1. Introduction
Fish has always been popular because of its high protein, low fat, and easy digestibility. Currently, half of the world’s fish production comes from aquaculture [
1]. In China, the price of wild fish is generally higher than that of farmed fish due to the general perception among consumers that wild fish are of better quality. Flesh quality, flavor, and appearance are the main judgment criteria to evaluate the quality of fresh commercial fish [
2], which has a great impact on the market price.
In order to improve the flesh quality of fish, researchers have conducted many studies. Diet, starvation, breeding environment, and season all have different degrees of influence on the nutritional index and flavor of fish muscle [
3,
4,
5,
6,
7]. In terms of the raising environment, there have been many studies that have found that wild fish have better flavor and firmer texture compared to farmed fish, in addition to differences in muscle nutrient composition [
8,
9,
10]. For example, wild sea bass (
Dicentrarchus labrax) had lower fat content, firmer flesh, richer flavored amino acids, and more polyunsaturated fatty acids than farmed sea bass [
11]. Grass carp (
Ctenopharyngodon idella) starved for 20 d in natural lakes showed reduced odoriferous volatiles and improved muscle hardness and elasticity [
12]. The above reports mainly focus on the comparison of morphology, quality, and flavor of artificially farmed and wild fish.
Silver carp (
Hypophthalmichthys molitrix) is the main freshwater aquaculture fish in China and is referred to as one of the four major Chinese carps, together with black carp (
Mylopharyngodon piceus), grass carp, and bighead carp (
Aristichthys nobilis) [
13]. As algae-feeding fish, the silver carp is often stocked in natural lakes in China and Southeast Asia as algae control fishes [
14]. Due to its low cost and eco-friendliness, silver carp is a major freshwater aquaculture target in China [
13], with Chinese silver carp accounting for 12.05% of total freshwater aquaculture production in 2021 [
15]. However, significant differences in flesh quality, texture, and flavor between artificially farmed and wild silver carps have been a constraint on the quality and market value of the farmed varieties. Investigating the causes of these differences and the effects of different farming methods on the flesh quality will contribute to improvement of the quality and market value of silver carp.
Therefore, three groups of silver carp were selected as experimental materials: pond-farmed; short-term stocked; and ranched, grown in a natural water ranch located in the Three Gorges Reservoir (TGR) of the Yangtze River. This study first examined muscle proximate composition and amino acid composition of the three groups, followed by a comparison of differences in food composition and digestive enzyme activity, and explored the effects of changes in living environment on the muscle nutrient composition of algal-feeding silver carp.
2. Materials and Methods
2.1. Experimental Design
The experiment was carried out in two water environments, one is a conventional aquaculture pond (water area 2 × 10
−2 km
2, average water depth 2 m) (N: 29°45′, E: 106°23′) and the other is a natural water ranch (water area 6 km
2, average water depth 40 m) in the TGR of Zhongxian County, Chongqing, China (N: 30°19′~30°21′, E: 108°01′~108°02′). There were three experimental groups: the first group was the farmed group, in which fish had been reared in a fish pond; the second group was the ranched group grown in the TGR water ranch; and the third group was the stocked group, in which silver carp from the same pond as the farmed group were stocked in a cage (5 m × 5 m) in a water ranch for 30 d. A total of 60 silver carp individuals were used, 20 per treatment (300–350 mm in length and 550–750 g in weight). The treatment time was 26 October to 26 November, and the pH of the aquaculture pond and the water ranch were 8.1~8.3 and 8.2~8.35, respectively; the DO were 4.3~4.65 mg/L and 6.33~6.8 mg/L, respectively; and the water temperature of both varied from 18.1 °C to 21.1 °C. The basic nutritional components of the artificial compound feed used in the farmed group are shown in
Table 1.
2.2. Animal Ethics
Handling and care of animals were conducted based on the Guiding Principles for the Care and Use of Laboratory Animals and were approved by the Committee for Laboratory Animal Experimentation at Southwest University, China on 18 July 2019 (Issue No. 2019071806).
2.3. Sample Collection
Fish in three treatment groups (n = 20) were anesthetized with 0.1 ppm MS-222. Their body weight and length were measured, they were dissected on ice, the intestine was taken for food composition analysis and digestive enzyme activity determination, and 10 g white muscles on both sides of the spine of each fish were taken in an aseptic environment for flesh quality determination. The above samples were stored at −80 °C for later use.
2.4. Experimental Methods
The determination of proximate composition was based on the method of Wu and Liu [
16,
17]. Moisture content was determined by drying at 105 °C; crude protein content was determined by the Kjeldahl method; crude fat content was determined by Soxhlet extraction; crude ash content was determined by high-temperature cautery; amino acid content was determined using a Hitachi L-8900 amino acid analyzer.
