Abstract
In external fertilizer fish, ovarian fluid (OF) seems to play a key role in fertilization success, improving spermatozoa swimming performance. These OF/sperm interaction mechanisms are frequently species-specific and/or population-specific and could decrease the risk of genetic introgression of wild populations from introduced or escaped zootechnical individuals. The Mediterranean brown trout (Salmo cettii) is threatened by genetic introgression with strains of domestic brown trout (Salmo trutta) that were introduced for recreational purposes. The aim of our study was to test if native S. cettii females, rather than zootechnical S. trutta, produce OF with a greater ability to upregulate the sperm motility of conspecific males. Thus, we compared the sperm swimming performances of males inhabiting the Biferno River (Molise region—Southern Italy) activated in native S. cettii vs. zootechnical S. trutta female’s OFs. In our study, native females’ OFs (20% diluted), compared to spring water, has the ability to significantly boost the sperm performance of the autochthonous males, while zootechnical S. trutta fails. These preliminary results suggest that OF-sperm interactions could potentially influence or direct the hybridization mechanisms involving the native Mediterranean trout inhabiting the Biferno River and the domestic lineage of brown trout introduced in the past.
Key Contribution:
During natural reproduction, the aqueous environment becomes hostile, decreasing the chances of fertilization success. Ovarian fluid promotes close contact between gametes, creating a stabilized fertilization microenvironment. Ovarian fluids of native S. cettii females improve sperm swimming performance of conspecific males compared to spring water, whilst ovarian fluids of zootechnical S. trutta fail.
1. Introduction
During natural reproduction, a common feature of all externally fertilizing freshwater fish is that they expel their gametes into the aqueous environment. Although the sperms need the water to activate their motility [], the aqueous environment is hostile and decreases the chances of fertilization success, due to its peculiar physical (turbulent flow, temperature variation) and chemical properties [,,,,]. Thus, specific mechanisms guiding sperm may have evolved to maximize the sperm–egg encounter under extreme environmental conditions [,,,]. In particular, after sperm ejaculation, OF promotes close contact between gametes, reducing their dispersion and creating a stabilized fertilization microenvironment [,]. Several studies have shown that ovarian fluid could significantly affect the swimming performances of sperm [,,,,,,,,,,] and could consequently influence the outcome of fertilization in terms of fertilized oocytes rate in different fish species (Chinook salmon, Pacific herring, Caspian brown trout, turbot, ocellated wrasse) [,,,,,]. According to some authors, these ovarian fluid–sperm interaction mechanisms could be species-specific and population-specific [,,]. In addition to parental genotypes, the interactions can be influenced by environmental factors (i.e., density and physical–chemical water parameters) and dietary factors, as stated by Beirão et al. []. These authors showed that sperm from wild males of Atlantic Cod was negatively affected by ovarian fluids of farmed females, suggesting possible relation to nutritional deficiencies of farmed individuals. Therefore, these phenomena could potentially play an important role in rivers inhabited by native salmonids where interspecific hybridization with allochthonous species/or strains represents a main threat to local populations.
The Mediterranean brown trout (1Salmo cettii) is an endemic freshwater species of the Mediterranean area whose conservation status is currently considered “near threatened” at the European level and “critical endangered” in Italy, according to IUCN Red Lists, mainly due to habitat degradation (dam building, river straightening, local pollution) and to the genetic introgression with zootechnical species introduced for recreational purposes. The introduction of S. trutta began at the end of the 19th century and had a severe impact on native populations inhabiting the Italian Peninsula and the main Mediterranean Islands [,,,]. This phenomenon has led to the introgression of domestic genes into the native genome and, in many cases, to its complete replacement. Indeed, Splendiani et al. reported that about 60% of the native Apennine populations are severely introgressed or have been completely replaced by S. trutta, and only 10% are pure or show an introgression degree lower than 0.10 [].
In particular, native populations of Mediterranean brown trout inhabiting the Biferno and Volturno river basins (Molise region—Southern Italy) are currently the target of the conservation project LIFE Nat. Sal. Mo. This project aims to recover the genetic integrity of wild S. cettii populations through the creation of the first semen cryobank of Mediterranean trout in Europe, which is used to maximize the genetic variability of the offspring in a supportive breeding program [,,,,,]. The populations of the main courses of Molise, characterized by a distinctive migratory tactic, were the least introgressed of the entire project area despite the massive introduction of zootechnical Salmo trutta that occurred throughout the past decades []. We hypothesized that this protective effect is mainly due to the peculiar migration patterns of the native population and to adaptive selection. Similarly, Jurlina et al. [] studied Adriatic streams of the Western Balkans and observed that migratory tactics and life-history plasticity preserves the original genetic structure of locally adapted populations, suggesting that these features act as a stabilizing population mechanism that protects them from introgression by allochthonous S. trutta strains.
