Every Fish Counts: Challenging Length–Weight Relationship Bias in Discards
Abstract
1. Introduction
- (1)
- Informing the development of size-based regulations: length–weight relationships can be used to determine the size at which a fish species reaches maturity [17]. This information can inform the establishment of minimum landing sizes, helping to protect juveniles and ensuring that fish can reproduce before being harvested [25].
- (2)
- Assessing the impact of fishing practices: comparing the length–weight relationships of discarded and retained fish can reveal the effects of fishing practices on size distribution and species composition [26]. This knowledge can be used to modify fishing gear, practices, or policies to minimize the capture of unwanted species or sizes, reducing discards and promoting sustainability.
- (3)
- Monitoring ecosystem health: regular analysis of length–weight relationships can provide insights into the overall health of an ecosystem [27]. Shifts in these relationships may signal changes in the abundance, growth, or reproductive success of a species, which could be linked to the impacts of fishing or other anthropogenic pressures [28]. Tracking these changes can inform adaptive management strategies to maintain ecosystem health and ensure the long-term sustainability of fisheries [29].
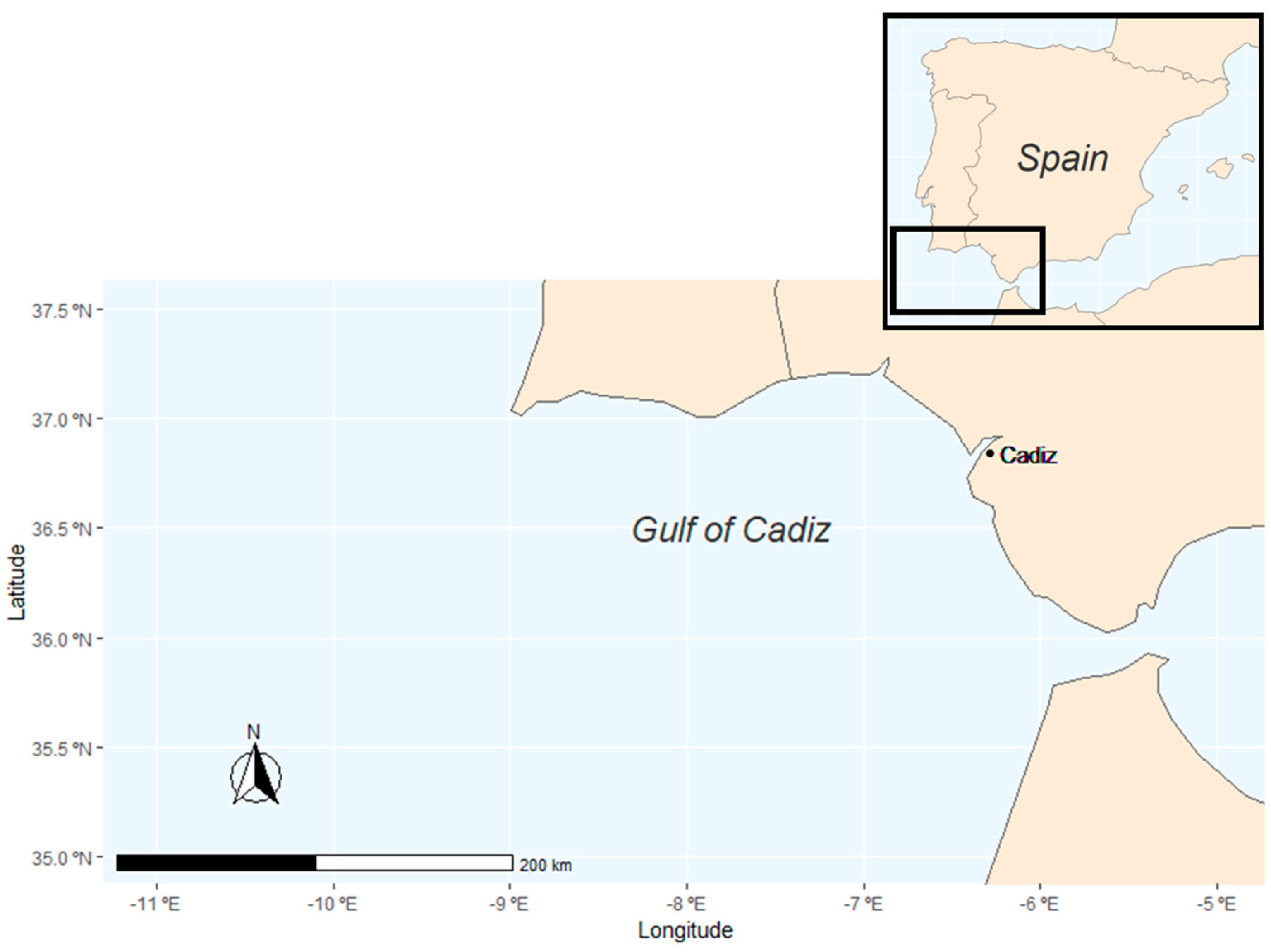
2. Materials and Methods
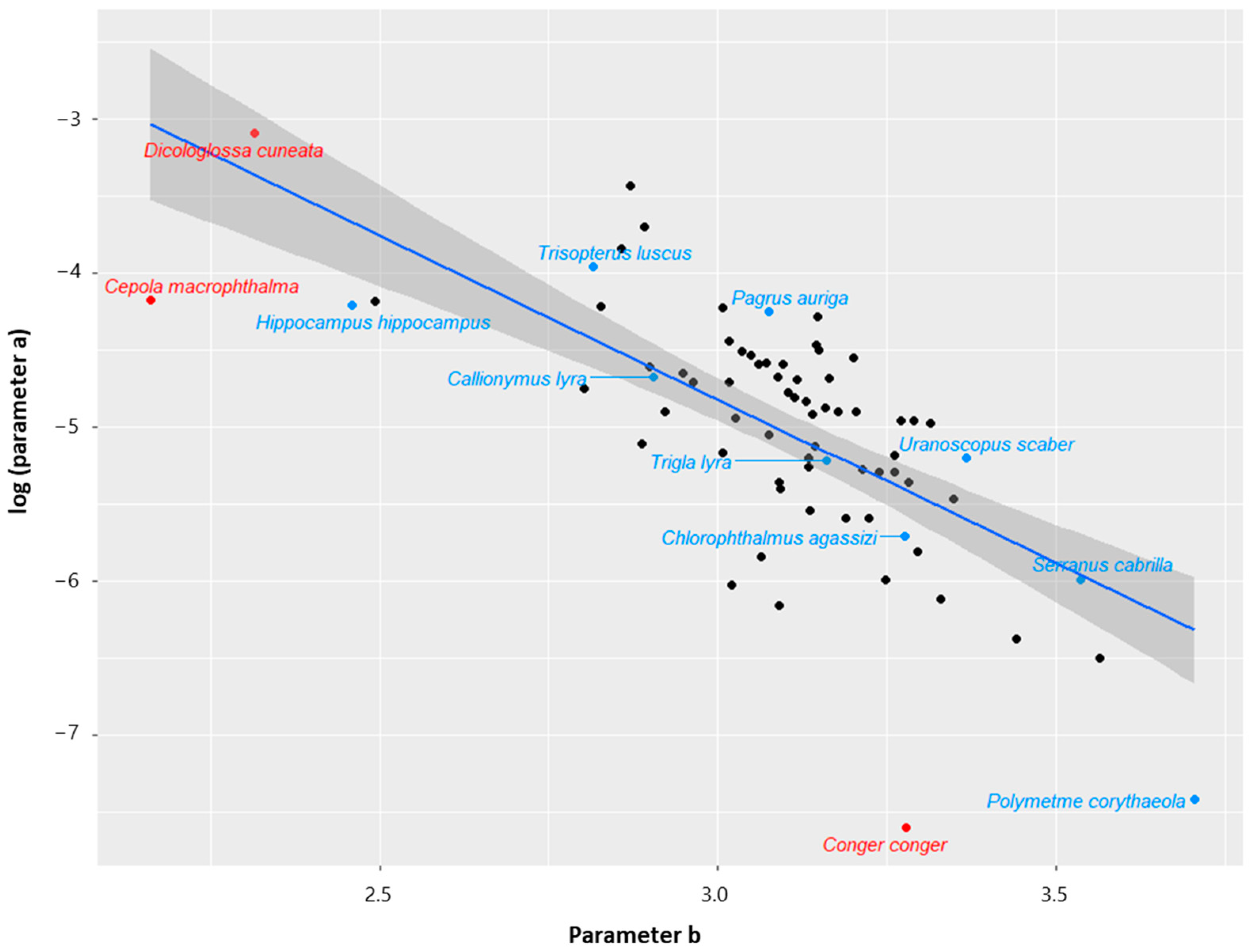
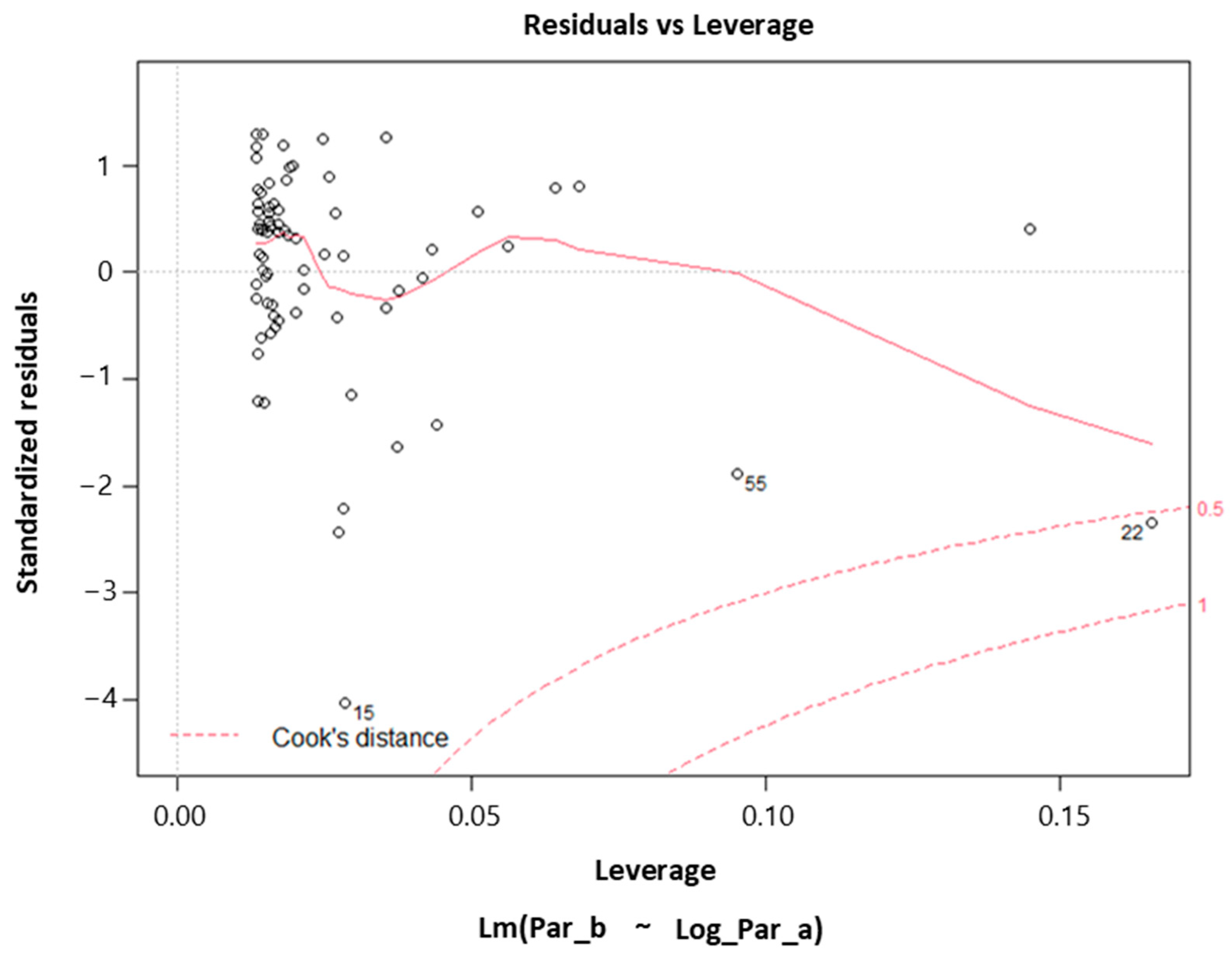
3. Results and Discussion
Low-Sample Specimens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellido, J.M.; Santos, M.B.; Pennino, M.G.; Valeiras, X.; Pierce, G.J. Fishery discards and bycatch: Solutions for an ecosystem approach to fisheries management? Hydrobiologia 2011, 670, 317–333. [Google Scholar] [CrossRef]
- Despoti, S.; Milisenda, G.; Ligas, A.; Bentes, L.; Maynou, F.; Vitale, S.; Garofalo, G.; Sbrana, M.; Erzini, K.; Tserpes, G.; et al. Marine spatial closures as a supplementary tool to reduce discards in bottom trawl fisheries: Examples from southern European waters. Fish. Res. 2020, 232, 105714. [Google Scholar] [CrossRef]
- Kelleher, K. Discards in the world’s marine fisheries: An update. In FAO Fisheries Technical Paper; FAO: Rome, Italy, 2005; p. 131. [Google Scholar]
- Despoti, S.; Stergiou, K.I.; Machias, A.; Vassilopoulou, V.; Tsagarakis, K.; Valavanis, V.; Adamidou, A.; Gionnoulaki, M. Assessing the spatial distribution of five non-commercial fish species in the Aegean Sea (Greece, Eastern Mediterranean Sea) based on discards data. Reg. Stud. Mar. Sci. 2021, 44, 101736. [Google Scholar] [CrossRef]
- Alverson, D.L.; Freeberg, M.H.; Murawski, S.A.; Pope, J.G. A Global Assessment of Fisheries Bycatch and Discards; Food and Agriculture Organization: Rome, Italy, 1994; Volume 339. [Google Scholar]
- Gamaza, M.A.; Torres, M.A.; Acosta, J.J.; Erzini, K.; Sobrino, I. Are we ready to implement a discard ban in the gulf of Cádiz? Stakeholders’ perceptions. Mar. Policy 2020, 116, 103711. [Google Scholar] [CrossRef]
- Catchpole, T.L.; Ribeiro-Santos, A.; Mangi, S.C.; Hedley, C.; Gray, T.S. The challenges of the landing obligation in EU fisheries. Mar. Policy 2017, 82, 76–86. [Google Scholar] [CrossRef]
- Catchpole, T.L.; Enever, R.; Maxwell, D.L.; Armstrong, M.J.; Reese, A.; Revill, A.S. Constructing indices to detect temporal trends in discarding. Fish. Res. 2011, 107, 94–99. [Google Scholar] [CrossRef]
