Gills of Molly Fish: A Potential Role in Neuro-Immune Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Histological and Histochemical Preparation
2.3. Semithin Sections and Transmission Electron Microscoy (TEM)
2.4. Scanning Electron Microscopy (SEM)
2.5. Immunohistochemical Analysis
2.6. Digitally Colored TEM Images
3. Results
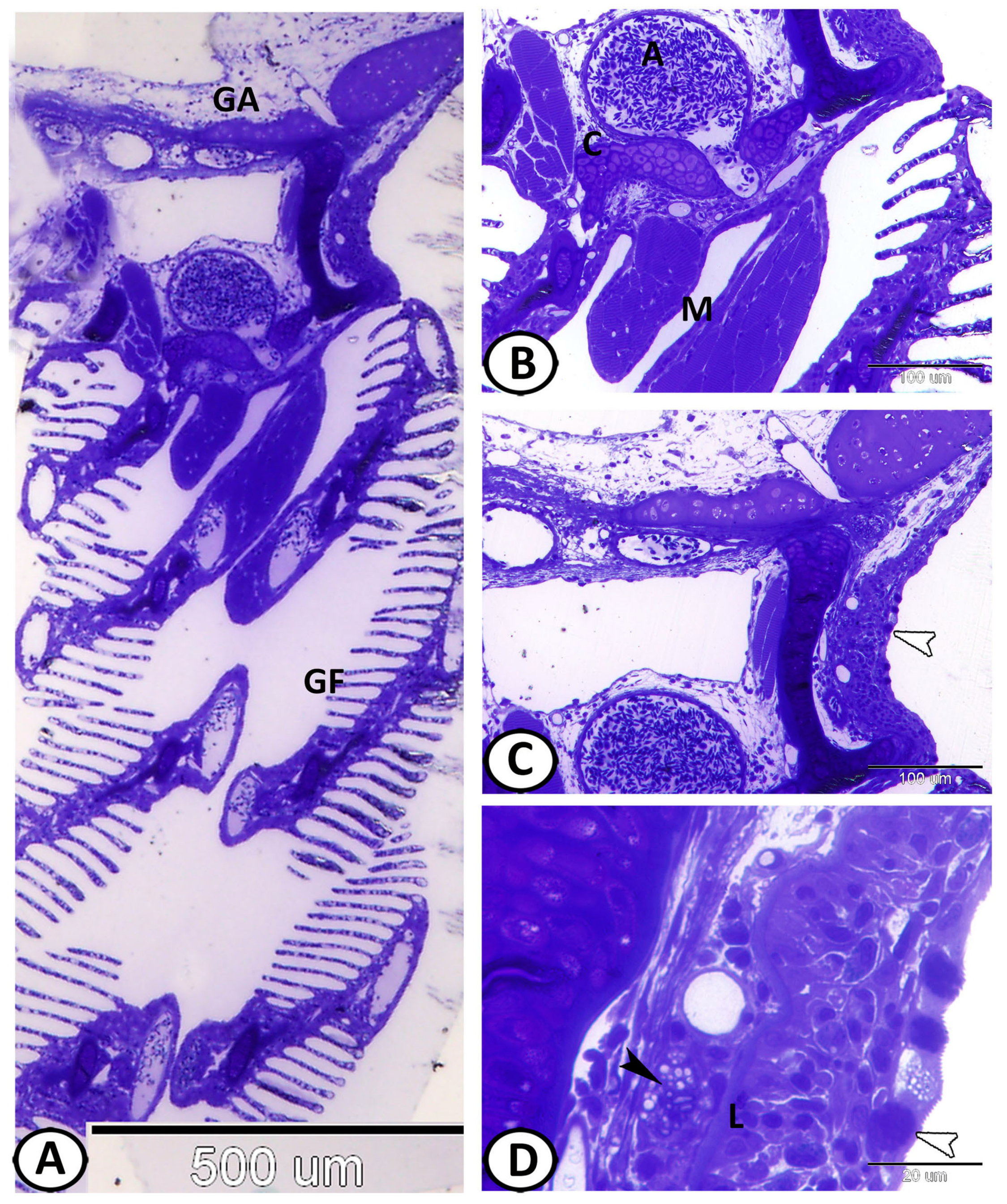
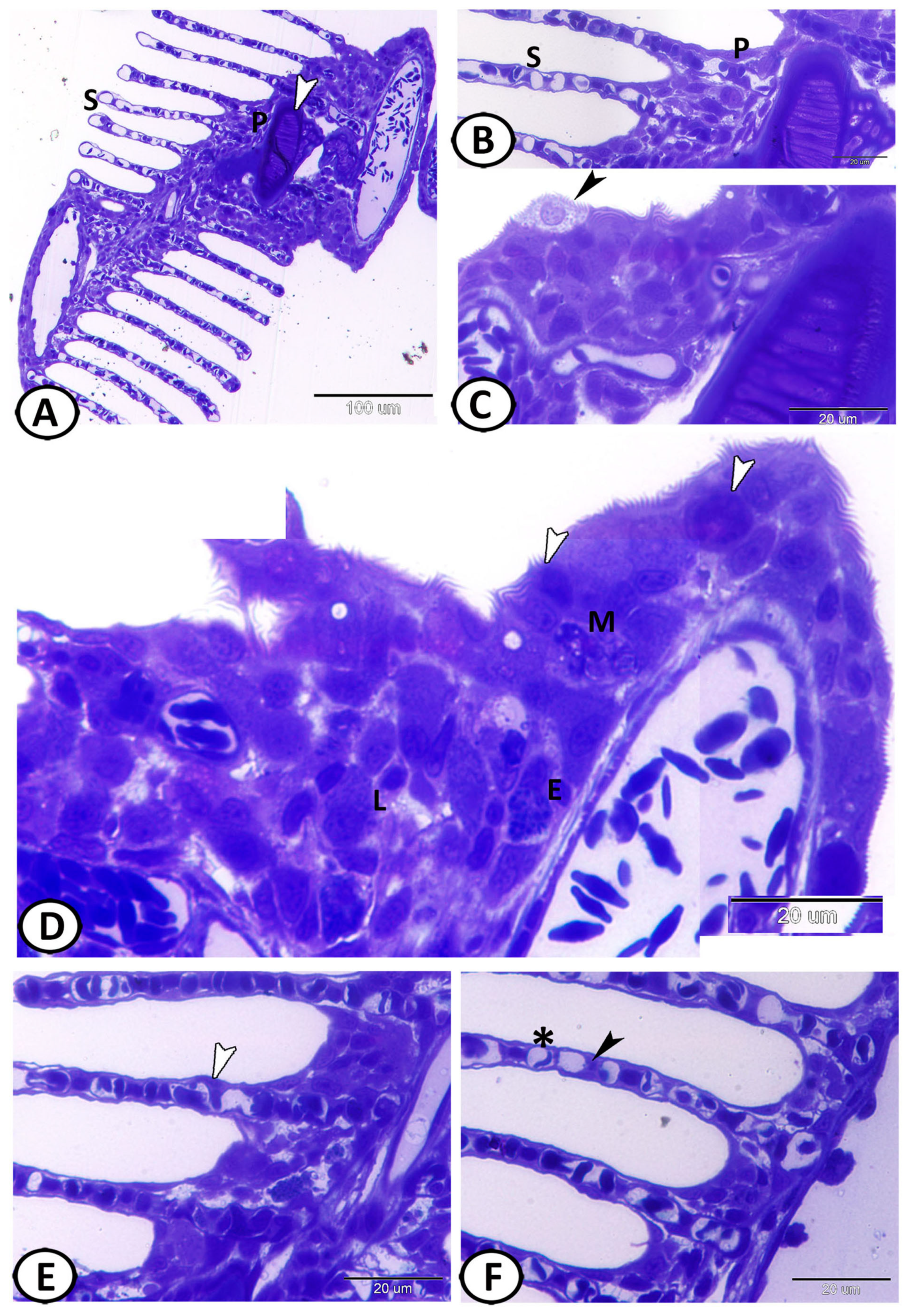
3.1. Histological Analysis
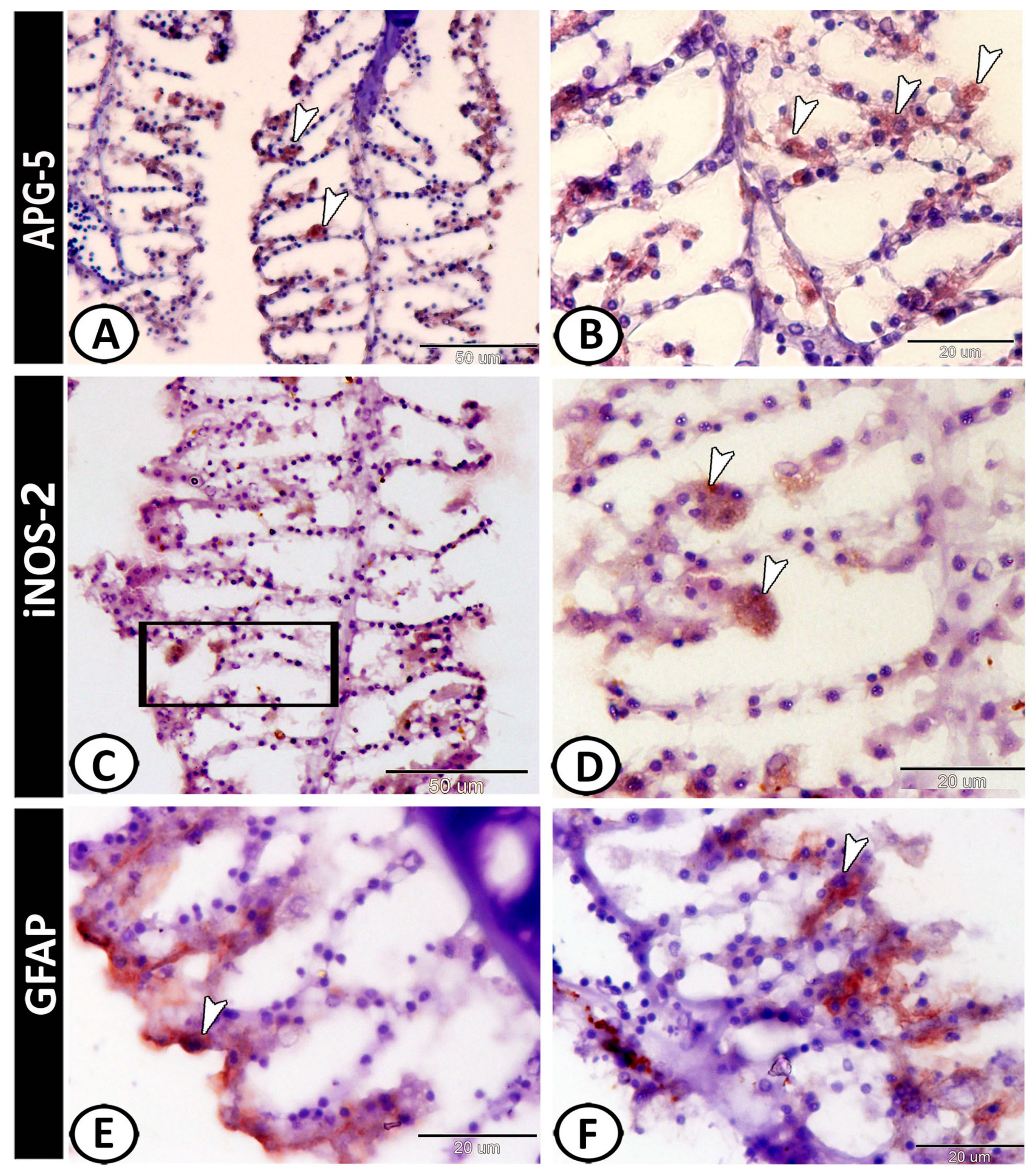
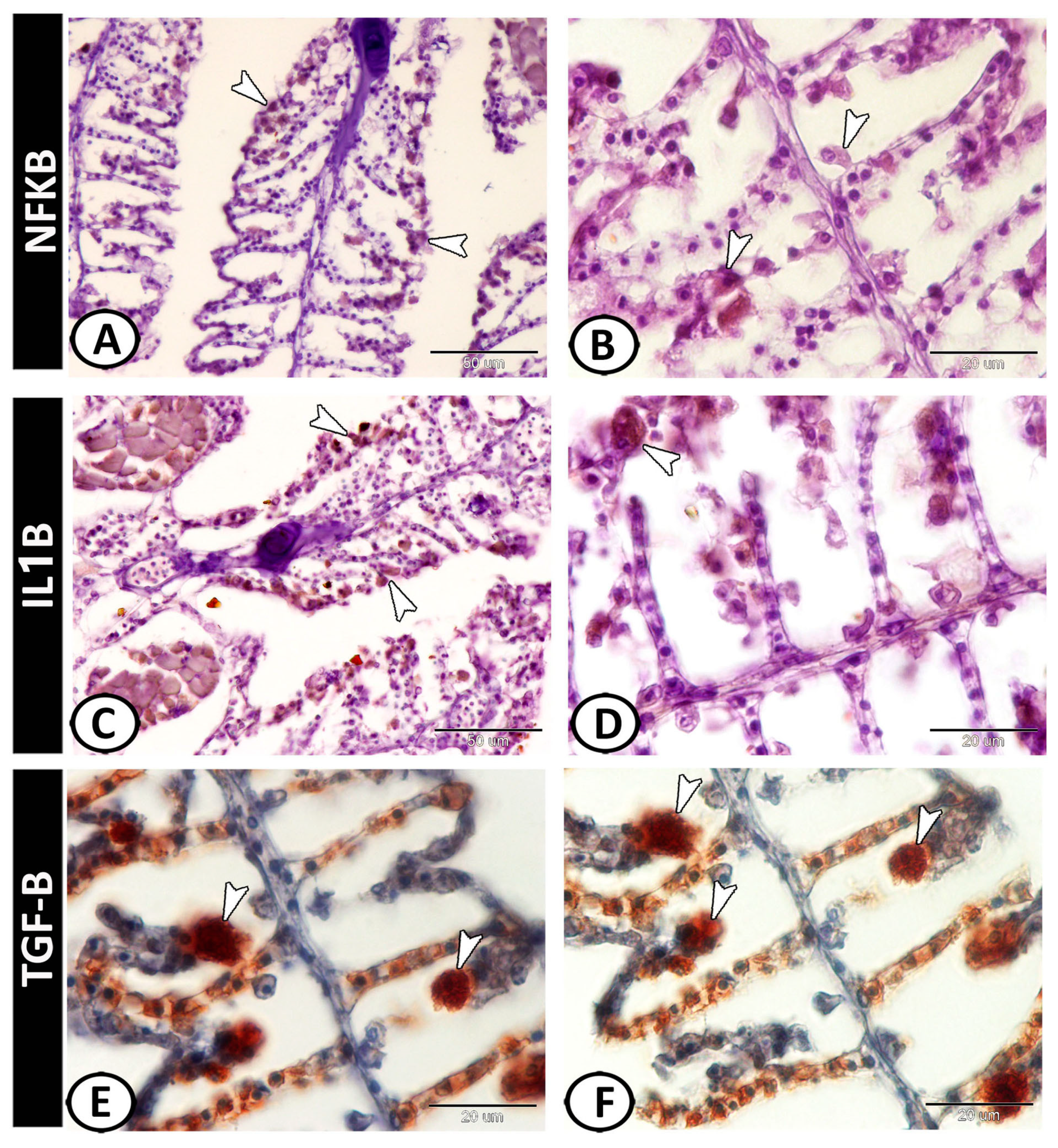
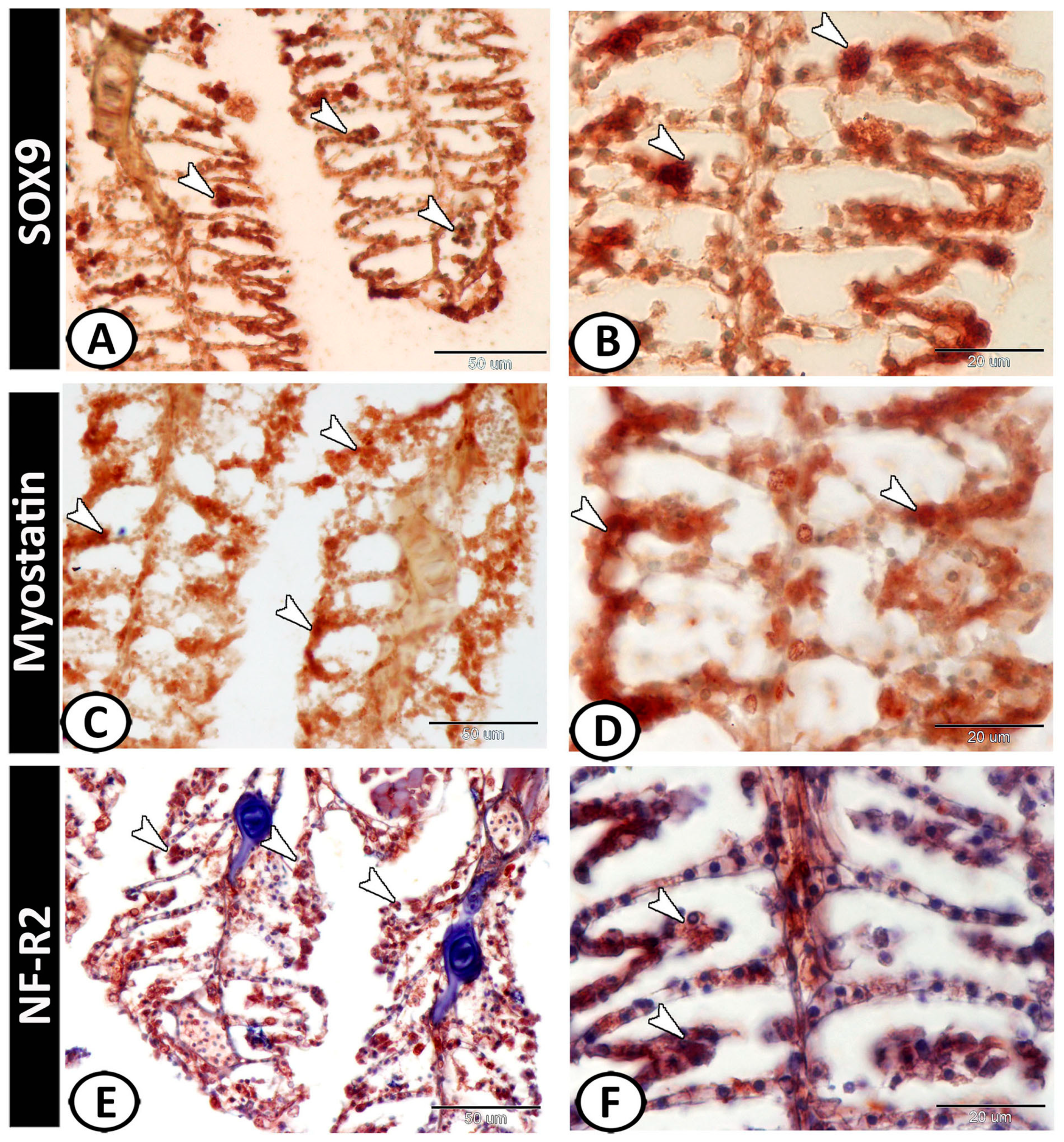
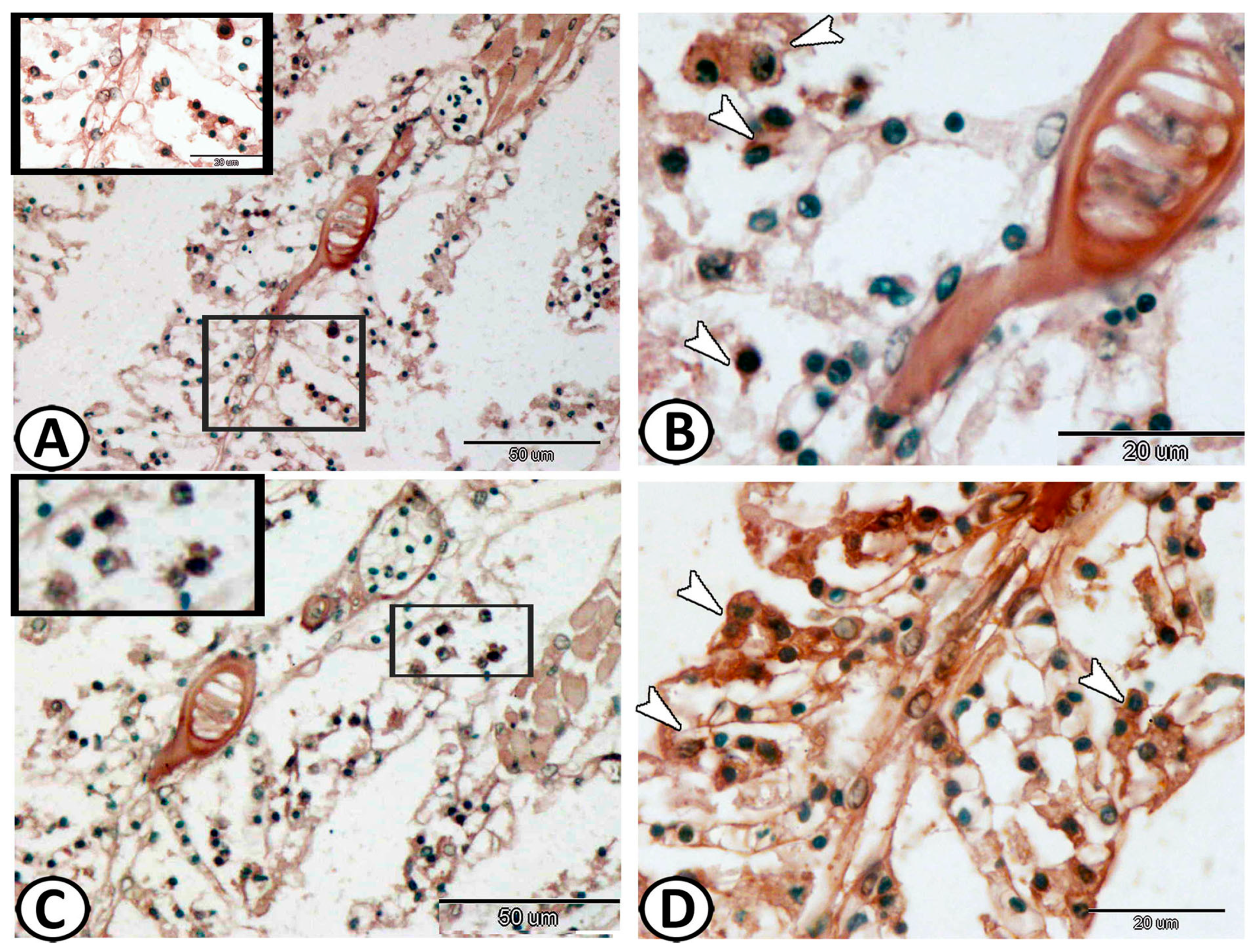
3.2. Immunohistochemical Analysis
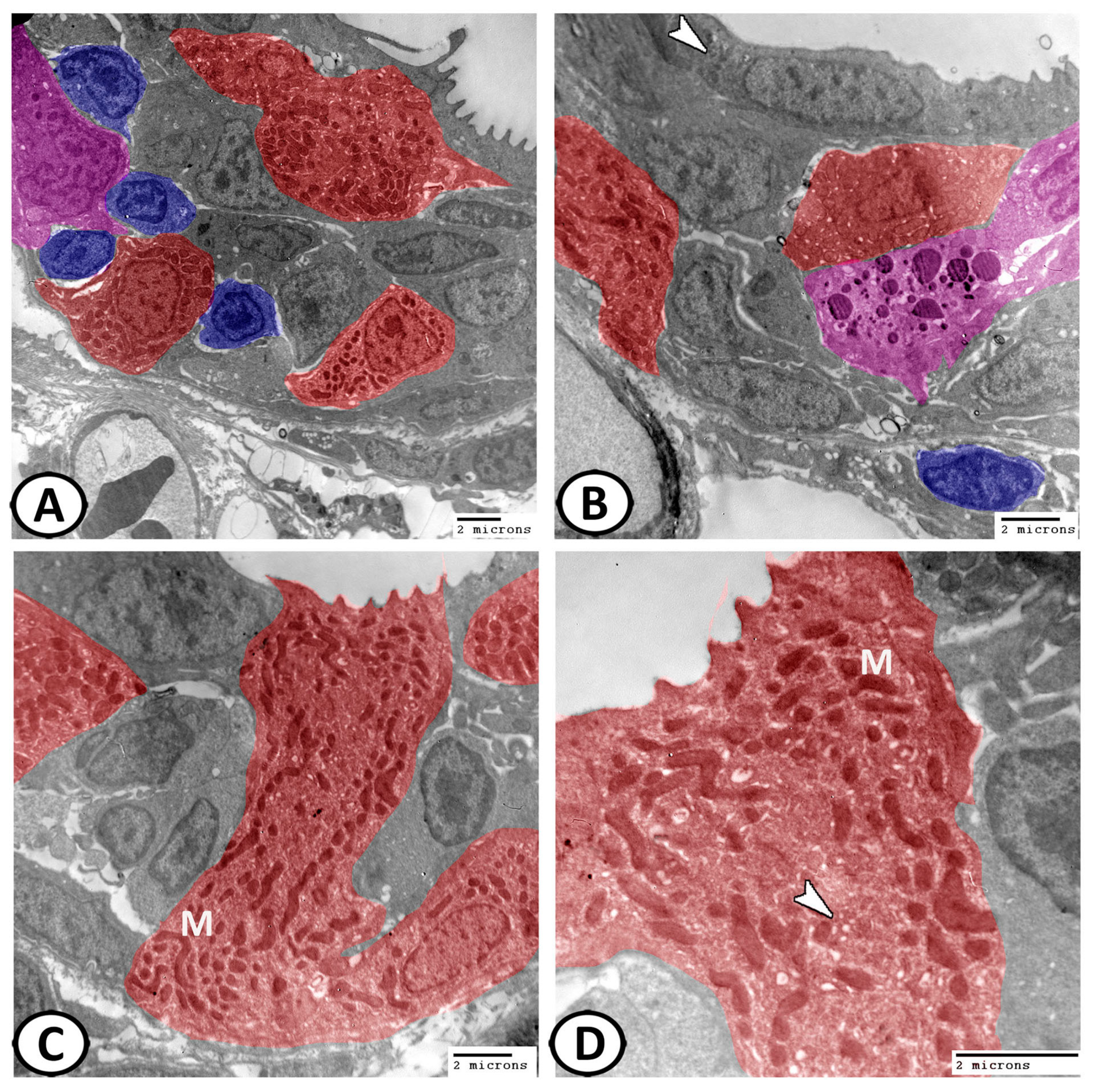
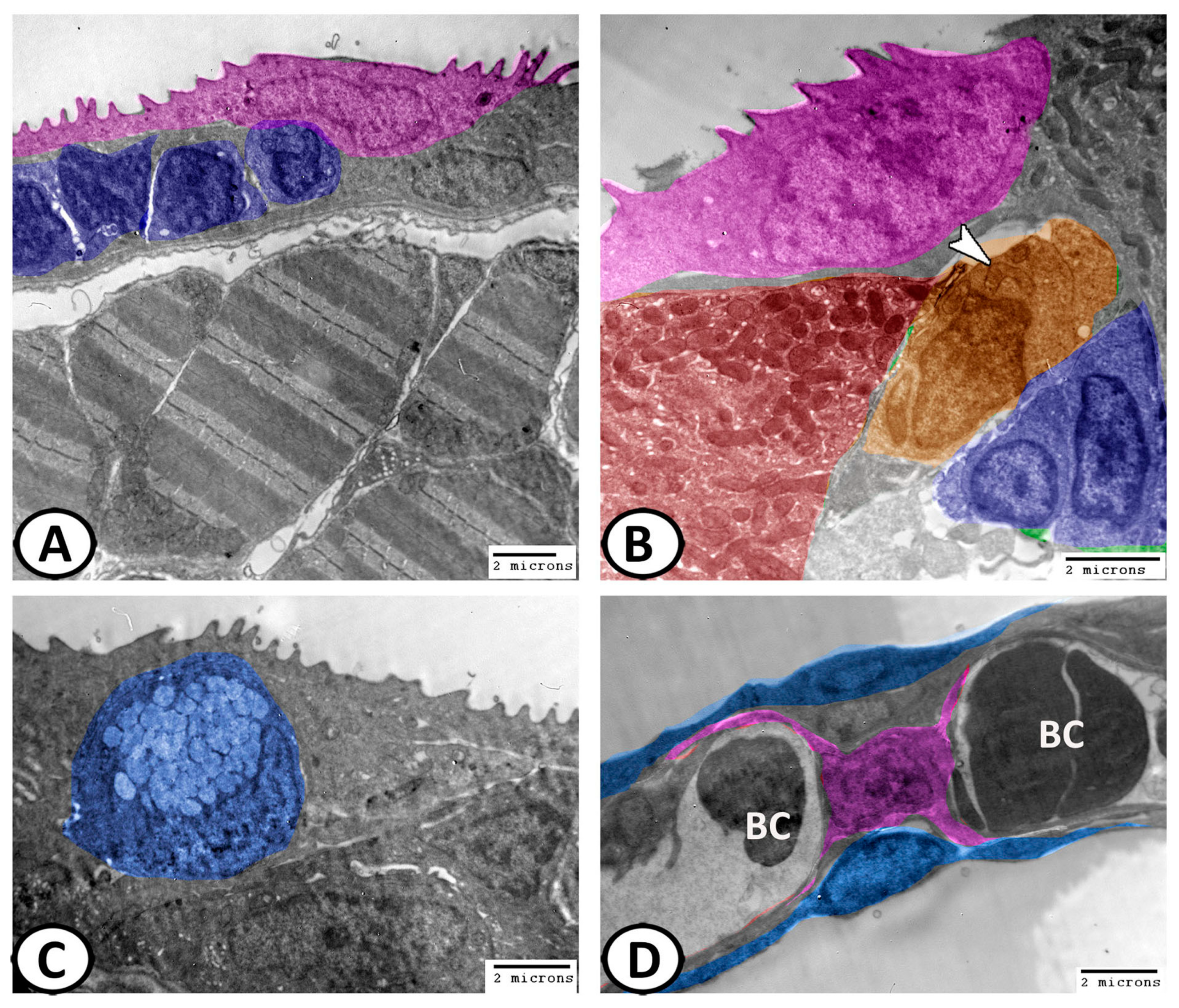
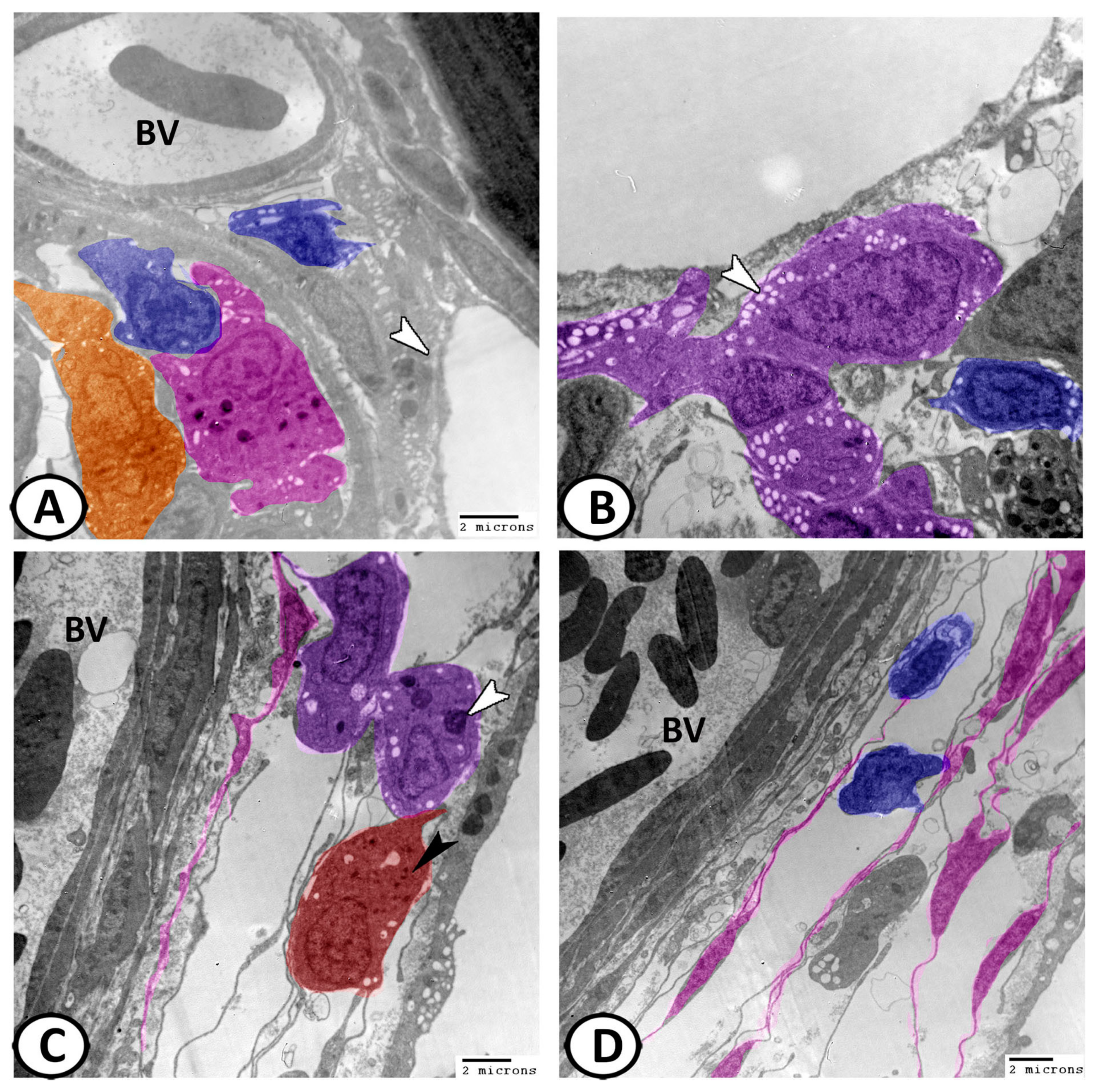
3.3. Transmission Electron Microscopy of Cellular Constituents of the Gill Filaments
3.3.1. Chloride Cells (Mitochondria-Rich Cells, MRCs)
3.3.2. Pavement Cells (PVC)
3.3.3. Mucous Cells
3.3.4. Pillar Cells
3.3.5. Other Cells
3.4. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tembo, R.N. The sublethal effects of low-pH exposure on the chemoreception of Poecilia sphenops. Arch. Environ. Contam. Toxicol. 2009, 57, 157–163. [Google Scholar] [PubMed]
- Alda, F.; Reina, R.G.; Doadrio, I.; Bermingham, E. Phylogeny and biogeography of the Poecilia sphenops species complex (Actinopterygii, Poeciliidae) in Central America. Mol. Phylogenetics Evol. 2013, 66, 1011–1026. [Google Scholar]
- Goss, G.G.; Perry, S.F.; Fryer, J.N.; Laurent, P. Gill morphology and acid-base regulation in freshwater fishes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [PubMed]