Furthermore, in the study area, Palombo et al. [] found discordance between nuclear (LDH-C1*) and mitochondrial (16 s) markers, showing the frequencies of foreign (maternal) mtDNA locus to be lower than expected. These anomalies are frequently driven by sex-biased asymmetries and selective introgression or by genetic drift on mtDNA []. Considering the massive supplementation of domestic strains carrying continuously allochthonous matrilinear haplotypes, we can speculate about the higher reproductive success of native females rather than the introduced ones.
In light of these considerations and previous research [,,,], the rationale of this study is to evaluate the potential ability of native S. cettii rather than zootechnical S. trutta ovarian fluids to upregulate the sperm motility parameters of native males, contributing to a potential increased fertilization chances in a flowing and turbulent environment for native females.
1Mediterranean brown trout is listed in Annex II of the Habitat Directive under the taxon Salmo macrostigma, but is currently reported as Salmo cettii in the Italian and European Red Lists and in the Member States’ conservation status assessments of the “habitats types and species of Community interest” (Art. 17). Recent genetic evidence suggests that Italian peninsular Mediterranean brown trout belong to a separate taxon named S. ghigii, limiting the name S. cettii to Sicilian trout [,,]. Although we agree with the recent observations, we still use the name S. cettii in the current paper, because Mediterranean brown trout populations are still protected by the Habitat Directive and subsequent conservation status updates under this taxon.
2. Materials and Methods
2.1. Animals
Breeders from the native Mediterranean brown trout (Salmo cettii) wild population were captured in the Biferno River (Molise region, Southern Italy—Adriatic basin) during the spawning season (January–February 2022) using electro-fishing and fixed traps. The fish were captured within LIFE Nat. Sal. Mo Project’s activities (LIFE17 NAT/IT/000547). The sampling sites coincide with a spawning area located close to the springs of the Biferno River at Bojano (CB) frequented by a native migrant population characterized by a mean introgression rate lower than 10%. We collected semen samples and OFs used in the experiment from ten wild males and five wild-tagged females genetically characterized as “native individuals” by Palombo et al. [] (Native admixture ancestry qi > 0.95). The males belonged to the 2+ and 3+ classes, with an average total length of 31.9 ± 3.2 cm (range = 25.5–36.0 cm). The females were 3+ and 5+ years-old and characterized by an average total length of 45.1 ± 4.7 cm (range = 39.0–53.5 cm).
In addition, OFs were collected from five zootechnical brown trout (S. trutta) females reared in a commercial hatchery that breeds domestic strain of S. trutta. Zootechnical S. trutta females were reared at a growth rate similar to that of wild Mediterranean trout of the Biferno River used in the experiment. S. trutta females were 4+ years-old, with an average total length of 50.2 ± 1.7 cm (range = 48.0–53.0 cm). No brood stock was sedated prior to sampling eggs or semen.
2.2. Ovarian Fluid and Sperm Collection and Analytical Measurements
Eggs were collected by an abdominal massage from five native S. cettii and five zootechnical S. trutta females. OF was separated from each egg batch directly with a syringe after egg decantation. After collection, each individual OF sample was subjected to the pH measurement using a BasiC 20 pH-meter (CRISON instruments, Barcelona, Spain). Then, OF samples were frozen because of the impossibility to collect all the sperm and ovarian fluid samples on the same day []. Moreover, OF samples were individually stored in at least five 1.5 mL microtubes in order to avoid freeze–thaw cycles.
In February, during the peak of the natural spawning season, semen samples were collected from ten native males by abdominal massage after careful cleaning of the urogenital papilla. Semen was stored on ice, transferred from the river to the laboratory and used within 4 h of collection. Sperm concentration was measured using a photometric method. Briefly, the optical density of the diluted semen in 0.9% NaCl with a ratio of 1:200 (v/v) was measured using a portable photometer DR 1900 (HACH Company, Loveland, CO, USA) at a wavelength of 530 nm. The value of the sperm concentration was extrapolated using a standard curve established previously by relating the optical density with the sperm concentration expressed as × 109 sperm/mL, following the procedure described by Nynca and Ciereszko []. Sperm viability was measured using the Muse® Cell Analyzer flow cytometer (Luminex corporation, 12212 Technology Blvd Suite 130, Austin, TX, USA) according to the manufacturer’s protocol. Semen samples were diluted in PBS to obtain concentrations in the range of 1 × 105 to 1 × 107 spermatozoa/mL. Subsequently, 20 μL of the diluted semen was mixed with 780 μL (dilution factor 1:40) of Muse Count and Viability Reagent in an Eppendorf tube (Luminex corporation 12212 Technology Blvd Suite 130, Austin, TX, USA), then incubated for 5 min at room temperature and analysed with the flow cytometry.
Nucleated cells were stained using a membrane-permeant DNA-staining dye in order to differentiate cells with a nucleus from debris and non-nucleated cells. A DNA binding Muse dye based on 7-aminoactinomycin D (7-AAD) stains cells that have lost their membrane integrity, allowing the dye to stain the nucleus of dead and dying cells. This parameter differentiates viable (live cells that do not stain) from non-viable (dead or dying cells that stain) cells. Sperm pH was determined by an electrode using a Basic 20 pH-meter.
