and chymotrypsin-like activities), zymograms were run according to the method of García-Carreño et al. [39], modified by Santigosa et al. [21]. Samples were mixed at a 1:1 ratio with a 20% glycerol loading buffer and loaded on a 12.5% polyacrylamide gel (10 × 10.5 × 0.1 cm); pure trypsin and albumin were used as controls. A commercial weight marker (Amersham GE Healthcare, Amersham, UK, RPN800E, 12000-225000Da) was also used to determine the molecular weight of protease fractions. Electrophoresis was performed at a constant current of 15 mA per gel for approximately 180 min at 4 °C (EPS 301 Power Supply). Gels were agitated in a 2% casein-TrisHCl solution at intestinal duct pH and 4 °C for 30 min. The temperature was then raised to 25 °C, and the gels were agitated for 90 min. Staining was carried out in a methanol:acetic:water (40:10:40) solution with 0.1% Brilliant Blue Coomassie R-250 for 25 min. The same solution without colorant was used for destaining for 10 min, and agitation was applied during both procedures.
Proteolytic characterization was performed by combining the homogenate with water or the corresponding inhibition solution for 45 min at a ratio of 4:1. Inhibition solutions selected were: TLCK (10 mM in HCl at 1 mM) as a trypsin-like activity inhibitor; TPCK (10 mM in methanol); and ZPCK (10 mM in dioxane) as chymotrypsin-like activity modifiers; and SBTI (250 µM in water) as a total serine protease activity inhibitor, according to Alarcon et al. [40]. Díaz et al. [41] demonstrated that the inhibition of used solvents is less than 5%. Zymogram results were analyzed using Quantity One 1-D Analysis Software 4.6.6 (BioRad).
For α-amylase determination, a kinetic assay was conducted to measure the rate of 2-chloro-4-nitrophenol formation at 405 nm, according to the kit manufacturer’s recommendation (Spinreact, Sant Esteve d’en Bas, Girona, Spain). One international unit (UI) is the amount of enzyme that hydrolyzes 1 μmol of substrate per minute in standard conditions. Activity was reported as mU per mg of protein.
For lipase determination, a kinetic assay was applied in which the increase in absorbance at 580 nm was measured, as performed by Santigosa et al. [27]. Briefly, previously homogenized samples stored at −80 °C were thawed on ice and mixed with a buffer containing (all values expressed in mM): 20.5 Tris, 3.6 taurodeoxycholate, 0.9 deoxycholate, 0.8 tartrate, 0.12 DGGR (1,2-Di-O-lauryl-rac-glycero-3-(glutaric acid 6-methylresorufin ester) as substrate, 0.05 CaCl2, 30 mannitol, and 1 mg·L−1 colipase (pH 8.3). Absorbance was registered every minute for 45 min, and the linear zone was determined between 10 and 20 min of reaction. Lipase (Sigma Aldrich, Spain, L0382, 33944 U·mg protein−1, 22980U·mg solid−1) was used as the standard and was previously diluted to 20 U·mL−1. One international unit (IU) is the amount of enzyme that hydrolyzes 1 µmol of substrate per minute in standard conditions. Lipase activity was calculated as mU per mg of protein.
All tests were conducted at 25 ± 0.5 °C using a microplate scanning spectrophotometer (Tecan Infinite 200 PRO, Tecan Austria).
To quantify protein concentration in proximal intestine homogenates, the Bradford (1976) method was applied with bovine serum albumin as the standard.
2.5. Lipid Peroxidation and Lipid Soluble Antioxidant System (l.s.a.s. with SOD, CAT, and GPx)
LPO levels were determined based on the concentration of malondialdehyde (MDA) calculated from a calibration curve [42]. Briefly, diluted samples were mixed with 5 µL of HCl 0.5 N and 50 µL of 0.12 M thiobarbituric acid (TBA) solution at pH 7. The mixture was heated to 95 °C for 10 min and then kept on ice for 5 min. Next, 300 µL of cold butanol were added and centrifuged for 10 min at 300× g and 4 °C (Eppendorf, 5418R). The absorbance of the supernatant was measured with a fluorimeter at 515–548 nm. MDA concentration was reported as pMol MDA per mg of protein.
SOD activity (EC 1.15.1.1) was determined by the ferricytochrome C method, with xanthine/xanthine oxidase as the source of superoxide radicals. A kinetic assay was performed according to Mccord and Fridovich [43], with some modifications. A reaction buffer was prepared, containing 50 mM potassium phosphate buffer at pH 7.8, 0.1 mM EDTA, 0.095 mM cytochrome C, and 0.015 mM xanthine. Afterwards, a diluted sample was mixed with xanthine oxidase (0.5 IU·mL−1) and the reaction buffer. Absorbance was measured at 550 nm. One unit of activity was defined as the amount of enzyme necessary to produce 50% inhibition of ferricytochrome C reduction rate. Activity was reported as U per mg of protein.
CAT activity (EC 1.11.1.6) was determined by measuring the decrease in H2O2 concentration at 240 nm. A kinetic assay was performed according to Aebi [44], with some modifications. The diluted sample was mixed with a reaction buffer containing 50 mM potassium phosphate buffer at pH 7.0 and 10 mM H2O2 One unit of enzyme activity was defined as the amount required to transform 1 µmol of substrate per minute under the above assay conditions. Activity was reported as U per mg of protein.
GPx activity (EC 1.11.1.9) was determined by measuring the NADPH consumption rate at 340 nm. A kinetic assay was performed according to Flohe and Gunzler [45], with some modifications. The following reaction mixture was loaded: 2 mM NADPH, Glutathione reductase (GR, 4 U·mL−1), H2O2 1 mM, diluted sample, GSH 40 mM, and a reaction buffer containing 50 mM potassium phosphate buffer at pH 7.2, sodium azide 2.66 mM, and EDTA 1.33 mM. One unit of enzyme activity was defined as the amount required to transform 1 µmol of substrate per minute under the above assay conditions. Activity was reported as U per mg of protein.
G6PDH activity (EC 1.1.1.49) was determined by a kit measuring the reduction of NADP+ at 340 nm (Spinreact, Sant Esteve d’en Bas, Girona, Spain) following the manufacturer’s recommendations. Activity was reported as mU per mg of protein.
All tests were conducted at 25 ± 0.5 °C using a microplate scanning spectrophotometer (Tecan Infinite 200 PRO, Tecan Austria).
To quantify protein concentration in proximal intestine homogenates, the Bradford (1976) method was applied with bovine serum albumin as the standard.
