Sex Ratio, Spawning Period, and Sexual Group Maturity of the Largehead Hairtail Trichiurus japonicus (Teleostei: Trichiuridae) in Korean Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Environmental Conditions
2.3. Histological Analysis
2.4. Sex Ratio
2.5. Gonadosomatic Index (GSI)
2.6. Gonadal Developmental Stage
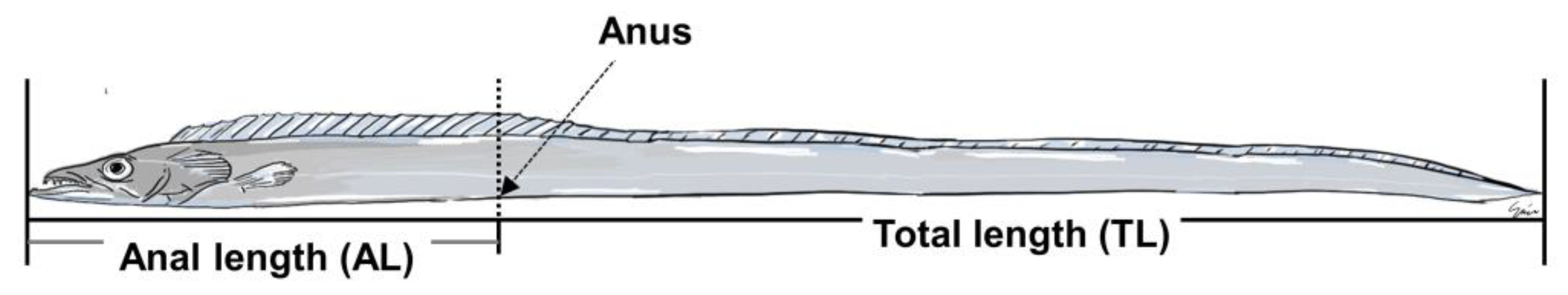
2.7. Sexual Group Maturity
3. Results
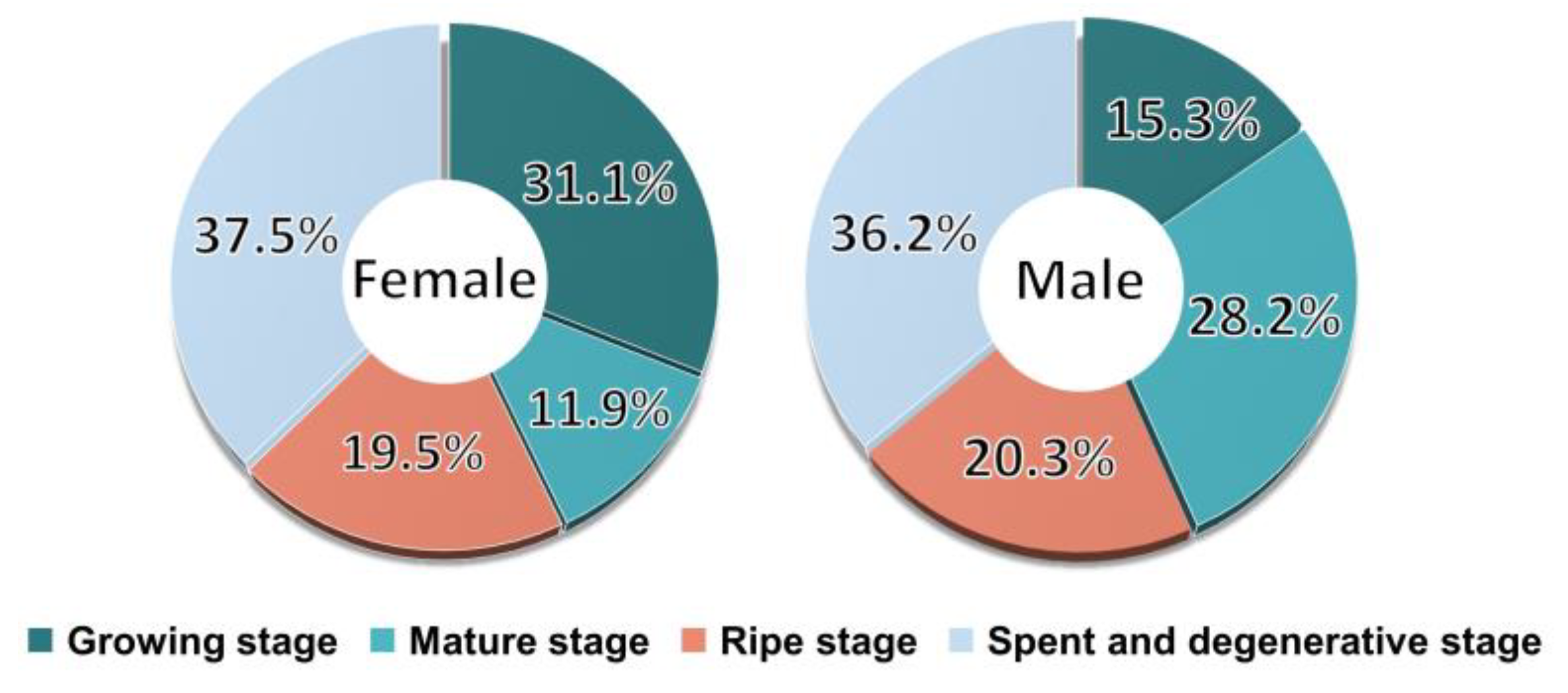
3.1. Sex Ratio
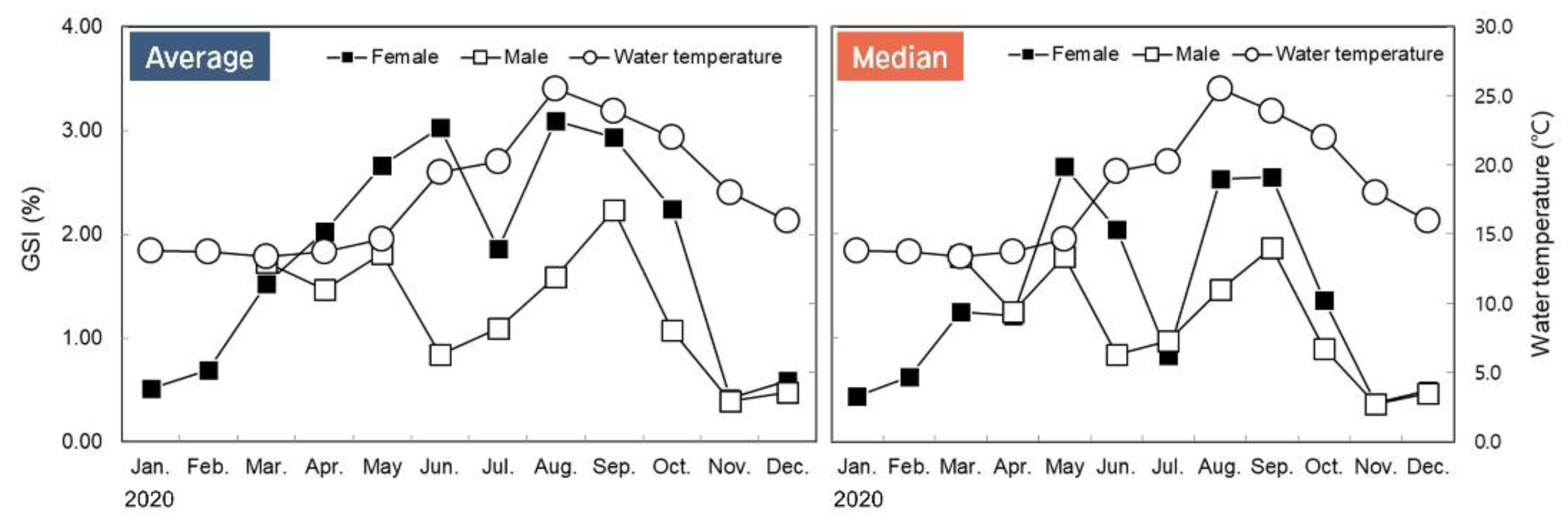
3.2. Monthly Change of Gonadosomatic Index (GSI)