2.3. Experimental Design
Each semen sample (N = 10) was analysed individually in the presence of OFs from five native S. cettii (NOF) and five zootechnical S. trutta females (ZOF) in a full factorial design (10 × 10). OF samples were diluted at 20% (v/v) in spring water (SW; pH = 8.1). SW was sampled at a source of the Biferno River near the spawning sites. The sperm are likely to encounter different OF concentrations when moving towards the eggs in natural environments. The OF dilution in the surrounding environment determines the physical and chemical properties of the activation media that could affect the behaviour of the sperm []. Many authors, for salmonids, have used dilution ranging from 10 to 100% OF [,,,,]. The choice of a 20% dilution rate was in accordance with Butts et al. [], which suggests that this OF dilution could represent the concentrations of OF during a natural spawning event of lake trout. Purchase and Rooke [] also used a dilution with an OF water ratio of 1:4 (20%), testing the utilization of frozen ovarian fluid to assess the OFs effect on sperm swimming performance of lake trout, brown trout and Atlantic salmon.
Sperm motility parameters recorded in 20%NOF and 20%ZOF (hereafter simply referred to as NOF and ZOF) were compared with each other and with that obtained in SW alone in order to reveal the changes that occur in swimming patterns when spermatozoa are released into the water and move to the eggs of both species. A saline activation medium (AM; pH = 8.85) consisting of 1 mM CaCl2, 20 mM Tris, 30 mM glycine and 125 mM NaCl, at pH 9.0 []), supplemented with 0.5% bovine serum albumin, was used as a control solution, in order check if the semen samples were suitable for their use in the experiment on the basis of their initial quality The experiment was conducted on five different days over the period of two weeks, dividing each day into two work sessions (morning and afternoon), one for each semen sample, collected respectively in the early morning and early afternoon (5 days × 2 males). Each day one microtube of OF from each female was thawed at 4 °C for 2 h and then kept on ice for the duration of the analysis.
2.4. Sperm Motility Analysis
Sperm motility analysis was performed using a Computer-Assisted Sperm Analysis (CASA) system coupled with a phase contrast microscope (Nikon model Ci-L negative contrast, Firenze, Italy) employing the Sperm Class Analyzer (SCA) software (VET Edition, Barcelona, Spain). An aliquot of 1 µL of semen from each male was activated in 300 µL of OF from each female in a randomized order, SW or AM (activation solutions), carrying out two replicate sperm-activations for each treatment combination (10 males × 12 activation solutions × 2 sperm-activations replicates = 240 observations). After rapid mixing, 0.7 µL of each dilution was immediately placed into a well (diameter 5 mm) of a 12-well multi-test glass slide (TEKDON Inc., Myakka City, FL, USA) and covered with a coverslip. Sperm motility parameters were analysed 10 s post-activation using a 25 fps rate during recording. The following sperm traits were evaluated: motile spermatozoa (MOT, %), curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s), average path velocity (VAP, µm/s), linearity (LIN, %), straightness (STR, %), beat cross frequency (BCF, Hz) and amplitude of lateral displacement of the spermatozoon head (ALH, µm).
The duration of sperm movement (DSM) was chronometer and calculated as the time from sperm activation until movement cessation of spermatozoa in the field of view. We consider spermatozoa vibration as a parameter to establish the cessation of movement.
The measurements took place in a lab with temperatures set at 17–18 °C. The loading of chambers and the recording of motility were both carried out by the same operator, taking care to standardize the operation timing from ice to observation. Although higher room temperature compared to the natural environment (10–12 °C) may have reasonably affected the duration of motility and velocity of spermatozoa, as reviewed by Dadras et al. []. This bias will have affected all measurements equally.
2.5. Statistical Analysis
All statistical analyses were performed using the statistical software R (Version 4.2.0, R Core Team, Vienna, Austraia), at significance levels of p ≤ 0.05. Replicates of each cross were treated as repeated measures, using its mean for statistical analysis to meet the independency of observations assumption. MOT (%) measurements were arcsine transformed prior to analysis.
The differences among treatments for all tested sperm motility parameters were analysed using Mixed Model ANOVAs with the activation medium as a fixed factor and the male’s identity (ID) as a random factor, followed by Tukey’s post hoc test for multiple comparisons between the groups (lme4, lmerTest and multcomp packages). The AIC test was used to compare different models (with or without males’ random effect) and determine which one was the best fit for the data (lmerTest package). The outliers were detected using the boxplot()$out function. Normality and homoscedasticity were tested by visual inspection of the residuals’ graphs, Shapiro–Wilk test and Levene’s test (rstatix and car packages). After transformation for normality, the model with MOT showed no homogeneity of variance (Levene’s test: df = 2, F = 13.7, p < 0.05). Then, MOT was analysed through GLMMs setting the Gaussian family (lme4 package).
Three-way ANOVAs were conducted, setting as fixed factors NOF vs. ZOF, males ID and females ID, to test the main effect of the males and females used in the experiment (stats package).