- Sasai, S.; Kaneko, T.; Hasegawa, S.; Tsukamoto, K. Morphological alteration in two types of gill chloride cells in Japanese eels (Anguilla japonica) during catadromous migration. Can. J. Zool. 1998, 76, 1480–1487. [Google Scholar] [CrossRef]
- Elsheikh, E. Scanning electron microscopic studies of gill arches and rakers in relation to feeding habits of some fresh water fishes. J. Basic Appl. Zool. 2013, 66, 121–130. [Google Scholar]
- Wilson, J.M.; Laurent, P. Fish gill morphology: Inside out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar]
- Perry, S.F. The chloride cell: Structure and function in the gills of freshwater fishes. Annu. Rev. Physiol. 1997, 59, 325–347. [Google Scholar]
- Zaccone, G.; Lauriano, E.R.; Kuciel, M.; Capillo, G.; Pergolizzi, S.; Alesci, A.; Ishimatsu, A.; Ip, Y.K.; Icardo, J.M. Identification and distribution of neuronal nitric oxide synthase and neurochemical markers in the neuroepithelial cells of the gill and the skin in the giant mudskipper, Periophthalmodon schlosseri. Zoology 2017, 125, 41–52. [Google Scholar]
- Zaccone, G.; Cupello, C.; Capillo, G.; Kuciel, M.; Nascimento, A.L.; Gopesh, A.; Germana, G.P.; Spanò, N.; Guerrera, M.C.; Aragona, M. Expression of acetylcholine-and G protein coupled muscarinic receptor in the neuroepithelial cells (NECs) of the obligated air-breathing fish, Arapaima gigas (Arapaimatidae: Teleostei). Zoology 2020, 139, 125755. [Google Scholar] [CrossRef]
- Blake, K.J.; Jiang, X.R.; Chiu, I.M. Neuronal regulation of immunity in the skin and lungs. Trends Neurosci. 2019, 42, 537–551. [Google Scholar]