3.3. Histological Change with Gonadal Developmental Stage
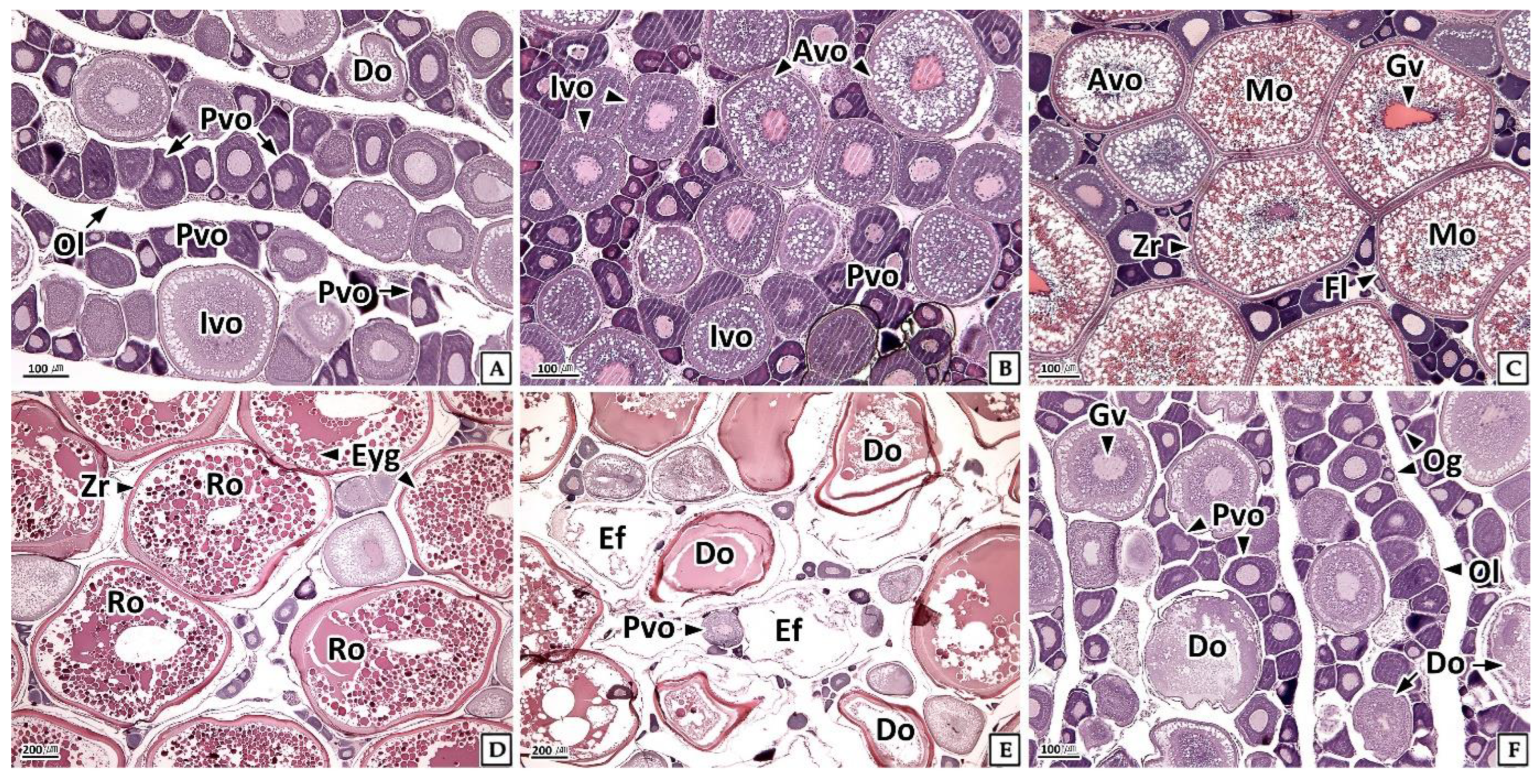
3.3.1. Ovary
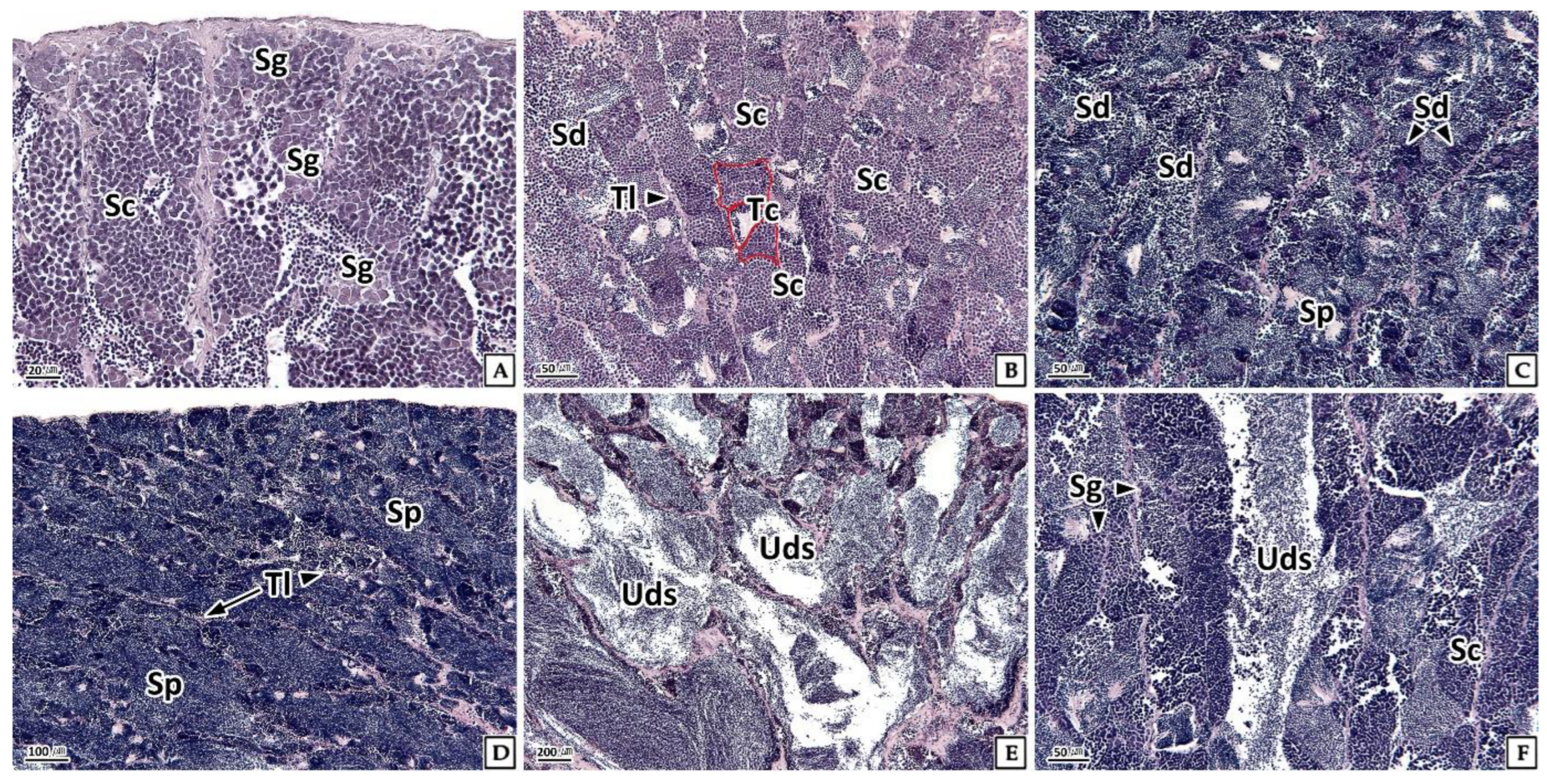
3.3.2. Testis
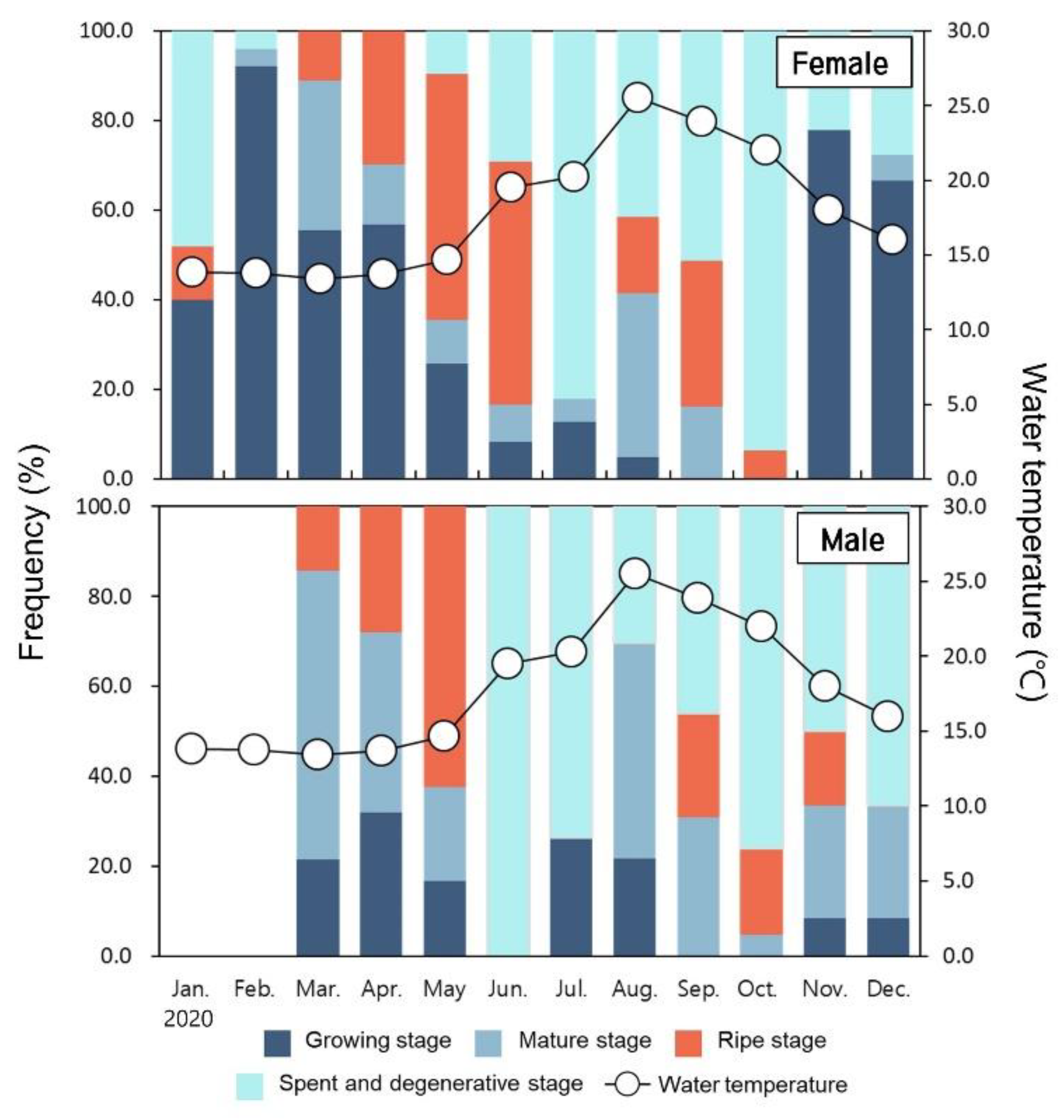
3.4. Monthly Change of Gonadal Developmental Stage
3.4.1. Ovary
3.4.2. Testis
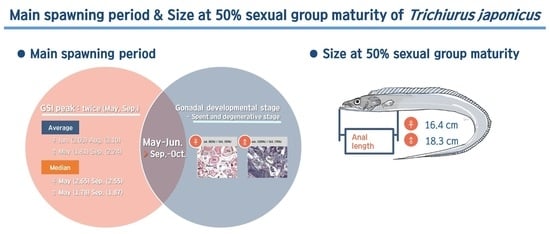
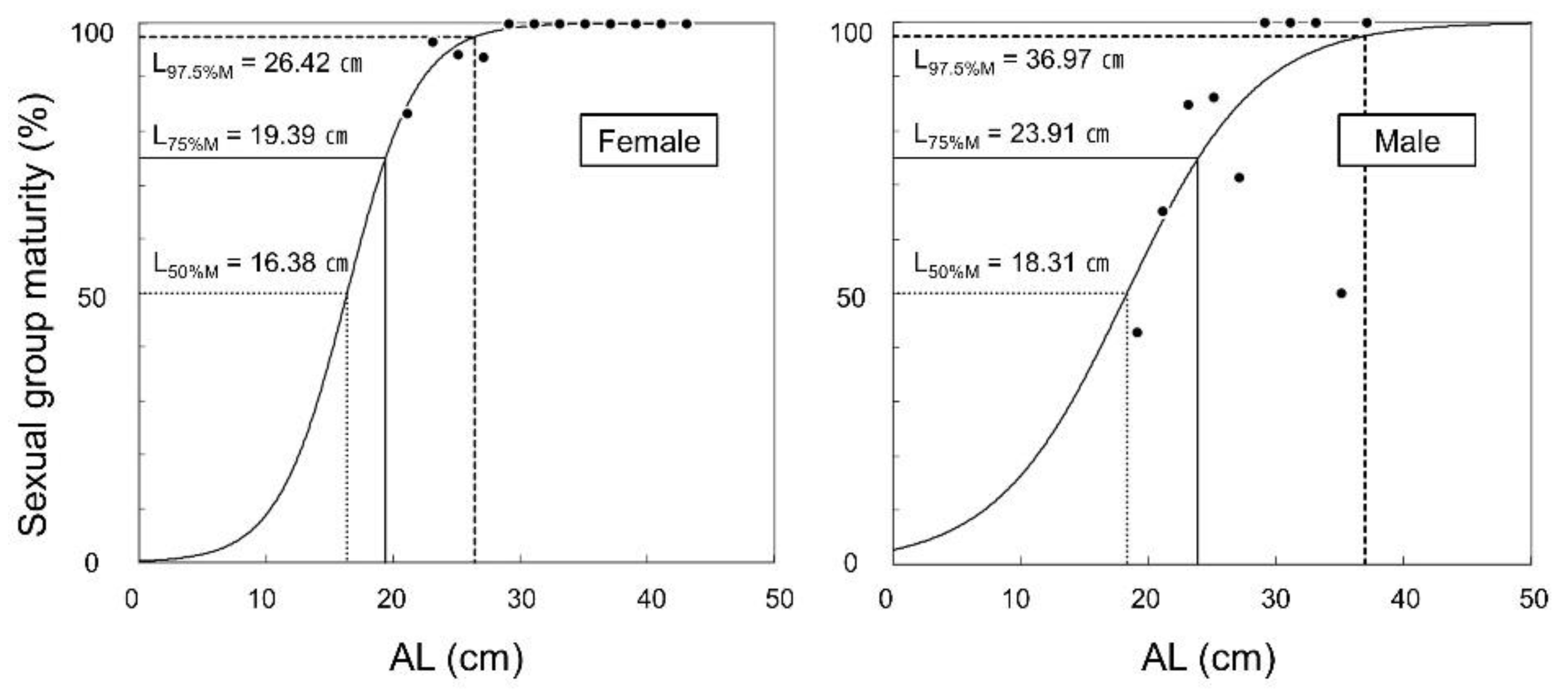
3.5. Sexual Group Maturity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fishbase. Available online: https://www.fishbase.se/summary/Trichiurus-lepturus.html (accessed on 5 January 2023).