To check if the individual addictive effect of males in OFs correlated with the VCL’s control values registered in AM, we tested the correlation using the cor.test() function, setting the Pearson’s method.
The graphics were generated by the ggplot2 package.
3. Results
3.1. Analytical Measurements of Semen and Ovarian Fluid
All sperm motility parameters recorded in AM to check the sperm’s initial quality are reported in the supplementary material (Table S1). The average sperm concentration and viability were 15.68 ± 7.19 × 109 sperm/mL and 94.69 ± 2.67% respectively. The average semen pH measured was 7.91 ± 0.19, while for native and zootechnical OFs it was 8.36 ± 0.09 and 8.56 ± 0.19, respectively. The pH values of each semen and OF sample were reported in the supplementary material (Table S2).
3.2. Comparison between Spring Water, ZOF and NOF as Activation Media
The random effect of males explained a significant proportion of variance for all tested parameters. Then, using Mixed Models with males’ random effect, we registered a significant increment of MOT, VCL, VAP, VSL and DSM in NOF compared to SW alone. In contrast, ZOF failed to significantly boost the swimming performances for the motility parameters reported above (Figure 1). For STR, LIN and ALH, we found significant differences among all groups. No significant difference between NOF and ZOF was found for BCF.
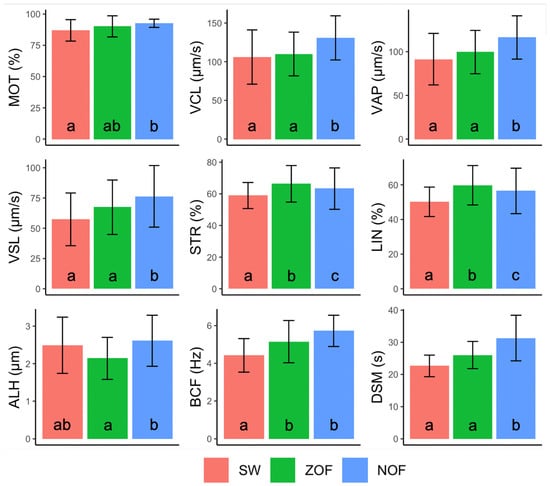
Figure 1.
Barplots displaying the variability among treatments (spring water—SW; 20% native ovarian fluid—NOF; 20% zootechnical ovarian fluid—ZOF) for each sperm motility parameter, presented as Means ± SD (observations number in: SW= 10; NOF = 50; ZOF = 50). Error bars represent standard deviation. Different letters show significant differences among treatments.
3.3. Exploring the Effects of Specific Males and Females Used in the Experiment
The three-way ANOVAs show significant differences between NOF and ZOF for each kinetic parameter (Table 1). All motility parameters except STR and LIN are higher (p < 0.05) in NOF than in ZOF. Further, the analysis shows a significant male effect on all sperm motility parameters, while the female effect was significant only for VCL, VAP and STR (Table 1).

Table 1.
Significant levels of the fixed effects of male ID, female ID and the effect of belonging of female to the native or zootechnical group (N-Z group) for all sperm traits.
The individual addictive effect of males—and the related inter-male variability—was correlated to the values registered with activation in AM (Figure 2), in accordance with the use of our activation medium as a proxy for the evaluation of semen quality. According to these findings, inter-male variability and female origin represent the main effects that explain most of the observed variability in our data.
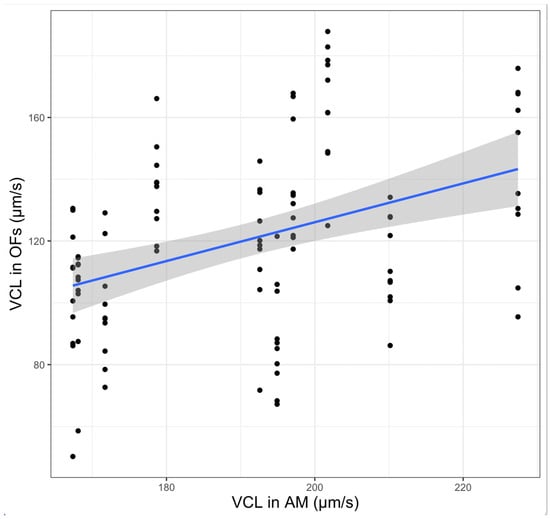
Figure 2.
The VCL (curvilinear velocity) values registered in all OFs (native and zootechnical) are significantly correlated to those registered in AM (p < 0.05). The blue line represents the fitted linear regression and the dark shadow shows the 95% confidence interval band.
4. Discussion
In this study, we showed that ovarian fluids of native S. cettii females have a significant enhancing effect on sperm swimming parameters of native wild males compared to SW alone, while zootechnical S. trutta OFs fail.