- Stone, J.C.; Glass, K.; Munn, Z.; Tugwell, P.; Doi, S.A. Comparison of bias adjustment methods in meta-analysis suggests that quality effects modeling may have less limitations than other approaches. J. Clin. Epidemiol. 2020, 117, 36–45. [Google Scholar] [CrossRef]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). Society 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Anderson, R.; Gutreuter, S. Length, weight, and associated structural indices. In Fisheries Techniques; Nielsen, L., Johnson, D., Eds.; American Fisheries Society: Bethesda, MD, USA, 1983; pp. 283–300. [Google Scholar]
- Pauly, D. Fishbyte section. Naga ICLARM Q. 1993, 16, 26. [Google Scholar]
- Petrakis, G.; Stergiou, K.I. Weight–length relationships for 33 fish species in Greek waters. Fish. Res. 1995, 21, 465–469. [Google Scholar] [CrossRef]
- Morato, T.; Afonso, P.; Lourinho, P.; Barreiros, J.P.; Santos, R.S.; Nash, R.D.M. Lenght-Weight relationships for 21 coastal fish species of the Azores, north-eastern Atlantic. Fish. Res. 2001, 50, 297–302. [Google Scholar] [CrossRef]
- Koutrakis, E.T.; Tsikliras, A.C. Length–weight relationships of fishes from three northern Aegean estuarine systems (Greece). J. Appl. Ichthyol. 2003, 19, 258–260. [Google Scholar] [CrossRef]
- Kimmerer, W.; Avent, S.R.; Bollens, S.M.; Feyrer, F.; Grimaldo, L.F.; Moyle, P.B.; Nobriga, M.; Visintainer, T. Variability in length–weight relationships used to estimate biomass of estuarine fish from survey data. Trans. Am. Fish. 2005, 134, 481–495. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Yapici, S.; Karachle, P.K.; Filiz, H. First length–weight relationships of 11 fish species in the Aegean Sea. J. Appl. Ichthyol. 2015, 31, 398–402. [Google Scholar] [CrossRef]
- Al Kamel, N.A.M.; Becheker, A.; Kara, H.M. Length-weight relationship of three commercially important fish species from Mocka water, southern Red Sea, Yemen. J. Appl. Ichthyol. 2020, 36, 366–368. [Google Scholar] [CrossRef]
- Coello, D.; Herrera, M.; Zambrano, R. Length-weight relationship of 74 fish species caught in the continental coast of Ecuador. J. Appl. Ichthyol. 2021, 37, 129–134. [Google Scholar] [CrossRef]
- Soykan, O.; Kinacigil, H.T. Length-weight relationship of some discarded fish species with emphasis on length at maturity from the Central Aegean Sea, Turkey. Thalassas 2021, 37, 505–511. [Google Scholar] [CrossRef]
- Lamprakis, M.K.; Kallianiotis, A.; Moutopoulos, D.K.; Stergiou, K.I. Weight–length relationships of fishes discarded by trawerls in the North Aegean Sea. Acta Ichthyol. Piscat. 2003, 33, 145–152. [Google Scholar] [CrossRef]
- Torres, M.A.; Ramos, F.; Sobrino, I. Length-weight relationships of 76 fish species from the Gulf of Cadiz (SW Spain). Fish. Res. 2012, 127–128, 171–175. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D.; FishBase. World Wide Web Electronic Publication. 2005. Available online: http://www.fishbase.org (accessed on 19 January 2023).
- Beverton, R.J.H.; Holt, S.J. On the dynamics of exploited fish populations. In Fishery Investigations Series II; Springer: Dordrecht, The Netherlands, 1957; Volume 19, pp. 1–533. [Google Scholar]
- Benoît, H.P.; Swain, D.P. Impacts of environmental change and direct and indirect harvesting effects on the dynamics of a marine fish community. Can. J. Fish. Aquat. Sci. 2008, 65, 2088–2104. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Rochet, M.J.; Trenkel, V.M. Factors for the variability of discards: Assumptions and field evidence. Can. J. Fish. Aquat. Sci. 2005, 62, 224–235. [Google Scholar] [CrossRef]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty; Springer Science & Business Media: Berlin, Germany, 1992. [Google Scholar]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcherm, T.J.; Rashid-Sumaila, U.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Gonçalves, J.M.S.; Bentes, L.; Lino, P.G.; Ribeiro, J.; Canario, A.V.M.; Erzini, K. Weight–length relationships for selected fish species of the small-scale demersal fisheries of the south and south-west coast of Portugal. Fish. Res. 1997, 30, 253–256. [Google Scholar] [CrossRef]