2.6. Statistical Analysis
Data were tested for normality by Shapiro-Wilk and homoscedasticity by Levene’s test, followed by an ANOVA and a post-hoc Tukey’s test to detect significant differences between experimental groups. For data that did not achieve normality, significant differences were then determined by Kruskal-Wallis H and Mann-Whitney U non-parametric tests. The software used was SPSS Statistics v25.0 (SPSS Inc., Chicago, IL, USA), and the one used for graphic representation was GraphPad 7.0 (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Growth Performance
The survival rate was calculated, and no significant differences were found between the experimental groups (87.8 ± 2.4%). No differences were found in final body weight, SGR, FCR, VFI, or PER among the experimental groups. Instead, K was significantly lower in L22S7 sea bass (Table 2), whereas HSI was significantly higher in sea bass fed low-protein diets.

Table 2.
Performance indicators when dietary protein was replaced by starch at levels above 20% or where lipid was included above 18%: final body weight (FBW), specific growth rate (SGR), condition factor (K), protein retention efficiency (PER), feed conversion ratio (FCR), voluntary feed intake (VFI), hepatosomatic index (HSI), and mesenteric fat index (MFI).
3.2. Anticipatory Digestive Enzyme Activities
Digestive enzyme activities and oxidative stress were studied in the proximal intestine 24 h post-feeding in sea bass fed with diets where protein and lipid were partially replaced by starch (P43S24, P40S29, and P36S36) or where lipid was included above 18% (L20S13 and L22S7).
Intestinal duct pH (Figure 1a) was between 6.92 ± 0.03 in P36S36 sea bass and 7.05 ± 0.01 in C fish, being above 7 in fish fed C and L22S7 diets. As pH affects enzyme activity, the protease activity for each experimental group was determined at the pH of its intestinal duct. Regarding TPA at 24 h post-feeding (Figure 1a), C and P43S24 sea bass showed a similar pattern of specific activity. Instead, a moderate decrease in dietary protein (P40S29) or an increase in dietary lipids (L20S13) provoked an up-regulation in anticipatory TPA versus that of C sea bass, with the increase being higher in the P40S29 fish (×2.95) than in the L20S13 fish (×1.51). By contrast, if dietary protein was limited (P36S36), the TPA was not significantly upregulated, and if dietary lipids were increased excessively (L22S7), the TPA presented a significant downregulation regarding the C group.
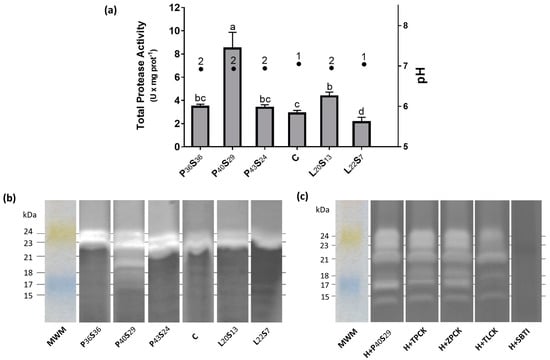
Figure 1.
(a) Total protease activity (TPA, gray bars) and pH (black dots) in the proximal intestine 24 h post-feeding in sea bass fed experimental diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%. Values are the mean ± SEM (n = 9). Significant differences in TPA between dietary conditions are shown by letters, and in pH by numbers (p < 0.05). (b) Model zymogram of protease activity in proximal intestine extract of sea bass fed diets when protein or lipid were modified. Figure 1 shows the molecular weight of each band with proteolytic activity and the molecular weight marker (MWM). All samples were analyzed individually; Figure 1 shows a representative result. (c) An inhibition zymogram made from the sample with the highest total protease activity in the proximal intestine of sea bass fed the P40S29 diet (22.69 U·mg prot−1). MWM = molecular weight marker; H = homogenate; TPCK and ZPCK = chymotrypsin activity modifiers; TLCK = trypsin inhibitor; SBTI = serine protease inhibitor.
Individual protease activity for each experimental group was characterized by zymography (Figure 1b). According to the TPA measured, P40S29 sea bass showed more activity in the zymogram than the other groups, presenting six different bands in a range from 24 to 15 KDa. Figure 1c shows the inhibition zymogram for P40S29 sea bass. The six bands detected had serine protease activity; the 23 and 17 kDa bands presented trypsin-like activity; and the 24, 21, 18, and 15 kDa bands had chymotrypsin-like activity. All the changes visually apparent in the inhibition zymogram were confirmed by Quantity One treatment. Zymography results and their quantification showed that the band with the highest proteolytic activity is that of 23 KDa with trypsin-like activity, followed by 24 and 21 KDa with chymotrypsin-like activity, and these three bands were detected in fish from all experimental conditions. 18 KDa chymotrypsin-like and 17 KDa trypsin-like activities present in P40S29 sea bass were also visualized as slight bands in P36S36 that presented intermediate TPA and L22S7 fish with the lowest TPA, whereas 15 KDa chymotrypsin-like activity was only detected in fish fed low protein diets (P40S29 and P36S36) (Figure 1b, Table 3).

Table 3.
Trypsin and chymotrypsin-like activities (U/mg prot−1) calculated from proximal intestine zymography analysis in sea bass fed experimental diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%.
Figure 2 shows amylase and lipase activities in the proximal intestine for each group. Significantly higher α-amylase activities (Figure 2a) were found in sea bass fed low protein diets (P36S36 and P40S29), being 73.4% and 309.6% higher than the C group, respectively. Animals fed 43–44% protein diets (P43S24, C, L20S13, and L22S7) showed similar amylase activity, regardless of their starch content (between 24 and 7%). Moreover, significant differences in lipase activity were found between sea bass fed low-protein diets (P36S36 and P40S29) and the C group. Furthermore, this enzyme activity tended to be more elevated in the P43S24, L20S13, and L22S7 groups versus C fish (Figure 2b).
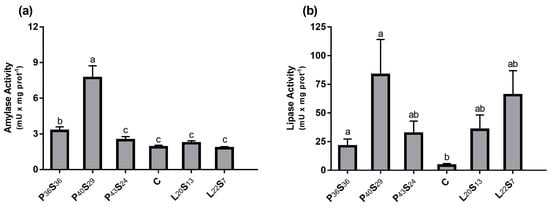
Figure 2.
(a) Amylase and (b) lipase activities in the proximal intestine 24 h post-feeding in sea bass fed experimental diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%. Values are the mean ± SEM (n = 9). Significant differences between dietary conditions are shown by letters (p < 0.05).
3.3. Lipid Peroxidation, the Lipid Soluble Antioxidant System, and Glucose-6-P-Dehydrogenase Enzyme Activities
The effects of dietary macronutrients on traditional markers of oxidative stress were also determined in the proximal intestine at 24 h post-feeding. Sea bass fed diets with 43–44% protein and 16–20% lipid (P43S24, C, and L20S13 fish) showed the lowest levels of LPO (Figure 3). However, both moderate substitution of dietary protein by starch (P40S29 fish) and increased dietary lipid (L22S7 fish) promoted a significant rise in LPO levels (+112.5% and +123.3%, respectively). When dietary protein was dropped to 36%, LPO tended to increase with respect to the C group (Figure 3).