The amino acid score (AAS), chemical score (CS), and essential amino acid index (EAAI) were calculated according to the amino acid scoring criteria (%, dry) recommended by FAO/WHO (1973) [
18] and the amino acid pattern (%, dry) of egg proteins proposed by the Institute of Nutrition and Food Hygiene, Chinese Academy of Preventive Medicine [
19].
The identification of intestinal contents was conducted according to the methods of Li [
20]. The food mass was diluted and mixed at a ratio of 1:1 using 0.65% saline, and 0.1 mL was taken for identification and counting of the plankton species under a microscope. The identification of plankton species was conducted by referring to the atlas of freshwater microorganisms and benthos edited by Zhou et al. [
21]. The weight of organisms was calculated according to the biomass criteria for each type of aquatic organism described by Zhao [
22].
The activities of trypsin, lipase, and amylase were measured with relevant kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Statistical Analysis
The data were initially processed using Excel 2019 and then statistically analyzed with SPSS 26.0 software using one-way analysis of variance (ANOVA). Duncan’s multiple range test was used if a statistical evaluation comparing different group values was necessary, with p < 0.05 indicating a significant difference. Data were expressed as “mean ± standard deviation”.
4. Discussion
The results of muscle proximate composition for the three groups show that the contents of crude protein and ash were significantly higher in the ranched group and the stocked group than in the farmed group, while the differences in the content of crude fat were not significant among the three groups. The main nutrition of fish is distributed in the muscle, and the content of crude protein, fat, and ash indicate the muscle nutritional value [
23]. This indicates that the nutritional value of silver carp was better in the ranched and stocked groups than in the pond-reared group, which is consistent with the results of the comparative analysis of the muscle nutritional composition of farmed and wild bass (
Dicentrarchus labrax) [
8].
In terms of essential amino acid and umami amino acid content, the stocked group was significant closer to the ranched group, both of which were significantly higher than the farmed group. The type, quantity, and composition ratio of amino acids are important indicators of the nutritional value of protein in food [
24]. For example, as one of the essential amino acids, the most important physiological function of Lys is to participate in the synthesis of proteins, so it is closely related to animal growth [
25]. Glu, Asp, Gly, and Ala are known as umami amino acids, and the high content of them was able to improve the quality and flavor of fish muscle [
26,
27]. In addition, Buchtov et al. [
28] suggested that high levels of essential amino acids could also improve the flavor and quality of fish muscle. This suggests that the muscle amino acid composition was superior and the flavor was better in the ranched group and the stocked group than in the farmed group. In addition, the muscle amino acid composition of all three groups conformed to the FAO/WHO ideal pattern, with WEAA/WTAA around 40% and WEAA/WNEAA both greater than 60% [
29]. AAS, CS, and EAAI are commonly used indicators for evaluating the composition of essential amino acids in fish muscle [
30]. The AAS was close to or greater than 1 and the CS was greater than 0.5 in all three groups, but the EAAI was greater in the ranched group and the stocked group than in the farmed group, indicating that the muscle essential amino acid composition of the three groups was relatively balanced and rich in high-quality protein, but the ranched group and the stocked group were better than the farmed group. This is in agreement with the results for the comparative analysis of muscle nutrient composition of farmed and simulated ecologically farmed loach (
Misgurnus anguillicaudatus) [
31]. In summary, after 30 days of being stocked in a natural water ranch, the muscle quality of the farmed silver carp was improved and close to that of the wild silver carp, with a more balanced amino acid composition, richer content, and better flavor.
Silver carp is a filter-feeding fish. It feeds mainly on diatoms (Bacillariophyta) in reservoirs and rivers, while it feeds mainly on blue-green algae (Cyanophyta) in ponds [
32,
33]. In this study, the main diets of the ranched group and the stocked group were detritus and diatoms, while the main diet of the farmed group was detritus and blue-green algae. For fish, diatoms are more digestible than blue-green algae [
34] and are more nutritious and richer in essential amino acids and polyunsaturated fatty acids [
35]. Changes in the food composition due to the different rearing waters may have contributed to the differences in flesh quality among the three groups.