- Kao, W.; Tomiyasu, M.; Takahashi, R.; Ogawa, M.; Hirose, T.; Kurosaka, K.; Tsuru, S.; Sanada, Y.; Minami, K.; Miyashita, K. Spatial and temporal distribution of hairtail (Trichiurus japonicus) in the Bungo Channel, Japan. J. Marine Acoust. Soc. Jpn. 2015, 42, 167–176. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J. Identification of Trichiurus (Pisces: Trichiuridae) eggs and larvae from Korea, with a taxonomic note. Fish. Aquat. Sci. 2014, 17, 137–143. [Google Scholar] [CrossRef]
- Marine Bio-Resource Information System (MBRIS). Available online: https://www.mbris.kr/pub/about/mbrisis/mbrisis.do (accessed on 5 January 2023).
- Korean Statistical Information Service (KOSIS). Available online: https://kosis.kr/index/index.do (accessed on 5 January 2023).
- Cha, H.K.; Lee, D.W. Reproduction of hairtail, Trichiurus lepturus Linnaeus in Korea waters. J. Korean Soc. Fish. Res. 2004, 6, 54–62. [Google Scholar]
- Kim, H.J.; Park, J.H.; Kwon, D.H.; Kim, Y. Maturation and spawning of largehead hairtail Trichiurus japonicus near Jeju Island, Korea. J. Fish. Aquat. Sci. 2020, 53, 1–8. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, J.T.; Lee, E.; Oh, T.Y.; Lee, D.W. Age and growth of largehead hairtail Trichiurus lepturus in the East China Sea. Kor. J. Fish. Aquat. Sci. 2011, 44, 695–700. [Google Scholar] [CrossRef]
- International Tribunal for the Law of the Sea (ITLOS). The United Nations Convention on the Law of the Sea. Available online: https://www.itlos.org/en/main/the-tribunal/unclos/ (accessed on 13 March 2023).
- Ministry of Government Legislation (MOLEG). Available online: https://www.moleg.go.kr/mpbleg/mpblegInfo.mo?mid=a10402020000&mpb_leg_pst_seq=129850 (accessed on 13 March 2023).