- Bailly, Y. Serotonergic neuroepithelial cells in fish gills: Cytology and innervation. In Airway Chemoreceptors in the Vertebrates; CRC Press: Boca Raton, FL, USA, 2009; pp. 61–97. [Google Scholar]
- Maina, J.N.; Icardo, J.M.; Zaccone, G.; Aragona, M.; Lauriano, E.R.; Alesci, A.; Albano, M.; Guerrera, M.C.; Germana, A.; Fernandes, J.M.O. Immunohistochemical and ultrastructural study of the immune cell system and epithelial surfaces of the respiratory organs in the bimodally breathing African sharptooth catfish (Clarias gariepinus Burchell, 1822). Anat. Rec. 2022, 305, 3212–3229. [Google Scholar] [CrossRef]
- Sayed, R.K.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and functional aspects of the spleen in molly fish Poecilia sphenops (Valenciennes, 1846): Synergistic interactions of stem cells, neurons, and immune cells. Biology 2022, 11, 779. [Google Scholar]
- Hussein, M.M.; Sayed, R.K.; Mokhtar, D.M. Structural and immunohistochemical analysis of the cellular compositions of the liver of molly fish (Poecilia sphenops), focusing on its immune role. Zool. Lett. 2023, 9, 1. [Google Scholar]
- Mokhtar, D.M.; Hussein, M.M.; Sayed, R.K. Novel Identification and Microscopy of the Intestinal Bulb of Molly Fish (Poecilia sphenops) with a Focus on Its Role in Immunity. Microsc. Microanal. 2022, 28, 1827–1839. [Google Scholar]
- Hirose, S.; Kaneko, T.; Naito, N.; Takei, Y. Molecular biology of major components of chloride cells. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 593–620. [Google Scholar]