Interestingly, the presence of NOF, rather than ZOF, induced a significant increase of VCL, VAP, VSL and ALH parameters and of DSM (longevity) compared to SW. The variation in sperm performances observed in our study within each group (NOF and ZOF) was mainly explained by the inter-male variability effect (male ID). Consistent with other similar studies [,,,], some males produce sperm with intrinsically superior motility features than others, resulting in better performances overall in the OFs. On the other hand, the effect of female ID was significant only for some parameters, such as VCL, VAP and STR, suggesting that some females produce OF with a higher capability to increase the sperm traits of males.
The differences that were observed in sperm motility patterns between groups NOF and ZOF could affect the fertilization success in relation to the female origin. It is known that, in fish with external fertilization, the sperm reproductive success could be strongly influenced by hostile environmental conditions, i.e., turbulent aqueous medium [,,] and by the spermatozoa’s ability to rapidly find the micropyle within the short time available []. The VCL seems to be an essential prerequisite for sperm to rapidly swim around the egg to find the micropylar canal and fertilize the eggs [,,]. Moreover, several authors reported the existence of a positive correlation between fertilization rate and some sperm motility parameters (VCL and longevity) in fish, including salmonids [,,,,]. The native ovarian fluid ability to upregulate the curvilinear velocity and the duration of progressive movement could promote the encounter between gametes in such unfavourable environments, thus increasing the fertilization rate. The zootechnical S. trutta OFs inability to increase the swimming performance of sperm compared to SW could lead to less efficient fertilization of the deposed eggs batch, potentially reducing the number of hybrid offspring. In conclusion, our results suggest that the native S. cettii females, rather than zootechnical S. trutta, have a superior ability to boost the sperm motility of Mediterranean trout males, as a potential fine-adapted mechanism to favour the egg-sperm encounter. However, to fully confirm the fertilization outcomes, it would be necessary to test the in vivo fertilization, reproducing the same critical natural conditions as the flowing water, turbulences and gamete’s expulsion dynamics. Conventional fertilization trials could easily lead to erroneous conclusions, suggesting that the static fertilization environment of artificial reproduction is very different from the challenging, natural one. Furthermore, a full-factorial experimental design involving 10 females × 10 males × 2 replicates scheme (n. of observations = 200) allowed us to make our main result robust, but it completely consumes the limited volumes of semen and OFs collected from the breeders involved in the experiment. Thus, the samples were not sufficient to conduct further analyses on semen and OFs composition. That could explain the found differences between ZOF and NOF regarding their boosting ability.
Therefore, in order to explore the nature of compatibility interaction between male and female gametes, we are planning to test how these interactions are affected by the chemical-physical and biological parameters of both semen and OFs. Further ionic composition and proteomic analysis could help to resolve the nature of the gametes compatibility mechanisms observed in our study.
5. Conclusions
The main outcomes of this preliminary study showed that, compared to spring water, NOF enhanced the sperm swimming performance of native males, whilst ZOF failed. A potential ovarian fluid-driven recognition mechanism might have evolved to increase the fertilization success in native populations of Mediterranean brown trout. Further studies are planned to elucidate the causes behind it.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8040190/s1, Table S1: Sperm motility parameters of Mediterranean trout (N = 10) activated in artificial medium (AM); Table S2: Seminal plasma and ovarian fluid pH measured for each male and female.
Author Contributions
Conceptualization, N.I., G.R. and S.E.; Data curation, N.I., S.E., M.D.I., V.P. and G.R.; Formal analysis, G.R., M.D.I., V.P. and S.E.; Methodology, G.R., M.D.I., P.G. and E.A.; Writing—original draft, G.R. and S.E.; Writing—review & editing, A.R., N.I. and M.D.I.; Funding Acquisition, N.I. and S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the LIFE Nat.Sal.Mo. project (LIFE17 NAT/IT/000547).