- Santos, M.N.; Gaspar, M.B.; Vasconcelos, P.; Monteiro, C.C. Weight–length relationships for 50 selected fish species of the Algarve (southern Portugal). Fish. Res. 2002, 59, 289–295. [Google Scholar] [CrossRef]
- Borges, T.C.; Olim, S.; Erzini, K. Weight–length relationships for fish species discarded in commercial fisheries of the Algarve (southern Portugal). J. Appl. Ichthyol. 2003, 19, 394–396. [Google Scholar] [CrossRef]
- Mata, A.J.; Morales, J.; Márquez, L. Weight-length relationships for 26 demersal fish species of the Spanish South-Atlantic coastal waters. J. Appl. Ichthyol. 2008, 24, 330–333. [Google Scholar] [CrossRef]
- Veiga, P.; Machado, D.; Almeida, C.; Bentes, L.; Monteiro, P.; Oliveira, F.; Ruano, M.; Erzini, K.; Gonçalves, J.M.S. Weight-length relationships for 54 species of the Arade estuary, southern Portugal. J. Appl. Ichthyol. 2009, 25, 493–496. [Google Scholar] [CrossRef]
- Compairé, J.C.; Soriguer, M.C. Lenght-weight relationships of seven fish species from tidepools of an intertidal rocky shore in the Gulf of Cadiz, Spain (NE Atlantic). J. Appl. Ichthyol. 2020, 36, 852–854. [Google Scholar] [CrossRef]
- Compairé, J.C.; Gómez-Cama, C.; Soriguer, M.C. Length-Weight relationships of six fish species of a rocky intertidal shore on the subtropical atlantic coast of Spain. Thalassas 2021, 37, 267–271. [Google Scholar] [CrossRef]
- Jiménez, M.P.; Sobrino, I.; Ramos, F. Distribution pattern, reproductive biology, and fishery of the wedge sole Dicologlossa cuneata in the Gulf of Cadiz, southwest Spain. Mar. Biol. 1998, 131, 173–187. [Google Scholar]
- Millán, M. Reproductive characteristics and condition status of anchovy Engraulis encrasicolus L. from the Bay of Cadiz (SW Spain). Fish. Res. 1999, 41, 73–86. [Google Scholar] [CrossRef]
- Velasco, E.M.; Del Árbol, J.; Baro, J.; Sobrino, I. Age and growth of the Spanish chub mackerel Scomber colias off southern Spain: A comparison between samples from the NE Atlantic and the SW Mediterranean. Rev. Biol. Mar. Oceanogr. 2011, 46, 27–34. [Google Scholar] [CrossRef]
- Whitehead, P.J.P.; Bauchot, M.L.; Jureau, J.C.; Nielsen, J.; Tortonese, E. Fishes of the North-Eastern Atlantic and the Mediterranean; UNESCO: Paris, France, 1986; Volume III. [Google Scholar]
- Lloris, D. Ictiofauna Marina: Manual de Identificación de los Peces Marinos de la Península Ibérica y Baleares; 954 Especies; Omega: Biel/Bienne, Switzerland, 2015. [Google Scholar]
- Ricker, W.E. Linear regressions in fishery research. Can. J. Fish. Aquat. Sci. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. In Bulletin of the Fish; Refugee Board of Canada: Ottawa, ON, Canada, 1975. [Google Scholar]
- Stergiou, K.I.; Moutopoulos, D.K. A review of length–weight relationships of fishes from Greek Marine Waters. ICLARM Q. 2001, 24, 23–39. [Google Scholar]
- Cook, R.D.; Weisberg, S. Residuals and Influence in Regression; Chapman and Hall: London, UK, 1982. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016; Available online: http://www.rstudio.com/ (accessed on 29 March 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 29 March 2023).