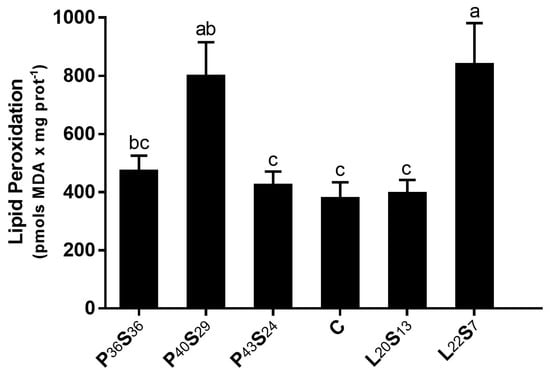
Figure 3.
Lipid peroxidation (LPO) in the proximal intestine 24 h post-feeding in sea bass fed experimental diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%. Values are the mean ± SEM (n = 9). Significant differences between dietary conditions are shown by letters (p < 0.05).
Antioxidant and G6PDH activities in the proximal intestine were determined (Figure 4). SOD and GPx activities were negatively affected by dietary low protein content, showing in P36S36 sea bass a significant reduction in their activities (−54.7% and −48.2%, respectively) relative to C fish (Figure 4a,b). CAT activity of the proximal intestine was higher than GPx activity by a factor of 102 (Figure 4c,b), suggesting that the former has a greater capacity to remove hydrogen peroxide. Regarding CAT activity, a significant increase was found in sea bass fed L20S13 and L22S7 diets versus C fish (+34.2% and +34.5%, respectively) (Figure 4c).
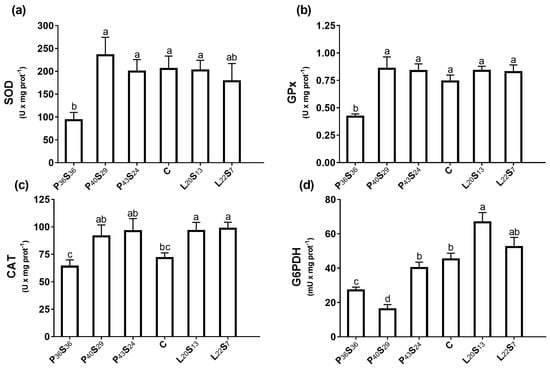
Figure 4.
(a) Superoxide dismutase (SOD), (b) glutathione peroxidase (GPx), (c) catalase (CAT), and (d) glucose-6-P-dehydrogenase (G6PDH) activities in the proximal intestine 24 h post-feeding in sea bass fed experimental diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%. Values are the mean ± SEM (n = 9). Significant differences between dietary conditions are shown by letters (p < 0.05).
Despite the trend toward increased CAT activity found in P40S29 and P43S24 groups, the higher individual variation prevents them from significantly differing from C sea bass. Moreover, CAT activity measured in the C and P36S36 groups was similar. Concerning G6PDH activity, an up-regulation was found in fish fed with high lipid content versus the C group (+47.9% in L20S13 fish and +15.9% in L22S7 fish); whereas in sea bass fed low protein and high starch diets, this enzymatic activity was significantly reduced versus the C fish by −64.5% in the P40S24 group and −39.9% in the P36S36 group (Figure 4d). Moreover, the proximal intestine of C sea bass presented the lowest CAT/GPx ratio, being significantly different from that calculated in P36S36 and P43S24 sea bass (Figure 5a).
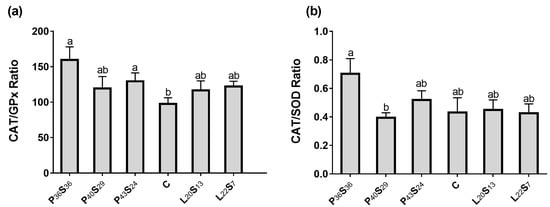
Figure 5.
(a) CAT/GPx and (b) CAT/SOD ratios in the proximal intestine 24 h post-feeding in sea bass fed experimental diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%. Values are the mean ± SEM (n = 9). Significant differences between dietary conditions are shown by letters (p < 0.05).
SOD, CAT, and GPx activities were normalized by LPO (Table 4). These ratios confirm that the oxidative status of C, P43S24, and L20S13 sea bass was similar. Instead, P36S36, P40S29, and L22S7 animals presented the lowest SOD/LPO, CAT/LPO, and GPx/LPO ratios, suggesting an antioxidant enzyme activity deficit regarding the LPO level found in the proximal intestine of these fish; the SOD activity of P36S36 sea bass was more significant, as was its high CAT/SOD ratio (Figure 5b).

Table 4.
SOD, CAT, and GPx activities normalized by LPO in the proximal intestine 24 h post-feeding in sea bass fed experimental diets where protein was replaced by starch at levels above 20% or where lipid was included above 18%.
Considering globally the oxidative stress markers studied in the proximal intestine, the P43S24 and L20S13 sea bass were able to maintain an oxidative status similar to that of C fish, increasing both groups CAT activity, and the L20S13 fish also their G6PDH activity. Instead, despite the high levels of proximal intestine LPO when sea bream was fed a diet with lower protein (P40S29) or high lipid content (L22S7), only an up-regulation of CAT activity was found. In fact, P40S29 sea bass presented the lowest GP6PDH activity. On the contrary, fish fed the protein-limiting diet (P36S36) showed a significant reduction in activity of the antioxidant enzymes studied, although their LPO tended to increase.
4. Discussion
Ingredients, macronutrient composition, and their ratios in the diet can affect the organism’s response against oxidative stress [37,46] and digestive capacities [12,24,27,28]. These processes are related to the protein-sparing effect, and their knowledge could lead to the improvement of sea bass diet formulation, which is crucial in achieving economically efficient and sustainable production systems in fish [47].
In the present study, fish fed low-protein diets did not show differences among the other experimental groups for final body weight; SGR and PER may have been involved because of the higher anticipatory digestive enzyme activities in P40S29 sea bass and the increase in anticipatory lipase and α-amylase activity in P36S36. This fact could be related to the initial body weight (95.4 ± 0.4 g), since growth parameters were more sensitive to dietary composition in the fingerlings and early juveniles, stages in which significant changes were detected [19,20,48]. The condition of the fish is indicated in terms of condition factor, MFI, and HSI. Thus, high lipid content significantly affects the condition factor (L22S7) and tends to increase the MFI (L20S13), as was also found in other species [49]. Instead, fish fed low-protein diets presented a significantly higher HSI, as has been described by Kim and coworkers in Oplegnathus fasciatus [48], probably due to excessive glycogen deposition caused by higher dietary carbohydrate inclusion.