Institutional Review Board Statement
The experiments were conducted in accordance with the Code of Ethics of the EU Directive 2010/63/EU for animal experiments. This study is part of a Nat. Sal. Mo LIFE project that received “a positive opinion” from the Ministry of the Environment and the Protection of the Territory and the Sea. The sampling and handling of fish followed animal welfare practices as reported in the Ministerial Protocol (ISPRA). All experiments were carried out with the appropriate authorizations from the Molise Region–Dipartimento Governo del Territorio, Mobilità e Risorse Naturali cod. DP.A4.02.4N.01 (protocol number 3969, 3 August 2018), according to the current regulations on the protection of the species, biosecurity, protocols of sampling of fresh water and animal welfare.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors thank Amber Burchell for the English language revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morisawa, M. Initiation mechanism of sperm motility at spawning in teleosts. Zool. Sci. 1985, 2, 605–615. [Google Scholar]
- Billard, R.; Bry, C.; Gillet, C. Stress, environment and reproduction in teleost fish. In Stress and Fish; Pickering, A.D., Ed.; Academic Press: London, UK, 1981; pp. 185–208. [Google Scholar]
- Petersen, C.W.; Warner, R.R.; Shapiro, D.Y.; Marconato, A. Components of fertilization success in the bluehead wrasse, Thalassoma bifasciatum. Behav. Ecol. 2001, 12, 237–245. [Google Scholar] [CrossRef]
- Hoysak, D.J.; Liley, N.R. Fertilization dynamics in sockeye salmon and a comparison of 450 sperm from alternative male phenotypes. J. Fish Biol. 2001, 58, 1286–1300. [Google Scholar] [CrossRef]
- Liao, W.B.; Huang, Y.; Zeng, Y.; Zhong, M.J.; Luo, Y.; Lüpold, S. Ejaculate evolution in external fertilizers: Influenced by sperm competition or sperm limitation? Evolution 2018, 72, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Kholodnyy, V.; Gadêlha, H.; Cosson, J.; Boryshpolets, S. How do freshwater fish sperm find the egg? The physicochemical factors guiding the gamete encounters of externally fertilizing freshwater fish. Rev. Aquac. 2020, 12, 1165–1192. [Google Scholar] [CrossRef]
- Lahnsteiner, F. The influence of ovarian fluid on the gamete physiology in the Salmonidae. Fish Physiol. Biochem. 2002, 27, 49–59. [Google Scholar] [CrossRef]
- Elofsson, H.; Van Look, K.J.W.; Sundell, K.; Sundh, H.; Borg, B. Stickleback sperm saved by salt in ovarian fluid. J. Exp. Biol. 2006, 209, 4230–4237. [Google Scholar] [CrossRef]
- Lehnert, S.J.; Butts, I.A.E.; Flannery, E.W.; Peters, K.M.; Heath, D.D.; Pitcher, T.E. Effects of ovarian fluid and genetic differences on sperm performance and fertilization success of alternative reproductive tactics in Chinook salmon. J. Evol. Biol. 2017, 30, 1236–1245. [Google Scholar] [CrossRef]
- Rosengrave, P.; Taylor, H.; Montgomerie, R.; Metcalf, V.; McBride, K.; Gemmell, N.J. Chemical composition of seminal and ovarian fluids of chinook salmon (Oncorhynchus tshawytscha) and their effects on sperm motility traits. Comp. Biochem. Physiol. 2009, 152, 123–129. [Google Scholar] [CrossRef]
- Turner, E.; Montgomerie, R. Ovarian fluid enhances sperm movement in Arctic charr. J. Fish Biol. 2002, 60, 1570–1579. [Google Scholar] [CrossRef]
- Urbach, D.; Folstad, I.; Rudolfsen, G. Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 2005, 57, 438–444. [Google Scholar] [CrossRef]
- Dietrich, G.J.; Wojtczak, M.; Słowińska, M.; Dobosz, S.; Kuźmiński, H.; Ciereszko, A. Effects of ovarian fluid on motility characteristics of rainbow trout (Oncorhynchus mykiss Walbaum) spermatozoa. J. Appl. Ichthyol. 2008, 24, 503–507. [Google Scholar] [CrossRef]
- Diogo, P.; Soares, F.; Dinis, M.T.; Cabrita, E. The influence of ovarian fluid on Solea senegalensis sperm motility. J. Appl. Ichthyol. 2010, 26, 690–695. [Google Scholar] [CrossRef]
- Galvano, P.M.; Johnson, K.; Wilson, C.C.; Pitcher, T.E.; Butts, I.A. Ovarian fluid influences sperm performance in lake trout, Salvelinus namaycush. Reprod. Biol. 2013, 13, 172–175. [Google Scholar] [CrossRef]
- Butts, I.A.E.; Johnson, K.; Wilson, C.C.; Pitcher, T.E. Ovarian fluid enhances sperm velocity based on relatedness in lake trout, Salvelinus namaycush. Theriogenology 2012, 78, 2105–2109. [Google Scholar] [CrossRef]
- Butts, I.A.E.; Prokopchuk, G.; Kašpar, V.; Cosson, J.; Pitcher, T.E. Ovarian fluid impacts flagellar beating and biomechanical metrics of sperm between alternative reproductive tactics. J. Exp. Biol. 2017, 220, 2210–2217. [Google Scholar] [CrossRef]
- Poli, F.; Immler, S.; Gasparini, C. Effects of ovarian fluid on sperm traits and its implications for cryptic female choice in zebrafish. Behav. Ecol. 2019, 30, 1298–1305. [Google Scholar] [CrossRef]