- Acosta, J.J.; Canoura, J.; Juárez, A. First record of Cynoscion nebulosus in the Spanish waters of the Gulf of Cadiz (ICES Division IXa South). Mar. Biodivers. Rec. 2013, 6, e112. [Google Scholar] [CrossRef]
- Bañon, R.; Arias, A.; Arana, D.; Cuesta, J.A. Identification of a non-native Cynoscion species (Perciformes: Sciaenidae) fron the Gulf of Cádiz (southwestern Spain) and data on its current status. Sci. Mar. 2017, 81, 19–26. [Google Scholar] [CrossRef]
- López-Pérez, C.; Olivar, M.P.; Hulley, P.A.; Tuset, V.M. Length–weight relationships of mesopelagic fishes from the equatorial and tropical Atlantic waters: Influence of environment and body shape. J. Fish Biol. 2020, 96, 1388–1398. [Google Scholar] [CrossRef]
- Ordines, F.; Quetglas, A.; Massutí, E.; Moranta, J. Habitat preferences and life history of the red scorpion fish, Scorpaena notata, in the Mediterranean. Estuar. Coast. Shelf Sci. 2009, 85, 537–546. [Google Scholar] [CrossRef]
- Matlock, G.C.; Strawn, K. Standard Length-Weight Relationships of 22 Fishes from Upper Galveston Bay; Texas Agricultural Experiment Station: College Station, TX, USA, 1976; Volume 1286, pp. 1–4. [Google Scholar]
- Vega-Cendejas, M.E.; Peralta-Meixuiero, M.A.; De Santillana, M.H. Length–weight relations of fishes inhabiting a hyperhaline coastal lagoon in Yucatan, Mexico. Acta Ichthyol. Piscat. 2017, 47, 411–415. [Google Scholar] [CrossRef]
- Bagenal, T.B.; Tesch, F.W. Age and growth. In Methods for Assessment of Fish in Freshwaters, 3rd ed.; Bagenal, T., Ed.; IBP Handbook No. 3; Blackwell Scientific Publications: Oxford, UK, 1978; pp. 101–136, Chapter 5. [Google Scholar]
- Pauly, D. Fish Population Dynamics in Tropical Waters: A Manual for Use with Programmable Calculators; ICLARM Studies and Reviews; ICLARM: Manila, Philippines, 1984; Volume 8, 325p. [Google Scholar]
- Safran, P. Theoretical analysis of the weight–length relationships in the juveniles. Mar. Biol. 1992, 112, 545–551. [Google Scholar] [CrossRef]
- Mendes, B.; Fonseca, P.; Campos, A. Weight-length relationships for 46 fish species of the Portuguese west coast. J. Appl. Ichthyol. 2004, 20, 355–361. [Google Scholar] [CrossRef]
- King, P.A.; Fives, J.M.; Mcgrath, D. Reproduction, growth and feeding of the dragonet, Callionymus lyra (Teleostei: Callionymidae), in Galway Bay, Ireland. J. Mar. Biol. Assoc. UK 1994, 74, 513–526. [Google Scholar] [CrossRef]
- Alonso-Fernández, A.; Domínguez-Petit, R.; Bao, M.; Rivas, C.; Saborido-Rey, F. Spawning pattern and reproductive strategy of female pouting Trisopterus luscus (Gadidae) on the Galicia shelf of north-western Spain. Aquat. Living Resour. 2008, 21, 383–393. [Google Scholar] [CrossRef]
- Agbali, M.; El-Mor, M. Some aspects of the Reproductive Biology of the Piper Gurnard Trigla Lyra (Linnaeus, 1758) in Dernah Coast-Eastern Libya. Int. J. Pharm. Life Sci. 2015, 6, 4445–4451. [Google Scholar]
- Marques, A. Some data on the biology of Polymetme corythaeola (phosich-thyidae), from off the Portuguese south coast, North East Atlantic. Cybium 2001, 25, 100–102. [Google Scholar]
- D’Onghia, G.; Sion, L.; Maiorano, P.; Mytilineou, C.; Dalessandro, S.; Carlucci, S.; Desantis, S. Population biology and life strategies of Chlorophthalmus agassizii Bonaparte, 1840 (Pisces: Osteichthyes) in the Mediterranean Sea. Mar. Biol. 2006, 149, 435–446. [Google Scholar] [CrossRef]
- Ilhan, D.; Akalin, S.; Tosunoglu, Z.; Özaydin, O. Growth characteristics and reproduction of comber, Serranus cabrilla (Actinopterygii, Perciformes, Serranidae), in the Aegean Sea. Acta Ichthyol. Piscat. 2010, 40, 55–60. [Google Scholar] [CrossRef]
- Orhan, A.K.; Kutlu, S.; Karayücel, I. Some reproductive characteristics of Uranoscopus scaber Linnaeus, 1758 (Pisces: Uranoscopidae) in the Black Sea (Turkey). Cah. Biol. Mar. 2011, 52, 253–260. [Google Scholar]
- Curtis, J.M.R.; Santos, S.V.; Nadeau, J.L.; Gunn, B.; Bigney-Wilner, K.; Balasubramanian, H.; Overington, S.; Lesage, C.M.; D’Entremont, J.; Wieckowski, K. Life history and ecology of the elusive European short-snouted seahorse Hippocampus hippocampus. J. Fish Biol. 2017, 91, 1603–1622. [Google Scholar] [CrossRef] [PubMed]
- Jisr, N.; Younes, G.; Sukhn, C.; El-Dakdouki, M.H. Length-weight relationships and relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. Egypt J. Aquat. Res. 2018, 44, 299–305. [Google Scholar] [CrossRef]