At 24 h post-feeding, the digestive activity detected is food-anticipatory activity that confers an adaptative regulation, so in the proximal intestine, the activity detected comes from the pancreatic enzyme release that takes place in the pyloric caeca region and reaches the proximal intestine [12,24]. Thus, an increase in these digestive activities could indicate an imbalance in digestion or absorption of the nutrients provided by the diet, which the fish try to compensate for by increasing digestive enzyme secretion to improve food acquisition and utilization and maintain growth [12]. When P40S29 and C groups are compared, the former showed a significant increase in TPA, which points to the idea that moderately low protein diets act as secretagogues, stimulating the anticipatory release of proteases. The present results in 95 g initial weight sea bass juveniles agreed with those found in a previous study in this species fingerlings, despite the difference in size [19]. In fact, in sea bass fingerlings, the effects of different protein-to-lipid ratios were studied, and higher TPA anticipatory activity was detected in fish fed diets with a 40:20 dietary protein-to-lipid ratio. Moreover, in European sea bass, like in sea bream [20], digestive enzyme activities diminished as fish grew according to the decline in protein requirements [50,51], but compensatory mechanisms related to the improvement of digestion are still evident in sea bass above 200 g, as the present data pointed out. It is also necessary to consider that both 36 and 40% of dietary protein are considered low for sea bass juveniles [13], whereas for P43S24 and C groups, the protein content was inside the range adequate for this species [11,13]. Despite this, sea bass fed a P36S36 diet, on the contrary to P40S29, showed similar anticipatory activity to those found in P43S24 and C groups. This fact could be related to the progressive increase in the total protease activity depending on the dietary protein described in sea bream when the diet protein content rose from 35 to 41% in a post-prandial study [24].
As it happened for protein, a slight increase in the dietary percentage of lipid upregulated TPA anticipatory activity in the L20S13 animals. Similar results were obtained in sea bream juveniles fed diets with moderate lipid content 7 h post-feeding [25], where the trypsin/chymotrypsin ratio tended to increase versus fish fed 17% lipid diets. Nevertheless, sea bass fed a L22S7 diet presented the lowest protease anticipatory activity. These animals were fed a diet containing the highest levels of total lipids (22.3%) and cellulose (12.5%). Dietary lipid and fiber could be related to opposite changes in the intestinal transit rate, and digestion time could make adaptive regulation of TPA unnecessary to maintain growth. In sea bream fed diets with 23% of dietary lipid and 11% of fiber, total protease activity in the proximal intestine was higher at 7 h post-feeding, probably due to a slow transit rate, suggesting that this elevated TPA could counteract the negative effect that lipid can have on protease activity [25], an idea that would reinforce the results obtained in this study.
Results from the proximal intestine pH show values close to those reported as normal in the literature [24,25,52]. Moreover, it is known that slight changes in intestinal duct pH can affect digestive enzyme activity considerably [53,54]. The differences found in intestinal pH between C and L22S7 fish and the other experimental groups can be attributed to slight changes in dietary proximate composition. In this sense, Lawlor et al. [55] showed that dietary ingredients can affect the buffering capacities of animals. A similar trend was also observed in sea bream fed with experimental diets where lipids were replaced by starch [25]. Data suggest that when protein requirements are met, both lipid and fiber dietary inclusions are able to modulate TPA anticipatory activity to improve the digestion process, allowing all groups to grow at comparable rates, as has been previously described in other carnivorous species [18,20,24,25].
Zymograms showed the activity of six proteases: 24, 21, 18, and 15 kDa with chymotrypsin activity and 23 and 17 kDA with trypsin activity in sea bass over 200 g. Individual protease profiles vary considerably between different developmental stages. Thus, García-Meilán et al. [19] previously reported activities of 23, 22, 20, and 17 kDa trypsins and an 18 kDa chymotrypsin in European sea bass fingerlings, but activities of 24, 21, and 15 kDa chymotrypsins in this species are reported in this paper for the first time. Irrespective of the administered diet, sea bass presented the high molecular weight proteases (24, 23, and 21 KDa). These were the only bands detected in C, P43S24, and L20S13 animals that presented low anticipatory levels of TPA. Moreover, a slight presence of proteolytic bands with lower molecular weights was detected in sea bass with intermediate and low TPA values (P36S36 and L22S7), and light bands were detected in animals P40S29 that had the highest anticipatory TPA. This suggests that a higher number of different proteases, presented in zymograms with different widths and/or definitions, were released by the pancreas as anticipatory proteolytic activity increased. Chymotrypsin-like (24 and 21 KDa) and trypsin-like (23 KDa) were constitutive proteases, since they were present in all experimental groups. Instead, a sequential release pattern related to the amount of TPA was observed in chymotrypsin-like (18 and 15 KDa) and trypsin-like (17 KDa) activities. These proteases release profile was different from that found in sea bream, where the same pattern has been detected with differences in the width and luminosity of the bands regardless of diet, size, or time post-ingesta [12,24,25].
Protease activities obtained in the present study were of the same magnitude as those obtained in sea bass fingerlings by García-Meilán et al. [19], but lipase activities were much higher in the proximal intestine of fingerlings (almost tenfold). In fact, it is known that younger fish have higher digestive enzyme activity, mainly lipase [20]. As it had been previously described in other species [12,24,27], lipase activity presented high individual variation. Despite this, and similar to the present study, a tendency to increase this enzyme activity was observed in sea bream as dietary lipids increased above 20%, and a similar profile was found in fish fed diets with moderate protein content (42%), enhancing lipid digestion to spare protein [25].
Castro et al. [37] found low values of α-amylase activity 24 h post-feeding in the anterior intestine of sea bass fed up to 20% of dietary starch. In the present study, slightly lower activity of this enzyme was found in sea bass fed diets containing up to 24% of dietary starch. These differences between the two studies could be due to the lower FM content in the present experiment (20% versus 63.3%) and differences in protein (43–44% versus 46%) and/or lipid dietary content (20% or more versus 18%). Moreover, an increase in α-amylase activity was detected in sea bass fingerlings fed with low starch diets [20]. In a similar study, conducted in sea bream, the same response was observed, but of a greater magnitude, which could be explained by different feeding habits since sea bass is strictly carnivorous whereas sea bream is an omnivorous species [25]. Furthermore, in sea bream, low-protein diets (35% protein) lead to an up-regulation in α-amylase 5 h after feeding [24], reinforcing the idea that fish need to improve digestion processes to achieve the same growth. In the present study, P36S36 and especially P40S24 sea bass presented an up-regulation in α-amylase activity.
The intestine has a high cell turnover that makes it more susceptible to oxidative stress [37]. In this sense, dietary composition promotes changes that could lead to an imbalance in the intestinal oxidative status due to low l.s.a.s. to cope with the LPO produced. These changes, in turn, can directly affect the integrity and fluidity of the membrane of the enterocytes that constitute the barrier, making both intestinal brush border nutrient digestion and absorption difficult [56].