- Li, J.; Xu, X.; Liu, C. A scanning electron microscopical observation of gill structure of largemouth bass Micropterus salmoides and blue gill Lepomis macrochirus. J. Dalian Fish. Univ. 2009, 24, 231–235. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Karnovsky, M. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137A. [Google Scholar]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; Apple Academic Press: Oakville, ON, Canada, 2021. [Google Scholar]
- Evans, D.H.; Piermarini, P.M.; Potts, W. Ionic transport in the fish gill epithelium. J. Exp. Zool. 1999, 283, 641–652. [Google Scholar]
- Watrin, A.; Mayer-Gostan, N. Simultaneous recognition of ionocytes and mucous cells in the gill epithelium of turbot and in the rat stomach. J. Exp. Zool. 1996, 276, 95–101. [Google Scholar]
- Heijden, A.; Verbost, P.; Eygensteyn, J.; Li, J.; Bonga, S.; Flik, G. Mitochondria-rich cells in gills of tilapia (Oreochromis mossambicus) adapted to fresh water or sea water: Quantification by confocal laser scanning microscopy. J. Exp. Biol. 1997, 200, 55–64. [Google Scholar] [CrossRef]
- Sturla, M.; Masini, M.; Prato, P.; Grattarola, C.; Uva, B. Mitochondria-rich cells in gills and skin of an African lungfish, Protopterus annectens. Cell Tissue Res. 2001, 303, 351–358. [Google Scholar] [CrossRef]
- Bettex-Galland, M.; Hughes, G. Contractile filamentous material in the pillar cells of fish gills. J. Cell Sci. 1973, 13, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Carmona, R.; García-Gallego, M.; Sanz, A.; Domezain, A.; Ostos-Garrido, M. Chloride cells and pavement cells in gill epithelia of Acipenser naccarii: Ultrastructural modifications in seawater-acclimated specimens. J. Fish Biol. 2004, 64, 553–566. [Google Scholar] [CrossRef]