- Yapıcı, S.; Filiz, H. Estimation of age, growth and reproduction of boarfish, Capros aper, in the South Aegean Sea. Pak. J. Zool. 2014, 46, 1061–1068. [Google Scholar]
| Family—Species | N | Length (cm) | Weight (g) | Parameters of the LWR | Growth Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean (±SD) | Min. | Max. | Mean (±SD) | a (95% CI) | b (95% CI) | R2 | p-Value | |||
| Batrachoididae | ||||||||||||
| Halobatrachus didactylus | 23 | 13.5 | 33.5 | 21.72 (±4.89) | 38.96 | 556.67 | 214.43 (±139.77) | 0.0114 (0.0036–0.0317) | 3.1464 (2.6152–3.6753) | 0.962 | <0.001 | Allometric + |
| Blennidae | ||||||||||||
| Blennius ocellaris | 29 | 4.3 | 14.7 | 11.18 (±2.39) | 0.83 | 45.91 | 21.76 (±11.90) | 0.0092 (0.0063–0.0133) | 3.1641 (3.0100–3.3183) | 0.985 | <0.001 | Allometric + |
| Callionymidae | ||||||||||||
| Callionymus lyra | 12 | 2.7 | 20.2 | 10.91 (±5.82) | 0.18 | 67.95 | 17.28 (±20.67) | 0.0093 (0.0040–0.0214) | 2.9038 (2.5409–3.2667) | 0.966 | <0.001 | Allometric − |
| Callionymus maculatus | 124 | 2.3 | 17.0 | 7.57 (±2.20) | 0.09 | 36.57 | 2.83 (±3.37) | 0.0152 (0.0124–0.0186) | 2.4930 (2.3914–2.5946) | 0.950 | <0.001 | Allometric − |
| Synchiropus phaeton | 21 | 8.6 | 17.6 | 11.72 (±2.16) | 3.75 | 44.71 | 10.87 (±9.01) | 0.0037 (0.0012–0.0108) | 3.1895 (2.7484–3.6305) | 0.919 | <0.001 | Allometric + |
| Caproidae | ||||||||||||
| Capros aper | 935 | 2.9 | 13.4 | 5.80 (±1.14) | 0.59 | 44.00 | 4.46 (±3.60) | 0.0246 (0.0228–0.0264) | 2.8909 (2.8487–2.9331) | 0.951 | <0.001 | Allometric − |
| Carangidae | ||||||||||||
| Trachurus mediterraneus | 326 | 8.6 | 32.0 | 18.21 (±3.05) | 4.83 | 239.30 | 48.72 (±31.07) | 0.0099 (0.0086–0.0114) | 2.8997 (2.8503–2.9491) | 0.976 | <0.001 | Allometric − |
| Trachurus picturatus | 95 | 12.2 | 17.5 | 14.34 (±1.31) | 14.1 | 50.65 | 26.09 (±8.06) | 0.0059 (0.0037–0.0093) | 3.1430 (2.9713–3.3148) | 0.934 | <0.001 | Allometric + |
| Trachurus trachurus | 2333 | 5.2 | 32.6 | 16.79 (±3.97) | 0.91 | 316.43 | 44.74 (±35.44) | 0.0064 (0.0061–0.0068) | 3.0752 (3.0562–3.0943) | 0.977 | <0.001 | Allometric + |
| Centracanthidae | ||||||||||||
| Spicara flexuosa | 36 | 9.1 | 20.4 | 16.83 (±2.30) | 6.78 | 101.76 | 57.62 (±22.96) | 0.0042 (0.0027–0.0076) | 3.3491 (3.1381–3.5602) | 0.967 | <0.001 | Allometric + |
| Spicara smaris | 33 | 6.1 | 18.7 | 14.95 (±2.82) | 2.12 | 73.39 | 40.56 (±18.54) | 0.0081 (0.0055–0.0117) | 3.1140 (2.9646–3.2533) | 0.985 | <0.001 | Allometric + |
| Centriscidae | ||||||||||||
| Macroramphosus scolopax | 17 | 5.6 | 13.2 | 9.21 (±2.30) | 0.95 | 10.91 | 4.93 (±3.12) | 0.0086 (0.0047–0.0155) | 2.8016 (2.5334–3.0697) | 0.969 | <0.001 | Allometric − |
| Cepolidae | ||||||||||||
| Cepola macrophthalma | 148 | 7.8 | 63.8 | 26.41 (±11.17) | 0.96 | 107.63 | 21.70 (±19.37) | 0.0153 (0.0126–0.0186) | 2.1607 (2.0999–2.2214) | 0.971 | <0.001 | Allometric − |
| Chlorophthalmidae | ||||||||||||
| Chlorophthalmus agassizi | 5 | 10.8 | 14.8 | 13.72 (±1.68) | 7.75 | 23.95 | 18.31 (±6.21) | 0.0033 (0.0000–0.2701) | 3.2756 (1.5900–4.9612) | 0.903 | 0.008 | Allometric + |
| Chimaeridae | ||||||||||||
| Chimaera monstrosa | 17 | 22.0 | 78.5 | 38.07 (±17.44) | 6.89 | 375.40 | 62.80 (±90.29) | 0.0152 (0.0124–0.0186) | 2.4930 (2.3914–2.5946) | 0.950 | <0.001 | Allometric − |
| Citharidae | ||||||||||||
| Citharus linguatula | 608 | 4.8 | 19.3 | 11.41 (±2.35) | 0.81 | 55.33 | 12.34 (±7.31) | 0.0052 (0.0047–0.0058) | 3.1339 (3.0904–3.1773) | 0.971 | <0.001 | Allometric + |
| Clupeidae | ||||||||||||
| Alosa alosa | 17 | 20.2 | 42.2 | 29.88 (±5.45) | 71.60 | 721.44 | 246.19 (±173.52) | 0.0030 (0.0001–0.0071) | 3.2949 (3.0383–3.5515) | 0.979 | <0.001 | Allometric + |
| Alosa fallax | 53 | 14.0 | 44.0 | 31.56 (±5.77) | 21.81 | 878.17 | 276.23 (±146.39) | 0.0090 (0.0058–0.0141) | 2.9626 (2.8330–3.0923) | 0.976 | <0.001 | Allometric − |
| Sardina pilchardus | 1026 | 8.0 | 20.9 | 13.97 (±3.09) | 2.64 | 83.89 | 24.22 (±16.39) | 0.0071 (0.0065–0.0078) | 3.0259 (2.9911–3.0676) | 0.966 | <0.001 | Isometric |
| Congridae | ||||||||||||
| Conger conger | 319 | 21.2 | 77.1 | 34.95 (±7.46) | 6.40 | 868.95 | 63.60 (±62.74) | 0.0005 (0.0003–0.0007) | 3.2778 (3.1199–3.4086) | 0.888 | <0.001 | Allometric + |
| Cynoglossidae | ||||||||||||