- Head, M.A.; Keller, A.A.; Bradburn, M. Maturity and growth of sablefish, Anoplopoma fimbria, along the U.S. West Coast. Fish. Res. 2014, 159, 56–67. [Google Scholar] [CrossRef]
- Head, M.A.; Stokes, G.L.; Thorson, J.T.; Keller, A.A. Techniques for improving estimates of maturity ogives in groundfish using double-reads and measurement error models. Fish. Res. 2016, 179, 251–258. [Google Scholar] [CrossRef]
- Misu, H. Studies on the fisheries biology on the ribbon fish (Trichiurus lepturus Linnaeus) in the East China and the Yellow Seas. (3) Distribution, migration and consideration of population of population. Bull. Seikai Reg. Fish. Res. Lab. 1961, 24, 115–131. [Google Scholar]
- Yamada, U. On the distribution and migration of the ribbon fish, Trichiurus lepturus Linnaeus, in the East China Sea and Yellow Seas by fish-size. Bull. Seikai Reg. Fish. Res. Lab. 1964, 32, 137–164. [Google Scholar]
- Munekiyo, M.; Kuwahara, A. Maturity and spawning of ribbon fish in the Western Wakasa Bay. Nippon Suisan Gakkaishi 1988, 54, 1315–1320. [Google Scholar] [CrossRef]
- Thiagarajan, R.; Lazarus, S.; Sastry, Y.A.; Khan, M.Z.; Kasim, H.M. Stock assessment of the ribbon fish, Trichiurus lepturus (Linnaeus), from the Indian waters. Indian J. Fish. 1992, 39, 182–194. [Google Scholar]
- Kim, S.H.; Lee, Y.D.; Rho, H.K. The study on the fisheries biological feature of hairtail, Trichiurus lepturus from the Cheju Strait. Korean J. Fish. Aquat. Sci. 1998, 31, 17–25. [Google Scholar]
- Kwok, K.Y.; Ni, I.H. Reproduction of cutlassfishes Trichiurus spp. from the South China Sea. Mar. Ecol. Prog. Ser. 1999, 176, 39–47. [Google Scholar] [CrossRef]
- Martins, A.S.; Haimovici, M. Reproduction of the cutlassfish Trichiurus lepturus in the southern Brazil subtropical convergence ecosystem. Sci. Mar. 2000, 64, 97–105. [Google Scholar] [CrossRef]
- Shih, N.T.; Hsu, K.C.; Ni, I.H. Age, growth and reproduction of cutlassfishes Trichiurus spp. in the southern East China Sea. J. Appl. Ichthyol. 2011, 27, 1307–1315. [Google Scholar] [CrossRef]
- Rajesh, K.M. Fishery, reproductive biology and stock status of the largehead hairtail Trichiurus lepturus Linnaeus, 1758 off Karnataka, south-west coast of India. Indian J. Fish. 2015, 62, 28–34. [Google Scholar]
- Clain, C.; Stewart, J.; Fowler, A.; Diamond, S. Reproductive biology of largehead hairtail (Trichiurus lepturus) in south-eastern Australia. Aquac. Fish. 2023, 8, 148–158. [Google Scholar] [CrossRef]
- Rideout, R.M.; Morgan, M.J.; Lilly, G.R. Variation in the frequency of skipped spawning in Atlantic cod (Gadus morhua) off Newfoundland and Labrador. ICES J. Mar. Sci. 2006, 63, 1101–1110. [Google Scholar] [CrossRef]
- Al-Nahdi, A.; Al-Marzouqi, A.; Al-Rasadi, E.; Groeneveld, J.C. The size composition, reproductive biology, age and growth of largehead cutlassfish Trichiurus lepturus Linnaeus from the Arabian Sea coast of Oman. Indian J. Fish. 2009, 56, 73–79. [Google Scholar]
- Korea Hydrogrphic and Oceanographic Administration (KHOA). Available online: http://www.khoa.go.kr/oceangrid/gis/category/reference/distribution.do (accessed on 5 January 2023).
- De la Cruz-Torres, J.; Martínez-Pérez, J.A.; Franco-López, J.; Ramírez-Villalobos, A.J. Biological and ecological aspects of Trichiurus lepturus Linnaeus, 1758 (Perciformes: Trichiuridae) in Boca Del Rio, Veracruz, Mexico. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 1058–1066. [Google Scholar] [CrossRef]
- Ghosh, S.; Rao, M.V.H.; Rohit, P.; Rammohan, K.; Maheswarudu, G. Reproductive biology, trophodynamics and stock structure of ribbon fish Trichiurus lepturus from northern Arabian Sea and northern Bay of Bengal. Indian J. Geo. Mar. Sci. 2014, 43, 755–771. [Google Scholar]
- Shin, S.R.; Kim, H.J.; Oh, H.Y.; Kim, J.W.; Lee, J.S. Histological description of oogenesis in largehead hairtail Trichiurus lepturus (Teleostei: Trichiuridae). J. Mar. Life Sci. 2022, 7, 55–59. [Google Scholar] [CrossRef]