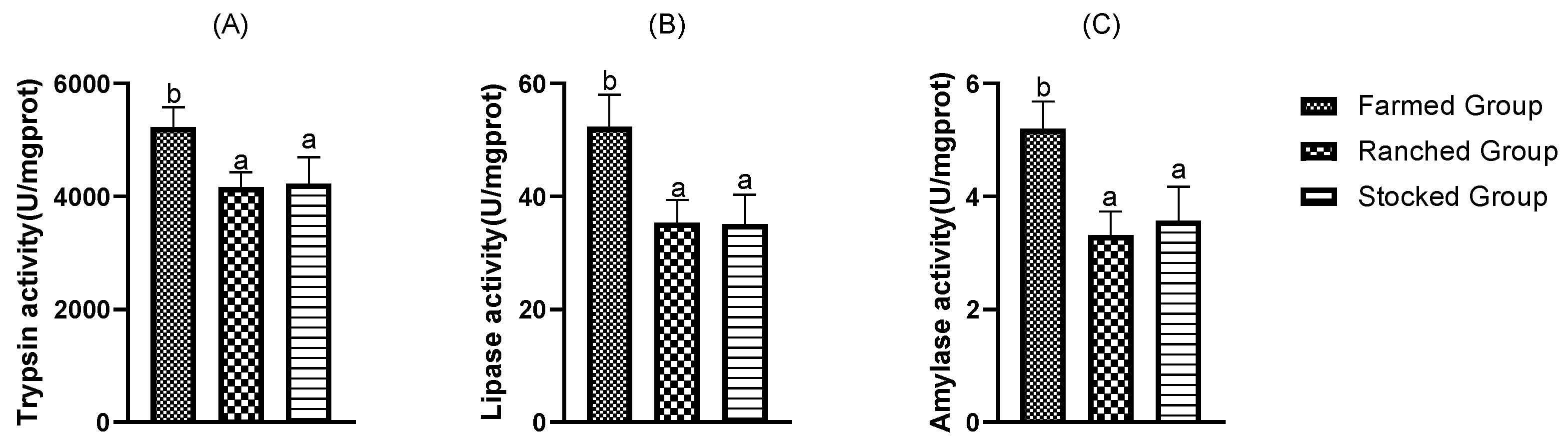
Determination of digestive enzyme activities in the three groups revealed that the lipase, amylase, and trypsin activities were significantly lower in the ranched group and the stocked group than in the farmed group, and the difference between the ranched group and the stocked group was not significant. Similar results were found in both indoor and wild tuna (
Euthynnus affinis) [
36]. Digestive enzymes are mainly secreted by the digestive glands and the digestive system plays a nutritional and digestive role; therefore, changes in digestive enzymes activity can affect the growth of fish [
37]. Changes in rearing water environment and food composition can affect digestive enzyme activity and cause changes in metabolism, thereby altering nutrients in the body [
38,
39]. This experiment conducted sampling at the end of November, when plankton was relatively unabundant in natural water and the ranched group and the stocked group were consuming less food and therefore had reduced secretion and low activity of digestive enzymes [
40]. When fish ingest less food, they consume their own stored fat and glycogen to sustain life activities, while amino acids are retained as functional substances and their levels tend to increase [
41]. It is therefore hypothesized that differences in environmental and food composition resulted in lower digestive enzyme activity in the ranched group and the stocked group. Both groups consumed their own fat and glycogen, in addition to ingestion and digestion, and they consumed more nutrient-rich diatoms, resulting in higher crude protein, ash, and amino acid content, which leads to higher flesh quality.
5. Conclusions
It has been shown that, for the same species of fish, both nutritional quality and palatability are superior to pond-cultured groups under wild and extensive water culture conditions, suggesting that we can improve the quality of fish by changing the culture environment, but the advantages of pond culture in terms of low cost and high output cannot be ignored. In this study, by stocking pond-cultured silver carp in natural waters for 30 d and comparing the changes in muscle quality of the stocked silver carp, the results showed that initially, in silver carp pond-reared through 30 days of short-term stocking in the natural ranch waters of the TGR, the basic nutrient composition and amino acid composition changed significantly, with no significant difference compared to the group grown for a longer time in ranch water, and all indexes were better than those of the farmed group. Analysis of food composition and digestive enzyme activity showed no significant differences between the ranched group and the stocked group, and significant differences for the farmed group. In summary, changes in some muscle nutrients caused by differences in environmental and food composition are responsible for the improved quality of farmed silver carp after short-term stocking in natural water. The transfer of farmed fish to natural water for a certain period can effectively improve the nutrient composition, quality, and flavor, indicating that the freshwater ranch-stocked model is a potentially viable eco-farming model, which can not only improve the muscle quality of cultured fish, but also make efficient use of reservoir resources. The present study provides a new method for improving the quality of commercial fish, especially filtering carps such as silver carp and bighead carp; exploring new eco-farming practices; and the rationally using reservoir resources to develop ecological fisheries in freshwater ranch environments.