- Rosengrave, P.; Gemmell, N.J.; Metcalf, V.; McBride, K.; Montgomerie, R. A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 2008, 19, 1178–1185. [Google Scholar] [CrossRef]
- Beirão, J.; Purchase, C.F.; Wringe, B.F.; Fleming, I.A. Wild Atlantic cod sperm motility is negatively affected by ovarian fluid of farmed females. Aquac. Environ. Interact. 2014, 5, 61–70. [Google Scholar] [CrossRef]
- Rosengrave, P.; Montgomerie, R.; Gemmell, N. Cryptic female choice enhances fertilization success and embryo survival in chinook salmon. Proc. R. Soc. B 2016, 283, 20160001. [Google Scholar] [CrossRef]
- Cherr, G.N.; Morisawa, M.; Vines, C.A.; Yoshida, K.; Smith, E.H.; Matsubara, T.; Pillai, M.C.; Griffin, F.J.; Yanagimachi, R. Two egg-derived molecules in sperm motility initiation and fertilization in the Pacific herring (Clupea pallasi). Int. J. Dev. Biol. 2008, 52, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Hatef, A.; Niksirat, H.; Alavi, S.M. Composition of ovarian fluid in endangered Caspian brown trout, Salmo trutta caspius, and its effects on spermatozoa motility and fertilizing ability compared to freshwater and a saline medium. Fish Physiol. Biochem. 2009, 35, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.D.; Niu, H.X.; Meng, Z.; Liu, X.F.; Lei, J.L. Biochemical composition of the ovarian fluid and its effects on the fertilization capacity of turbot Scophthalmus maximus during the spawning season. J. Fish Biol. 2015, 86, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, S.H.; Stiver, K.A.; Marsh-Rollo, S.E. Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat. Commun. 2016, 16, 12452. [Google Scholar] [CrossRef]
- Yeates, S.E.; Diamond, S.E.; Einum, S.; Emerson, B.C.; Holt, W.V.; Gage, M.J. Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming. Behav. Evol. 2013, 67, 3523–3536. [Google Scholar] [CrossRef]
- Beirão, J.; Purchase, C.F.; Wringe, B.F.; Fleming, I.A. Inter-population ovarian fluid variation differentially modulates sperm motility in Atlantic cod Gadus morhua. J. Fish Biol. 2015, 87, 54–68. [Google Scholar] [CrossRef]
- Zadmajid, V.; Myers, J.N.; Sørensen, S.R.; Butts, I.A.E. Ovarian fluid and its impacts on spermatozoa performance in fish: A review. Theriogenology 2019, 1, 144–152. [Google Scholar] [CrossRef]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Pesaresi, S.; Occhipinti, G.; Fioravanti, T.; Lorenzoni, M.; Nisi Cerioni, P.; Caputo Barucchi, V. Alien brown trout invasion of the Italian peninsula: The role of geological, climate and anthropogenic factors. Biol. Invasions 2016, 18, 2029–2044. [Google Scholar] [CrossRef]
- Berrebi, P. Three brown trout Salmo trutta lineages in Corsica described through allozyme variation. J. Fish Biol. 2015, 86, 60–73. [Google Scholar] [CrossRef]
- Sabatini, A.; Cannas, R.; Follesa, M.C.; Palmas, F.; Manunza, A.; Matta, G.; Pendugiu, A.A.; Serra, P.; Cau, A. Genetic characterization and artificial reproduction attempt of endemic Sardinian trout Salmo trutta L., 1758 (Osteichthyes, Salmonidae): Experiences in captivity. Ital. J. Zool. 2011, 78, 20–26. [Google Scholar] [CrossRef]
- Splendiani, A.; Fioravanti, T.; Giovannotti, M.; Olivieri, L.; Ruggeri, P.; Nisi Cerioni, P.; Vanni, S.; Enrichetti, F.; Caputo Barucchi, V. Museum samples could help to reconstruct the original distribution of Salmo trutta complex in Italy. J. Fish Biol. 2017, 90, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Esposito, S.; Rusco, G.; Roncarati, A.; Miranda, M.; Gibertoni, P.P.; Cerolini, S.; Iaffaldano, N. Semen cryopreservation for the Mediterranean brown trout of the Biferno River (Molise-Italy): Comparative study on the effects of basic extenders and cryoprotectants. Sci. Rep. 2019, 9, 9703. [Google Scholar] [CrossRef] [PubMed]
- Rusco, G.; Di Iorio, M.; Gibertoni, P.P.; Esposito, S.; Penserini, M.; Roncarati, A.; Cerolini, S.; Iaffaldano, N. Optimization of Sperm Cryopreservation Protocol for Mediterranean Brown Trout: A Comparative Study of Non-Permeating Cryoprotectants and Thawing Rates In Vitro and In Vivo. Animals 2019, 9, 304. [Google Scholar] [CrossRef]
- Rusco, G.; Di Iorio, M.; Iampietro, R.; Esposito, S.; Gibertoni, P.P.; Penserini, M.; Roncarati, A.; Iaffaldano, N. A Simple and Efficient Semen Cryopreservation Method to Increase the Genetic Variability of Endangered Mediterranean Brown Trout Inhabiting Molise Rivers. Animals 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Rusco, G.; Di Iorio, M.; Iampietro, R.; Roncarati, A.; Esposito, S.; Iaffaldano, N. Cryobank of Mediterranean Brown Trout Semen: Evaluation of the Use of Frozen Semen up to Six Hours Post-Collection. Fishes 2021, 6, 26. [Google Scholar] [CrossRef]