- Sayed, R.K.; Abd-El Aziz, N.A.; Ibrahim, I.A.; Mokhtar, D.M. Structural, ultrastructural, and functional aspects of the skin of the upper lip of silver carp (Hypophthalmichthys molitrix). Microsc. Res. Tech. 2021, 84, 1821–1833. [Google Scholar] [CrossRef]
- Zaccone, G. Immunity and Neuroimmune Interactions at the Mucosal Barriers in Fish. Fishes 2022, 7, 381. [Google Scholar] [CrossRef]
- Zaccone, G.; Lauweryns, J.; Fasulo, S.; Tagliafierro, G.; Ainis, L.; Licata, A. Immunocytochemical localization of serotonin and neuropeptides in the neuroendocrine paraneurons of teleost and lungfish gills. Acta Zool. 1992, 73, 177–183. [Google Scholar] [CrossRef]
- Zaccone, G.; Fasulo, S.; Ainis, L.; Licata, A. Paraneurons in the skin and gills of fishes. In Ichthyology: Recent Research Advances; Saksena, D.N., Ed.; Science Publishers Inc.: Enfield, NH, USA, 1997; pp. 417–447. [Google Scholar]
- Zaccone, G.; Lauriano, E.R.; Capillo, G.; Kuciel, M. Air-breathing in fish: Air-breathing organs and control of respiration: Nerves and neurotransmitters in the air-breathing organs and the skin. Acta Histochem. 2018, 120, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Mauceri, A.; Maisano, M.; Giannetto, A.; Parrino, V.; Fasulo, S. Innervation and neurotransmitter localization in the lung of the Nile bichir Polypterus bichir bichir. Anat. Rec. 2007, 290, 1166–1177. [Google Scholar] [CrossRef]
- Zachar, P.C.; Jonz, M.G. Neuroepithelial cells of the gill and their role in oxygen sensing. Respir. Physiol. Neurobiol. 2012, 184, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, D.; Icardo, J.M.; Kuciel, M.; Alesci, A.; Pergolizzi, S.; Satora, L.; Lauriano, E.R.; Zaccone, G. Polymorphous granular cells in the lung of the primitive fish, the bichir P olypterus senegalus. Acta Zool. 2017, 98, 13–19. [Google Scholar] [CrossRef]