| Symphurus nigrescens | 132 | 5.2 | 12.3 | 9.12 (±1.54) | 1.03 | 16.77 | 7.48 (±3.80) | 0.0050 (0.0038–0.0065) | 3.2620 (3.1403–3.3837) | 0.956 | <0.001 | Allometric + |
| Engraulidae | ||||||||||||
| Engraulis encrasicolus | 3231 | 4.0 | 16.7 | 10.10 (±2.08) | 0.31 | 24.46 | 6.58 (±4.18) | 0.0045 (0.0042–0.0048) | 3.0916 (3.0629–3.1202) | 0.933 | <0.001 | Allometric + |
| Etmopteridae | ||||||||||||
| Etmopterus spinax | 412 | 9.3 | 33.7 | 16.11 (±4.75) | 2.85 | 144.48 | 19.76 (±23.34) | 0.0029 (0.0025–0.0035) | 3.0652 (3.0018–3.1285) | 0.957 | <0.001 | Allometric + |
| Gadidae | ||||||||||||
| Gadiculus argenteus | 158 | 6.4 | 16.1 | 9.49 (±1.60) | 1.71 | 48.98 | 8.55 (±5.33) | 0.0047 (0.0037–0.0061) | 3.2816 (3.1694–3.3938) | 0.955 | <0.001 | Allometric + |
| Micromesistius poutassou | 208 | 12.8 | 29.1 | 20.77 (±2.95) | 11.32 | 158.3 | 56.66 (±25.58) | 0.0022 (0.0016–0.0028) | 3.3292 (3.2412–3.4171) | 0.964 | <0.001 | Allometric + |
| Trisopterus luscus | 8 | 18.1 | 23.0 | 20.41 (±1.66) | 62.00 | 126.15 | 94.21 (±21.56) | 0.0190 (0.0030–0.1204) | 2.8157 (2.2034–3.4280) | 0.947 | <0.001 | Allometric − |
| Gobiidae | ||||||||||||
| Aphia minuta | 293 | 3.0 | 5.7 | 4.55 (±0.47) | 0.16 | 1.16 | 0.50 (±0.17) | 0.0060 (0.0043–0.0083) | 2.8875 (2.6734–3.1016) | 0.707 | <0.001 | Allometric − |
| Gobius niger | 167 | 4.7 | 10.0 | 6.96 (±1.18) | 0.78 | 11.17 | 3.53 (±2.06) | 0.0056 (0.0047–0.0067) | 3.2623 (3.1687–3.3559) | 0.966 | <0.001 | Allometric + |
| Haemulidae | ||||||||||||
| Pomadasys incisus | 613 | 4.5 | 22.9 | 11.28 (±4.33) | 0.88 | 166.60 | 27.06 (±28.30) | 0.0074 (0.0071–0.0077) | 3.2049 (3.1875–3.2222) | 0.995 | <0.001 | Allometric + |
| Lophiidae | ||||||||||||
| Lophius budegassa | 241 | 4.3 | 27.7 | 9.74 (±3.42) | 1.09 | 348.68 | 17.07 (±34.35) | 0.0110 (0.0094–0.0129) | 3.0354 (2.9650–3.1059) | 0.968 | <0.001 | Isometric |
| Lophius piscatorius | 68 | 5.0 | 28.5 | 10.78 (±4.08) | 1.51 | 426.83 | 25.14 (±53.21) | 0.0102 (0.0074–0.0139) | 3.0723 (2.9394–3.2052) | 0.927 | <0.001 | Allometric + |
| Merluccidae | ||||||||||||
| Merluccius merluccius | 396 | 5.0 | 27.1 | 10.61 (±2.90) | 0.69 | 128.95 | 8.96 (±10.68) | 0.0047 (0.0042–0.0052) | 3.0906 (3.0421–3.1390) | 0.976 | <0.001 | Allometric + |
| Mugilidae | ||||||||||||
| Chelon ramada | 48 | 24.3 | 43.6 | 30.59 (±4.38) | 97.30 | 716.75 | 244.44 (±134.11) | 0.0017 (0.0009–0.0034) | 3.4406 (3.2398–3.6413) | 0.962 | <0.001 | Allometric + |
| Pentanchidae | ||||||||||||
| Galeus melastomus | 169 | 12.7 | 57.7 | 21.21 (±6.12) | 5.33 | 492.47 | 31.57 (±44.53) | 0.0024 (0.0018–0.0031) | 3.0201 (2.9315–3.1087) | 0.964 | <0.001 | Isometric |
| Peristediidae | ||||||||||||
| Peristedion cataphractum | 16 | 11.1 | 25.1 | 16.36 (±4.07) | 6.33 | 87.19 | 27.45 (±24.98) | 0.0025 (0.0015–0.0042) | 3.2481 (3.0700–3.4262) | 0.990 | <0.001 | Allometric + |
| Phycidae | ||||||||||||
| Phycis blennoides | 24 | 6.3 | 16.2 | 12.74 (±2.33) | 1.34 | 26.46 | 12.58 (±5.69) | 0.0039 (0.0026–0.0058) | 3.1366 (2.9813–3.2920) | 0.987 | <0.001 | Allometric + |
| Phosichthydae | ||||||||||||
| Polymetme corythaeola | 5 | 15.6 | 21.5 | 17.98 (±2.23) | 15.09 | 49.71 | 26.89 (±13.51) | 0.0006 (0.0002–0.0016) | 3.7040 (3.3378–4.0702) | 0.996 | <0.001 | Allometric + |
| Sciaenidae | ||||||||||||
| Cynoscion nebulosus | 36 | 16.6 | 25.5 | 19.86 (±1.8) | 52.10 | 194.85 | 89.49 (±30.1) | 0.0073 (0.0033–0.0160) | 3.1388 (2.8755–3.4020) | 0.945 | <0.001 | Allometric + |
| Umbrina canariensis | 18 | 12.2 | 27.3 | 20.27 (±4.76) | 20.17 | 243.59 | 118.31 (±72.17) | 0.0093 (0.0061–0.0144) | 3.0887 (2.9449–3.2326) | 0.992 | <0.001 | Allometric + |
| Umbrina ronchus | 62 | 10.2 | 26.3 | 17.03 (±3.13) | 11.27 | 211.46 | 66.04 (±42.14) | 0.0101 (0.0070–0.0145) | 3.0603 (2.9312–3.1893) | 0.974 | <0.001 | Allometric + |
| Scombridae | ||||||||||||
| Scomber colias | 52 | 17.4 | 28.0 | 21.87 (±2.10) | 38.35 | 206.52 | 87.51 (±28.95) | 0.0095 (0.0033–0.0274) | 2.9487 (2.6048–3.2926) | 0.853 | <0.001 | Allometric − |
| Scomber scombrus | 48 | 13.4 | 31.0 | 25.58 (±4.77) | 12.07 | 241.2 | 140.52 (±63.81) | 0.0037 (0.0022–0.0060) | 3.2227 (3.0708–3.3746) | 0.975 | <0.001 | Allometric + |
| Scophthalmidae | ||||||||||||
| Lepidorhombus whiffiagonis | 55 | 7.8 | 13.8 | 11.29 (±1.48) | 1.89 | 17.26 | 9.24 (±3.74) | 0.0015 (0.0009–0.0026) | 3.5651 (3.3406–3.7896) | 0.949 | <0.001 | Allometric + |