- Lee, J.S.; Huh, S.H. Reproductive biology of the slimy, Leiognathus nuchalis (Teleostei: Leiognathidae). Korean J. Ichthyol. 2000, 12, 192–202. [Google Scholar]
- Martins, A.S.; Haimovici, M.; Palacios, R. Diet and feeding of the cutlassfish Trichiurus lepturus in the subtropical convergence ecosystem of southern Brazil. J. Mar. Biol. Assoc. 2005, 85, 1223–1229. [Google Scholar] [CrossRef]
- Chiou, W.D.; Chen, C.Y.; Wang, C.M.; Chen, C.T. Food and feeding habits of ribbonfish Trichiurus lepturus in coastal waters of south-western Taiwan. Fish. Sci. 2006, 72, 373–381. [Google Scholar] [CrossRef]
- Rideout, R.M.; Burton, M.P.; Rose, G.A. Observations on mass atresia and skipped spawning in northern Atlantic cod, from Smith Sound, Newfoundland. J. Fish Biol. 2000, 57, 1429–1440. [Google Scholar] [CrossRef]
- Rideout, R.M.; Rose, G.A.; Burton, M.P. Skipped spawning in female iteroparous fishes. Fish. Fish. 2005, 6, 50–72. [Google Scholar] [CrossRef]
- Jørgensen, C.; Ernande, B.; Fiksen, Ø.; Dieckmann, U. The logic of skipped spawning in fish. Can. J. Fish. Aquat. Sci. 2006, 63, 200–211. [Google Scholar] [CrossRef]
- Eckelbarger, K.J.; Watling, L. Role of phylogenetic constraints in determining reproductive patterns in deep-sea invertebrates. Invertebr. Biol. 1995, 114, 256–269. [Google Scholar] [CrossRef]
- Wallace, R.A.; Selman, K. Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zool. 1981, 21, 325–343. [Google Scholar] [CrossRef]
- Reuben, S.; Vijayakumaran, K.; Achayya, P.; Prabhakar, R.V.D. Biology and exploitation of Trichiurus lepturus Linnaeus from Visakhapatnam waters. Indian J. Fish. 1997, 44, 101–110. [Google Scholar]
- Bellini, A.T. Biologia e Bionomia de Trichiurus lepturus (Linneu, 1758) (Trichiuridae; Perciformes; Teleostei), da Costa Brasileira, entra Cabo Frio (23°00) eTorres (29°21’). M.S. Thesis, Universidade de Sao Paolo, Sao Paolo, Brazil, 1980. [Google Scholar]
- Sheridan, P.F.; Trimm, D.L.; Baker, B.M. Reproduction and food habits of seven species of northern Gulf of Mexico fishes. Contr. Mar. Sci. 1984, 27, 175–204. [Google Scholar]
| Months (2020) | Number of Samples (Total Length, TL; Total Weight, TW) | |
|---|---|---|
| Sex Ratio | Histological Analysis of the Gonads | |
| January | 30 (TL 85.5 ± 6.9 cm, TW 346.6 ± 128.0 g) | 30 (TL 85.5 ± 6.9 cm, TW 346.6 ± 128.0 g) |
| February | 30 (TL 77.1 ± 4.6 cm, TW 253.4 ± 57.1 g) | 30 (TL 77.1 ± 4.6 cm, TW 253.4 ± 57.1 g) |
| March | 70 (TL 83.8 ± 7.9 cm, TW 308.2 ± 126.8 g) | 32 (TL 85.0 ± 8.7 cm, TW 343.3 ± 145.5 g) |
| April | 185 (TL 79.1 ± 12.6 cm, TW 281.7 ± 204.1 g) | 62 (TL 79.0 ± 15.3 cm, TW 300.8 ± 258.2 g) |
| May | 95 (TL 78.3 ± 9.9 cm, TW 252.6 ± 130.1 g) | 60 (TL 74.8 ± 19.0 cm, TW 279.0 ± 195.5 g) |
| June | 108 (TL 80.3 ± 11.9 cm, TW 323.8 ± 172.8 g) | 25 (TL 90.2 ± 12.9 cm, TW 420.9 ± 188.3 g) |
| July | 70 (TL 78.8 ± 10.9 cm, TW 316.0 ± 179.2 g) | 62 (TL 78.7 ± 11.4 cm, TW 206.7 ± 43.0 g) |
| August | 228 (TL 79.6 ± 5.8 cm, TW 284.8 ± 92.4 g) | 64 (TL 79.6 ± 7.4 cm, TW 294.5 ± 102.6 g) |
| September | 209 (TL 83.1 ± 10.0 cm, TW 328.7 ± 163.8 g) | 64 (TL 81.7 ± 10.4 cm, TW 317.7 ± 150.9 g) |
| October | 272 (TL 84.9 ± 8.2 cm, TW 327.5 ± 159.7 g) | 60 (TL 87.6 ± 10.9 cm, TW 388.9 ± 225.6 g) |
| November | 286 (TL 83.6 ± 8.5 cm, TW 314.6 ± 197.4 g) | 30 (TL 84.2 ± 11.1 cm, TW 320.1 ± 188.0 g) |
| December | 280 (TL 83.7 ± 7.2 cm, TW 305.4 ± 120.5 g) | 30 (TL 84.6 ± 9.3 cm, TW 322.4 ± 138.4 g) |
| Total Average | 1863 TL 81.9 ± 9.2 cm, TW 303.2 ± 155.3 g | 549 TL 81.7 ± 11.3 cm, TW 322.1 ± 185.8 g |
| Total Length (cm) | Number | Sex Ratio (F:M) | Female (%) | ||
|---|---|---|---|---|---|
| Total | Female | Male | |||
| 50.1–55.0 | 3 | 2 | 1 | 1:0.50 | 66.7 |
| 55.1–60.0 | 4 | 3 | 1 | 1:0.33 | 75.0 |
| 60.1–65.0 | 32 | 13 | 19 | 1:1.46 | 40.6 |