- Zaccone, G.; Mauceri, A.; Fasulo, S. Neuropeptides and nitric oxide synthase in the gill and the air-breathing organs of fishes. J. Exp. Zool. Part A Comp. Exp. Biol. 2006, 305, 428–439. [Google Scholar] [CrossRef]
- Jonz, M.G.; Zaccone, G. Nervous control of the gills. Acta Histochem. 2009, 111, 207–216. [Google Scholar] [CrossRef]
- Jonz, M.G.; Buck, L.T.; Perry, S.F.; Schwerte, T.; Zaccone, G. Sensing and surviving hypoxia in vertebrates. Ann. N. Y. Acad. Sci. 2016, 1365, 43–58. [Google Scholar] [CrossRef]
- Porteus, C.S.; Pollack, J.; Tzaneva, V.; Kwong, R.W.; Kumai, Y.; Abdallah, S.J.; Zaccone, G.; Lauriano, E.R.; Milsom, W.K.; Perry, S.F. A role for nitric oxide in the control of breathing in zebrafish (Danio rerio). J. Exp. Biol. 2015, 218, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Capillo, G.; Fernandes, J.M.O.; Kiron, V.; Lauriano, E.R.; Alesci, A.; Lo Cascio, P.; Guerrera, M.C.; Kuciel, M.; Zuwala, K. Expression of the antimicrobial peptide piscidin 1 and neuropeptides in fish gill and skin: A potential participation in neuro-immune interaction. Mar. Drugs 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.R.; Capillo, G.; Icardo, J.M.; Fernandes, J.M.O.; Kiron, V.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Zaccone, G. Neuroepithelial cells (NECs) and mucous cells express a variety of neurotransmitters and neurotransmitter receptors in the gill and respiratory air-sac of the catfish Heteropneustes fossilis (Siluriformes, Heteropneustidae): A possible role in local immune defence. Zoology 2021, 148, 125958. [Google Scholar] [PubMed]