Most of the studies related to antioxidant capacity by environmental or nutritional factors have been carried out in the liver, due to its involvement in metabolism and detoxification [15,57,58,59,60,61,62,63,64,65]. However, the intestine acts as a barrier, preventing the entry of possible pathogens, in addition to being the place where digestion and absorption processes take place. In this sense, effects of diet composition on oxidative status seem to be species-specific and tissue-specific; the liver is a more sensitive tissue to changes than the intestine, and the response against oxidative stress in both organs can be different in European sea bass and in sea bream [17,37]. Nevertheless, intestinal oxidative stress in relation to nutritional status has hardly been studied, despite the negative effects it may have on enterocyte membranes, affecting nutrient digestion and absorption [66,67,68].
Dietary lipid content and its level of unsaturation increase lipid peroxidation [36]. Several studies reported that LPO levels at the intestinal level were lower in fish-fed diets containing vegetable oils than those fed with fish oil [17,37,69]. However, in the present study, most of the dietary lipids (88–94%) come from fish oil and, to a lesser extent, from fish meal, which are rich in EPA and DHA and prone to being easily oxidized. According to this, fish fed a L22S7 diet presented high MDA levels, whereas in L20S13, those were similar to C fish. Furthermore, we are not aware of any studies that have determined the effects that the main composition of the diet may have on intestinal oxidative stress when fish were fed with low levels of FM (20% in all diets). In the liver, dietary carbohydrates administration was associated with oxidative stress protection in European sea bass up to 20% of inclusion [17,37,58], in common dentex between 24% and 28% [61,64] and in yellow catfish with inclusion levels of up to 38% [70], probably because a higher carbohydrate content in the diet implies a lower lipid or protein content, while in Senegalese sole this protection effect was not found [57]. In the intestine, carbohydrates did not have oxidative stress preventive effects, neither in gilthead sea bream and European sea bass fed diets up to 20% starch inclusion [37,69] nor in sea bass fed diets up to 24% starch inclusion (present work); but C, P43S24, and L20S13 fish can control LPO. Instead, the P40S29 fish proximal intestine tended to trigger LPO, despite having l.s.a.s. (SOD, CAT, and GPx) similar to those found in C, P43S24, and L20S13. Accordingly, higher peroxidation levels were also found in the plasma of rats fed a protein-deficient diet [71]. Moreover, a deficient protein diet could also be associated with a decrease in l.s.a.s. synthesis and higher superoxide anion release [72], as also described in this study for animals P36S36. Furthermore, a higher peroxidation level could also be related to less oxidative reducing power since the intestine is a major consumer of GSH and an adequate GSSG:GSH ratio must be maintained [73]. The key enzyme in this process is G6PDH, which provides the NADPH required for GSH reduction [35,36,74]. In the present study, G6PDH activity decreased as dietary starch content rose. Data reinforce the low G6PDH activity at intestinal levels found in sea bream fed diets containing 20% carbohydrates [73]. Moreover, NADPH, produced by G6PDH, also contributes to the recycling of other antioxidant systems involving thioredoxins, glutaredoxins, and peroxiredoxins [75] and maintains the activity of an important ROS-detoxifying enzyme: CAT [76]. The higher levels of LPO in P40S29 could be due to lower GSH availability, and the GPx and CAT activities detected could be downregulated by lower NADPH production through G6PDH. Instead, low G6PDH and lower antioxidant enzyme activities found in P36S36 did not affect LPO production.
Higher MDA levels could indicate an overproduction of ROS or reduced efficacy of the l.s.a.s. [70], and both the antioxidant enzyme activities and these normalized by LPO levels can be helpful to clarify if a specific diet could be associated with a deficit of a certain antioxidant enzyme. In this sense, moderate and severe limitations of dietary protein content or a higher level of lipid contribute to a lower detoxification capacity of superoxide anion in the proximal intestine of sea bass.
The main by-product of the SOD catalytic reaction is hydroperoxide (ROOH), which in turn can be detoxified through different pathways by both CAT and GPx; with GPx being induced by the low H2O2 levels in the basal antioxidant situation and CAT being preferentially activated by high H2O2 production responding to an oxidative stress situation [16,36,77]. This idea was reinforced by the present results, where CAT activity was 100× higher than GPx. In fact, the elimination of hydrogen peroxide via CAT is more energy efficient since it is independent of glutathione recycling [78]. Thereby, it could be expected that the proximal intestine of sea bass fed with the different tested diets would show preference for one or another detoxification route, making the ratio CAT/GPx a good alternative to evaluate this trait. In this sense, the proximal intestine of the C fish showed the lower value for this ratio. Instead, the proximal intestine of P36S36 sea bass presented an elevated CAT/GPx ratio and an altered CAT/SOD ratio, suggesting that the P36S36 diet can induce oxidative damage in the intestine [79,80].
5. Conclusions
In conclusion, the present study shows the effects of different levels of dietary macronutrients on anticipatory digestive enzymes and oxidative status in the proximal intestine of European sea bass. Data reveal that the inclusion of carbohydrates up to 24% or lipids up to 20% is possible for this species if protein requirements are met without negative effects on the digestive enzymes’ activities or oxidative status. This knowledge could lead to the improvement of sea bass diet formulation, which is crucial to achieving economically efficient and sustainable production systems in fish.
Author Contributions
Conceptualization, R.F. and Á.G.; methodology, all authors carried out the sampling; I.G.-M., J.I.H.-M., B.O.-G. and Á.G. performed laboratory analyses; I.G.-M., J.I.H.-M. and Á.G. analyzed and interpreted the data; I.G.-M., J.I.H.-M. and Á.G. writing—original draft preparation, writing—review, editing, drafted and critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Ministerio de Ciencia, Innovación y Universidades” (MCIUN) through the project: Modulation of skin mucus properties by functional factors and culture conditions in sea bream and trout: regulation, control, and applications in aquaculture, PID2019-106878-RB-I00. IP: Antonio Ibarz Valls 01/06/2020-31/05/2024, and the “Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR). Generalitat de Catalunya” (2017SGR-1574). The project leading to these results has received funding from the “la Caixa” Foundation (ID 100010434), under agreement (LCF/PR/PR06/51010001). The authors would also like to acknowledge the financial support given to JIHM by the University of Costa Rica through the Postgraduate Studies Abroad scholarship.
Institutional Review Board Statement
The animal study protocol was approved by the Experimental Animal Commission of the Generalitat de Catalunya (Project 11172) following the principles and legislation assigned by the European Union (Directive 2010/63/EU).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the personnel from IRTA for the maintenance of the fish during the in vivo trial.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- The State of Food and Agriculture 2020; FAO: Rome, Italy, 2020.