| Scorpaenidae | ||||||||||||
| Scorpaena notata | 71 | 5.8 | 17.0 | 11.85 (±2.17) | 3.35 | 103.32 | 36.70 (±20.77) | 0.0137 (0.0102–0.0186) | 3.1475 (3.0255–3.2695) | 0.974 | <0.001 | Allometric + |
| Scyliorhinidae | ||||||||||||
| Scyliorhinus canicula | 261 | 9.5 | 56.5 | 26.54 (±10.78) | 2.44 | 651.10 | 91.06 (±126.76) | 0.0021 (0.0017–0.0027) | 3.0907 (3.0188–3.1626) | 0.965 | <0.001 | Allometric + |
| Sebastidae | ||||||||||||
| Helicolenus dactylopterus | 109 | 4.4 | 16.9 | 7.93 (±2.33) | 1.24 | 80.78 | 10.18 (±11.92) | 0.0111 (0.0097–0.0127) | 3.1499 (3.0833–3.2165) | 0.988 | <0.001 | Allometric + |
| Serranidae | ||||||||||||
| Serranus cabrilla | 3 | 18.4 | 23.8 | 20.23 (±3.09) | 72.78 | 181.95 | 109.9 (±62.41) | 0.0025 (0–1.3845) | 3.5373 (1.4275–5.6471) | 0.996 | 0.030 | Allometric + |
| Serranus hepatus | 1519 | 3.4 | 14.5 | 9.20 (±1.74) | 0.52 | 44.06 | 14.33 (±7.83) | 0.0105 (0.0098–0.0112) | 3.1996 (3.1696–3.2296) | 0.966 | <0.001 | Allometric + |
| Soleidae | ||||||||||||
| Dicologlossa cuneata | 89 | 6.4 | 21.5 | 11.61 (±3.27) | 2.70 | 72.22 | 15.07 (±10.58) | 0.0454 (0.0328–0.0629) | 2.3162 (2.1823–2.4501) | 0.931 | <0.001 | Allometric − |
| Microchirus boscanion | 822 | 4.9 | 17.4 | 8.87 (±1.37) | 1.14 | 38.42 | 8.26 (±3.91) | 0.0074 (0.0066–0.0083) | 3.1777 (3.1232–3.2321) | 0.941 | <0.001 | Allometric + |
| Microchirus ocellatus | 27 | 5.2 | 15.4 | 12.04 (±2.24) | 2.17 | 55.67 | 28.34 (±11.51) | 0.0146 (0.0090–0.0240) | 3.0070 (2.8067–3.2074) | 0.974 | <0.001 | Isometric |
| Microchirus variegatus | 88 | 4.9 | 15.8 | 9.31 (±2.31) | 0.99 | 39.46 | 10.32 (±8.31) | 0.0084 (0.0055–0.0128) | 3.1033 (2.9134–3.2932) | 0.924 | <0.001 | Allometric + |
| Solea solea | 20 | 9.3 | 19.8 | 13.87 (±2.75) | 7.49 | 81.99 | 28.08 (±17.38) | 0.0090 (0.0054–0.0151) | 3.0161 (2.8189–3.2132) | 0.982 | <0.001 | Isometric |
| Sparidae | ||||||||||||
| Boops boops | 422 | 6.7 | 31.2 | 19.96 (±4.40) | 2.19 | 346.72 | 91.15 (±63.12) | 0.0051 (0.0046–0.0057) | 3.2137 (3.1789–3.2485) | 0.987 | <0.001 | Allometric + |
| Dentex canariensis | 28 | 11.9 | 23.7 | 16.33 (±3.21) | 25.35 | 172.78 | 69.13 (±43.38) | 0.0214 (0.0150–0.0305) | 2.8567 (2.7290–2.9842) | 0.987 | <0.001 | Allometric − |
| Diplodus annularis | 698 | 4.7 | 18.6 | 10.81 (±2.62) | 1.05 | 113.72 | 22.41 (±16.30) | 0.0069 (0.0064–0.0074) | 3.3140 (3.2849–3.3430) | 0.986 | <0.001 | Allometric + |
| Diplodus bellottii | 923 | 4.7 | 20.0 | 10.55 (±2.82) | 1.10 | 130.16 | 20.88 (±18.51) | 0.0070 (0.0066–0.0073) | 3.2896 (3.2689–3.3103) | 0.991 | <0.001 | Allometric + |
| Diplodus vulgaris | 181 | 10.6 | 22.8 | 18.31 (±2.60) | 15.36 | 185.72 | 98.52 (±39.93) | 0.0068 (0.0056–0.0083) | 3.2699 (3.2001–3.3396) | 0.979 | <0.001 | Allometric + |
| Pagellus acarne | 58 | 12.3 | 22.6 | 17.60 (±2.08) | 20.13 | 143.09 | 69.94 (±25.60) | 0.0107 (0.0069–0.0167) | 3.0477 (2.8926–3.2029) | 0.965 | <0.001 | Allometric + |
| Pagellus bellottii | 596 | 5.3 | 23.4 | 15.34 (±2.73) | 1.76 | 196.04 | 49.71 (±23.80) | 0.0091 (0.0084–0.0098) | 3.1171 (3.0875–3.1467) | 0.986 | <0.001 | Allometric + |
| Pagellus erythrinus | 401 | 4.7 | 27.8 | 14.20 (±4.34) | 1.33 | 195.94 | 44.98 (±36.33) | 0.0117 (0.0107–0.0128) | 3.0155 (2.9810–3.0499) | 0.987 | <0.001 | Isometric |
| Pagrus auriga | 6 | 15.0 | 22.3 | 18.12 (±2.56) | 57.77 | 192.16 | 110.69 (±47.79) | 0.0142 (0.0044–0.0454) | 3.0760 (2.6735–3.4786) | 0.989 | <0.001 | Allometric + |
| Spondyliosoma cantharus | 281 | 9.4 | 26.4 | 17.96 (±4.17) | 10.14 | 347.63 | 91.57 (±57.74) | 0.0101 (0.0086–0.0119) | 3.0967 (3.0413–3.1521) | 0.977 | <0.001 | Allometric + |
| Syngnathidae | ||||||||||||
| Hippocampus hippocampus | 6 | 7.2 | 11.1 | 9.47 (±1.62) | 1.72 | 6.14 | 3.97 (±1.74) | 0.0148 (0.0002–0.9529) | 2.4595 (0.5570–4.3621) | 0.704 | 0.023 | Allometric − |
| Torpedinidae | ||||||||||||
| Torpedo marmorata | 97 | 10.9 | 45.0 | 20.87 (±7.17) | 28.35 | 2288.71 | 262.32 (±320.93) | 0.0322 (0.0258–0.0401) | 2.8698 (2.7963–2.9433) | 0.984 | <0.001 | Allometric − |
| Trachinidae | ||||||||||||
| Echiichthys vipera | 42 | 9.9 | 27.7 | 16.91 (±3.09) | 6.02 | 166.17 | 32.03 (±24.63) | 0.0057 (0.0035–0.0094) | 3.0084 (2.8350–3.1818) | 0.968 | <0.001 | Isometric |
| Trachinus draco | 156 | 5.7 | 25.0 | 16.43 (±3.30) | 1.05 | 100.23 | 29.15 (±14.87) | 0.0074 (0.0063–0.0086) | 2.9211 (2.8647–2.9775) | 0.985 | <0.001 | Allometric − |