- Saltys, H.A.; Jonz, M.G.; Nurse, C.A. Comparative study of gill neuroepithelial cells and their innervation in teleosts and Xenopus tadpoles. Cell Tissue Res. 2006, 323, 1–10. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdelhafez, E.A. An overview of the structural and functional aspects of immune cells in teleosts. Histol. Histopathol. 2021, 34, 399–414. [Google Scholar]
- Yao, Z.; Delorme-Axford, E.; Backues, S.K.; Klionsky, D.J. Atg41/Icy2 regulates autophagosome formation. Autophagy 2015, 11, 2288–2299. [Google Scholar] [CrossRef]
- Pierdominici, M.; Vomero, M.; Barbati, C.; Colasanti, T.; Maselli, A.; Vacirca, D.; Giovannetti, A.; Malorni, W.; Ortona, E. Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. FASEB J. 2012, 26, 1400–1412. [Google Scholar] [CrossRef]
- Pasparakis, M. Role of NF-κB in epithelial biology. Immunol. Rev. 2012, 246, 346–358. [Google Scholar] [CrossRef]
- Kato, G.; Miyazawa, H.; Nakayama, Y.; Ikari, Y.; Kondo, H.; Yamaguchi, T.; Sano, M.; Fischer, U. A novel antigen-sampling cell in the teleost gill epithelium with the potential for direct antigen presentation in mucosal tissue. Front. Immunol. 2018, 9, 2116. [Google Scholar] [CrossRef] [PubMed]
- Huminiecki, L.; Goldovsky, L.; Freilich, S.; Moustakas, A.; Ouzounis, C.; Heldin, C.-H. Emergence, development and diversification of the TGF-β signalling pathway within the animal kingdom. BMC Evol. Biol. 2009, 9, 28. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Cheng, X.; Li, W.; Lyu, S.; Chen, X.; Li, Q.; Wang, H. Molecular Evolution of Transforming Growth Factor-β (TGF-β) Gene Family and the Functional Characterization of Lamprey TGF-β2. Front. Immunol. 