- Pinotti, L.; Giromini, C.; Ottoboni, M.; Tretola, M.; Marchis, D. Review: Insects and former foodstuffs for upgrading food waste biomasses/streams to feed ingredients for farm animals. Animal 2019, 13, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Robaina, L.; Caballero, M.J.; Montero, D.; Calandra, G.; Mompel, D.; Karalazos, V.; Kaushik, S.; Izquierdo, M.S. Combined replacement of fishmeal and fish oil in European sea bass (Dicentrarchus labrax): Production performance, tissue composition and liver morphology. Aquaculture 2017, 474, 101–112. [Google Scholar] [CrossRef]
- Campos, I.; Matos, E.; Maia, M.R.G.; Marques, A.; Valente, L.M.P. Partial and total replacement of fish oil by poultry fat in diets for European seabass (Dicentrarchus labrax) juveniles: Effects on nutrient utilization, growth performance, tissue composition and lipid metabolism. Aquaculture 2019, 502, 107–120. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Messina, M.; Bruno, M.; Tulli, F.; Poli, B.M.; Giorgi, G.; Chini-Zittelli, G.; Tredici, M.; Tibaldi, E. Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 2018, 485, 173–182. [Google Scholar] [CrossRef]
- Eroldoğan, T.; Turchini, G.M.; Yılmaz, A.H.; Taşbozan, O.; Engin, K.; Ölçülü, A.; Özşahinoğlu, I.; Mumoğullarında, P. Potential of cottonseed oil as fish oil replacer in European sea bass feed formulation. Turk. J. Fish. Aquat. Sci. 2012, 12, 787–797. [Google Scholar] [CrossRef]
- Peixoto, M.J.; Magnoni, L.; Gonçalves, J.F.M.; Twijnstra, R.H.; Kijjoa, A.; Pereira, R.; Palstra, A.P.; Ozório, R.O.A. Effects of dietary supplementation of Gracilaria Sp. extracts on fillet quality, oxidative stress, and immune responses in European seabass (Dicentrarchus labrax). J. Appl. Phycol. 2019, 31, 761–770. [Google Scholar] [CrossRef]
- Silva-Brito, F.; Timoteo, F.; Magnoni, L. Impact of the replacement of dietary fish oil by animal fats and environmental salinity on the metabolic response of European seabass (Dicentrarchus labrax). Comp. Biochem. Physiol. 2019, 233, 46–59. [Google Scholar] [CrossRef]
- Medale, F.; Le Boucher, R.; Panserat, S. Plant based diets for farmed fish. INRA Prod. Anim. 2013, 26, 303–315. [Google Scholar]
- Tibaldi, E.; Hakim, Y.; Uni, Z.; Tulli, F.; de Francesco, M.; Luzzana, U.; Harpaz, S. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax). Aquaculture 2006, 261, 182–193. [Google Scholar] [CrossRef]
- Tibaldi, E.; Chini Zittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freeze-dried Isochrysis Sp. (T-ISO) biomass as a source of protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Gallardo, M.A. Meal timing affects protein-sparing effect by carbohydrates in sea bream: Effects on digestive and absorptive processes. Aquaculture 2014, 434, 121–128. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Sether, B.S.; Albrektsen, S.; Noble, C. Review on European sea bass (Dicentrarchus labrax, Linnaeus, 1758) nutrition and feed management: A practical guide for optimizing feed formulation and farming protocols. Aquac. Nutr. 2015, 21, 129–151. [Google Scholar] [CrossRef]
- Vandeputte, M.; Gagnaire, P.A.; Allal, F. The European sea bass: A key marine fish model in the wild and in aquaculture. Anim. Genet. 2019, 50, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Furné, M.; García-Gallego, M.; Hidalgo, M.C.; Morales, A.E.; Domezain, A.; Domezain, J.; Sanz, A. Oxidative stress parameters during starvation and refeeding periods in Adriatic sturgeon (Acipenser naccarii) and rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2009, 15, 587–595. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Halliwell, B. Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010, 393, 561–564. [Google Scholar] [CrossRef]
- Castro, C.; Diógenes, A.F.; Coutinho, F.; Panserat, S.; Corraze, G.; Pérez-Jiménez, A.; Peres, H.; Oliva-Teles, A. Liver and intestine oxidative status of gilthead sea bream fed vegetable oil and carbohydrate rich diets. Aquaculture 2016, 464, 665–672. [Google Scholar] [CrossRef]
- Bakke, A.M.; Glover, C.; Krogdahl, A. Feeding, digestion and absorption of nutrients. In Multifunctional Gut of Fish; Grosell, M., Farrell, A., Brauner, C., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 57–75. [Google Scholar]
- García-Meilán, I.; Ordóñez-Grande, B.; Machahua, C.; Buenestado, S.; Fontanillas, R.; Gallardo, M.A. Effects of dietary protein-to-lipid ratio on digestive and absorptive processes in sea bass fingerlings. Aquaculture 2016, 463, 163–173. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Valentín, J.M.; Hernández, M.D.; García, B.; Fontanillas, R.; Gallardo, M.A. Modulation of digestive and absorptive processes with age and/or after a dietary change in gilthead sea bream. Aquaculture 2016, 459, 54–64. [Google Scholar] [CrossRef]
- Santigosa, E.; Sánchez, J.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J.; Gallardo, M.A. Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 282, 68–74. [Google Scholar] [CrossRef]
- Furné, M.; Hidalgo, M.C.; López, A.; García-Gallego, M.; Morales, A.E.; Domezain, A.; Domezainé, J.; Sanz, A. Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A Comparative Study. Aquaculture 2005, 250, 391–398. [Google Scholar] [CrossRef]
- Magalhães, R.; Díaz-Rosales, P.; Diógenes, A.F.; Enes, P.; Oliva-Teles, A.; Peres, H. Improved digestibility of plant ingredient-based diets for European seabass (Dicentrarchus labrax) with exogenous enzyme supplementation. Aquac. Nutr. 2018, 24, 1287–1295. [Google Scholar] [CrossRef]
- García-Meilán, I.; Valentín, J.M.; Fontanillas, R.; Gallardo, M.A. Different protein to energy ratio diets for gilthead sea bream (Sparus aurata): Effects on digestive and absorptive processes. Aquaculture 2013, 412–413, 1–7. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Valentín, J.M.; Fontanillas, R.; Gallardo, Á. High dietary carbohydrate inclusion by both protein and lipid replacement in gilthead sea bream. Changes in digestive and absorptive processes. Aquaculture 2020, 520, 734977. [Google Scholar] [CrossRef]
- Santigosa, E.; Sáenz de Rodrigáñez, M.Á.; Rodiles, A.; Barroso, F.G.; Alarcón, F.J. Effect of diets containing a purified soybean trypsin inhibitor on growth performance, digestive proteases and intestinal histology in juvenile sea bream (Sparus aurata L.). Aquac. Res. 2010, 41, e187–e198. [Google Scholar] [CrossRef]