| 65.1–70.0 | 77 | 46 | 31 | 1:0.67 | 59.7 |
| 70.1–75.0 | 262 | 153 | 109 | 1:0.71 | 58.4 |
| 75.1–80.0 | 496 | 293 | 203 | 1:0.69 | 59.1 |
| 80.1–85.0 | 456 | 311 | 145 | 1:0.47 | 68.2 |
| 85.1–90.0 | 222 | 177 | 45 | 1:0.25 | 79.7 |
| 90.1–95.0 | 117 | 102 | 15 | 1:0.15 | 87.2 |
| 95.1–100.0 | 113 | 102 | 11 | 1:0.11 | 90.3 |
| 100.1–105.0 | 55 | 48 | 7 | 1:0.15 | 87.3 |
| 105.1–110.0 | 14 | 12 | 2 | 1:0.17 | 85.7 |
| 110.1–115.0 | 7 | 7 | - | - | 100 |
| 115.1–120.0 | 5 | 5 | - | - | 100 |
| Total/Average | 1863 | 1274 | 589 | 1:0.46 | 68.4 |
| Anal Length (cm) | Female | Male | ||||
|---|---|---|---|---|---|---|
| Examined Individuals | Mature Individuals | Maturity (%) | Examined Individuals | Mature Individuals | Maturity (%) | |
| 18.1–20.0 | 5 | 5 | 100 | 7 | 3 | 42.9 |
| 20.1–22.0 | 37 | 32 | 86.6 | 25 | 16 | 64.0 |
| 22.1–24.0 | 68 | 66 | 97.1 | 51 | 43 | 84.3 |
| 24.1–26.0 | 72 | 69 | 95.8 | 44 | 38 | 86.4 |
| 26.1–28.0 | 74 | 70 | 94.6 | 29 | 22 | 75.9 |
| 28.1–30.0 | 23 | 23 | 100 | 7 | 7 | 100 |
| 30.1–32.0 | 28 | 28 | 100 | 1 | 1 | 100 |
| 32.1–34.0 | 34 | 34 | 100 | 12 | 12 | 100 |
| 34.1–36.0 | 14 | 14 | 100 | 3 | 2 | 66.7 |
| 36.1–38.0 | 6 | 6 | 100 | 2 | 2 | 100 |
| 38.1–40.0 | 2 | 2 | 100 | - | - | - |
| 40.1–42.0 | 3 | 3 | 100 | - | - | - |
| 42.1–44.0 | 2 | 2 | 100 | - | - | - |
| Total | 368 | 354 | 96.2 | 181 | 146 | 80.7 |
| Region | Species | Sampling Area | Sex Ratio (F:M) | Size (cm) | Citation |
|---|---|---|---|---|---|
| Tropical to subtropical | T. lepturus | Visakhapatnam Waters, India | - | TL 42.5 | Reuben et al., 1997 [37] |
| All-India | - | TL 60 | Thiagarajan et al., 1992 [16] | ||
| Karnataka Coast, India | 1:0.85 | TL 55.4 | Rajesh et al., 2015 [21] | ||
| Arabian Sea, Oman | 1:0.12 | TL 79 | Al-Nahdi et al., 2009 [24] | ||
| Northern Arabian Sea | 1:0.75 | TL 61.2 | Ghosh et al., 2014 [27] | ||
| Northern Bay of Bengal | 1:0.81 | TL 52.9 | |||
| South-eastern Brazil | - | TL 39 | Bellini, 1980 [38] | ||
| Gulf of Mexico | - | TL 35 | Sheridan et al., 1984 [39] | ||
| Southern Brazil | 1:1 | TL 69.3 | Martins and Haimovici, 2000 [19] | ||
| Temperate | T. nanhaiensis | South China Sea | 1:1 | AL 28.2 | Kwok and Ni, 1999 [18] |
| T. lepturus | South China Sea | 1:1 | AL 25.5 | ||
| South-eastern Australia | 1:0.4 | TL 108.0 | Clain et al., 2023 [22] | ||
| Jeju Island, Korea | - | AL 26.0 | Cha and Lee, 2004 [6] | ||
| T. japonicus | Southern East China Sea | 1:1 | PL 26.4 | Shih et al., 2011 [20] | |
| Jeju Island, Korea | 1:0.38 | AL 25.0 | Kim et al., 2020 [7] | ||
| Jeju Island, Korea | 1:0.46 | AL 16.4 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.R.; Kim, H.J.; Kim, J.W.; Kwon, D.-H.; Choi, J.; Park, J.J.; Lee, J.S. Sex Ratio, Spawning Period, and Sexual Group Maturity of the Largehead Hairtail Trichiurus japonicus (Teleostei: Trichiuridae) in Korean Waters. Fishes 2023, 8, 194. https://doi.org/10.3390/fishes8040194
Shin SR, Kim HJ, Kim JW, Kwon D-H, Choi J, Park JJ, Lee JS. Sex Ratio, Spawning Period, and Sexual Group Maturity of the Largehead Hairtail Trichiurus japonicus (Teleostei: Trichiuridae) in Korean Waters. Fishes. 2023; 8(4):194. https://doi.org/10.3390/fishes8040194
Chicago/Turabian StyleShin, So Ryung, Hyeon Jin Kim, Jae Won Kim, Dae-Hyeon Kwon, Junghwa Choi, Jung Jun Park, and Jung Sick Lee. 2023. "Sex Ratio, Spawning Period, and Sexual Group Maturity of the Largehead Hairtail Trichiurus japonicus (Teleostei: Trichiuridae) in Korean Waters" Fishes 8, no. 4: 194. https://doi.org/10.3390/fishes8040194
APA StyleShin, S. R., Kim, H. J., Kim, J. W., Kwon, D.-H., Choi, J., Park, J. J., & Lee, J. S. (2023). Sex Ratio, Spawning Period, and Sexual Group Maturity of the Largehead Hairtail Trichiurus japonicus (Teleostei: Trichiuridae) in Korean Waters. Fishes, 8(4), 194. https://doi.org/10.3390/fishes8040194