2022, 13, 836226. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, G.; Rowlerson, A.; Mascarello, F.; Patruno, M.; Funkenstein, B. Myostatin precursor is present in several tissues in teleost fish: A comparative immunolocalization study. Cell Tissue Res. 2003, 311, 239–250. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, B.; Du, H. A review on sox genes in fish. Rev. Aquac. 2021, 13, 1986–2003. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Zhang, F.-L.; Tang, X.-L.; Yang, X.-J. Telocytes enhances M1 differentiation and phagocytosis while inhibits mitochondria-mediated apoptosis via activation of NF-κB in macrophages. Cell Transplant. 2021, 30, 09636897211002762. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, D.M.; Hussein, M.M.; Zaccone, G.; Alesci, A.; Lauriano, E.R.; Sayed, R.K.A. Gills of Molly Fish: A Potential Role in Neuro-Immune Interaction. Fishes 2023, 8, 195. https://doi.org/10.3390/fishes8040195
Mokhtar DM, Hussein MM, Zaccone G, Alesci A, Lauriano ER, Sayed RKA. Gills of Molly Fish: A Potential Role in Neuro-Immune Interaction. Fishes. 2023; 8(4):195. https://doi.org/10.3390/fishes8040195
Chicago/Turabian StyleMokhtar, Doaa M., Marwa M. Hussein, Giacomo Zaccone, Alessio Alesci, Eugenia Rita Lauriano, and Ramy K. A. Sayed. 2023. "Gills of Molly Fish: A Potential Role in Neuro-Immune Interaction" Fishes 8, no. 4: 195. https://doi.org/10.3390/fishes8040195
APA StyleMokhtar, D. M., Hussein, M. M., Zaccone, G., Alesci, A., Lauriano, E. R., & Sayed, R. K. A. (2023). Gills of Molly Fish: A Potential Role in Neuro-Immune Interaction. Fishes, 8(4), 195. https://doi.org/10.3390/fishes8040195