- Ferguson, A.; Reed, T.E.; Cross, T.F.; McGinnity, P.; Prodöhl, P.A. Anadromy, potamodromy and residency in brown trout Salmo trutta: The role of genes and the environment. J. Fish Biol. 2019, 95, 692–718. [Google Scholar] [CrossRef]
- Di Iorio, M.; Rusco, G.; Esposito, S.; D′Andrea, M.; Roncarati, A.; Iaffaldano, N. The role of semen cryobanks for protecting endangered native salmonids: Advantages and perspectives as outlined by the LIFE Nat.Sal.Mo. project on Mediterranean brown trout (Molise region-Italy). Front. Mar. Sci. 2023, 9, 1075498. [Google Scholar] [CrossRef]
- Palombo, V.; De Zio, E.; Salvatore, G.; Esposito, S.; Iaffaldano, N.; D′Andrea, M. Genotyping of Two Mediterranean Trout Populations in Central-Southern Italy for Conservation Purposes Using a Rainbow-Trout-Derived SNP Array. Animals 2021, 11, 1803. [Google Scholar] [CrossRef]
- Škraba Jurlina, D.; Marić, A.; Mrdak, D.; Kanjuh, T.; Špelić, I.; Nikolić, V.; Piria, M.; Simonović, P. Alternative life-history in native trout (Salmo spp.) suppresses the invasive effect of alien trout strains introduced into streams in the Western part of the Balkans. Front. Ecol. Evol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Toews, D.P.; Brelsford, A. The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 2012, 21, 3907–3930. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Carosi, A.; Giovannotti, M.; La Porta, G.; Splendiani, A.; Barucchi, V.C. Ecology and conservation of the Mediterranean trout in the central Apennines (Italy). J. Limnol. 2019, 78. [Google Scholar] [CrossRef]
- D′Agaro, E.; Gibertoni, P.; Marroni, F.; Messina, M.; Tibaldi, E.; Esposito, S. Genetic and Phenotypic Characteristics of the Salmo trutta Complex in Italy. Appl. Sci. 2022, 12, 3219. [Google Scholar] [CrossRef]
- Polgar, G.; Iaia, M.; Righi, T.; Volta, P. The Italian Alpine and Subalpine trouts: Taxonomy, Evolution, and Conservation. Biology 2022, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Purchase, C.F.; Rooke, A.C. Freezing ovarian fluid does not alter how it affects fish sperm swimming performance: Creating a cryptic female choice ‘spice rack’ for use in split-ejaculate experimentation. J. Fish Biol. 2020, 96, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Nynca, J.; Ciereszko, A. Measurement of concentration and viability of brook trout (Salvelinus fontinalis) spermatozoa using computer-aided fluorescent microscopy. Aquacilture 2009, 292, 256–258. [Google Scholar] [CrossRef]
- Billard, R. Reproduction in rainbow trout: Sex differentiation, dynamics of gametogenesis, biology and preservation of gametes. Aquaculture 1992, 100, 263–298. [Google Scholar] [CrossRef]
- Dadras, H.; Dzyuba, B.; Cosson, J.; Golpour, A.; Siddique, M.A.M.; Linhart, O. Effect of water temperature on the physiology of fish spermatozoon function: A brief review. Aquac. Res. 2016, 48, 729–740. [Google Scholar] [CrossRef]
- Petersen, C.W.; Warner, R.R.; Cohen, S.; Hess, H.C.; Sewell, A.T. Variable pelagic fertilization success: Implications for mate choice and spatial patterns of mating. Ecology 1992, 73, 391–401. [Google Scholar] [CrossRef]
- Petersen, C.W.; Warner, R.R. Sperm competition in fishes. In Sperm Competition and Sexual Selection; Birkhead, T.R., MØller, A.P., Eds.; Academic Press: San Jose, CA, USA, 1998; pp. 435–463. [Google Scholar]
- Kime, D.E.; Van Look, K.J.W.; McAllister, B.G.; Huyskens, G.; Rurangwa, E.; Ollevier, F. Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp. Biochem. Physiol. 2001, 130, 425–433. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Cherr, G.; Matsubara, T.; Andoh, T.; Harumi, T.; Vines, C.; Pillai, M.; Griffin, F.; Matsubara, H.; Weatherby, T. Sperm attractant in the micropyle region of fish and insect eggs. Biol. Reprod. 2013, 88, 1–11. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Harumi, T.; Matsubara, H.; Yan, W.; Yuan, S.; Hirohashi, N.; Iida, T.; Yamaha, E.; Arai, K.; Matsubara, T. Chemical and physical guidance of fish spermatozoa into the egg through the micropyle. Biol. Reprod. 2017, 96, 780–799. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Berger, B.; Weismann, T.; Patzner, R.A. Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture 1998, 163, 163–181. [Google Scholar] [CrossRef]
- Gage, M.J.G.; Macfarlane, C.P.; Yeates, S.; Ward, R.G.; Searle, J.B.; Parker, G.A. Spermatozoal traits and sperm competition in Atlantic salmon: Relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 2004, 14, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Linhart, O.; Rodina, M.; Gela, D.; Kocour, M.; Vandeputte, M. Spermatozoal competition in common carp (Cyprinus carpio): What is the primary determinant of competition success? Reprod. Colch. 2005, 130, 705–711. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).