| Triglidae | ||||||||||||
| Chelidonichthys lastoviza | 12 | 11.4 | 22.8 | 19.17 (±3.73) | 14.75 | 141.15 | 80.33 (±43.12) | 0.0050 (0.0008–0.0321) | 3.2378 (2.6009–3.8747) | 0.921 | <0.001 | Allometric + |
| Chelidonichthys lucerna | 24 | 4.1 | 34.0 | 14.66 (±10.01) | 0.74 | 494.31 | 70.93 (±117.70) | 0.0147 (0.0108–0.0200) | 2.8274 (2.7067–2.9480) | 0.990 | <0.001 | Allometric − |
| Chelidonichthys obscurus | 51 | 8.6 | 22.5 | 16.62 (±2.76) | 4.67 | 87.59 | 40.07 (±19.61) | 0.0055 (0.0038–0.0079) | 3.1343 (3.0046–3.2641) | 0.979 | <0.001 | Allometric + |
| Lepidotrigla cavillone | 207 | 3.9 | 22.5 | 8.845 (±2.23) | 0.54 | 93.29 | 9.00 (±9.17) | 0.0079 (0.0070–0.0090) | 3.1298 (3.0721–3.1874) | 0.982 | <0.001 | Allometric + |
| Lepidotrigla dieuzeidei | 160 | 2.7 | 13.9 | 10.26 (±2.54) | 0.24 | 30.00 | 14.32 (±8.55) | 0.0076 (0.0067–0.0087) | 3.1594 (3.1036–3.2151) | 0.988 | <0.001 | Allometric + |
| Trigla lyra | 4 | 16.0 | 26.6 | 21.07 (±4.39) | 34.74 | 169.89 | 91.33 (±57.93) | 0.0054 (0.0006–0.0481) | 3.1603 (2.4390–3.8815) | 0.992 | 0.003 | Allometric + |
| Uranoscopidae | ||||||||||||
| Uranoscopus scaber | 8 | 14.7 | 24.5 | 21.00 (±3.17) | 49.01 | 286.15 | 170.04 (±77.61) | 0.0055 (0.0007–0.0446) | 3.3674 (2.6799–4.0550) | 0.953 | <0.001 | Allometric + |
| Species | Number of Studies | Mean N | Geometric Mean a | Mean b |
|---|---|---|---|---|
| Polymetme corythaeola | 1 | 9 | 0.0034 | 3.1160 |
| Pagrus auriga | 1 | 1 | 0.0191 | 3.0000 * |
| Hippocampus hippocampus | 4 | 49 | 0.0023 | 3.0000 |
| Chlorophthalmus agassizi | 8 | 226 | 0.0049 | 3.1100 |
| Callionymus lyra | 11 | 262 | 0.0166 | 2.6900 |
| Trisopterus luscus | 14 | 395 | 0.0081 | 3.1400 |
| Trigla lyra | 15 | 742 | 0.0110 | 2.9400 |
| Uranoscopus scaber | 21 | 95 | 0.0141 | 3.0500 |
| Serranus cabrilla | 35 | 243 | 0.0170 | 2.8600 |
| Our Work | [32] | [33] | [34] | [59] | [36] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | n | a | b | n | a | b | n | a | b | n | a | b | n | a | b | n | a | b |
| Serranus cabrilla | 3 | 0.0025 | 3.5373 | 171 | 0.00007337 | 2.6610 | 51 | 0.0729 | 2.4100 | - | - | - | 95 | 0.0213 | 2.7760 | - | - | - |
| Trigla lyra | 4 | 0.0054 | 3.1603 | - | - | - | 15 | 0.0217 | 2.7350 | 7 | 0.00858 | 3.1380 | 42 | 0.0056 | 3.1230 | - | - | - |
| Chlorophthalmus agassizi | 5 | 0.0033 | 3.2756 | - | - | - | - | - | - | 6 | 0.00786 | 2.9090 | - | - | - | - | - | - |
| Polymetme corythaeola | 5 | 0.0006 | 3.7040 | - | - | - | - | - | - | 9 | 0.00337 | 3.1160 | - | - | - | - | - | - |
| Pagrus auriga | 6 | 0.0142 | 3.0760 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Hippocampus hippocampus | 6 | 0.0148 | 2.4595 | - | - | - | - | - | - | - | - | - | - | - | - | 9 | 0.0064 | 2.7300 |
| Trisopterus luscus | 8 | 0.0190 | 2.8157 | 22 | 0.00001921 | 2.9310 | 56 | 0.0031 | 3.4400 | - | - | - | 1700 | 0.0089 | 3.0850 | - | - | - |
| Uranoscopus scaber | 8 | 0.0055 | 3.3674 | - | - | - | - | - | - | - | - | - | 33 | 0.0305 | 2.8290 | - | - | - |
| Callionymus lyra | 12 | 0.0093 | 2.9038 | 24 | 0.00084177 | 2.1170 | 235 | 0.1053 | 2.1070 | 31 | 0.05630 | 2.3100 | 60 | 0.0800 | 2.1710 | 24 | 0.0078 | 3.0200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-García, C.; Castro-Gutiérrez, J.; Domínguez-Bustos, Á.R.; García-González, A.; Cabrera-Castro, R. Every Fish Counts: Challenging Length–Weight Relationship Bias in Discards. Fishes 2023, 8, 222. https://doi.org/10.3390/fishes8050222
Rodríguez-García C, Castro-Gutiérrez J, Domínguez-Bustos ÁR, García-González A, Cabrera-Castro R. Every Fish Counts: Challenging Length–Weight Relationship Bias in Discards. Fishes. 2023; 8(5):222. https://doi.org/10.3390/fishes8050222
Chicago/Turabian StyleRodríguez-García, Carlos, Jairo Castro-Gutiérrez, Ángel Rafael Domínguez-Bustos, Alberto García-González, and Remedios Cabrera-Castro. 2023. "Every Fish Counts: Challenging Length–Weight Relationship Bias in Discards" Fishes 8, no. 5: 222. https://doi.org/10.3390/fishes8050222
APA StyleRodríguez-García, C., Castro-Gutiérrez, J., Domínguez-Bustos, Á. R., García-González, A., & Cabrera-Castro, R. (2023). Every Fish Counts: Challenging Length–Weight Relationship Bias in Discards. Fishes, 8(5), 222. https://doi.org/10.3390/fishes8050222