- Santigosa, E.; García-Meilán, I.; Valentín, J.M.; Navarro, I.; Pérez-Sánchez, J.; Gallardo, M.Á. Plant oils’ inclusion in high fish meal-substituted diets: Effect on digestion and nutrient absorption in gilthead sea bream (Sparus aurata L.). Aquac. Res. 2011, 42, 962–974. [Google Scholar] [CrossRef]
- Santigosa, E.; García-Meilán, I.; Valentin, J.M.; Pérez-Sánchez, J.; Médale, F.; Kaushik, S.; Gallardo, M.A. Modifications of intestinal nutrient absorption in response to dietary fish meal replacement by plant protein sources in sea bream (Sparus aurata) and rainbow trout (Onchorynchus mykiss). Aquaculture 2011, 317, 146–154. [Google Scholar] [CrossRef]
- Dias, J.; Alvarez, M.J.; Diez, A.; Arzel, J.; Corraze, G.; Bautista, J.M.; Kaushik, S.J.; Francé, F. Regulation of hepatic lipogenesis by dietary protein energy in juvenile European seabass (Dicentrarchus labrax). Aquaculture 1998, 161, 169–186. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Influence of temperature on protein utilization in juvenile European seabass (Dicentrarchus labrax). Aquaculture 1999, 170, 337–348. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Effect of dietary lipid level on growth performance and feed utilization by European sea bass juveniles (Dicentrarchus labrax). Aquaculture 1999, 179, 325–334. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Dietary carbohydrate utilization by European sea bass (Dicentrarchus labrax L.) and gilthead sea bream (Sparus aurata L.) juveniles. Rev. Fish. Sci. 2011, 19, 201–215. [Google Scholar] [CrossRef]
- Marnett, L.J.; Hancock, A.B. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res.-Fundam. Mol. Mech. Mutagen 1999, 424, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Babaei, S.; Abedian Kenari, A.; Hedayati, M.; Yazdani Sadati, M.A.; Metón, I. Effect of diet composition on growth performance, hepatic metabolism and antioxidant activities in Siberian sturgeon (Acipenser baerii, Brandt, 1869) submitted to starvation and refeeding. Fish. Physiol. Biochem. 2016, 42, 1509–1520. [Google Scholar] [CrossRef]
- Pandolfi, P.P.; Sonati, F.; Rivi, R.; Mason, P.; Grosveld, F.; Luzzatto, L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995, 14, 5209–5215. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Castro, C.; Peréz-Jiménez, A.; Coutinho, F.; Díaz-Rosales, P.; Serra, C.A.D.R.; Panserat, S.; Corraze, G.; Peres, H.; Oliva-Teles, A. Dietary carbohydrate and lipid sources affect differently the oxidative status of European sea bass (Dicentrarchus labrax) juveniles. Br. J. Nutr. 2015, 114, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Lupatsch, I.; Kissil, G.W. Predicting aquaculture waste from gilthead sea bream (Sparus aurata) culture using a nutritional approach. Aquac. Living Resour. 1998, 11, 265–268. [Google Scholar] [CrossRef]
- García-Carreño, F.L.; Dimes, L.E.; Haard, N.F. Substrate gel-electroforesis for composition and molecular-weight of proteinases of proteinaceous proteinase-inhibitors. Anal Biochem 1993, 214, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, F.J.; Diaz, M.; Moyano, F.J.; Abellan, E. Characterization and functional properties of digestive proteases in two sparids; gilthead seabream (Sparus aurata) and common dentex (Dentex dentex). Fish. Physiol. Biochem. 1998, 19, 257–267. [Google Scholar] [CrossRef]
- Diaz, M.; Moyano, F.J.; Garcia Carreno, F.L.; Alarcon, F.J.; Sarasquete, M.C. Substrate-SDS-PAGE determination of protease activity through larval development in sea bream. Aquac. Int. 1997, 5, 461–471. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Mccords, J.M.; Fridovich, I. Superoxide dismutase an enzymic function for erythrocuprein-hemocuprein. J. Biol. Chem 1969, 244, 6049–6055. [Google Scholar]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W. Assays of glutathione-peroxidase. Methods Enzym. 1984, 105, 114–121. [Google Scholar]
- Babaei, S.; Abedian-Kenari, A.; Hedayati, M.; Yazdani-Sadati, M.A. Growth response, body composition, plasma metabolites, digestive and antioxidant enzymes activities of Siberian sturgeon (Acipenser baerii, Brandt, 1869) fed different dietary protein and carbohydrate: Lipid ratio. Aquac. Res. 2017, 48, 2642–2654. [Google Scholar] [CrossRef]
- Medale, F.; Kaushik, S. Evolution of INRA research in the field of fish nutrition: Exploring alternatives to marine fishery-derived ingredients. Prod. Anim. 2008, 21, 87–94. [Google Scholar]
- Kim, K.W.; Moniruzzaman, M.; Kim, K.D.; Han, H.S.; Yun, H.; Lee, S.; Bai, S.C. Effects of dietary protein levels on growth performance and body composition of juvenile parrot fish, Oplegnathus fasciatus. Int. Aquat. Res. 2016, 8, 239–245. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Du, Z.Y.; Wang, Y.; Yang, H.J. Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture 2005, 249, 439–447. [Google Scholar] [CrossRef]
- Masser, M.P.; Grant, W.E.; Neill, W.H.; Robinson, E.H. A simulation model representing effects on dietary energy/protein ratio and water temperature on growth of channel catfish (Ictalurus punctatus). Ecol. Model. 1991, 53, 17–35. [Google Scholar] [CrossRef]
- Kaushik, S.; Luquet, P. Relationship between protein-intake and voluntary energy-intake as affected by body-weight with an estimation of maintenance needs in rainbow-trout. Z. Tierphysiol. Tierernahr. Futterm. 1984, 51, 57–69. [Google Scholar] [CrossRef]
- Nikolopoulou, D.; Moutou, K.A.; Fountoulaki, E.; Venou, B.; Adamidou, S.; Alexis, M.N. Patterns of gastric evacuation, digesta characteristics and pH changes along the gastrointestinal tract of gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 406–414. [Google Scholar] [CrossRef]
- Deguara, S.; Jauncey, K.; Agius, C. Enzyme activities and pH variations in the digestive tract of gilthead sea bream. J. Fish. Biol. 2003, 62, 1033–1043. [Google Scholar] [CrossRef]
- Guillaume, J.; Choubert, G. Digestive physiology and nutrient digestibility in fishes. In Nutrition and Feeding of Fish and Crustaceans; Guillaume, J., Ed.; Springer Praxis: Chichester, UK, 2001. [Google Scholar]
- Lawlor, P.G.; Lynch, P.B.; Caffrey, P.J.; O’Reilly, J.J.; O’Connell, M.K. Measurements of the acid-binding capacity of ingredients used in pig diets. Ir. Vet. J. 2005, 58, 447–452. [Google Scholar] [CrossRef]
- Morais, S.; Silva, T.; Cordeiro, O.; Rodrigues, P.; Guy, D.R.; Bron, J.E.; Taggart, J.B.; Bell, J.G.; Tocher, D.R. Effects of genotype and dietary fish oil replacement with vegetable oil on the intestinal transcriptome and proteome of Atlantic salmon (Salmo salar). BMC Genom. 2012, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Pérez-Jiménez, A.; Guerreiro, I.; Peres, H.; Castro-Cunha, M.; Oliva-Teles, A. Effects of temperature and dietary protein level on hepatic oxidative status of Senegalese sole juveniles (Solea senegalensis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Peréz-Jiménez, A.; Coutinho, F.; Corraze, G.; Panserat, S.; Peres, H.; Teles, A.O.; Enes, P. Nutritional history does not modulate hepatic oxidative status of European sea bass (Dicentrarchus labrax) submitted to handling stress. Fish. Physiol. Biochem. 2018, 44, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Kjær, M.A.; Aursnes, I.A.; Berge, G.M.; Sørensen, M.; Marchenko, Y.; Gjøen, T.; Ruyter, B. The influence of different dietary oil qualities on growth rate, feed utilization and oxidative stress in Atlantic cod. Aquac. Nutr. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Morales, A.E.; Pérez-Jiménez, A.; Carmen Hidalgo, M.; Abellán, E.; Cardenete, G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 153–161. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Hidalgo, M.C.; Morales, A.E.; Arizcun, M.; Abellán, E.; Cardenete, G. Antioxidant enzymatic defenses and oxidative damage in Dentex dentex fed on different dietary macronutrient levels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 537–545. [Google Scholar] [CrossRef]
- Saera-Vila, A.; Benedito-Palos, L.; Sitjà-Bobadilla, A.; Nácher-Mestre, J.; Serrano, R.; Kaushik, S.; Pérez-Sánchez, J. Assessment of the health and antioxidant trade-off in gilthead sea bream (Sparus aurata L.) fed alternative diets with low levels of contaminants. Aquaculture 2009, 296, 87–95. [Google Scholar] [CrossRef]
- Giannetto, A.; Fernandes, J.M.O.; Nagasawa, K.; Mauceri, A.; Maisano, M.; De Domenico Cappello, E.T.; Oliva, S.; Fasulo, S. Influence of continuous light treatment on expression of stress biomarkers in Atlantic cod. Dev. Comp. Immunol. 2014, 44, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, A.; Abellán, E.; Arizcun, M.; Cardenete, G.; Morales, A.E.; Hidalgo, M.C. Dietary carbohydrates improve oxidative status of common dentex (Dentex dentex) juveniles, a carnivorous fish species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 203, 17–23. [Google Scholar] [CrossRef]
- Sánchez-Nuño, S.; Sanahuja, I.; Fernández-Alacid, L.; Ordóñez-Grande, B.; Carbonell, T.; Ibarz, A. Oxidative attack during temperature fluctuation challenge compromises liver protein homeostasis of a temperate fish model. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 236, 110311. [Google Scholar] [CrossRef]
- Enes, P.; Pérez-Jiménez, A.; Peres, H.; Couto, A.; Pousão-Ferreira, P.; Oliva-Teles, A. Oxidative status and gut morphology of white sea bream, Diplodus sargus fed soluble non-starch polysaccharide supplemented diets. Aquaculture 2012, 358–359, 79–84. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, D.; Zhou, X.Q.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.D.; Zhao, Y. Effects of glutamate on growth, antioxidant capacity, and antioxidant-related signaling molecule expression in primary cultures of fish enterocytes. Fish. Physiol. Biochem. 2015, 41, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, H.; Mokrani, A.; Ji, K.; Yu, H.; Ge, X.; Ren, M.; Xie, J.; Pan, L.; Sun, A. Dietary histidine affects intestinal antioxidant enzyme activities, antioxidant gene expressions and inflammatory factors in juvenile blunt Snout bream (Megalobrama amblycephala). Aquac. Nutr. 2019, 25, 249–259. [Google Scholar] [CrossRef]
- Gao, J.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Ren, T.; Komilus, C.F.; Han, Y. Effects of dietary palm oil supplements with oxidized and non-oxidized fish oil on growth performances and fatty acid compositions of juvenile Japanese sea bass, Lateolabrax japonicus. Aquaculture 2012, 324–325, 97–103. [Google Scholar] [CrossRef]
- Wang, L.N.; Liu, W.B.; Lu, K.L.; Xu, W.N.; Cai, D.S.; Zhang, C.N.; Qian, Y. Effects of dietary carbohydrate/lipid ratios on non-specific immune responses, oxidative status and liver histology of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2014, 426–427, 41–48. [Google Scholar] [CrossRef]
- Prada, F.J.A.; Macedo, D.V.; Mello, M.A.R. Evaluation of a protein deficient diet in rats through blood oxidative stress biomarkers. Res. Commun. Mol. Pathol. Pharmacol. 2003, 113, 213–228. [Google Scholar] [PubMed]
- Arrigo, T.; Leonardi, S.; Cuppari, C.; Manti, S.; Lanzafame, A.; D’Angelo, G.; Gitto, E.; Marseglia, L.; Salpietro, C. Role of the diet as a link between oxidative stress and liver diseases. World J. Gastroenterol. 2015, 21, 384–395. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Rufino-Palomares, E.; Ordóñez-Grande, B.; Gallardo, M.A.; Oliva-Teles, A.; Peres, H. Dietary glutamine supplementation effects on amino acid metabolism, intestinal nutrient absorption capacity and antioxidant response of gilthead sea bream (Sparus aurata) juveniles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 9–17. [Google Scholar] [CrossRef]
- Jain, S.K.; Palmer, M. Effect of glucose-6-phosphate dehydrogenase deficiency on reduced and oxidized glutathione and lipid peroxide levels in the blood of African-Americans. Clin. Chim. Acta Int. J. Clin. Chem. 1996, 253, 181–183. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Hillar, A.; Nicholls, P. A Mechanism for NADPH inhibition of catalase compound II formation. FEBS Lett. 1992, 314, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Box, A.; Sureda, A.; Galgani, F.; Pons, A.; Deudero, S. Assessment of environmental pollution at Balearic islands applying oxidative stress biomarkers in the mussel Mytilus galloprovincialis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxidative Med. Cell. Longev. 2010, 3, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Agostini, J.F.; Toé, H.C.Z.D.; Vieira, K.M.; Baldin, S.L.; Costa, N.L.F.; Cruz, C.U.; Longo, L.; Machado, M.M.; da Silveira, T.R.; Schuck, P.F.; et al. Cholinergic system and oxidative stress changes in the brain of a zebrafish model chronically exposed to ethanol. Neurotox. Res. 2018, 33, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Fridovich, I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982, 257, 5751–5754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).